Usefulness of the New Hematological Parameter: Reactive Lymphocytes RE-LYMP with Flow Cytometry Markers of Inflammation in COVID-19
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Study Groups: Clinical and Blood Count Parameters
3.2. Analysis of Leukocyte and Plasmablast Subpopulations by Flow Cytometry
3.3. Lymphocyte T Subtypes with Expression of Activation Markers: CD25, CD45RO, CD38 and HLA-DR
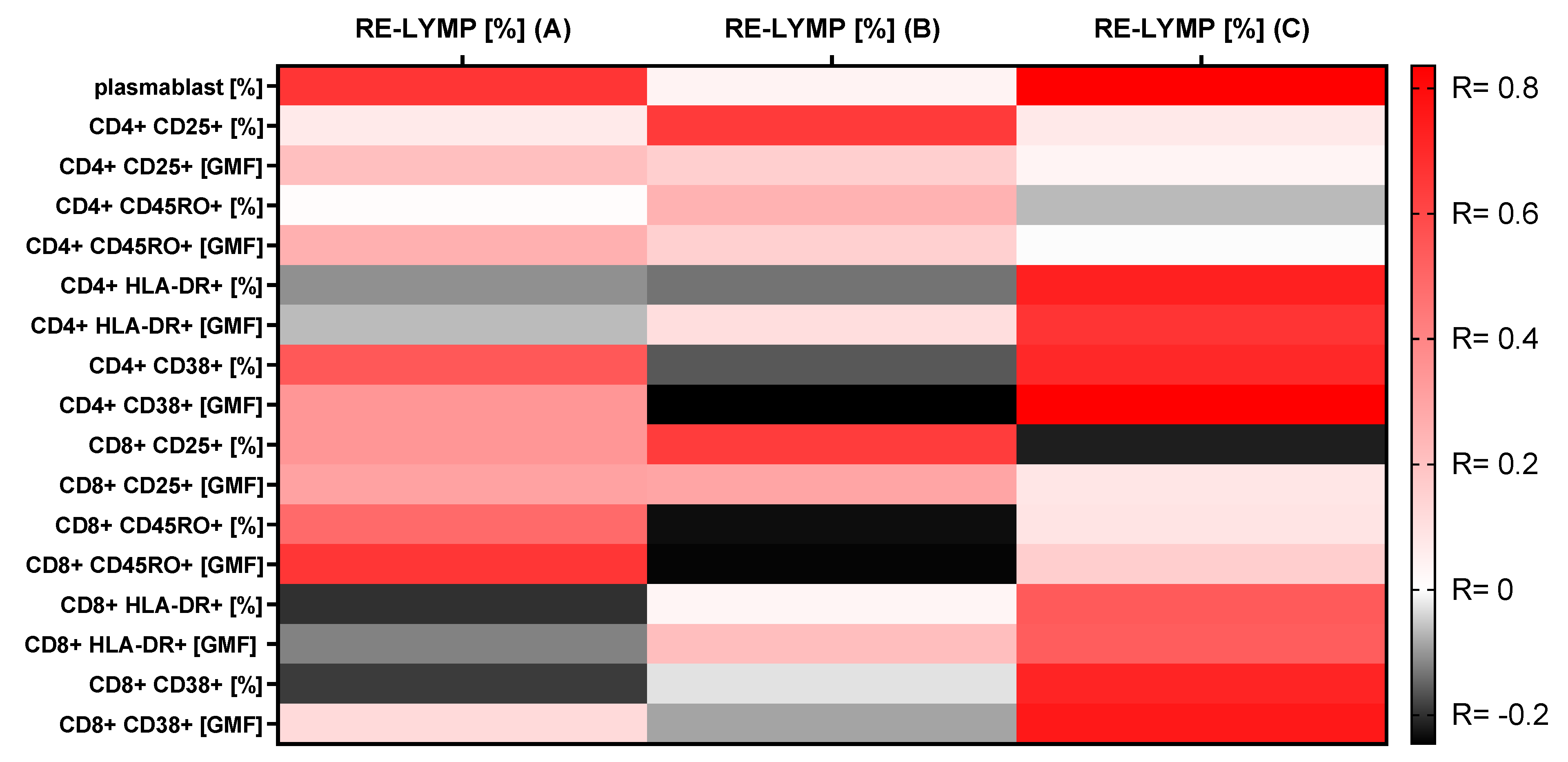
3.4. Correlations between the RE-LYMP Parameter and Activation Lymphocyte Markers
4. Discussion
4.1. Basic Morphological Parameters with Reactive Lymphocytes (RE-LYMP)
4.2. Basic Lymphocyte Subpopulations by Flow Cytometry
4.3. Plasmablast and Activation T Lymphocyte Markers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| ID | Age | f/m | Symptoms | PEMC | Oxygen Suplementation |
|---|---|---|---|---|---|
| 1 | 80 | m | Fever, dyspnea, diarrhea, fatigue | yes | no |
| 2 | 78 | f | Fever, cough, dyspnea, fatigue | yes | yes |
| 3 | 63 | m | Fever | yes | yes |
| 4 | 42 | m | Fatigue | no | no |
| 5 | 39 | f | Fatigue | no | no |
| 6 | 44 | m | Fever, cough, dyspnea, | yes | no |
| 7 | 37 | f | Fever, cough, dyspnea, diarrhea | yes | no |
| 8 | 35 | f | Fever, cough, dyspnea, fatigue | no | no |
| 9 | 57 | m | Fever, cough, dyspnea, | no | no |
| 10 | 78 | f | Fever, cough | yes | no |
| 11 | 39 | f | Fatigue | yes | no |
| 12 | 72 | m | Fever, cough, dyspnea, diarrhea, fatigue | yes | yes |
| 13 | 28 | m | Fever, cough, dyspnea, fatigue | no | no |
| 14 | 63 | m | Fever, cough | yes | no |
| 15 | 43 | m | Fatigue | yes | no |
| 16 | 33 | m | Fever, cough, diarrhea, fatigue | no | no |
| 17 | 67 | f | Fever, cough, dyspnea, fatigue | no | yes |
| 18 | 72 | m | Fever, cough, dyspnea, diarrhea, fatigue | no | no |
| 19 | 47 | f | Fever, cough, dyspnea, diarrhea, fatigue | no | no |
| 20 | 34 | m | Fever, cough, dyspnea, diarrhea, fatigue | no | no |


References
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Henriot, I.; Launay, E.; Boubaya, M.; Cremet, L.; Illiaquer, M.; Caillon, H.; Desjonqueres, A.; Gillet, B.; Bene, M.C.; Eveillard, M. New parameters on the hematology analyzer XN-10 (SysmexTM) allow to distinguish childhood bacterial and viral infections. Int. J. Lab. Hematol. 2017, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sysmex, E.G. Novel Haematological Parameters for Rapidly Monitoring the Immune System Response. Sysmex White Paper Infection/Inflammation; Sysmex Europe GmbH: Norderstedt, Germany, 2017; Volume 27, pp. 1–5. [Google Scholar]
