Discovery, Function, and Therapeutic Targeting of Siglec-8
Abstract
:1. Introduction
2. Siglecs and the Discovery of Siglec-8
3. Expression Pattern of Siglec-8
4. Siglec-8 Function on Eosinophils and Mast Cells
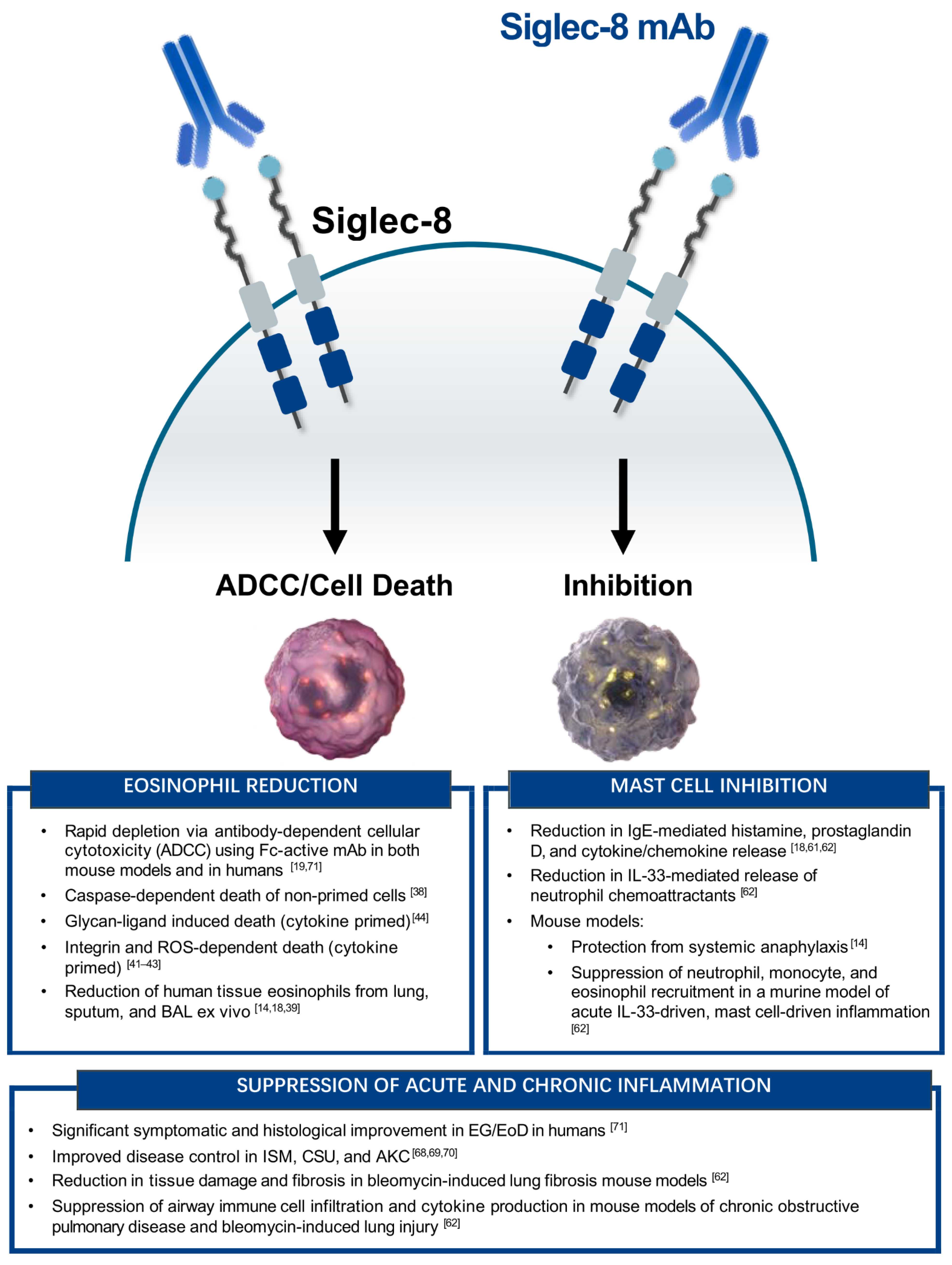
4.1. Anti-Eosinophil Activity
4.2. Anti-Mast Cell Activity
5. Development of AK002, a Humanized Anti-Siglec-8 mAb
6. AK002 Efficacy in Eosinophil- and Mast Cell-Mediated Diseases
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varki, A.; Angata, T. Siglecs—the major subfamily of I-type lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, J.A.; Chang, A.T.; Youngblood, B.A.; Bochner, B.S. Eosinophil and mast cell Siglecs: From biology to drug target. J. Leukoc. Biol. 2020, 108, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Simon, H.U.; von Gunten, S. Targeting siglecs—A novel pharmacological strategy for immuno-and glycotherapy. Biochem. Pharmacol. 2011, 82, 323–332. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.K.; Paulson, J.C. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol. Sci. 2009, 30, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Barenwaldt, A.; Laubli, H. The sialoglycan-Siglec glyco-immune checkpoint—a target for improving innate and adaptive anti-cancer immunity. Expert Opin. Ther. Targets 2019, 23, 839–853. [Google Scholar] [CrossRef]
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of Eosinophils to Human Health and Disease. Annu. Rev. Pathol. 2020, 15, 179–209. [Google Scholar] [CrossRef] [Green Version]
- Robida, P.A.; Puzzovio, P.G.; Pahima, H.; Levi-Schaffer, F.; Bochner, B.S. Human eosinophils and mast cells: Birds of a feather flock together. Immunol. Rev. 2018, 282, 151–167. [Google Scholar] [CrossRef]
- Floyd, H.; Ni, J.; Cornish, A.L.; Zeng, Z.; Liu, D.; Carter, K.C.; Steel, J.; Crocker, P.R. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J. Biol. Chem. 2000, 275, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Kikly, K.K.; Bochner, B.S.; Freeman, S.D.; Tan, K.B.; Gallagher, K.T.; D’Alessio, K.J.; Holmes, S.D.; Abrahamson, J.A.; Erickson-Miller, C.L.; Murdock, P.R.; et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J. Allergy Clin. Immunol. 2000, 105, 1093–1100. [Google Scholar] [CrossRef]
- Crocker, P.R.; Clark, E.A.; Filbin, M.; Gordon, S.; Jones, Y.; Kehrl, J.H.; Kelm, S.; Le Douarin, N.; Powell, L.; Roder, J.; et al. Siglecs: A family of sialic-acid binding lectins. Glycobiology 1998, 8, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Foussias, G.; Yousef, G.M.; Diamandis, E.P. Molecular characterization of a Siglec-8 variant containing cytoplasmic tyrosine-based motifs, and mapping of the Siglec-8 gene. Biochem. Biophys. Res. Commun. 2000, 278, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Plitt, J.; Bochner, B.S. Human eosinophils express two Siglec-8 splice variants. J. Allergy Clin. Immunol. 2002, 109, 176. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Falahati, R.; Bryce, P.J.; Bright, J.; Williams, J.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; et al. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int. Arch. Allergy Immunol. 2019, 180, 91–102. [Google Scholar] [CrossRef]
