Influence of Magnetic Field with Schumann Resonance Frequencies on Photosynthetic Light Reactions in Wheat and Pea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
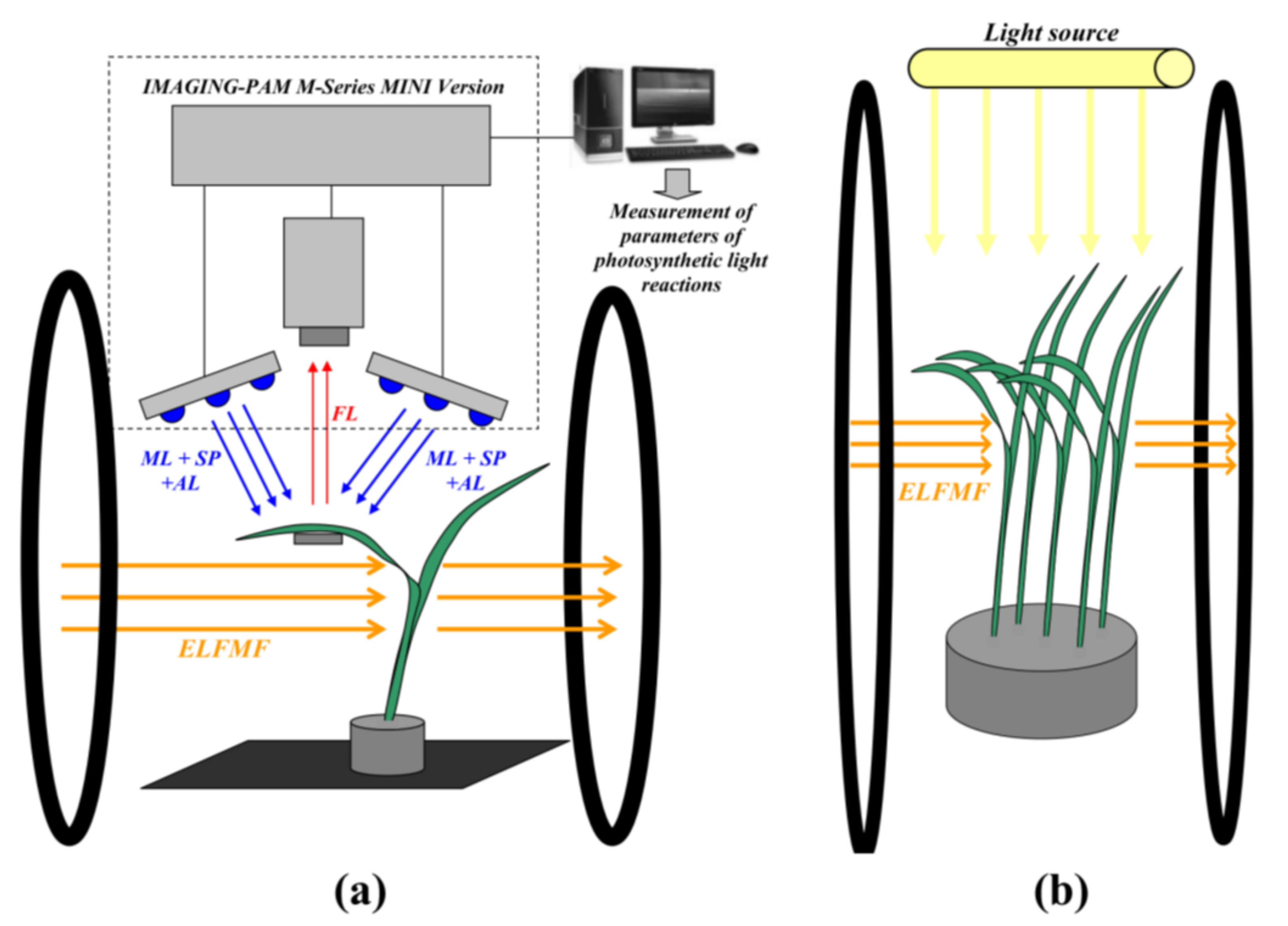
2.2. Short-Term and Chronic Treatments by Magnetic Fields with Schumann Resonance Frequencies
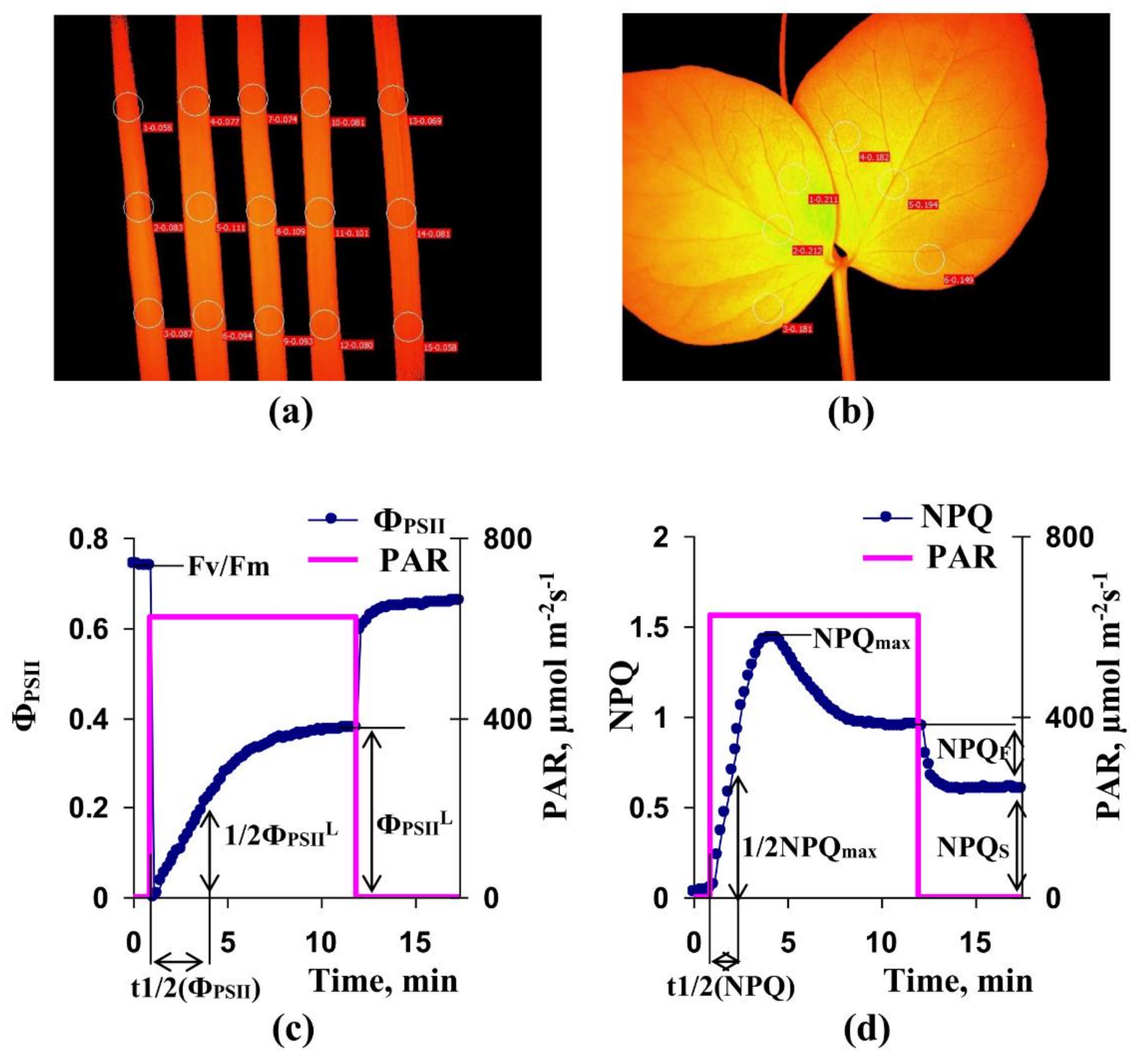
2.3. Measurements of Parameters of Photosynthetic Light Reactions
2.4. Statistics
3. Results
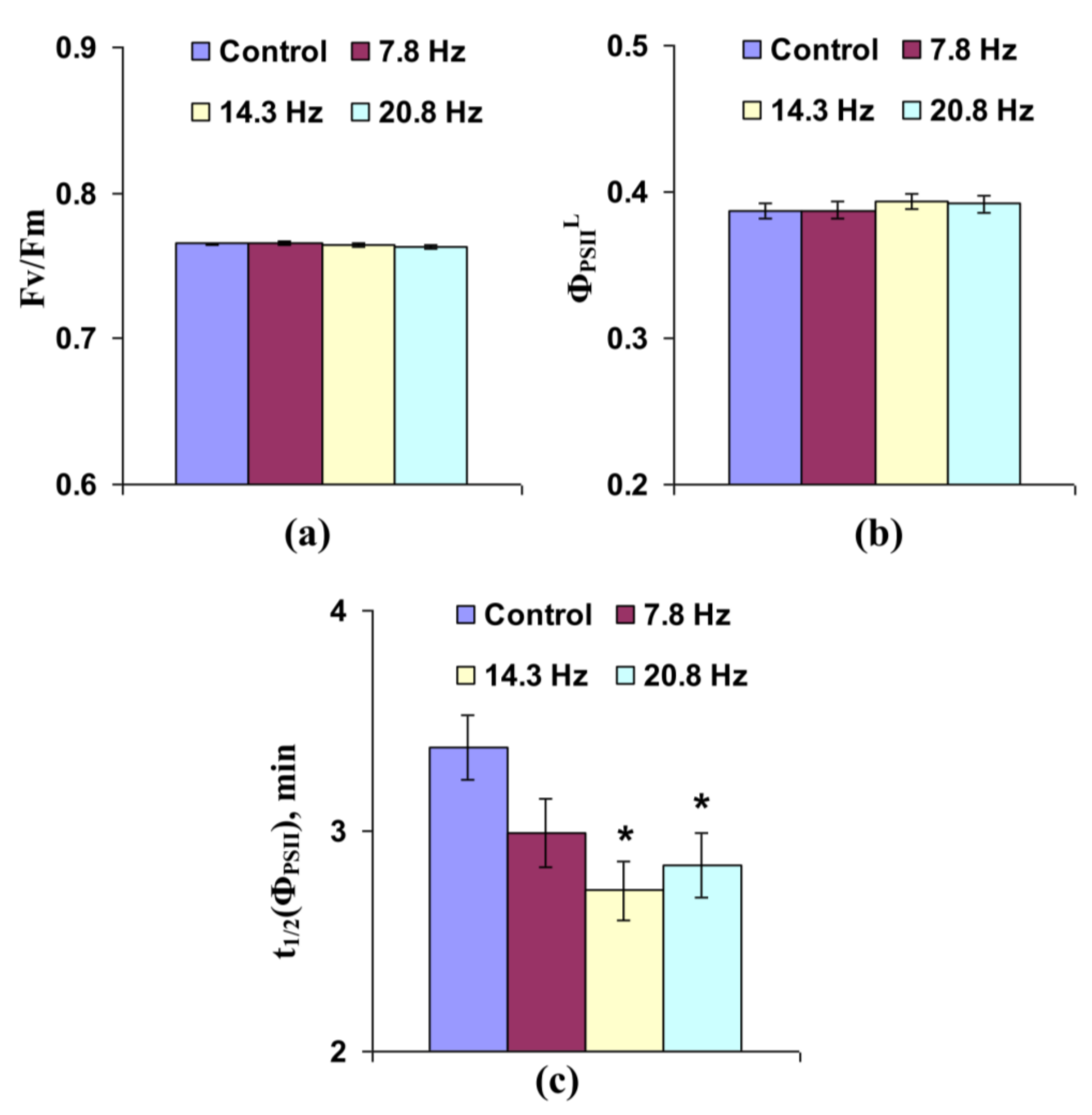
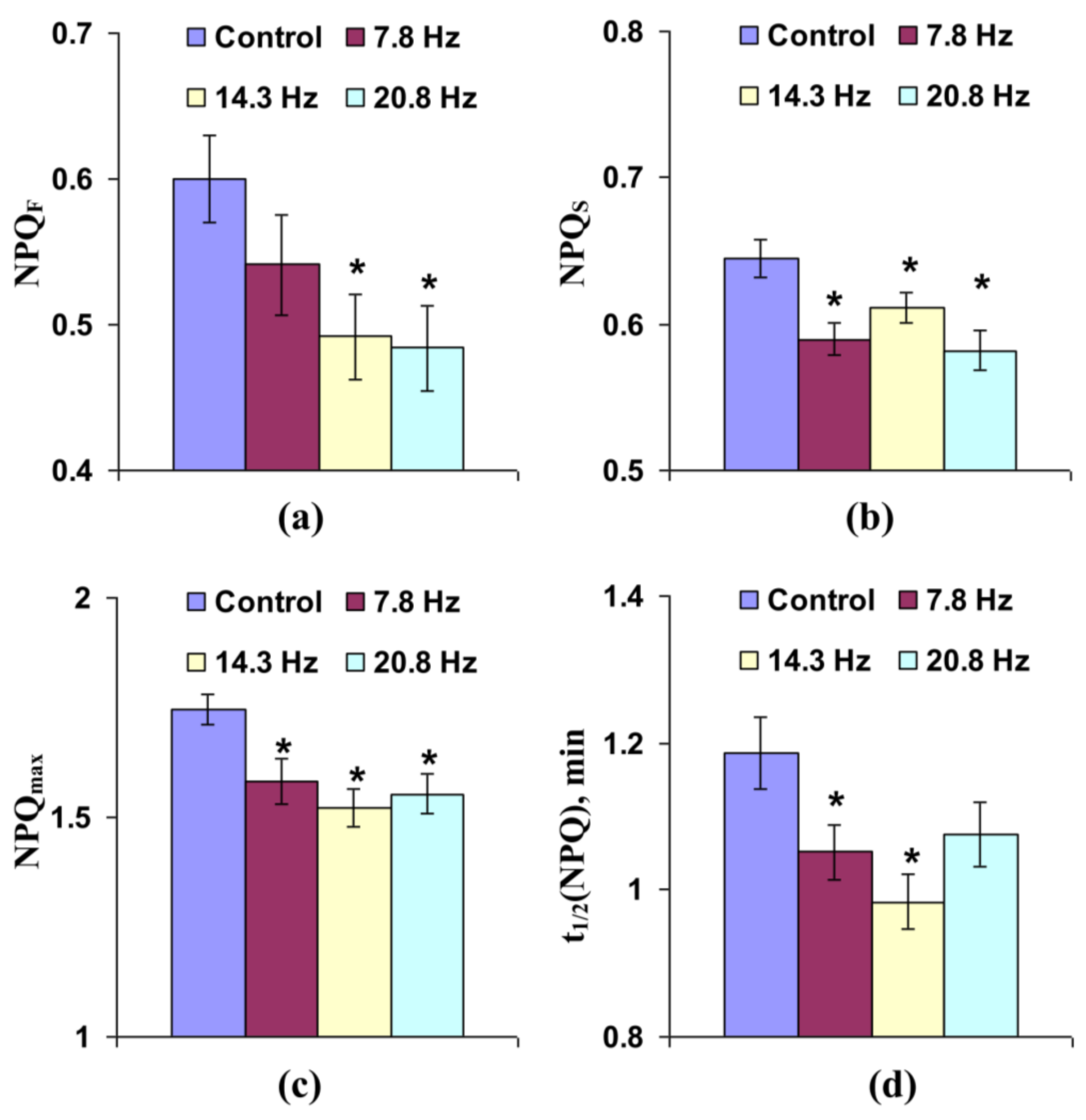
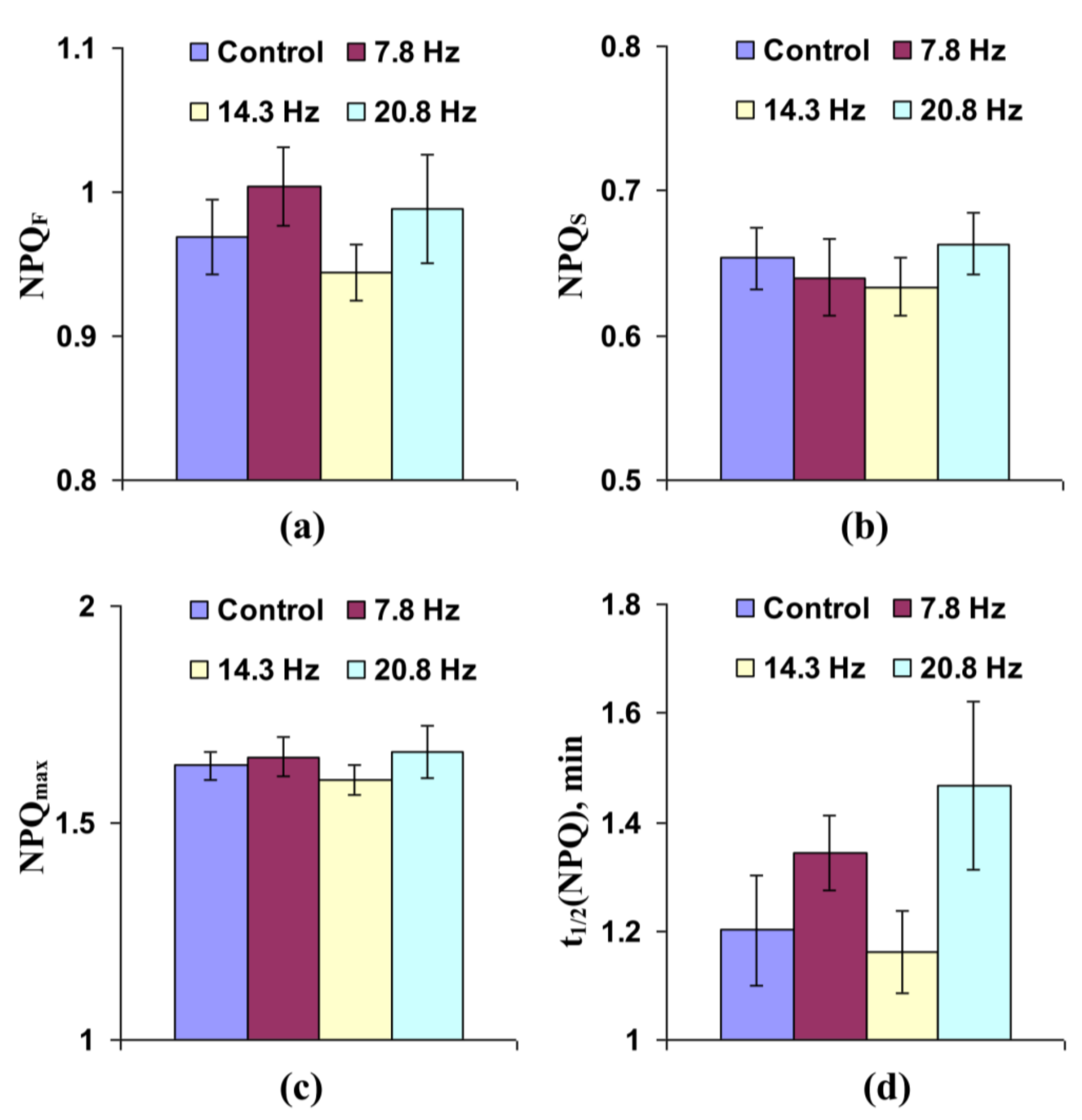
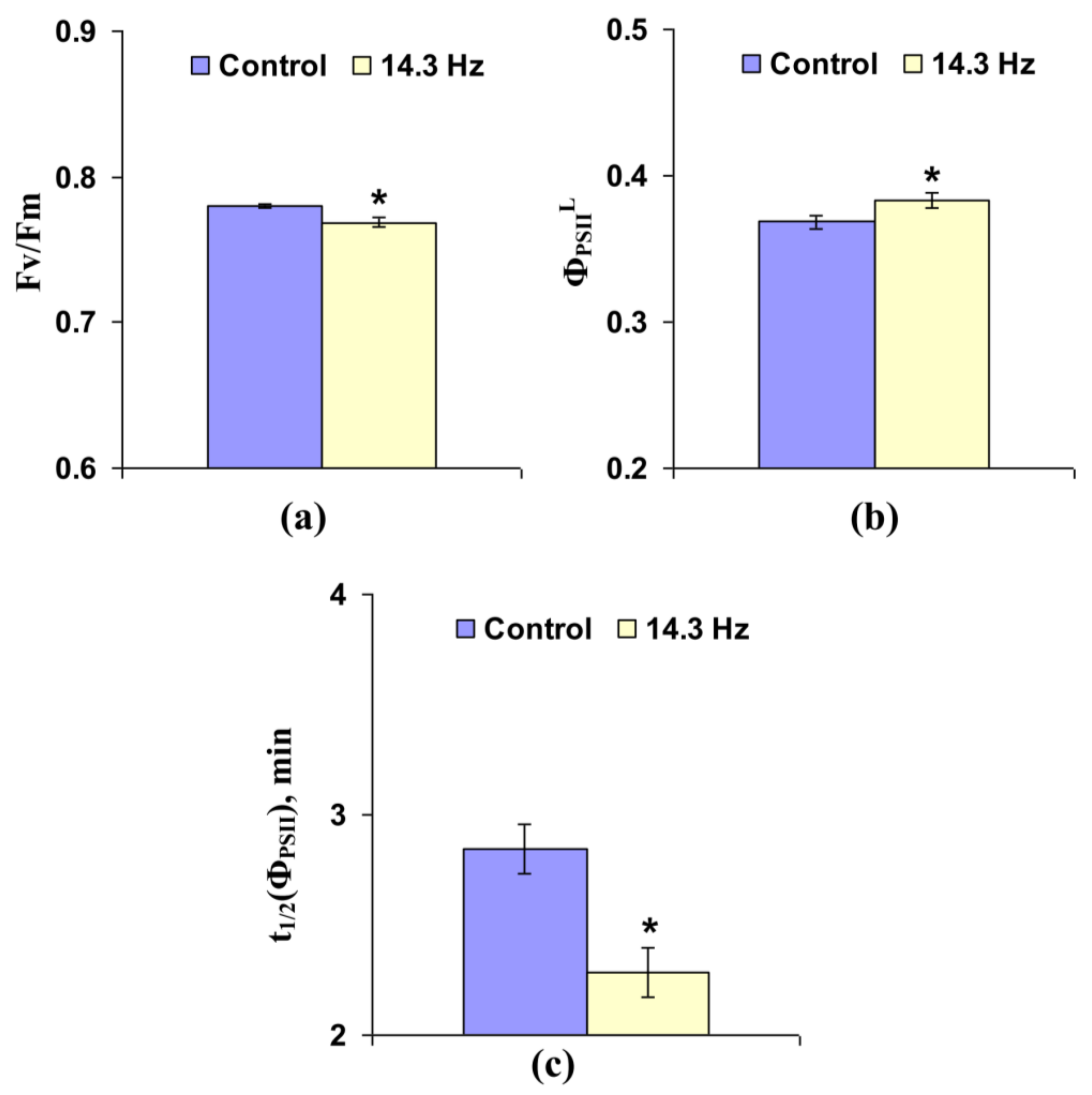
3.1. Investigation of the Influence of Short-Term Treatment by Magnetic Fields with Schumann Resonance Frequencies on Parameters of Photosynthetic Light Reactions
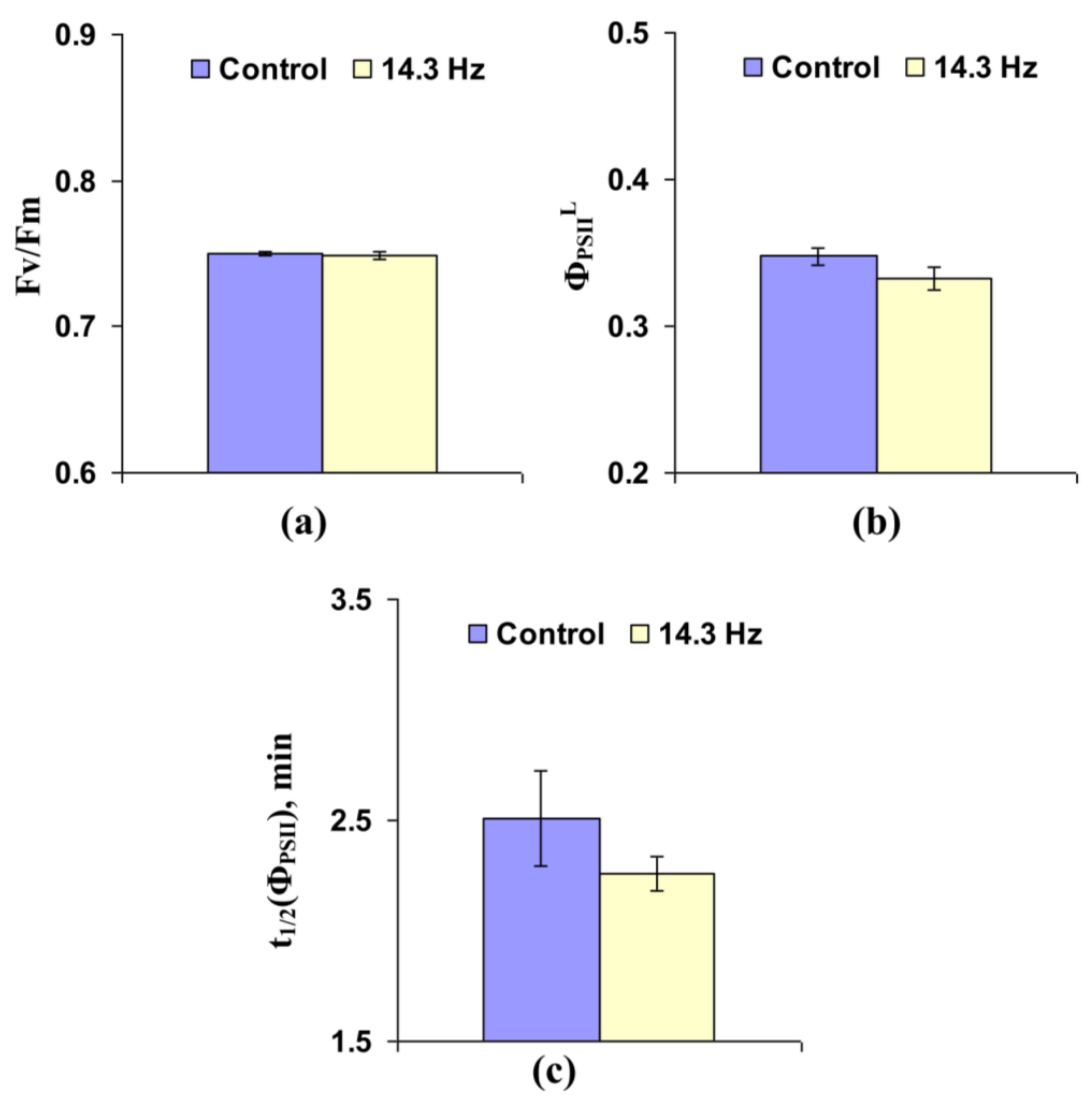
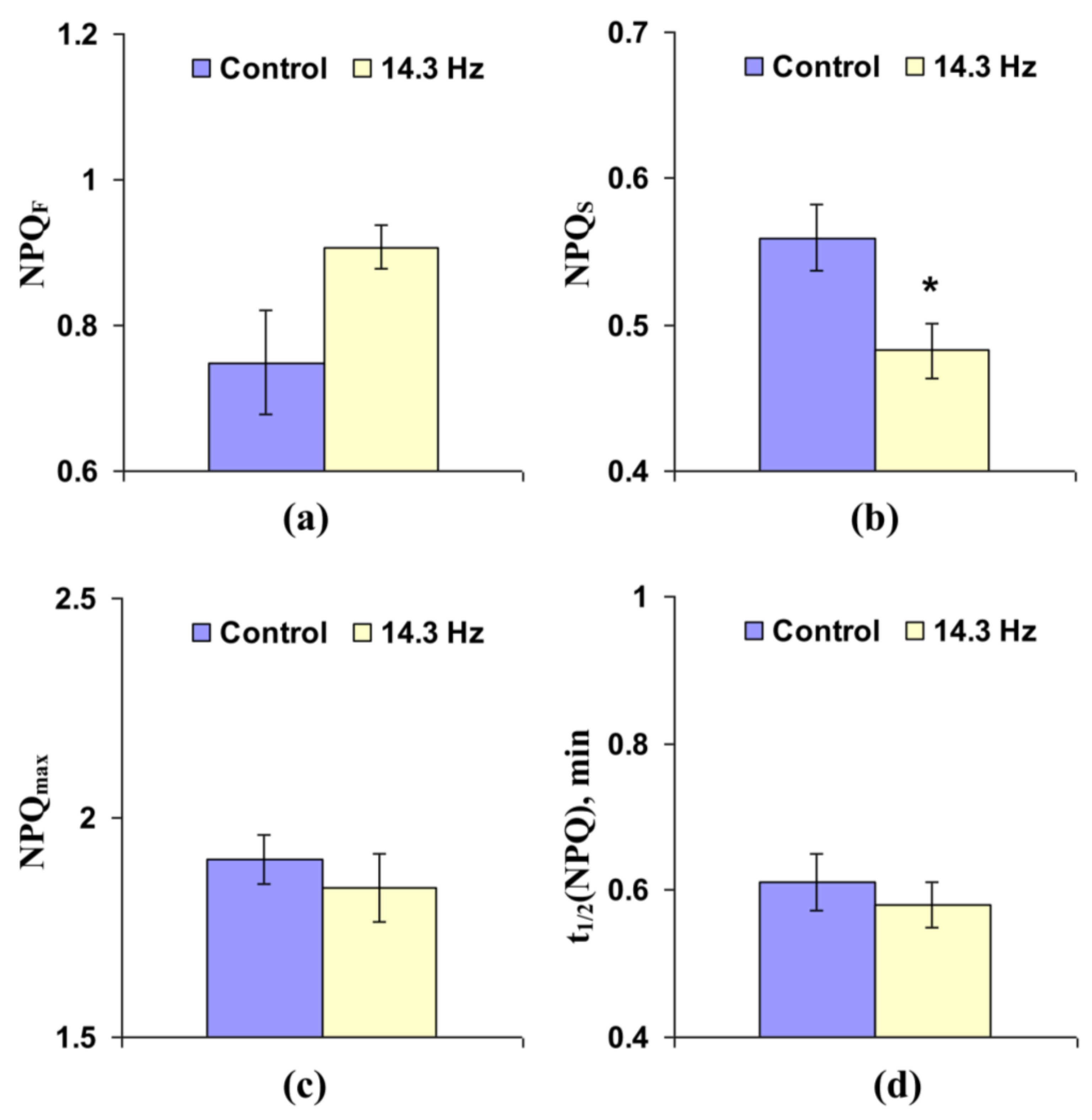
3.2. Investigation of the Influence of Chronic Treatment by Magnetic Fields with the Second Harmonic in Schumann Resonance Frequencies on Parameters of Photosynthetic Light Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quiles, M.J.; López, N.I. Photoinhibition of photosystems I and II induced by exposure to high light intensity during oat plant growth. Effects on the chloroplast NADH dehydrogenase complex. Plant Sci. 2004, 166, 815–823. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B 2012, 112, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Allakhverdiev, S.I.; Nishiyama, Y. The mechanism of photoinhibition in vivo: Re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochim. Biophys. Acta 2012, 1817, 1127–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanervo, E.; Tasaka, Y.; Murata, N.; Aro, E.M. Membrane lipid unsaturation modulates processing of the photosystem II reaction center protein D1 at low temperatures. Plant Physiol. 