- Linssen, J.; Jennissen, V.; Hildmann, J.; Reisinger, E.; Schindler, J.; Malchau, G.; Nierhaus, A.; Wielckens, K. Identification and quantification of high fluorescence-stained lymphocytes as antibody synthesizing/secreting cells using the automated routine hematology analyzer XE-2100. Cytom. Part B Clin. Cytom. 2007, 72, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.Y.C.; Yap, E.S.; De Mel, S.; Teo, W.Z.Y.; Lee, C.T.; Kan, S.; Lee, M.C.C.; Loh, W.N.H.; Lim, E.L.; Lee, S.Y. Temporal changes in immune blood cell parameters in COVID-19 infection and recovery from severe infection. Br. J. Haematol. 2020, 190, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Licenziati, S.; Corulli, M.; Canaris, A.D.; De Francesco, M.A.; Fiorentini, S.; Peroni, L.; Fallacara, F.; Dima, F.; Balsari, A.; et al. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry 1997, 27, 71–76. [Google Scholar] [CrossRef]
- Sandoval-Montes, C.; Santos-Argumedo, L. CD38 is expressed selectively during the activation of a subset of mature T cells with reduced proliferation but improved potential to produce cytokines. J. Leukoc. Biol. 2005, 77, 513–521. [Google Scholar] [CrossRef]
- Reddy, M.; Eirikis, E.; Davis, C.; Davis, H.M.; Prabhakar, U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: An in vitro model to monitor cellular immune function. J. Immunol. Methods 2004, 293, 127–142. [Google Scholar] [CrossRef]
- Norton, A.J.; Ramsay, A.D.; Smith, S.H.; Beverley, P.C.; Isaacson, P.G. Monoclonal antibody (UCHL1) that recognises normal and neoplastic T cells in routinely fixed tissues. J. Clin. Pathol. 1986, 39, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Akbar, A.N.; Terry, L.; Timms, A.; Beverley, P.C.; Janossy, G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J. Immunol. 1988, 140, 2171–2178. [Google Scholar]
- Picker, L.J.; Treer, J.R.; Ferguson-Darnell, B.; Collins, P.A.; Buck, D.; Terstappen, L.W. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory cell transition. J. Immunol. 1993, 150, 1105–1121. [Google Scholar]
- Partida-Sanchez, S.; Cockayne, D.A.; Monard, S.; Jacobson, E.L.; Oppenheimer, N.; Garvy, B.; Kusser, K.; Goodrich, S.; Howard, M.; Harmsen, A.; et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 2001, 7, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.M.; Martin, F.; Kearney, J.F. Mouse CD38 is down-regulated on germinal center B cells and mature plasma cells. J. Immunol. 1997, 158, 1108–1115. [Google Scholar] [PubMed]
