Human Adipose-Derived Stem Cells in Madelung’s Disease: Morphological and Functional Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Donors
2.2. Primary Cell Line Isolation, Culture, and Conditioning
2.3. Cell Proliferation Assays
2.4. Flow-Cytometry
2.5. Adipogenic Differentiation
2.6. Immunofluorescence
2.7. DNA Extraction and Amplification of Mitochondrial Genome
2.8. SNP Genotyping Analysis by Sanger Sequencing
- mtDNA FW: 5′-TAGCATTAACCTTTTAAGTT
- mtDNA RV: 5′-CCTTTAGTGTTGTGTATGGT
2.9. Statistical Analysis
3. Results
3.1. Clinical and Histological Features of Madelung Patients
3.2. Morphological and Functional Characterization of MD-ASC and ASC
3.3. MD-ASC Secretome Is Able to Trigger ASC/MD-ASC Switch
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iglesias, L.; Pérez-Llantada, E.; Saro, G.; Pino, M.; Hernández, J.L. Benign Symmetric Lipomatosis (Madelung’s Disease). Eur. J. Intern. Med. 2000, 11, 171–173. [Google Scholar] [CrossRef]
- González-García, R.; Rodríguez-Campo, F.J.; Sastre-Pérez, J.; Muñoz-Guerra, M.F. Benign Symmetric Lipomatosis (Madelung’s Disease): Case Reports and Current Management. Aesth. Plast. Surg. 2004, 28, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.-C.; Huang, M.-H.; Perng, C.-K.; Liao, W.-C. Madelung Disease: Analysis of Clinicopathological Experience in Taipei Veterans General Hospital. Ann. Plast. Surg. 2019, 82, S66–S71. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, L.; Yang, W.; Wang, C.; Hu, G.; Mo, Z. Profiling of Differentially Expressed Genes in Adipose Tissues of Multiple Symmetric Lipomatosis. Mol. Med. Rep. 2017, 16, 6570–6579. [Google Scholar] [CrossRef] [Green Version]
- Szewc, M.; Sitarz, R.; Moroz, N.; Maciejewski, R.; Wierzbicki, R. Madelung’s Disease—Progressive, Excessive, and Symmetrical Deposition of Adipose Tissue in the Subcutaneous Layer: Case Report and Literature Review. Diabetes Metab. Syndr. Obes. 2018, 11, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Herbst, K.L. Subcutaneous Adipose Tissue Diseases: Dercum Disease, Lipedema, Familial Multiple Lipomatosis, and Madelung Disease. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, H.J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Schiltz, D.; Tschernitz, S.; Ortner, C.; Anker, A.; Klein, S.; Felthaus, O.; Biermann, N.; Schreml, J.; Prantl, L.; Schreml, S. Adipose Tissue in Multiple Symmetric Lipomatosis Shows Features of Brown/Beige Fat. Aesthetic Plast. Surg. 2020, 44, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Enzi, G.; Busetto, L.; Sergi, G.; Coin, A.; Inelmen, E.M.; Vindigni, V.; Bassetto, F.; Cinti, S. Multiple Symmetric Lipomatosis: A Rare Disease and Its Possible Links to Brown Adipose Tissue. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 347–353. [Google Scholar] [CrossRef]
- Lindner, A.; Marbach, F.; Tschernitz, S.; Ortner, C.; Berneburg, M.; Felthaus, O.; Prantl, L.; Kye, M.J.; Rappl, G.; Altmüller, J.; et al. Calcyphosine-like (CAPSL) Is Regulated in Multiple Symmetric Lipomatosis and Is Involved in Adipogenesis. Sci. Rep. 2019, 9, 8444. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; He, H.; Xie, Y.; Zhao, L.; Zhao, S.; Wan, X.; Yang, W.; Mo, Z. MiR-125a-3p and MiR-483-5p Promote Adipogenesis via Suppressing the RhoA/ROCK1/ERK1/2 Pathway in Multiple Symmetric Lipomatosis. Sci. Rep. 2015, 5, 11909. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, B.M.; Roychowdhury, S.; Tang, H.; Hillian, A.D.; Feldstein, A.E.; Stahl, G.L.; Takahashi, K.; Nagy, L.E. Identification of a Cytochrome P4502E1/Bid/C1q-Dependent Axis Mediating Inflammation in Adipose Tissue after Chronic Ethanol Feeding to Mice. J. Biol. Chem. 2011, 286, 35989–35997. [Google Scholar] [CrossRef] [Green Version]