1997, 114, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Battisti, D.S.; Naylor, R.L. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef] [Green Version]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef] [Green Version]
- Stepien, P.; Johnson, G.N. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: Role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Kraslavsky, V.; Bharti, S.; Allakhverdiev, S.I.; Jajoo, A. Analysis of salt stress induced changes in Photosystem II heterogeneity by prompt fluorescence and delayed fluorescence in wheat (Triticum aestivum) leaves. J. Photochem. Photobiol. B. 2011, 104, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Pavlovič, A. The effect of electrical signals on photosynthesis and respiration. In Plant Electrophysiology; Volkov, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 33–62. [Google Scholar]
- Szechyńska-Hebda, M.; Lewandowska, M.; Karpiński, S. Electrical signaling, photosynthesis and systemic acquired acclimation. Front Physiol. 2017, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V.; Sukhova, E.; Vodeneev, V. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Prog. Biophys. Mol. Biol. 2019, 146, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [Green Version]
- Cruz, J.A.; Avenson, T.J.; Kanazawa, A.; Takizawa, K.; Edwards, G.E.; Kramer, D.M. Plasticity in light reactions of photosynthesis for energy production and photoprotection. J. Exp. Bot. 2005, 56, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Alte, F.; Stengel, A.; Benz, J.P.; Petersen, E.; Soll, J.; Groll, M.; Bölter, B. Ferredoxin: NADPH oxidoreductase is recruited to thylakoids by binding to a polyproline type II helix in a pH-dependent manner. Proc. Natl. Acad. Sci. USA 2010, 107, 19260–19265. [Google Scholar] [CrossRef] [Green Version]
- Benz, J.P.; Stengel, A.; Lintala, M.; Lee, Y.H.; Weber, A.; Philippar, K.; Gügel, I.L.; Kaieda, S.; Ikegami, T.; Mulo, P.; et al. Arabidopsis Tic62 and ferredoxin-NADP(H) oxidoreductase form light-regulated complexes that are integrated into the chloroplast redox poise. Plant Cell. 2010, 21, 3965–3983. [Google Scholar] [CrossRef] [Green Version]
- Pottosin, I.; Shabala, S. Transport across chloroplast membranes: Optimizing photosynthesis for adverse environmental conditions. Mol. Plant 2016, 9, 356–370. [Google Scholar] [CrossRef] [Green Version]
- Szabò, I.; Spetea, C. Impact of the ion transportome of chloroplasts on the optimization of photosynthesis. J. Exp. Bot. 2017, 68, 3115–3128. [Google Scholar] [CrossRef] [Green Version]
- Price, C.; Williams, E.; Elhalel, G.; Sentman, D. Natural ELF fields in the atmosphere and in living organisms. Int. J. Biometeorol. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Melnikov, A. Diurnal, seasonal and inter-annual variations in the Schumann resonance parameters. J. Atmos. Sol. Terr. Phys. 2004, 66, 1179–1185. [Google Scholar] [CrossRef]