- Hartman, W.R.; Pelleymounter, L.L.; Moon, I.; Kalari, K.; Liu, M.; Wu, T.Y.; Escande, C.; Nin, V.; Chini, E.N.; Weinshilboum, R.M. CD38 expression, function, and gene resequencing in a human lymphoblastoid cell line-based model system. Leuk. Lymphoma 2010, 51, 1315–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, H.E.; Yoshida, T.; Sime, W.; Hiepe, F.; Thiele, K.; Manz, R.A.; Radbruch, A.; Dorner, T. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 2009, 113, 2461–2469. [Google Scholar] [CrossRef]
- Tomkinson, B.E.; Wagner, D.K.; Nelson, D.L.; Sullivan, J.L. Activated lymphocytes during acute Epstein-Barr virus infection. J. Immunol. 1987, 139, 3802–3807. [Google Scholar]
- Arbiol-Roca, A.; Imperiali, C.E.; Montserrat, M.M.; Cerro, A.S.; de Basea, A.C.B.; Navarro, L.S.; Dot Bach, D.; Politi, J.V. Reference intervals for a complete blood count on an automated haematology analyser Sysmex XN in healthy adults from the southern metropolitan area of Barcelona. Ejifcc 2018, 29, 48–54. [Google Scholar]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Liu, W.; Tao, Z.W.; Wang, L.; Yuan, M.L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.G.; et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. (Engl.) 2020, 133, 1032–1038. [Google Scholar] [CrossRef]
- Lindsley, A.W.; Schwartz, J.T.; Rothenberg, M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H.; Wong, V.W.; Wong, C.K.; Chan, P.K.; Chu, C.M.; Hui, D.S.; Suen, M.W.; Sung, J.J.; Chung, S.S.; Lam, C.W. Serum LD1 isoenzyme and blood lymphocyte subsets as prognostic indicators for severe acute respiratory syndrome. J. Intern. Med. 2004, 255, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, R.; Li, Z.; Wang, Y.; Liu, Y.; Zheng, X.; Gao, C.; Li, X.; Wang, C. Imbalance between Th17 and regulatory T cells in patients with systemic lupus erythematosus combined EBV/CMV viraemia. Clin. Exp. Rheumatol. 2019, 38, 864–873. [Google Scholar] [PubMed]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef] [Green Version]
- Moratto, D.; Chiarini, M.; Giustini, V.; Serana, F.; Magro, P.; Roccaro, A.M.; Imberti, L.; Castelli, F.; Notarangelo, L.D.; Quiros-Roldan, E. Flow Cytometry Identifies Risk Factors and Dynamic Changes in Patients with COVID-19. J. Clin. Immunol. 2020. [Google Scholar] [CrossRef]