- Harvey, N.L.; Srinivasan, R.S.; Dillard, M.E.; Johnson, N.C.; Witte, M.H.; Boyd, K.; Sleeman, M.W.; Oliver, G. Lymphatic Vascular Defects Promoted by Prox1 Haploinsufficiency Cause Adult-Onset Obesity. Nat. Genet. 2005, 37, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Klopstock, T.; Naumann, M.; Seibel, P.; Shalke, B.; Reiners, K.; Reichmann, H. Mitochondrial DNA Mutations in Multiple Symmetric Lipomatosis. Mol. Cell. Biochem. 1997, 174, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Klopstock, T.; Naumann, M.; Schalke, B.; Bischof, F.; Seibel, P.; Kottlors, M.; Eckert, P.; Reiners, K.; Toyka, K.V.; Reichmann, H. Multiple Symmetric Lipomatosis: Abnormalities in Complex IV and Multiple Deletions in Mitochondrial DNA. Neurology 1994, 44, 862–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, S.L.; Cheuk-Him Ng, A.; Innes, A.M.; Wagner, J.D.; Dyment, D.A.; Tetreault, M.; Care4Rare Canada Consortium; Majewski, J.; Boycott, K.M.; Screaton, R.A.; et al. Homozygous Mutations in MFN2 Cause Multiple Symmetric Lipomatosis Associated with Neuropathy. Hum. Mol. Genet. 2015, 24, 5109–5114. [Google Scholar] [CrossRef] [Green Version]
- Zolotov, S.; Xing, C.; Mahamid, R.; Shalata, A.; Sheikh-Ahmad, M.; Garg, A. Homozygous LIPE Mutation in Siblings with Multiple Symmetric Lipomatosis, Partial Lipodystrophy, and Myopathy. Am. J. Med. Genet. 2017, 173, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Bergamin, N.; Dardis, A.; Beltrami, A.; Cesselli, D.; Rigo, S.; Zampieri, S.; Domenis, R.; Bembi, B.; Beltrami, C.A. A Human Neuronal Model of Niemann Pick C Disease Developed from Stem Cells Isolated from Patient’s Skin. Orphanet J. Rare Dis. 2013, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Bourkoula, E.; Mangoni, D.; Ius, T.; Pucer, A.; Isola, M.; Musiello, D.; Marzinotto, S.; Toffoletto, B.; Sorrentino, M.; Palma, A.; et al. Glioma-Associated Stem Cells: A Novel Class of Tumor-Supporting Cells Able to Predict Prognosis of Human Low-Grade Gliomas. Stem Cells 2014, 32, 1239–1253. [Google Scholar] [CrossRef]
- Manini, I.; Ruaro, M.E.; Sgarra, R.; Bartolini, A.; Caponnetto, F.; Ius, T.; Skrap, M.; Di Loreto, C.; Beltrami, A.P.; Manfioletti, G.; et al. Semaphorin-7A on Exosomes: A Promigratory Signal in the Glioma Microenvironment. Cancers 2019, 11, 758. [Google Scholar] [CrossRef] [Green Version]
- Avolio, E.; Meloni, M.; Spencer, H.L.; Riu, F.; Katare, R.; Mangialardi, G.; Oikawa, A.; Rodriguez-Arabaolaza, I.; Dang, Z.; Mitchell, K.; et al. Combined Intramyocardial Delivery of Human Pericytes and Cardiac Stem Cells Additively Improves the Healing of Mouse Infarcted Hearts through Stimulation of Vascular and Muscular Repair. Circ. Res. 2015, 116, e81–e94. [Google Scholar] [CrossRef] [Green Version]
- Avolio, E.; Gianfranceschi, G.; Cesselli, D.; Caragnano, A.; Athanasakis, E.; Katare, R.; Meloni, M.; Palma, A.; Barchiesi, A.; Vascotto, C.; et al. Ex Vivo Molecular Rejuvenation Improves the Therapeutic Activity of Senescent Human Cardiac Stem Cells in a Mouse Model of Myocardial Infarction. Stem Cells 2014, 32, 2373–2385. [Google Scholar] [CrossRef]