- Price, C. ELF Electromagnetic waves from lightning: The Schumann resonances. Atmosphere 2016, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Elhalel, G.; Price, C.; Fixler, D.; Shainberg, A. Cardioprotection from stress conditions by weak magnetic fields in the Schumann Resonance band. Sci. Rep. 2019, 9, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, T.; Yoshimoto, M.; Koyama, S.; Miyakoshi, J. Exposure a extremely low frequency magnetic fields affects insulin-secreting cells. Bioelectromagnetics 2008, 29, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, J.A.T.; Dobránszki, J. Magnetic fields: How is plant growth and development impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef]
- Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic field (MF) applications in plants: An overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Davies, M.S. Effects of 60 Hz electromagnetic fields on early growth in three plant species and a replication of previous results. Bioelectromagnetics 1996, 17, 154–161. [Google Scholar] [CrossRef]
- Aksyonov, S.J.; Bulychev, A.A.; Grunina, T.Y.; Goryachev, S.N.; Turovetsky, V.B. Effects of ELF-EMF treatment on wheat seeds at different stages of germination and possible mechanism of their origin. Electromagn. Biol. Med. 2001, 20, 231–253. [Google Scholar]
- Iqbal, M.; Haq, Z.U.; Jamil, Y.; Ahmad, M.R. Effect of presowing magnetic treatment on properties of pea. Int. Agrophys. 2012, 26, 25–31. [Google Scholar] [CrossRef]
- Fischer, G.; Tausz, M.; Köck, M.; Grill, D. Effects of weak 16 3/2 Hz magnetic fields on growth parameters of young sunflower and wheat seedlings. Bioelectromagnetics 2004, 25, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Rochalska, M. Influence of frequent magnetic field on chlorophyll content in leaves of sugar beet plants. Nukleonika 2005, 50, S25–S28. [Google Scholar]