- Liu, Z.; Long, W.; Tu, M.; Chen, S.; Huang, Y.; Wang, S.; Zhou, W.; Chen, D.; Zhou, L.; Wang, M.; et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Fink, K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front. Immunol. 2012, 3, 78. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, T.; Bela-Ong, D.B.; Toh, Y.X.; Flamand, M.; Devi, S.; Koh, M.B.; Hibberd, M.L.; Ooi, E.E.; Low, J.G.; Leo, Y.S.; et al. Dengue virus activates polyreactive, natural IgG B cells after primary and secondary infection. PLoS ONE 2011, 6, e29430. [Google Scholar] [CrossRef]
- Wrammert, J.; Onlamoon, N.; Akondy, R.S.; Perng, G.C.; Polsrila, K.; Chandele, A.; Kwissa, M.; Pulendran, B.; Wilson, P.C.; Wittawatmongkol, O.; et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J. Virol. 2012, 86, 2911–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holling, T.M.; Schooten, E.; van Den Elsen, P.J. Function and regulation of MHC class II molecules in T-lymphocytes: Of mice and men. Hum. Immunol. 2004, 65, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Glaria, E.; Valledor, A.F. Roles of CD38 in the Immune Response to Infection. Cells 2020, 9, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, S.; Hodge, G.; Flower, R.; Han, P. Surface activation markers of T lymphocytes: Role in the detection of infection in neonates. Clin. Exp. Immunol. 1998, 113, 33–38. [Google Scholar] [CrossRef]
- Wong, R.S.; Wu, A.; To, K.F.; Lee, N.; Lam, C.W.; Wong, C.K.; Chan, P.K.; Ng, M.H.; Yu, L.M.; Hui, D.S.; et al. Haematological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. BMJ 2003, 326, 1358–1362. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Qiu, Z.; Zhang, L.; Han, Y.; He, W.; Liu, Z.; Ma, X.; Fan, H.; Lu, W.; Xie, J.; et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 2004, 189, 648–651. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Zeng, X.; Ou, A.H.; Huang, Y.; Zhang, X. Study on T cell subsets and their activated molecules from the convalescent SARS patients during two follow-up surveys. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2004, 20, 322–324. [Google Scholar]
- Letourneau, S.; Krieg, C.; Pantaleo, G.; Boyman, O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J. Allergy Clin. Immunol. 2009, 123, 758–762. [Google Scholar] [CrossRef]