- Ius, T.; Ciani, Y.; Ruaro, M.E.; Isola, M.; Sorrentino, M.; Bulfoni, M.; Candotti, V.; Correcig, C.; Bourkoula, E.; Manini, I.; et al. An NF-ΚB Signature Predicts Low-Grade Glioma Prognosis: A Precision Medicine Approach Based on Patient-Derived Stem Cells. Neuro Oncol. 2018, 20, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A.; et al. Adipose Tissue Derived Stem Cells: In Vitro and in Vivo Analysis of a Standard and Three Commercially Available Cell-Assisted Lipotransfer Techniques. Stem Cell Res. Ther. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeppieri, M.; Salvetat, M.L.; Beltrami, A.P.; Cesselli, D.; Bergamin, N.; Russo, R.; Cavaliere, F.; Varano, G.P.; Alcalde, I.; Merayo, J.; et al. Human Adipose-Derived Stem Cells for the Treatment of Chemically Burned Rat Cornea: Preliminary Results. Curr. Eye Res. 2013, 38, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Bergamin, N.; Gianfranceschi, G.; Vascotto, C.; Romanello, M.; Rigo, S.; Vagnarelli, G.; Faggiani, M.; Parodi, P.; Kelley, M.R.; et al. The Redox Function of APE1 Is Involved in the Differentiation Process of Stem Cells toward a Neuronal Cell Fate. PLoS ONE 2014, 9, e89232. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Diaz, Z.T.; Singer, H.J.; Mert, K.B.; Bunnell, B.A. Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in Vitro from Adipose-Derived Stem Cells. Cells 2020, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghadban, S.; Pursell, I.A.; Diaz, Z.T.; Herbst, K.L.; Bunnell, B.A. 3D Spheroids Derived from Human Lipedema ASCs Demonstrated Similar Adipogenic Differentiation Potential and ECM Remodeling to Non-Lipedema ASCs In Vitro. Int. J. Mol. Sci. 2020, 21, 8350. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Cesselli, D.; Bergamin, N.; Marcon, P.; Rigo, S.; Puppato, E.; D’Aurizio, F.; Verardo, R.; Piazza, S.; Pignatelli, A.; et al. Multipotent Cells Can Be Generated in Vitro from Several Adult Human Organs (Heart, Liver, and Bone Marrow). Blood 2007, 110, 3438–3446. [Google Scholar] [CrossRef]
- Andrews, R.M.; Kubacka, I.; Chinnery, P.F.; Lightowlers, R.N.; Turnbull, D.M.; Howell, N. Reanalysis and Revision of the Cambridge Reference Sequence for Human Mitochondrial DNA. Nat. Genet. 1999, 23, 147. [Google Scholar] [CrossRef]
- Zielińska-Kaźmierska, B.; Lewicki, M.; Manowska, B. Madelung Disease. Postep. Derm. Alergol. 2015, 5, 400–403. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, A.; Han, S.-A.; Ryu, J.-K.; Song, J.-Y. Multiple Symmetric Lipomatosis (Madelung’s Disease) Presenting as Bilateral Huge Gynecomastia. J. Breast Cancer 2014, 17, 397. [Google Scholar] [CrossRef] [Green Version]
- Lauvrud, A.T.; Kelk, P.; Wiberg, M.; Kingham, P.J. Characterization of Human Adipose Tissue-Derived Stem Cells with Enhanced Angiogenic and Adipogenic Properties. J. Tissue Eng. Regen. Med. 2017, 11, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L. Rare Adipose Disorders (RADs) Masquerading as Obesity. Acta Pharmacol. Sin. 2012, 33, 155–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitochondrial Dysfunction in Multiple Symmetrical Lipomatosis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/1650162/ (accessed on 14 October 2020).