- Hwang, S.M.; Uhm, T.G.; Lee, S.K.; Kong, S.K.; Jung, K.H.; Binas, B.; Chai, Y.G.; Park, S.W.; Chung, I.Y. Olig2 is expressed late in human eosinophil development and controls Siglec-8 expression. J. Leukoc. Biol. 2016, 100, 711–723. [Google Scholar] [CrossRef]
- Legrand, F.; Cao, Y.; Wechsler, J.B.; Zhu, X.; Zimmermann, N.; Rampertaap, S.; Monsale, J.; Romito, K.; Youngblood, B.A.; Brock, E.C.; et al. Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic disorders: Receptor expression and targeting using chimeric antibodies. J. Allergy Clin. Immunol. 2019, 143, 2227–2237. [Google Scholar] [CrossRef]
- Johansson, M.W.; Kelly, E.A.; Nguyen, C.L.; Jarjour, N.N.; Bochner, B.S. Characterization of Siglec-8 Expression on Lavage Cells after Segmental Lung Allergen Challenge. Int. Arch. Allergy Immunol. 2018, 177, 16–28. [Google Scholar] [CrossRef]
- Kerr, S.C.; Gonzalez, J.R.; Schanin, J.; Peters, M.C.; Lambrecht, B.N.; Brock, E.C.; Charbit, A.; Mark Ansel, K.; Youngblood, B.A.; Fahy, J.V. An anti-siglec-8 antibody depletes sputum eosinophils from asthmatic subjects and inhibits lung mast cells. Clin. Exp. Allergy 2020. [Google Scholar] [CrossRef]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Falahati, R.; Bochner, B.S.; Rasmussen, H.S.; Peterson, K.; Bebbington, C.; Tomasevic, N. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Chang, A.T.; Bebbington, C.; Tomasevic, N. AK002, a novel humanized monoclonal antibody to siglec-8, inhibits mast cell activity and depletes eosinophils in ex vivo bone marrow tissue from patients with systemic mastocytosis. Blood 2018, 132, 1104. [Google Scholar] [CrossRef]
- Hudson, S.A.; Herrmann, H.; Du, J.; Cox, P.; El Haddad, B.; Butler, B.; Crocker, P.R.; Ackerman, S.J.; Valent, P.; Bochner, B.S. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. J. Clin. Immunol. 2011, 31, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoi, H.; Myers, A.; Matsumoto, K.; Crocker, P.R.; Saito, H.; Bochner, B.S. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy 2006, 61, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Zimmermann, N.; Carrigan, P.E.; Lee, J.J.; Rothenberg, M.E.; Bochner, B.S. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: Identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G). Genomics 2003, 82, 521–530. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Biedermann, B.; Nitschke, L.; Crocker, P.R. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur. J. Immunol. 2004, 34, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Crocker, P.R.; Paulson, J.C. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6’-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology 2005, 15, 1125–1135. [Google Scholar] [CrossRef] [Green Version]