- Rouas, R.; Merimi, M.; Najar, M.; El Zein, N.; Fayyad-Kazan, M.; Berehab, M.; Agha, D.; Bron, D.; Burny, A.; Rachidi, W.; et al. Human CD8(+) CD25 (+) CD127 (low) regulatory T cells: MicroRNA signature and impact on TGF-beta and IL-10 expression. J. Cell. Physiol. 2019, 234, 17459–17472. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Liu, Y.; Zhou, R.; Deng, X.; Li, F.; Liang, K.; Shi, Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020, 160, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.; Vijayalakshmi, D.; Dhivya, K.; Janane, M. Memory T Cells (CD45RO) Role and Evaluation in Pathogenesis of Lichen Planus and Lichenoid Mucositis. J. Clin. Diagn. Res. JCDR 2017, 11, ZC84–ZC86. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, I.; Rutkowska, E.; Klos, K.; Wiesik-Szewczyk, E.; Jahnz-Rozyk, K.; Rzepecki, P.; Chcialowski, A. Maturation of T and B Lymphocytes in the Assessment of the Immune Status in COVID-19 Patients. Cells 2020, 9, 2615. [Google Scholar] [CrossRef] [PubMed]
- Song, C.B.; Zhang, L.L.; Wu, X.; Fu, Y.J.; Jiang, Y.J.; Shang, H.; Zhang, Z.N. CD4(+)CD38(+) central memory T cells contribute to HIV persistence in HIV-infected individuals on long-term ART. J. Transl. Med. 2020, 18, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamadia, L.E.; Remmerswaal, E.B.; Weel, J.F.; Bemelman, F.; van Lier, R.A.; Ten Berge, I.J. Primary immune responses to human CMV: A critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 2003, 101, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Kestens, L.; Vanham, G.; Vereecken, C.; Vandenbruaene, M.; Vercauteren, G.; Colebunders, R.L.; Gigase, P.L. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin. Exp. Immunol. 1994, 95, 436–441. [Google Scholar] [CrossRef]
- Wursch, D.; Ormsby, C.E.; Romero-Rodriguez, D.P.; Olvera-Garcia, G.; Zuniga, J.; Jiang, W.; Perez-Patrigeon, S.; Espinosa, E. CD38 Expression in a Subset of Memory T Cells Is Independent of Cell Cycling as a Correlate of HIV Disease Progression. Dis. Markers 2016, 2016, 9510756. [Google Scholar] [CrossRef]
- Musyoki, S.; Mining, S.; Nyongesa, P. Level of CD8 T Lymphocytes Activation in HIV-Infected Pregnant Women: In the Context of CD38 and HLA-DR Activation Markers. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 715279. [Google Scholar] [CrossRef]
- Benito, J.M.; Lopez, M.; Lozano, S.; Martinez, P.; Gonzalez-Lahoz, J.; Soriano, V. CD38 expression on CD8 T lymphocytes as a marker of residual virus replication in chronically HIV-infected patients receiving antiretroviral therapy. AIDS Res. Hum. Retrovir. 2004, 20, 227–233. [Google Scholar] [CrossRef]