- Aleman, E.I.; Mbogholi, A.; Boix, Y.F.; Gonzalez-Ohnedo, J.; Chalfun, A. Effects of EMFs on some biological parameters in coffee plants (Coffea arabica L.) obtained by in vitro propagation. Polish, J. Environ. Stud. 2014, 23, 95–101. [Google Scholar]
- Kornarzyński, K.; Dziwulska-Hunek, A.; Kornarzyńska-Gregorowicz, A.; Sujak, A. Effect of electromagnetic stimulation of amaranth seeds of different initial moisture on the germination parameters and photosynthetic pigments content. Sci. Rep. 2018, 8, 14023. [Google Scholar] [CrossRef]
- De Souza-Torres, A.; Sueiro-Pelegrín, L.; Zambrano-Reyes, M.; Macías-Socarras, I.; González-Posada, M.; García-Fernández, D. Extremely low frequency non-uniform magnetic fields induce changes in water relations, photosynthesis and tomato plant growth. Int. J. Radiat. Biol. 2020, 96, 951–957. [Google Scholar] [CrossRef]
- Yano, A.; Ohashi, Y.; Hirasaki, T.; Fujiwara, K. Effects of a 60 Hz magnetic field on photosynthetic CO2 uptake and early growth of radish seedlings. Bioelectromagnetics 2004, 25, 572–581. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhova, E.; Khlopkov, A.; Vodeneev, V.; Sukhov, V. Simulation of a nonphotochemical quenching in plant leaf under different light intensities. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148138. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Analysis of light-induced changes in the photochemical reflectance index (PRI) in leaves of pea, wheat, and pumpkin using pulses of green-yellow measuring light. Remote Sens. 2019, 11, 810. [Google Scholar] [CrossRef] [Green Version]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Ruban, A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.P.; Björkman, O.; Shih, C.; Grossman, A.R.; Rosenquist, M.; Jansson, S.; Niyogi, K.K. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 2000, 403, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Gilmore, A.M.; Caffarri, S.; Bassi, R.; Golan, T.; Kramer, D.; Niyogi, K.K. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 2004, 279, 22866–22874. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Adams III, W.W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Jajoo, A.; Mekala, N.R.; Tongra, T.; Tiwari, A.; Grieco, M.; Tikkanen, M.; Aro, E.M. Low pH-induced regulation of excitation energy between the two photosystems. FEBS Lett. 2014, 588, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Singh-Rawal, P.; Jajoo, A.; Mathur, S.; Mehta, P.; Bharti, S. Evidence that pH can drive state transitions in isolated thylakoid membranes from spinach. Photochem. Photobiol. Sci. 2010, 9, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V. Electrical signals as mechanism of photosynthesis regulation in plants. Photosynth. Res. 2016, 130, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Flügge, U.I.; Freisl, M.; Heldt, H.W. The mechanism of the control of carbon fixation by the pH in the chloroplast stroma: Studies with acid mediated proton transfer across the envelope. Planta 1980, 149, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Wolosiuk, R.A.; Ballicora, M.A.; Hagelin, K. The reductive pentose phosphate cycle for photosynthetic CO2 assimilation: Enzyme modulation. FASEB J. 1993, 7, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, T.; Yamamoto, H. Contribution of cyclic and pseudo-cyclic electron transport to the formation of proton motive force in chloroplasts. Mol. Plant. 