- López-Gallardo, E.; Cammarata-Scalisi, F.; Emperador, S.; Hernández-Ainsa, C.; Habbane, M.; Vela-Sebastián, A.; Bayona-Bafaluy, M.P.; Montoya, J.; Ruiz-Pesini, E. Mitochondrial DNA Pathogenic Mutations in Multiple Symmetric Lipomatosis. Clin. Genet. 2020, 97, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Lipomatosis Incidence and Characteristics in an Italian Cohort of Mitochondrial Patients—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30873109/ (accessed on 14 October 2020).
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. Isolation of Adipose and Bone Marrow Mesenchymal Stem Cells Using CD29 and CD90 Modifies Their Capacity for Osteogenic and Adipogenic Differentiation. J. Tissue Eng. 2015, 6, 204173141559235. [Google Scholar] [CrossRef] [PubMed]
- Tremp, M.; Menzi, N.; Tchang, L.; di Summa, P.G.; Schaefer, D.J.; Kalbermatten, D.F. Adipose-Derived Stromal Cells from Lipomas: Isolation, Characterisation and Review of the Literature. Pathobiology 2016, 83, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yu, J.M.; Joo, H.J.; Kim, H.K.; Cho, H.H.; Bae, Y.C.; Jung, J.S. Role of CD9 in Proliferation and Proangiogenic Action of Human Adipose-Derived Mesenchymal Stem Cells. Pflugers Arch. Eur. J. Physiol. 2007, 455, 283–296. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, E.-C.; Son, Y.; Lee, D.-W.; Park, Y.S.; Choi, J.H.; Cho, K.-H.; Kwon, K.-S.; Kim, J.-R. CD9 Induces Cellular Senescence and Aggravates Atherosclerotic Plaque Formation. Cell Death Differ. 2020, 27, 2681–2696. [Google Scholar] [CrossRef]