- Zidovec Lepej, S.; Vince, A.; Dakovic Rode, O.; Remenar, A.; Jeren, T. Increased numbers of CD38 molecules on bright CD8+ T lymphocytes in infectious mononucleosis caused by Epstein-Barr virus infection. Clin. Exp. Immunol. 2003, 133, 384–390. [Google Scholar] [CrossRef]
- Kim, T.S.; Shin, E.C. The activation of bystander CD8(+) T cells and their roles in viral infection. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.M.; Jamieson, S.E.; Burgner, D. HLA and infectious diseases. Clin. Microbiol. Rev. 2009, 22, 370–385. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhu, L.; Nguyen, T.H.O.; Wan, Y.; Sant, S.; Quinones-Parra, S.M.; Crawford, J.C.; Eltahla, A.A.; Rizzetto, S.; Bull, R.A.; et al. Clonally diverse CD38(+)HLA-DR(+)CD8(+) T cells persist during fatal H7N9 disease. Nat. Commun. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Kestens, L.; Vanham, G.; Gigase, P.; Young, G.; Hannet, I.; Vanlangendonck, F.; Hulstaert, F.; Bach, B.A. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS 1992, 6, 793–797. [Google Scholar] [CrossRef] [PubMed]
| Patients n = 40 | ||
|---|---|---|
| Sex: f/m (n) | 25/15 | |
| Age (mean ± SD years) | 46.2 ± 19.1 | |
| Women (mean ± SD years) | 46.1 ± 17.3 | |
| Men (mean ± SD years) | 46.4 ± 24.1 | |
| Clinical symptoms and diseases comorbidities (%) (no/yes) | ||
| 35.7/64.3 | |
| 65.2/34.8 | |
| 72.1/27.9 | |
| 84.3/15.7 | |
| 88.6/11.4 | |
| 97.2/2.8 | |
| Groups (n) | ||
| 20 | |
| 20 | |
| 9 | |
| 6 | |
| 2 | |
| 1 | |
| 2 | |
| Sysmex parameters [103/µL] [median (Q1–Q3)] | Reference values [3,17] | |
| 5.96 (3.90–7.89) | 3.9–9.5 |
| 3.24 (2.39–4.34) | 1.53–4.98 |
| 1.57 (1.12–2.54) | 1.13–3.00 |
| 0.53 (0.36–0.68) | 0.22–0.63 |
| 0.09 (0.04–0.18) | 0.03–0.29 |
| 0.03 (0.02–0.05) | 0.02–0.07 |
| 0.02 (0.01- 0.04) | 0.01–0.04 |
| 243.0 (188.0–287.0) | 153–368 |
| 0.10 (0.06–0.18) | 0–0.5 × 109/L |
| 6.5 (4.10–11.70) ↑ | 0–5% |
| Sysmex Parameters [Median (Q1–Q3)] | A. COVID-19(+) n = 20 | B. HC n = 20 | C. COVID-19(−) Viruses n = 20 | * p < 0.05 A-B-C Anova Kruskal-Wallis | * p < 0.05 in Groups Post-Hoc |
|---|---|---|---|---|---|
| 4.25 (3.59–6.72) | 6.08 (5.06–8.02) | 6.72 (4.14–9.34) | ||
| 2.84 (2.20–3.38) | 3.44 (3.12–4,70) | 3.51 (2.18–4.90) | ||
| 0.85 (0.69–1.56) | 1.72 (1.52–2.32) | 1.95 (1.25–2.93) | * p = 0.0250 | A-B, A-C |
| 0.36 (0.26–0.64) | 0.56 (0.42–0.69) | 0.57 (0.36–0.69) | ||
