Microvascular Density, Endothelial Area, and Ki-67 Proliferative Index Correlate Each Other in Cat Post-Injection Fibrosarcoma
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Hematoxylin–Eosin and Immunohistochemistry
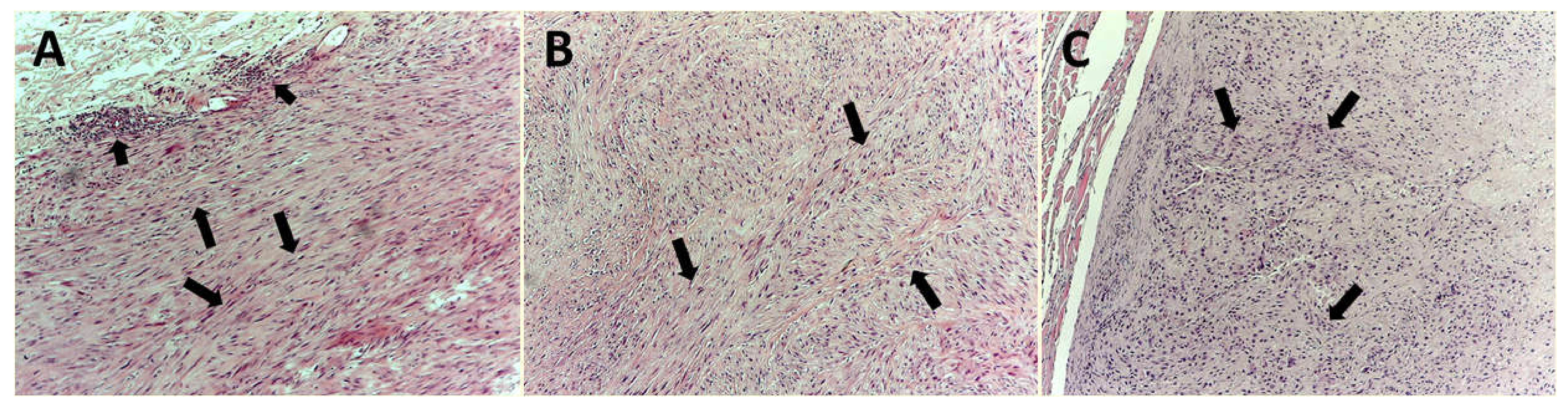
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paoloni, M.; Khanna, C. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 2008, 8, 147–156. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Palmieri, B.; De Vico, G.; Iannitti, T. Onco-epidemiology of domestic animals and targeted therapeutic attempts: Perspectives on human oncology. J. Cancer Res. Clin. Oncol. 2014, 140, 1807–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esplin, D.G.; McGill, L.D.; Meininger, A.C.; Wilson, S.R. Postvaccination sarcomas in cats. J. Am. Vet. Med. Assoc. 1993, 202, 1245–1247. [Google Scholar] [PubMed]
- Kass, P.H.; Barnes, W.G., Jr.; Spangler, W.L.; Chomel, B.B.; Culbertson, M.R. Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. J. Am. Vet. Med Assoc. 1993, 203, 396–405. [Google Scholar] [PubMed]
- Macy, D.W. The potential role and mechanisms of FeLV vaccine-induced neoplasms. Semin. Vet. Med. Surg. 1995, 10, 234–237. [Google Scholar]
- Martin, M. Vaccine-associated fibrosarcoma in a cat. Can. Vet. J. 2003, 44, 660–663. [Google Scholar]
- Martano, M.; Morello, E.; Buracco, P. Feline injection-site sarcoma: Past, present and future perspectives. Vet. J. 2011, 188, 136–141. [Google Scholar] [CrossRef]
- Doddy, F.D.; Glickman, L.T.; Glickman, N.W.; Janovitz, E.B. Feline fibrosarcomas at vaccination sites and non-vaccination sites. J. Comp. Pathol. 1996, 114, 165–174. [Google Scholar] [CrossRef]
- Kliczkowska, K.; Jankowska, U.; Jagielski, D.; Czopowicz, M.; Sapierzyński, R. Epidemiological and morphological analysis of feline injection site sarcomas. Pol. J. Vet. Sci. 2015, 18, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Rocchi, L.; Caraffi, S.; Perris, R.; Mangieri, D. The angiogenic asset of soft tissue sarcomas: A new tool to discover new therapeutic targets. Biosci. Rep. 2014, 34, e00147. [Google Scholar] [CrossRef]
- Vascellari, M.; Melchiotti, E.; Bozza, M.A.; Mutinelli, F. Fibrosarcomas at presumed sites of injection in dogs: Characteristics and comparison with non-vaccination site fibrosarcomas and feline post-vaccinal fibrosarcomas. J. Vet. Med. Aphysiologypathol. Med. 2003, 50, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Macy, D.W.; Hendrick, M.J. The Potential Role of Inflammation in the Development of Postvaccinal Sarcomas in Cats. Vet. Clin. N. Am. Small Anim. Pract. 1996, 26, 103–109. [Google Scholar] [CrossRef]
- Saba, C.F. Vaccine-associated feline sarcoma: Current perspectives. Vet. Med. 2017, 8, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patruno, R.; Marech, I.; Zizzo, N.; Ammendola, M. c-Kit expression, angiogenesis, and grading in canine mast cell tumour: A unique model to study c-Kit driven human malignancies. BioMed Res. Int. 2014, 2014, 730246. [Google Scholar] [CrossRef]
- Girolamo, R. Editorial [ Hot Topic: Targeting Tumor Angiogenesis: An Update (Guest Editor: Girolamo Ranieri)]. Curr. Med. Chem. 2012, 19, 937. [Google Scholar] [CrossRef]