- Saller, M.M.; Prall, W.C.; Docheva, D.; Schönitzer, V.; Popov, T.; Anz, D.; Clausen-Schaumann, H.; Mutschler, W.; Volkmer, E.; Schieker, M.; et al. Increased Stemness and Migration of Human Mesenchymal Stem Cells in Hypoxia Is Associated with Altered Integrin Expression. Biochem. Biophys. Res. Commun. 2012, 423, 379–385. [Google Scholar] [CrossRef]
- Dong, Q.; Gao, H.; Shi, Y.; Zhang, F.; Gu, X.; Wu, A.; Wang, D.; Chen, Y.; Bandyopadhyay, A.; Yeh, I.-T.; et al. Aging Is Associated with an Expansion of CD49fhi Mammary Stem Cells That Show a Decline in Function and Increased Transformation Potential. Aging (Albany NY) 2016, 8, 2754–2776. [Google Scholar] [CrossRef] [Green Version]
- Rider, D.A.; Nalathamby, T.; Nurcombe, V.; Cool, S.M. Selection Using the Alpha-1 Integrin (CD49a) Enhances the Multipotentiality of the Mesenchymal Stem Cell Population from Heterogeneous Bone Marrow Stromal Cells. J. Mol. Histol. 2007, 38, 449–458. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, J.; Liu, J.; Liu, Y.; Wang, L.; Jiang, R.; Diao, Z.; Yan, G.; Pèault, B.; Sun, H.; et al. Different Angiogenic Potentials of Mesenchymal Stem Cells Derived from Umbilical Artery, Umbilical Vein, and Wharton’s Jelly. Stem Cells Int. 2017, 2017, 3175748. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojanović, S.; Najman, S.; Korać, A. Stem Cells Derived from Lipoma and Adipose Tissue—Similar Mesenchymal Phenotype but Different Differentiation Capacity Governed by Distinct Molecular Signature. Cells 2018, 7, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teshima, T.; Matsuoka, A.; Shiba, M.; Dairaku, K.; Matsumoto, H.; Suzuki, R.; Koyama, H. Comparison of Properties of Stem Cells Isolated from Adipose Tissue and Lipomas in Dogs. Stem Cells Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesselli, D.; Beltrami, A.P.; D’Aurizio, F.; Marcon, P.; Bergamin, N.; Toffoletto, B.; Pandolfi, M.; Puppato, E.; Marino, L.; Signore, S.; et al. Effects of Age and Heart Failure on Human Cardiac Stem Cell Function. Am. J. Pathol. 2011, 179, 349–366. [Google Scholar] [CrossRef] [PubMed]
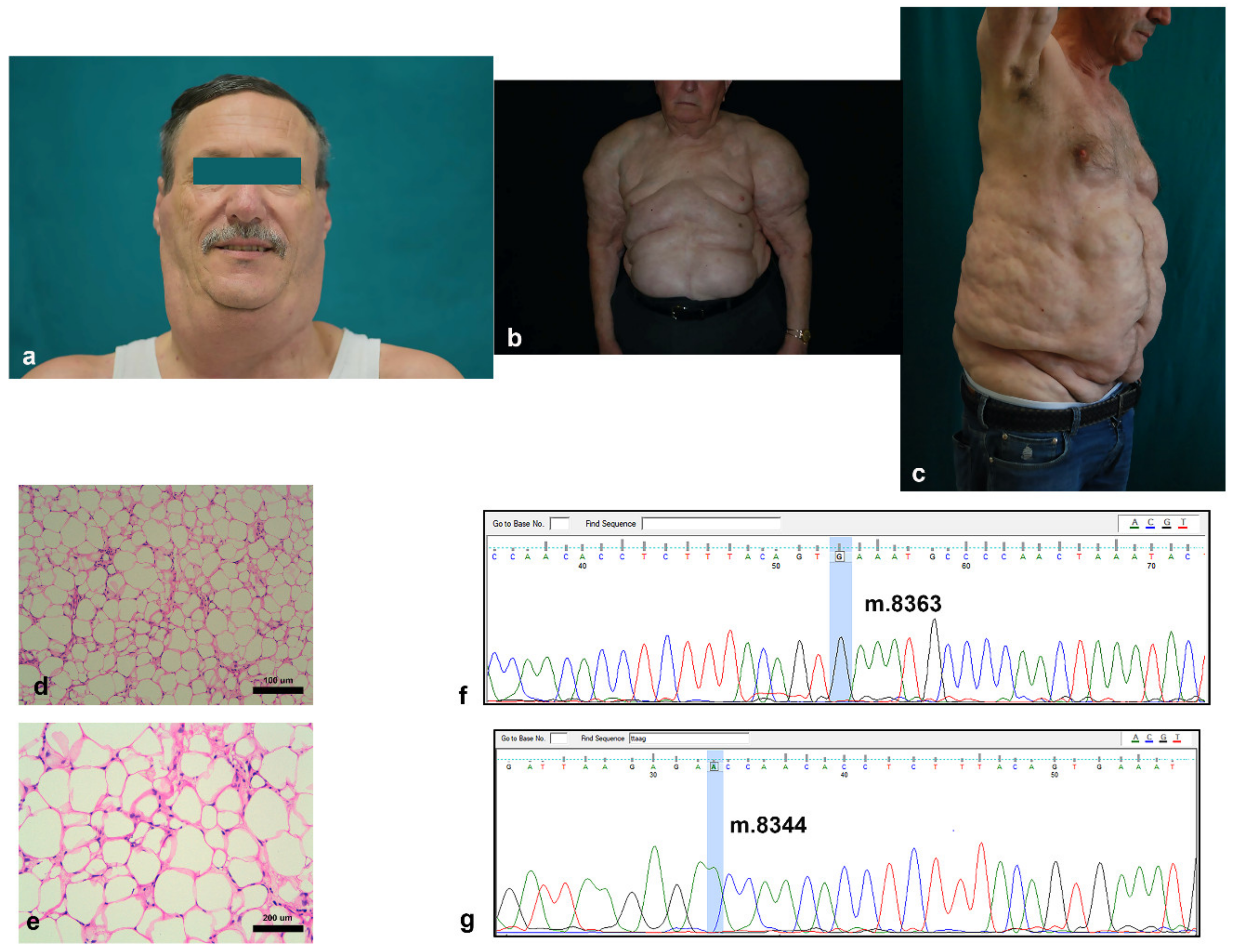
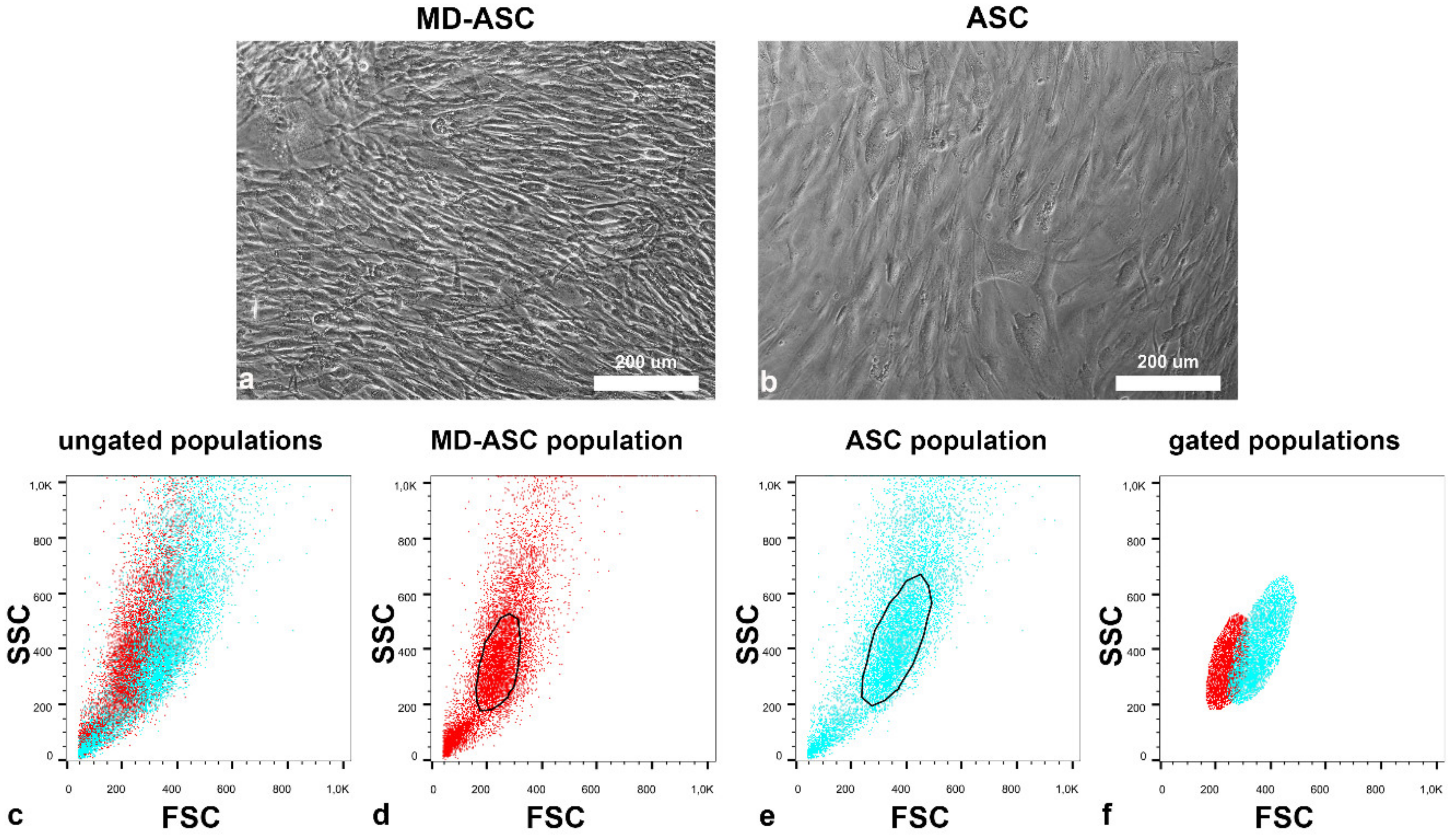


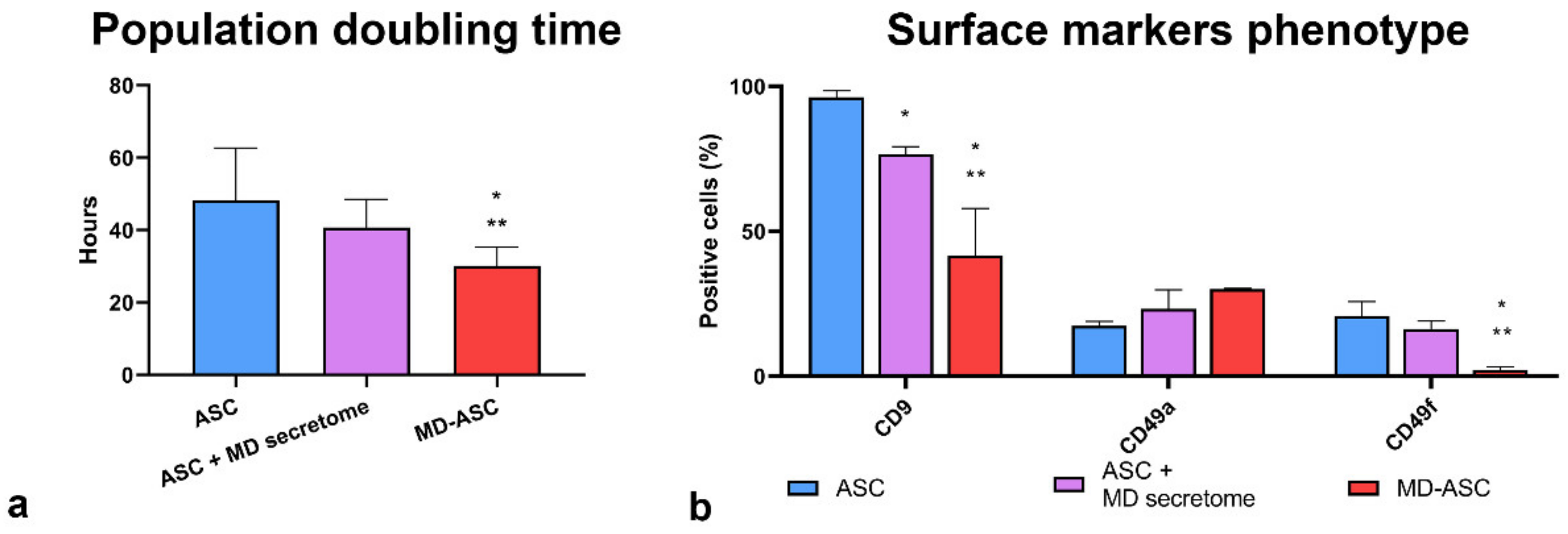
| Madelung Patients | ||
|---|---|---|
| N. of subjects | 8 | |
| Age of onset (years) (mean ± SD) | 49 ± 25 | |
| Sex; n (%) | ||
| Male | 6 (75%) | |
| Female | 2 (25%) | |
| BMI (body mass index) (kg/m2) (mean ± SD) | 29.8 ± 3.7 | |
| Localization of excess fat; n (%) | ||
| Cervical | 2 (25%) | |
| Upper chest | 4 (50%) | |
| Abdominal | 2 (25%) | |
| Smoker; n (%) | ||
| Yes | 2 (25%) | |
| No | 6 (75%) | |
| Comorbidities; n (%) | ||
| Hepatic disease | 3 (37.5%) | |
| Hypertension | 2 (25%) | |