| 0.05 (0.00–0.08) | 0.18 (0.07–0.26) | 0.10 (0.05–0.16) | * p = 0.0038 | A-B |
| 0.02 (0.02–0.04) | 0.04 (0.02–0.05) | 0.04 (0.02–0.06) | * p = 0.0003 | A-B |
| 0.02 (0.01–0.09) | 0.01 (0.01–0.02) | 0.02 (0.01–0.04) | ||
| 196.00 (177–290) | 261.50 (230–287) | 226.50 (190–286) | ||
| 0.05 (0.04–0.09) | 0.08 (0.05–0.10) | 0.21 (0.13–0.37) | ||
| 5.45 (2.80–8.20) | 4.20 (3.10–5.00) | 11.05 (7.75–25.2) | * <0.0001 | A-C, B-C |
| Lymphocyte Subset (%) [Median (Q1–Q3)] | A. COVID-19(+) n = 20 | B. HC n = 20 | C. COVID-19(−) Viruses n = 20 | * p < 0.05 A-B-C Anova | * p < 0.05 in Group Post Hoc |
|---|---|---|---|---|---|
| Lymphocytes subsets: (% of all cells) | |||||
| Lymphocytes [%] [103/µL] | 32.6 (21.1–49.3) 0.98 (0.76–2.99) | 39.7 (34.2–44.7) 2.16 (1.75–2.73) | 36.9 (28.7–45.5) 2.25 (1.64–3.08) | * 0.0350 | A-B, A-C |
| Lymphocytes T [%] [103/µL] | 24.3 (13.9–37.5) 0.65 (0.57–2.24) | 29.6 (25.6–35.0) 1.73 (1.39–2.13) | 28.1 (23.6–34.3) 1.88 (1.25–2.40) | - - | |
| Lymphocytes T CD4 [%] [103/µL] | 13.3 (6.3–23.1) 0.48 (0.26–1.11) | 18.8 (16.1–20.7) 1.04 (0.84–1.27) | 16.5 (12.8–26.0) 1.08 (0.70–1.38) | - - | |
| Lymphocytes T CD8 [%] [103/µL] | 9.9 (4.2–12.6) 0.33 (0.16–0.86) | 11.7 (8.1–14.4) 0.70 (0.50–0.90) | 13.5 (10.1–7.4) 0.57 (0.45–0.85) | - - | |
| CD4/CD8 | 1.3 (1.0–3.5) | 1.4 (1.3–2.1) | 1.5 (1.3–0.7) | - | |
| Lymphocytes B [%] [103/µL] | 2.0 (1.4–4.7) 0.13 (0.03–0.18) | 3.3 (2.5–4.1) 0.23 (0.12–0.28) | 2.8 (2.0–4.2) 0.20 (0.12–0.28) | - - | |
| NK cells [%] [103/µL] | 5.0 (4.1–9.1) 0.18 (0.10–0.40) | 3.4 (2.5–5.6) 0.21 (0.13–0.38) | 5.0 (2.5–6.5) 0.25 (0.14–0.44) | - - | |
| Plasmablasts (% of B CD19+ cells) | 8.8 (6.1–26.5) | 2.7 (1.8–3.5) | 11.1 (2.2–26.2) | * 0.0001 | * A-B * B-C |
| Lymphocyte Subset (%) [Median (Q1–Q3)] | A. COVID-1(+) n = 20 | B. HC n = 20 | C. COVID-19(−) Viruses n = 20 | * p < 0.05 A-B-C Anova | * p < 0.05 in Group Post Hoc |
|---|---|---|---|---|---|
| CD4+ subpopulation: (% of CD4+ cells) | |||||
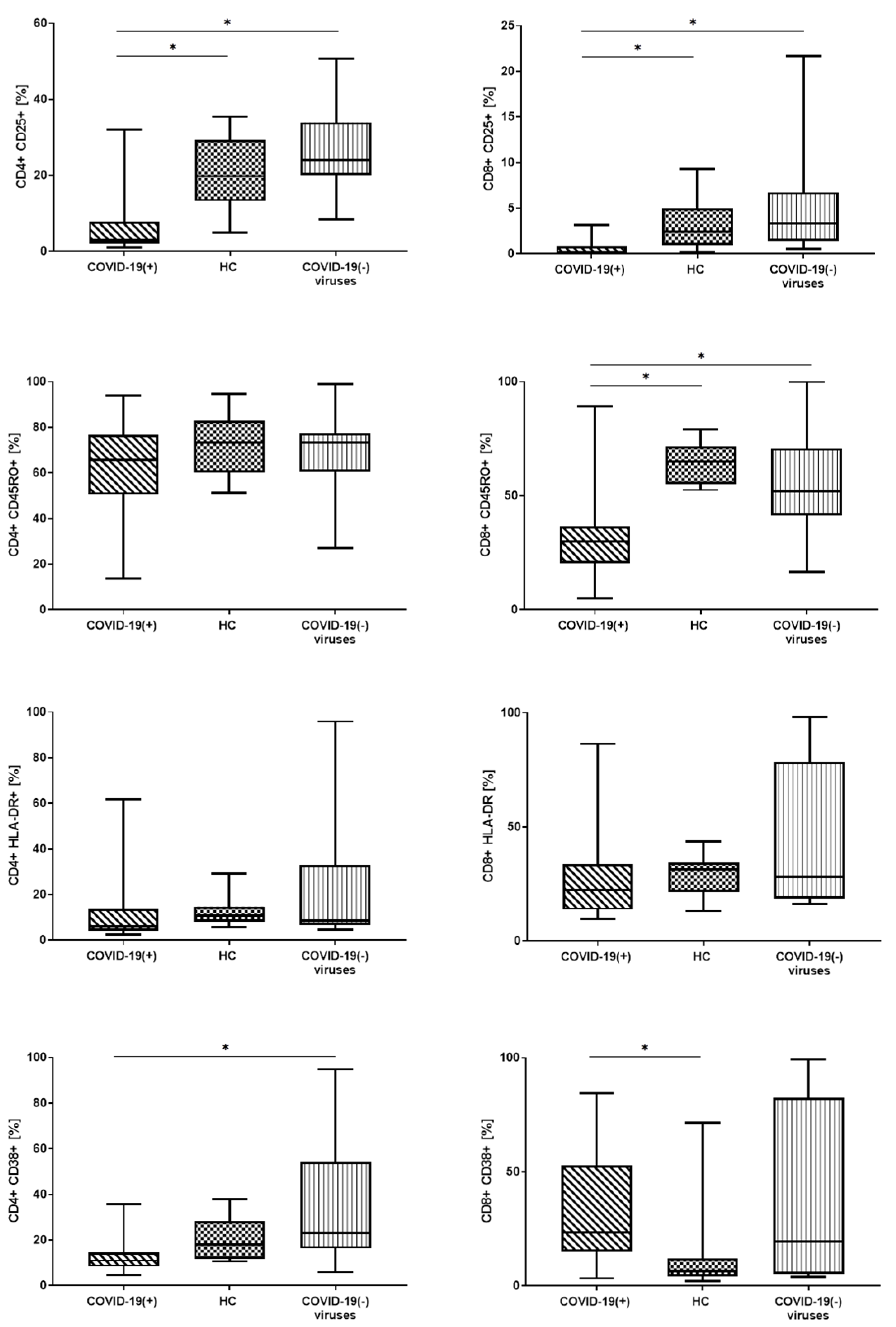
| CD4+ CD25 % | 3.0 (2.0–6.5) | 19.8 (13.2–29.1) | 24.1 (20.3–33.2) | * 0.0000 | * A-C * A-B |
| CD4+ CD25 GMF | 108.0 (96.0–135.0) | 304.5 (229.5–352.0) | 290.0 (251.0–366.5) | * 0.0120 | * A-C * A-B |
| CD4+ CD45RO % | 65.7 (52.2–75.3) | 73.2 (61.3–82.3) | 73.3 (61.4–77.0) | - | - |
| CD4+ CD45RO GMF | 2437.5 (1647.0–4373.0) | 4807.5 (3194.5–7098.5) | 5280.5 (3651.0–7793.5) | * 0.0129 | * A-C * A-B |