- Dyer, K.D.; Moser, J.M.; Czapiga, M.; Siegel, S.J.; Percopo, C.M.; Rosenberg, H.F. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 2008, 181, 4004–4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Gicheva, N.; Macauley, M.S.; Arlian, B.M.; Paulson, J.C.; Kawasaki, N. Siglec-F is a novel intestinal M cell marker. Biochem. Biophys. Res. Commun. 2016, 479, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bolden, J.E.; Lucas, E.C.; Zhou, G.; O’Sullivan, J.A.; de Graaf, C.A.; McKenzie, M.D.; Di Rago, L.; Baldwin, T.M.; Shortt, J.; Alexander, W.S.; et al. Identification of a Siglec-F+ granulocyte-macrophage progenitor. J. Leukoc. Biol. 2018, 104, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Pfirschke, C.; Engblom, C.; Gungabeesoon, J.; Lin, Y.; Rickelt, S.; Zilionis, R.; Messemaker, M.; Siwicki, M.; Gerhard, G.M.; Kohl, A.; et al. Tumor-Promoting Ly-6G(+) SiglecF(high) Cells Are Mature and Long-Lived Neutrophils. Cell Rep. 2020, 32, 108164. [Google Scholar] [CrossRef]
- O’Sullivan, J.A.; Wei, Y.; Carroll, D.J.; Moreno-Vinasco, L.; Cao, Y.; Zhang, F.; Lee, J.J.; Zhu, Z.; Bochner, B.S. Frontline Science: Characterization of a novel mouse strain expressing human Siglec-8 only on eosinophils. J. Leukoc. Biol. 2018, 104, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chhiba, K.D.; Zhang, F.; Ye, X.; Wang, L.; Zhang, L.; Robida, P.A.; Moreno-Vinasco, L.; Schnaar, R.L.; Roers, A.; et al. Mast Cell-Specific Expression of Human Siglec-8 in Conditional Knock-in Mice. Int. J. Mol. Sci. 2018, 20, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuplez, E.; Krier-Burris, R.; Cao, Y.; Marsche, G.; O’Sullivan, J.; Bochner, B.S. Frontline Science: Superior mouse eosinophil depletion in vivo targeting transgenic Siglec-8 instead of endogenous Siglec-F: Mechanisms and pitfalls. J. Leukoc. Biol. 2020, 108, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Nycholat, C.M.; Duan, S.; Knuplez, E.; Worth, C.; Elich, M.; Yao, A.; O’Sullivan, J.; McBride, R.; Wei, Y.; Fernandes, S.M.; et al. A Sulfonamide Sialoside Analogue for Targeting Siglec-8 and -F on Immune Cells. J. Am. Chem. Soc. 2019, 141, 14032–14037. [Google Scholar] [CrossRef] [PubMed]
- Bryce, P.J.; Falahati, R.; Kenney, L.L.; Leung, J.; Bebbington, C.; Tomasevic, N.; Krier, R.A.; Hsu, C.L.; Shultz, L.D.; Greiner, D.L.; et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J. Allergy Clin. Immunol. 2016, 138, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef] [Green Version]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef] [Green Version]
- Nutku, E.; Aizawa, H.; Hudson, S.A.; Bochner, B.S. Ligation of Siglec-8: A selective mechanism for induction of human eosinophil apoptosis. Blood 2003, 101, 5014–5020. [Google Scholar] [CrossRef] [Green Version]
- von Gunten, S.; Vogel, M.; Schaub, A.; Stadler, B.M.; Miescher, S.; Crocker, P.R.; Simon, H.U. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J. Allergy Clin. Immunol. 2007, 119, 1005–1011. [Google Scholar] [CrossRef]