- Ranieri, G.; Patruno, R.; Lionetti, A.; Di Summa, A.; Mattioli, E.; Bufo, P.; Pellecchia, A.; Ribatti, D.; Zizzo, N. Endothelial area and microvascular density in a canine non-Hodgkin’s lymphoma: An interspecies model of tumor angiogenesis. Leuk. Lymphoma 2005, 46, 1639–1643. [Google Scholar] [CrossRef]
- Berse, B.; Hunt, J.A.; Diegel, R.J.; Morganelli, P.; Yeo, K.; Brown, F.; Fava, R.A. Hypoxia augments cytokine (transforming growth factor-beta (TGF-beta) and IL-1)-induced vascular endothelial growth factor secretion by human synovial fibroblasts. Clin. Exp. Immunol. 1999, 115, 176–182. [Google Scholar] [CrossRef]
- Patruno, R.; Zizzo, N.; Zito, A.F.; Catalano, V.; Valerio, P.; Pellecchia, V.; D’Errico, E.; Mazzone, F.; Ribatti, D.; Ranieri, G. Microvascular density and endothelial area correlate with Ki-67 proliferative rate in the canine non-Hodgkin’s lymphoma spontaneous model. Leuk. Lymphoma 2006, 47, 1138–1143. [Google Scholar] [CrossRef]
- Crawford, Y.; Kasman, I.; Yu, L.; Zhong, C.; Wu, X.; Modrusan, Z.; Kaminker, J.; Ferrara, N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 2009, 15, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Marech, I.; Ammendola, M.; Gadaleta, C.; Zizzo, N.; Oakley, C.; Gadaleta, C.D.; Ranieri, G. Possible biological and translational significance of mast cells density in colorectal cancer. World J. Gastroenterol. 2014, 20, 8910–8920. [Google Scholar] [CrossRef]
- Sammarco, G.; Gadaleta, C.D.; Zuccalà, V.; Albayrak, E.; Patruno, R.; Milella, P.; Sacco, R.; Ammendola, M. Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients. Int. J. Mol. Sci. 2018, 19, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limoge, M.; Safina, A.; Beattie, A.; Kapus, L.; Truskinovsky, A.M.; Bakin, A.V. Tumor-fibroblast interactions stimulate tumor vascularization by enhancing cytokine-driven production of MMP9 by tumor cells. Oncotarget 2017, 8, 35592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppé, J.P.; Kauser, K.; Campisi, J.; Beauséjour, C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006, 281, 29568–29574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laforgia, M.; Calabrò, C.; Scattone, A.; Laface, C.; Porcelli, M.; Gadaleta, C.D.; Nardulli, P.; Ranieri, G. Pharmacotherapy in Mast Cell Leukemia. Expert Opin. Pharmacother. 2020, 21, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, M.J.; Goldschmidt, M.H.; Shofer, F.S.; Wang, Y.Y.; Somlyo, A.P. Postvaccinal sarcomas in the cat: Epidemiology and electron probe microanalytical identification of aluminum. Cancer Res. 1992, 52, 5391–5394. [Google Scholar] [PubMed]
- Couto, S.S.; Griffey, S.M.; Duarte, P.C.; Madewell, B.R. Feline vaccine-associated fibrosarcoma: Morphologic distinctions. Vet. Pathol. 2002, 39, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Powers, B.E.; Hoopes, P.J.; Ehrhart, E.J. Tumor diagnosis, grading, and staging. Semin. Vet. Med. Surg. 1995, 10, 158–167. [Google Scholar]
- Ammendola, M.; Sacco, R.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Patruno, R.; Giordano, M.; Ruggieri, E.; Zizzo, N.; et al. Microvascular density and endothelial area correlate with Ki-67 proliferative index in surgically-treated pancreatic ductal adenocarcinoma patients. Oncol. Lett. 2015, 10, 967–971. [Google Scholar] [CrossRef] [Green Version]