2017, 10, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettinger, W.F.; Clear, A.M.; Fanning, K.J.; Peck, M.L. Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol. 1999, 119, 1379–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stange, B.C.; Rowland, R.E.; Rapley, B.I.; Podd, J.V. ELF magnetic fields increase amino acid uptake into Vicia faba L. roots and alter ion movement across the plasma membrane. Bioelectromagnetics 2002, 23, 347–354. [Google Scholar] [CrossRef]
- Pazur, A.; Rassadina, V.; Dandler, J.; Zoller, J. Growth of etiolated barley plants in weak static and 50 Hz electromagnetic fields tuned to calcium ion cyclotron resonance. Biomagn. Res. Technol. 2006, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Betti, L.; Trebbi, G.; Fregola, F.; Zurla, M.; Mesirca, P.; Brizzi, M.; Borghini, F. Weak static and extremely low frequency magnetic fields affect in vitro pollen germination. Sci. World J. 2011, 11, 875–890. [Google Scholar] [CrossRef] [Green Version]
- Belyavskaya, N.A. Biological effects due to weak magnetic field on plants. Adv. Space Res. 2004, 34, 1566–1574. [Google Scholar] [CrossRef]
- Goldsworthy, A. Effects of electrical and electromagnetic fields on plants and related topics. In Plant Electrophysiology. Theory and Methods; Volkov, A.G., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Pazur, A.; Rassadina, V. Transient effect of weak electromagnetic fields on