- Na, H.J.; Hudson, S.A.; Bochner, B.S. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine 2012, 57, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Nutku, E.; Hudson, S.A.; Bochner, B.S. Mechanism of Siglec-8-induced human eosinophil apoptosis: Role of caspases and mitochondrial injury. Biochem. Biophys. Res. Commun. 2005, 336, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Nutku-Bilir, E.; Hudson, S.A.; Bochner, B.S. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am. J. Respir. Cell Mol. Biol. 2008, 38, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, D.J.; O’Sullivan, J.A.; Nix, D.B.; Cao, Y.; Tiemeyer, M.; Bochner, B.S. Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating beta2-integrin-dependent function in human eosinophils. J. Allergy Clin. Immunol. 2018, 141, 2196–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, S.A.; Bovin, N.V.; Schnaar, R.L.; Crocker, P.R.; Bochner, B.S. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6’-sulfated sialyl Lewis x. J. Pharmacol. Exp. Ther. 2009, 330, 608–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochner, B.S.; Alvarez, R.A.; Mehta, P.; Bovin, N.V.; Blixt, O.; White, J.R.; Schnaar, R.L. Glycan array screening reveals a candidate ligand for Siglec-8. J. Biol. Chem. 2005, 280, 4307–4312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiwamoto, T.; Brummet, M.E.; Wu, F.; Motari, M.G.; Smith, D.F.; Schnaar, R.L.; Zhu, Z.; Bochner, B.S. Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J. Allergy Clin. Immunol. 2014, 133, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiwamoto, T.; Katoh, T.; Evans, C.M.; Janssen, W.J.; Brummet, M.E.; Hudson, S.A.; Zhu, Z.; Tiemeyer, M.; Bochner, B.S. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J. Allergy Clin. Immunol. 2015, 135, 1329–1340. [Google Scholar] [CrossRef] [Green Version]
- Propster, J.M.; Yang, F.; Rabbani, S.; Ernst, B.; Allain, F.H.; Schubert, M. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc. Natl. Acad. Sci. USA 2016, 113, E4170–E4179. [Google Scholar] [CrossRef] [Green Version]
- Kroezen, B.S.; Conti, G.; Girardi, B.; Cramer, J.; Jiang, X.; Rabbani, S.; Muller, J.; Kokot, M.; Luisoni, E.; Ricklin, D.; et al. A Potent Mimetic of the Siglec-8 Ligand 6’-Sulfo-Sialyl Lewis(x). ChemMedChem 2020, 15, 1706–1719. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, H.; Fernandes, S.M.; Wei, Y.; Gonzalez-Gil, A.; Motari, M.G.; Vajn, K.; Stevens, W.W.; Peters, A.T.; Bochner, B.S.; et al. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J. Allergy Clin. Immunol. 2015, 135, 799–810. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Gonzalez-Gil, A.; Wei, Y.; Fernandes, S.M.; Porell, R.N.; Vajn, K.; Paulson, J.C.; Nycholat, C.M.; Schnaar, R.L. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology 2017, 27, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.; Porell, R.N.; Fernandes, S.M.; Wei, Y.; Yu, H.; Carroll, D.J.; McBride, R.; Paulson, J.C.; Tiemeyer, M.; Aoki, K.; et al. Sialylated keratan sulfate proteoglycans are Siglec-8 ligands in human airways. Glycobiology 2018, 28, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.; Li, T.A.; Porell, R.N.; Fernandes, S.M.; Tarbox, H.E.; Lee, H.S.; Aoki, K.; Tiemeyer, M.; Kim, J.; Schnaar, R.L. Isolation, identification, and characterization of the human airway ligand for the eosinophil and mast cell immunoinhibitory receptor Siglec-8. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Schleimer, R.P.; Schnaar, R.L.; Bochner, B.S. Regulation of airway inflammation by Siglec-8 and Siglec-9 sialoglycan ligand expression. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Angata, T.; Cho, J.Y.; Miller, M.; Broide, D.H.; Varki, A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 2007, 109, 4280–4287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, N.; McBride, M.L.; Yamada, Y.; Hudson, S.A.; Jones, C.; Cromie, K.D.; Crocker, P.R.; Rothenberg, M.E.; Bochner, B.S. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 2008, 63, 1156–1163. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, E.; Cho, J.Y.; Rosenthal, P.; Chao, J.; Miller, M.; Pham, A.; Aceves, S.S.; Varki, A.; Broide, D.H. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Song, D.J.; Cho, J.Y.; Lee, S.Y.; Miller, M.; Rosenthal, P.; Soroosh, P.; Croft, M.; Zhang, M.; Varki, A.; Broide, D.H. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J. Immunol. 2009, 183, 5333–5341. [Google Scholar] [CrossRef] [Green Version]
- Song, D.J.; Cho, J.Y.; Miller, M.; Strangman, W.; Zhang, M.; Varki, A.; Broide, D.H. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin. Immunol. 2009, 131, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Kano, G.; Hudson, S.A.; Brummet, M.; Zimmermann, N.; Zhu, Z.; Bochner, B.S. Mechanisms of Siglec-F-induced eosinophil apoptosis: A role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS ONE 2013, 8, e68143. [Google Scholar] [CrossRef]
- Yokoi, H.; Choi, O.H.; Hubbard, W.; Lee, H.S.; Canning, B.J.; Lee, H.H.; Ryu, S.D.; von Gunten, S.; Bickel, C.A.; Hudson, S.A.; et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J. Allergy Clin. Immunol. 2008, 121, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Schanin, J.; Gebremeskel, S.; Korver, W.; Falahati, R.; Butuci, M.; Haw, T.J.; Nair, P.M.; Liu, G.; Hansbro, N.G.; Hansbro, P.M.; et al. A monoclonal antibody to Siglec-8 suppresses non-allergic airway inflammation and inhibits IgE-independent mast cell activation. Mucosal. Immunol. 2020. [Google Scholar] [CrossRef]
- Gebremeskel, S.; Davis, T.; Wong, A.; Brock, E.; Leung, J.; Schanin, J.; Youngblood, B. A Siglec-8 antibody reduces substance P-induced inflammation by inhibiting MRGPR-mediated mast cell activation. Allergy 2020, 75, 66–67. [Google Scholar] [CrossRef]