| Diabetes mellitus | 2 (25%) | |
| Overweight | 4 (50%) | |
| Hyperuricemia | 3 (37.5%) | |
| Hyperthyroidism | 1 (12.5%) | |
| Obstructive sleep apnea | 2 (25%) | |
| Peptic ulcer | 4 (50%) |
| MD- ASC | ASC | p-Value | |
|---|---|---|---|
| CD90 | 94.43 ± 2.15 | 99.9 ± 0.01 | 0.011789 |
| CD44 | 91.36 ± 8.80 | 99.93 ± 0.05 | 0.167086 |
| CD29 | 92.56 ± 9.18 | 100 ± 0 | 0.233574 |
| CD73 | 82.16 ± 4.19 | 98.73 ± 1.59 | 0.006143 |
| CD49B | 87.5 ± 9.89 | 99.4 ± 2.17 | 0.106926 |
| CD51 | 89.6 ± 9.49 | 99.93 ± 0.05 | 0.132482 |
| CD59 | 64.86 ± 30.80 | 90 ± 3.9 | 0.231021 |
| CD146 | 45.23 ± 13.00 | 21.73 ± 11.35 | 0.077795 |
| CD49D | 46.75 ± 3.04 | 57 ± 5.65 | 0.174691 |
| CD10 | 69.6 ± 14.71 | 51.56 ± 6.85 | 0.126587 |
| CD9 | 32.25 ± 0.35 | 96.16 ± 2.4 | 0.004540 |
| CD49A | 30.05 ± 0.21 | 17.35 ± 1.48 | 0.006903 |
| CD49F | 2.13 ± 1.02 | 20.76 ± 4.98 | 0.003157 |
| CD66E | 6.26 ± 3.32 | 1.2 ± 0.70 | 0.136762 |
| CD45 | 1.33 ± 0.72 | 2.16 ± 1.87 | 0.512728 |
| CXCR4 | 4.56 ± 4.7 | 2.76 ± 3.93 | 0.637976 |
| CD38 | 0 | 0 | - |
| CD144 | 0 | 0 | - |
| CD271 | 0 | 0 | - |
| CD133 | 0 | 0 | - |
| HLA-ABC | 0 | 0 | - |
| CD117 | 0 | 0 | - |
| CD34 | 0 | 0 | - |
| ABCG2 | 0 | 0 | - |
| KDR | 0 | 0 | - |
| HLA-DR | 0 | 0 | - |
| Concentration | m.8363G ≥ A Status | m.8344A ≥ G Status | |
|---|---|---|---|
| ASC 1 | 23 ng/µL | Wildtype | Wildtype |
| ASC 2 | 50 ng/µL | Wildtype | Wildtype |
| ASC 3 | 15 ng/µL | Wildtype | Wildtype |