- Kanno, A.; Hatori, M.; Hosaka, M.; Kishimoto, K.N.; Watanuki, M.; Watanabe, M.; Itoi, E. Multiple bone metastasis of sclerosing epithelioid fibrosarcoma 12 years after initial surgery-increasing ki-67 labeling index. Sarcoma 2009, 2009, 953750. [Google Scholar] [CrossRef]
- Lin, X.Y.; Wang, L.; Zhang, Y.; Dai, S.D.; Wang, E.H. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn. Pathol. 2013, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Hendrick, M.J.; Brooks, J.J. Postvaccinal sarcomas in the cat: Histology and immunohistochemistry. Vet. Pathol. 1994, 31, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Labriola, A.; Achille, G.; Florio, G.; Zito, A.F.; Grammatica, L.; Paradiso, A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int. J. Oncol. 2002, 21, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Passantino, L.; Patruno, R.; Passantino, G.; Jirillo, F.; Catino, A.; Mattioli, V.; Gadaleta, C.; Ribatti, D. The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol. Rep. 2003, 10, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Montemurro, S.; Ruggieri, E.; Patruno, R.; Marech, I.; Cariello, M.; Vacca, A.; et al. Correlation between serum tryptase, mast cells positive to tryptase and microvascular density in colo-rectal cancer patients: Possible biological-clinical significance. PLoS ONE 2014, 9, e99512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammendola, M.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Zizzo, N.; Patruno, R.; Crovace, A.; Ruggieri, E.; Zito, A.F.; et al. Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. Int. J. Mol. Sci. 2015, 16, 3237–3250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammendola, M.; Patruno, R.; Sacco, R.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Zizzo, N.; Gadaleta, C.; Porcelli, M.; et al. Mast cells positive to tryptase and tumour-associated macrophages correlate with angiogenesis in locally advanced colorectal cancer patients undergone to surgery. Expert Opin. Ther. Targets 2016, 20, 533–540. [Google Scholar] [CrossRef]
- Ranieri, G.; Ammendola, M.; Patruno, R.; Celano, G.; Zito, F.A.; Montemurro, S.; Rella, A.; Di Lecce, V.; Gadaleta, C.D.; Battista De Sarro, G.; et al. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int. J. Oncol. 2009, 35, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Lyu, H.G.; Haider, A.H.; Landman, A.B.; Raut, C.P. The opportunities and shortcomings of using big data and national databases for sarcoma research. Cancer 2019, 125, 2926–2934. [Google Scholar] [CrossRef]
- Wibmer, C.; Leithner, A.; Zielonke, N.; Sperl, M.; Windhager, R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann. Oncol. 2010, 21, 1106–1111. [Google Scholar] [CrossRef]
- Folpe, A.L. Fibrosarcoma: A review and update. Histopathology 2014, 64, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Therapeutic Targets for Bone and Soft-Tissue Sarcomas. Int. J. Mol. Sci. 2019, 20, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, C.M. Cats, Cancer and Comparative Oncology. Vet. Sci. 2015, 2, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Paoloni, M.; Mazcko, C.; Khanna, C. The Comparative Oncology Trials Consortium: Using Spontaneously Occurring Cancers in Dogs to Inform the Cancer Drug Development Pathway. PLoS Med. 2009, 6, e1000161. [Google Scholar] [CrossRef]