calcium ion concentration in Arabidopsis thaliana. BMC Plant Biol. 2009, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupenina, N.A.; Bulychev, A.A. Action potential in a plant cell lowers the light requirement for non-photochemical energy-dependent quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 2007, 1767, 781–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhov, V.; Surova, L.; Morozova, E.; Sherstneva, O.; Vodeneev, V. Changes in H+-ATP synthase activity, proton electrochemical gradient, and pH in pea chloroplast can be connected with variation potential. Front Plant Sci. 2016, 7, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhov, V.; Sukhova, E.; Sinitsyna, Y.; Gromova, E.; Mshenskaya, N.; Ryabkova, A.; Ilin, N.; Vodeneev, V.; Mareev, E.; Price, C. Influence of Magnetic Field with Schumann Resonance Frequencies on Photosynthetic Light Reactions in Wheat and Pea. Cells 2021, 10, 149. https://doi.org/10.3390/cells10010149
Sukhov V, Sukhova E, Sinitsyna Y, Gromova E, Mshenskaya N, Ryabkova A, Ilin N, Vodeneev V, Mareev E, Price C. Influence of Magnetic Field with Schumann Resonance Frequencies on Photosynthetic Light Reactions in Wheat and Pea. Cells. 2021; 10(1):149. https://doi.org/10.3390/cells10010149
Chicago/Turabian StyleSukhov, Vladimir, Ekaterina Sukhova, Yulia Sinitsyna, Ekaterina Gromova, Natalia Mshenskaya, Anastasiia Ryabkova, Nikolay Ilin, Vladimir Vodeneev, Evgeny Mareev, and Colin Price. 2021. "Influence of Magnetic Field with Schumann Resonance Frequencies on Photosynthetic Light Reactions in Wheat and Pea" Cells 10, no. 1: 149. https://doi.org/10.3390/cells10010149