| CD4+ HLA-DR+ % | 6.1(4.2–12.9) | 10.7 (8.3–14.5) | 8.7 (7.0–31.0) | - | - |
| CD4+ HLA-DR+ GMF | 118.0 (105.0–158.0) | 137.5 (124.5–159.0) | 148.5 (122.5–386.0) | - | - |
| CD4+ CD38+ % | 11.0 (9.0–13.5) | 18.0 (11.9–27.4) | 23.0 (16.5–53.1) | * 0.0050 | * A-C |
| CD4+ CD38+ GMF | 313.0 (262.0–390.0) | 232.5 (185.5–286.0) | 317.0 (216.5–865.0) | - | - |
| CD8+ subpopulation: (% of CD8+ cells) | |||||
| CD8+ CD25+ % | 0.2 (0.0–1.2) | 2.5 (0.9–4.5) | 3.4 (1.4–6.6) | * 0.0009 | * A-C * A-B |
| CD8+ CD25+ GMF | 80.5 (63.0–101.0) | 121.0 (112.0–138.0) | 127.5 (119.0–144.5) | * 0.0001 | * A-C * A-B |
| CD8+ CD45RO+ % | 30.0 (20.8–35.9) | 65.0 (55.9–71.5) | 51.9 (42.9–66.4) | * 0.0001 | * A-C * A-B |
| CD8+ CD45RO+ GMF | 796.0 (571.0–1293.0) | 2683.5 (1922.0–3735.5) | 2136.0 (1313.5–3061) | * 0.0002 | * A-C * A-B |
| CD8+ HLA-DR+ % | 22.5 (14.5–31.3) | 31.5 (23.3–34.3) | 28.2 (18.6–76.3) | - | - |
| CD8+ HLA-DR+ GMF | 200.5 (165.0–307.0) | 262.5 (206.5–298.0) | 246.0 (183.0–3613.5) | - | - |
| CD8+ CD38+ % | 23.3 (15.1–49.5) | 6.3 (4.3–11.0) | 19.6 (5.4–80.0) | * 0.0222 | * A-B |
| CD8+ CD38+ GMF | 349.0 (233.0–589.0) | 137.0 (127.0–177.0) | 229.0 (131.5–2000.5) | * 0.0050 | * A-B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, E.; Kwiecień, I.; Kulik, K.; Chełstowska, B.; Kłos, K.; Rzepecki, P.; Chciałowski, A. Usefulness of the New Hematological Parameter: Reactive Lymphocytes RE-LYMP with Flow Cytometry Markers of Inflammation in COVID-19. Cells 2021, 10, 82. https://doi.org/10.3390/cells10010082
Rutkowska E, Kwiecień I, Kulik K, Chełstowska B, Kłos K, Rzepecki P, Chciałowski A. Usefulness of the New Hematological Parameter: Reactive Lymphocytes RE-LYMP with Flow Cytometry Markers of Inflammation in COVID-19. Cells. 2021; 10(1):82. https://doi.org/10.3390/cells10010082
Chicago/Turabian StyleRutkowska, Elżbieta, Iwona Kwiecień, Katarzyna Kulik, Beata Chełstowska, Krzysztof Kłos, Piotr Rzepecki, and Andrzej Chciałowski. 2021. "Usefulness of the New Hematological Parameter: Reactive Lymphocytes RE-LYMP with Flow Cytometry Markers of Inflammation in COVID-19" Cells 10, no. 1: 82. https://doi.org/10.3390/cells10010082
APA StyleRutkowska, E., Kwiecień, I., Kulik, K., Chełstowska, B., Kłos, K., Rzepecki, P., & Chciałowski, A. (2021). Usefulness of the New Hematological Parameter: Reactive Lymphocytes RE-LYMP with Flow Cytometry Markers of Inflammation in COVID-19. Cells, 10(1), 82. https://doi.org/10.3390/cells10010082