- Paoloni, M.C.; Khanna, C. Comparative oncology today. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 1023–1032. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, G.; Gadaleta, C.D.; Patruno, R.; Zizzo, N.; Daidone, M.G.; Hansson, M.G.; Paradiso, A.; Ribatti, D. A model of study for human cancer: Spontaneous occurring tumors in dogs. Biological features and translation for new anticancer therapies. Crit. Rev. Oncol. Hematol. 2013, 88, 187–197. [Google Scholar] [CrossRef]
- Marech, I.; Gadaleta, C.D.; Ranieri, G. Possible prognostic and therapeutic significance of c-Kit expression, mast cell count and microvessel density in renal cell carcinoma. Int. J. Mol. Sci. 2014, 15, 13060–13076. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. Lond. Ser. Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Marech, I.; Ammendola, M.; Leporini, C.; Patruno, R.; Luposella, M.; Zizzo, N.; Passantino, G.; Sacco, R.; Farooqi, A.A.; Zuccalà, V.; et al. C-Kit receptor and tryptase expressing mast cells correlate with angiogenesis in breast cancer patients. Oncotarget 2018, 9, 7918–7927. [Google Scholar] [CrossRef] [Green Version]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Zuccalà, V.; Romano, R.; Luposella, M.; Patruno, R.; Vallicelli, C.; Verdecchia, G.M.; et al. Mast Cells Positive to Tryptase and c-Kit Receptor Expressing Cells Correlates with Angiogenesis in Gastric Cancer Patients Surgically Treated. Gastroenterol. Res. Pract. 2013, 2013, 703163. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zou, S.; Wang, C.; Xun, J. Clinicopathological implications of VEGF/VEGFR2 expression and microvessel density in soft tissue sarcoma. Int. J. Clin. Exp. Med. 2017, 10, 13500–13508. [Google Scholar]
- Yudoh, K.; Kanamori, M.; Ohmori, K.; Yasuda, T.; Aoki, M.; Kimura, T. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br. J. Cancer 2001, 84, 1610–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; O’Reilly, M.S.; Marshall, B.; Flynn, E.; Ji, R.W.; Folkman, J. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J. Clin. Investig. 1998, 101, 1055–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.; Al-Saleem, T.; Brooks, J.J.; Rogatko, A.; Kraybill, W.G.; Eisenberg, B. Vascular endothelial growth factor and soft tissue sarcomas: Tumor expression correlates with grade. Ann. Surg. Oncol. 2001, 8, 260–267. [Google Scholar] [CrossRef]
- Ranieri, G.; Ammendola, M.; Marech, I.; Laterza, A.; Abbate, I.; Oakley, C.; Vacca, A.; Sacco, R.; Gadaleta, C.D. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J. Gastroenterol. 2015, 21, 6018–6025. [Google Scholar] [CrossRef]
- Patruno, R.; Arpaia, N.; Gadaleta, C.D.; Passantino, L.; Zizzo, N.; Misino, A.; Lucarelli, N.M.; Catino, A.; Valerio, P.; Ribatti, D.; et al. VEGF concentration from plasma-activated platelets rich correlates with microvascular density and grading in canine mast cell tumour spontaneous model. J. Cell. Mol. Med. 2009, 13, 555–561. [Google Scholar] [CrossRef]