- O’Sullivan, J.A.; Carroll, D.J.; Cao, Y.; Salicru, A.N.; Bochner, B.S. Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells. J. Allergy Clin. Immunol. 2018, 141, 1774–1785. [Google Scholar] [CrossRef] [Green Version]
- Satoh, M.; Iida, S.; Shitara, K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin. Biol. Ther. 2006, 6, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef] [Green Version]
- Poznanski, S.M.; Ashkar, A.A. What Defines NK Cell Functional Fate: Phenotype or Metabolism? Front. Immunol. 2019, 10, 1414. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Bonnekoh, H.; Hawro, T.; Hawro, M.; Michaelis, E.; Rasmussen, H.; Singh, B.; Kantor, A.; Chang, A.; Maurer, M. Safety and efficacy data of AK002, an anti-Siglec-8 monoclonal antibody, in patients with indolent systemic mastocytosis (ISM): Results from a first-in-human, open-label phase 1 study. Allergy 2019, 74, 854–915. [Google Scholar] [CrossRef] [Green Version]
- Altrichter, S.; Staubach, P.; Pasha, M.; Rasmussen, H.; Singh, B.; Chang, A.; Bernstein, J.; Siebenhaar, F.; Maurer, M. Efficacy and safety data of AK002, an anti-siglec-8 monoclonal antibody, in patients with multiple forms of uncontrolled chronic urticaria (CU): Results from an open-label phase 2a study. Allergy 2019, 74, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Levine, H.T.; Tauber, J.; Nguyen, Q.; Anesi, S.D. Phase 1b Study of AK002, an Anti-Siglec-8 Monoclonal Antibody, in Patients with Severe Allergic Conjunctivitis (KRONOS Study). J. Allergy Clin. Immunol. 2020, 145, AB185. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.M.; Collins, M.H.; Stucke, E.M.; Putnam, P.E.; Franciosi, J.P.; Kushner, J.P.; Abonia, J.P.; Rothenberg, M.E. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J. Allergy Clin. Immunol. 2014, 134, 1114–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoda, T.; Wen, T.; Caldwell, J.M.; Collins, M.H.; Besse, J.A.; Osswald, G.A.; Abonia, J.P.; Arva, N.C.; Atkins, D.; Capocelli, K.E.; et al. Molecular, endoscopic, histologic, and circulating biomarker-based diagnosis of eosinophilic gastritis: Multi-site study. J. Allergy Clin. Immunol. 2020, 145, 255–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Siglec-8 | Siglec-F | |
|---|---|---|
| Surface Expression | ||
| Eosinophils | Yes | Yes |
| Mast cells | Yes | No |
| Basophils | Yes; weak | No |
| Alveolar macrophages | No | Yes |
| Neutrophils | No | Sometimes |
| T cells | No | No or minimal |
| Monocytes | No | No |
| Intestinal tuft/M cells | No | Yes |
| Expression is at least in part regulated by the transcription factor Olig2 | Yes | Unknown |
| Ligands | ||
| 6’-S-Sialyl-LacNac | Yes | Yes |
| 6’-S-Sialyl-Lewis X | Yes | Yes |
| Tri and tetra-antennary bisected glycans containing α2,3-linked terminal sialic acid | No | Yes |
| Sialylated keratan sulfate chains on human aggrecan | Yes | Unknown |
| Sialylated keratan sulfate chains on human DMBT1 | Yes | Unknown |