| MD-ASC 1 | 41 ng/µL | Wildtype | Wildtype |
| MD-ASC 2 | 18 ng/µL | Wildtype | Wildtype |
| MD-ASC 3 | 34 ng/µL | Wildtype | Wildtype |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caponnetto, F.; Manini, I.; Bulfoni, M.; Zingaretti, N.; Miotti, G.; Di Loreto, C.; Cesselli, D.; Mariuzzi, L.; Parodi, P.C. Human Adipose-Derived Stem Cells in Madelung’s Disease: Morphological and Functional Characterization. Cells 2021, 10, 44. https://doi.org/10.3390/cells10010044
Caponnetto F, Manini I, Bulfoni M, Zingaretti N, Miotti G, Di Loreto C, Cesselli D, Mariuzzi L, Parodi PC. Human Adipose-Derived Stem Cells in Madelung’s Disease: Morphological and Functional Characterization. Cells. 2021; 10(1):44. https://doi.org/10.3390/cells10010044
Chicago/Turabian StyleCaponnetto, Federica, Ivana Manini, Michela Bulfoni, Nicola Zingaretti, Giovanni Miotti, Carla Di Loreto, Daniela Cesselli, Laura Mariuzzi, and Pier Camillo Parodi. 2021. "Human Adipose-Derived Stem Cells in Madelung’s Disease: Morphological and Functional Characterization" Cells 10, no. 1: 44. https://doi.org/10.3390/cells10010044
APA StyleCaponnetto, F., Manini, I., Bulfoni, M., Zingaretti, N., Miotti, G., Di Loreto, C., Cesselli, D., Mariuzzi, L., & Parodi, P. C. (2021). Human Adipose-Derived Stem Cells in Madelung’s Disease: Morphological and Functional Characterization. Cells, 10(1), 44. https://doi.org/10.3390/cells10010044