- Zizzo, N.; Patruno, R.; Zito, F.A.; Di Summa, A.; Tinelli, A.; Troilo, S.; Misino, A.; Ruggieri, E.; Goffredo, V.; Gadaleta, C.D.; et al. Vascular endothelial growth factor concentrations from platelets correlate with tumor angiogenesis and grading in a spontaneous canine non-Hodgkin lymphoma model. Leuk. Lymphoma 2010, 51, 291–296. [Google Scholar] [CrossRef]
- Ranieri, G.; Coviello, M.; Patruno, R.; Valerio, P.; Martino, D.; Milella, P.; Catalano, V.; Scotto, F.; De Ceglie, A.; Quaranta, M.; et al. Vascular endothelial growth factor concentrations in the plasma-activated platelets rich (P-APR) of healthy controls and colorectal cancer patients. Oncol. Rep. 2004, 12, 817–820. [Google Scholar] [CrossRef]
- Ranieri, G.; Laface, C.; Laforgia, M.; De Summa, S.; Porcelli, M.; Macina, F.; Ammendola, M.; Molinari, P.; Lauletta, G.; Di Palo, A.; et al. Bevacizumab Plus FOLFOX-4 Combined With Deep Electro-Hyperthermia as First-line Therapy in Metastatic Colon Cancer: A Pilot Study. Front. Oncol. 2020, 10, 590707. [Google Scholar] [CrossRef]
- Jang, H.K.; Kim, B.S.; Han, J.; Yoon, J.K.; Lee, J.R.; Jeong, G.J.; Shin, J.Y. Therapeutic angiogenesis using tumor cell-conditioned medium. Biotechnol. Prog. 2016, 32, 456–464. [Google Scholar] [CrossRef]
- Hanyu, A.; Kojima, K.; Hatake, K.; Nomura, K.; Murayama, H.; Ishikawa, Y.; Miyata, S.; Ushijima, M.; Matsuura, M.; Ogata, E.; et al. Functional in vivo optical imaging of tumor angiogenesis, growth, and metastasis prevented by administration of anti-human VEGF antibody in xenograft model of human fibrosarcoma HT1080 cells. Cancer Sci. 2009, 100, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, S.-A.; Lee, H.-J.; Jeong, S.-J.; Han, I.; Jung, J.H.; Lee, E.-O.; Zhu, S.; Chen, C.-Y.; Kim, S.-H. Paeonol Oxime Inhibits bFGF-Induced Angiogenesis and Reduces VEGF Levels in Fibrosarcoma Cells. PLoS ONE 2010, 5, e12358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, A.; Arii, S.; Furutani, M.; Hanaki, K.; Takeda, Y.; Moriga, T.; Kondo, Y.; Gorrin Rivas, M.J.; Imamura, M. Vascular endothelial growth factor-induced tumor angiogenesis and tumorigenicity in relation to metastasis in a HT1080 human fibrosarcoma cell model. Int. J. Cancer 1999, 80, 738–743. [Google Scholar] [CrossRef]
- Ranieri, G.; Patruno, R.; Ruggieri, E.; Montemurro, S.; Valerio, P.; Ribatti, D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: From the biology to the clinic. Curr. Med. Chem. 2006, 13, 1845–1857. [Google Scholar] [CrossRef]
- Eisenthal, A.; Schwartz, I.; Issakov, J.; Klausner, Y.; Misonzhnik, F.; Lifschitz-Mercer, B. Immunohistochemistry Evaluation of the Effect in Vivo of Tumor Necrosis Factor (TNF)-alpha on Blood Vessel Density in Murine Fibrosarcoma. Sarcoma 2003, 7, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Nissen, L.J.; Cao, R.; Hedlund, E.M.; Wang, Z.; Zhao, X.; Wetterskog, D.; Funa, K.; Bråkenhielm, E.; Cao, Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J. Clin. Investig. 2007, 117, 2766–2777. [Google Scholar] [CrossRef] [Green Version]
- Gadbail, A.R.; Hande, A.; Chaudhary, M.; Nikam, A.; Gawande, M.; Patil, S.; Tekade, S.; Gondivkar, S. Tumor angiogenesis in keratocystic odontogenic tumor assessed by using CD-105 antigen. J. Oral Pathol. Med. 2011, 40, 263–269. [Google Scholar] [CrossRef]