| Mouse Muc5b glycans | No | Yes |
| 9-N-(2-naphthyl-sulfonyl)-Neu5Acα2-3-[6-O-sulfo]-Galβ1-4GlcNAc (6’-O-sulfo (NSA)Neu5Ac) | Yes | Yes |
| 6′-sulfo-sialyl Lewis X mimetic retaining the neuraminic acid core, but with a carbocyclic mimetic of the Gal moiety and a sulfonamide substituent in the 9-position | Yes | Unknown |
| Function | ||
| Eosinophils in vitro: Non-cytokine primed | ||
| Crosslinking with antibody induces eosinophil death in non-cytokine-primed cells | Yes; modest | Yes; weak |
| Death that is caspase-dependent | Yes | Yes |
| Death that is integrin- and ROS-dependent | No | No |
| Death that is NADPH oxidase-dependent | No | No |
| Death is associated with mitochondrial membrane damage | Yes | Yes |
| Receptor internalized after ligation | Yes | Yes |
| Eosinophils in vitro: Cytokine primed | ||
| Crosslinking with antibody or multivalent ligand induces eosinophil death in cytokine-primed cells | Yes; marked | Yes; weak |
| Death that is caspase-dependent | No | Yes |
| Death that is beta-2 integrin- and ROS-dependent | Yes | No |
| Death that is NADPH oxidase-dependent | Yes | No |
| Death that is associated with mitochondrial membrane-damage | Yes | Yes |
| Role for SHP-1 phosphatase in cell death | No | No |
| Role for MAP kinases in cell death | Yes | Unknown |
| Mast cells in vitro | ||
| Crosslinking induces cell death | No | Not applicable |
| Inhibition of IgE receptor-mediated degranulation | Yes | Not applicable |
| Inhibition of IL-33-stimulated responses | Yes | Not applicable |
| Receptor internalized after ligation | Yes | Not applicable |
| Internalization of a toxic payload after ligation causes cell death | Yes | Not applicable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youngblood, B.A.; Leung, J.; Falahati, R.; Williams, J.; Schanin, J.; Brock, E.C.; Singh, B.; Chang, A.T.; O’Sullivan, J.A.; Schleimer, R.P.; et al. Discovery, Function, and Therapeutic Targeting of Siglec-8. Cells 2021, 10, 19. https://doi.org/10.3390/cells10010019
Youngblood BA, Leung J, Falahati R, Williams J, Schanin J, Brock EC, Singh B, Chang AT, O’Sullivan JA, Schleimer RP, et al. Discovery, Function, and Therapeutic Targeting of Siglec-8. Cells. 2021; 10(1):19. https://doi.org/10.3390/cells10010019
Chicago/Turabian StyleYoungblood, Bradford A., John Leung, Rustom Falahati, Jason Williams, Julia Schanin, Emily C. Brock, Bhupinder Singh, Alan T. Chang, Jeremy A. O’Sullivan, Robert P. Schleimer, and et al. 2021. "Discovery, Function, and Therapeutic Targeting of Siglec-8" Cells 10, no. 1: 19. https://doi.org/10.3390/cells10010019
APA StyleYoungblood, B. A., Leung, J., Falahati, R., Williams, J., Schanin, J., Brock, E. C., Singh, B., Chang, A. T., O’Sullivan, J. A., Schleimer, R. P., Tomasevic, N., Bebbington, C. R., & Bochner, B. S. (2021). Discovery, Function, and Therapeutic Targeting of Siglec-8. Cells, 10(1), 19. https://doi.org/10.3390/cells10010019