- Sharma, S.G.; Aggarwal, N.; Gupta, S.D.; Singh, M.K.; Gupta, R.; Dinda, A.K. Angiogenesis in renal cell carcinoma: Correlation of microvessel density and microvessel area with other prognostic factors. Int. Urol. Nephrol. 2011, 43, 125–129. [Google Scholar] [CrossRef]
- Hershey, A.E.; Sorenmo, K.U.; Hendrick, M.J.; Shofer, F.S.; Vail, D.M. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986–1996). J. Am. Vet. Med. Assoc. 2000, 216, 58–61. [Google Scholar] [CrossRef]
- Rudmann, D.G.; Van Alstine, W.G.; Doddy, F.; Sandusky, G.E.; Barkdull, T.; Janovitz, E.B. Pulmonary and mediastinal metastases of a vaccination-site sarcoma in a cat. Vet. Pathol. 1996, 33, 466–469. [Google Scholar] [CrossRef]




| Variable | No. of Patients |
|---|---|
| Age years | |
| <8 | 34 |
| >8 | 65 |
| Gender | |
| Male | 44 |
| Female | 55 |
| Tumor site | Interscapular area (40) |
| Dorsal area (21) | |
| Costal area (15) | |
| Left Scapular area (12) | |
| Right Scapular area (7) | |
| Left neck (2) | |
| Thigh sx (2) | |
| Histological grade | |
| G1 | 32 |
| G2 | 38 |
| G3 | 29 |
| Average time from vaccine injection to diagnosis | 2.1 years |
| No. of Patients (%) | MVD ×400 | EA ×400 | Ki-67 Index in Terms of MIB-1 Positive Nuclei ×400 |
|---|---|---|---|
| G1 (32/99–32.3%) | 9 ± 4 | 81.96 × 10−2 ± 20.30 μm2 | 9.35 ± 5.82 |
| G2 (38/99–38.4%) | 12 ± 5 | 83.01 × 10−2 ± 25.48 μm2 | 15.16 ± 8.91 |
| G3 (29/99–29.3%) | 25 ± 9 | 151.77 × 10−2 ± 33.52 μm2 | 44.33 ± 17.91 |
| ANOVA test | G1 vs. G2 | G1 vs. G2 | G1 vs. G2 |
| n.s. | n.s. | n.s. | |
| G1 vs. G3 | G1 vs. G3 | G1 vs. G3 | |
| f = 282.80 | f = 288.75 | f = 340.83 | |
| p = 3.53 × 10−24 | p = 2.12× 10−24 | p = 3.41 × 10−26 | |
| G2 vs. G3 | G2 vs. G3 | G2 vs. G3 | |
| f = 196.54 | f = 247.24 | f = 214.29 | |
| p = 2.53 × 10−21 | p = 7.79. × 10−24 | p = 2.97 × 10−22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patruno, R.; Passantino, G.; Laface, C.; Tinelli, A.; Zito, A.; Ruggieri, R.; Luposella, F.; Gadaleta, P.; Laforgia, M.; Lacitignola, L.; et al. Microvascular Density, Endothelial Area, and Ki-67 Proliferative Index Correlate Each Other in Cat Post-Injection Fibrosarcoma. Cells 2021, 10, 31. https://doi.org/10.3390/cells10010031
Patruno R, Passantino G, Laface C, Tinelli A, Zito A, Ruggieri R, Luposella F, Gadaleta P, Laforgia M, Lacitignola L, et al. Microvascular Density, Endothelial Area, and Ki-67 Proliferative Index Correlate Each Other in Cat Post-Injection Fibrosarcoma. Cells. 2021; 10(1):31. https://doi.org/10.3390/cells10010031
Chicago/Turabian StylePatruno, Rosa, Giuseppe Passantino, Carmelo Laface, Antonella Tinelli, Alfredo Zito, Roberta Ruggieri, Francesco Luposella, Pietro Gadaleta, Mariarita Laforgia, Luca Lacitignola, and et al. 2021. "Microvascular Density, Endothelial Area, and Ki-67 Proliferative Index Correlate Each Other in Cat Post-Injection Fibrosarcoma" Cells 10, no. 1: 31. https://doi.org/10.3390/cells10010031
APA StylePatruno, R., Passantino, G., Laface, C., Tinelli, A., Zito, A., Ruggieri, R., Luposella, F., Gadaleta, P., Laforgia, M., Lacitignola, L., Ammendola, M., Ranieri, G., & Zizzo, N. (2021). Microvascular Density, Endothelial Area, and Ki-67 Proliferative Index Correlate Each Other in Cat Post-Injection Fibrosarcoma. Cells, 10(1), 31. https://doi.org/10.3390/cells10010031








