Dual Inhibition of FAAH and MAGL Counteracts Migraine-like Pain and Behavior in an Animal Model of Migraine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Treatment
2.3. Open Field Test
2.4. Orofacial Formalin Test
2.5. Tissue and Blood Sample Collection
2.6. rt-PCR
2.7. Statistical Analysis
3. Results
3.1. Open Field Test
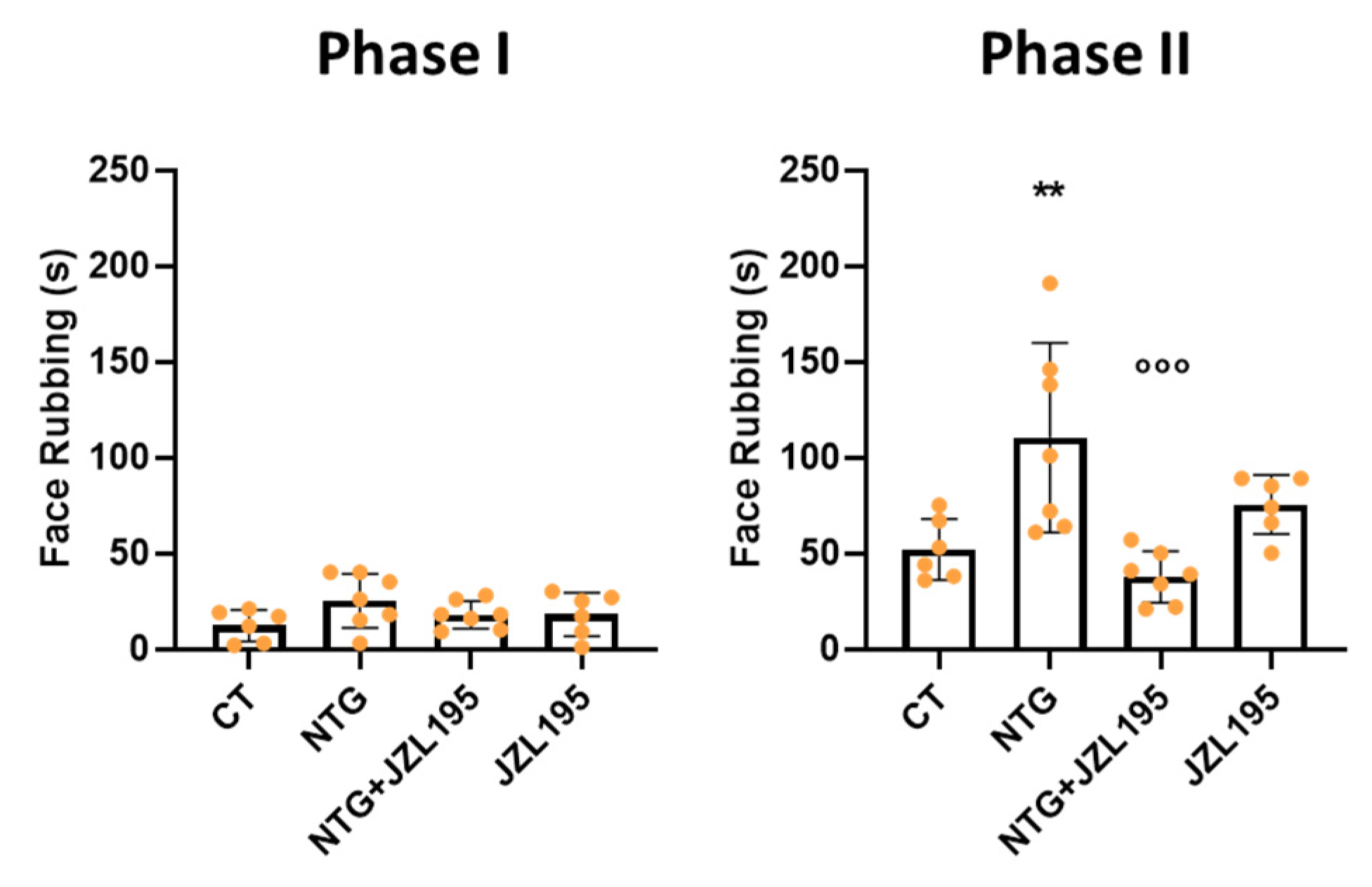
3.2. Orofacial Formalin Test
3.3. Molecular and Biochemical Changes Induced by JZL195
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guindon, J.; Hohmann, A. The Endocannabinoid System and Pain. CNS Neurol. Disord. Drug Targets 2012, 8, 403–421. [Google Scholar] [CrossRef]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and pain: A clinical review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertwee, R.G. Cannabinoid receptors and pain. Prog. Neurobiol. 2001, 63, 569–611. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Guindon, J.; Desroches, J.; Beaulieu, P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br. J. Pharmacol. 2007, 150, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Kathuria, S.; Gaetani, S.; Fegley, D.; Valiño, F.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; La Rana, G.; Calignano, A.; et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003, 9, 76–81. [Google Scholar] [CrossRef]
- Long, J.Z.; Li, W.; Booker, L.; Burston, J.J.; Kinsey, S.G.; Schlosburg, J.E.; Pavón, F.J.; Serrano, A.M.; Selley, D.E.; Parsons, L.H.; et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009, 5, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.; Smith, S.E.; Liimatta, M.B.; Beidler, D.; Sadagopan, N.; Dudley, D.T.; Young, T.; Wren, P.; Zhang, Y.; Swaney, S.; et al. Mechanistic and pharmacological characterization of PF-04457845: A highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J. Pharmacol. Exp. Ther. 2011, 338, 114–124. [Google Scholar] [CrossRef]
- Anderson, W.B.; Gould, M.J.; Torres, R.D.; Mitchell, V.A.; Vaughan, C.W. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine inflammatory pain model. Neuropharmacology 2014, 81, 224–230. [Google Scholar] [CrossRef]
- Sakin, Y.S.; Dogrul, A.; Ilkaya, F.; Seyrek, M.; Ulas, U.H.; Gulsen, M.; Bagci, S. The effect of FAAH, MAGL, and Dual FAAH/MAGL inhibition on inflammatory and colorectal distension-induced visceral pain models in Rodents. Neurogastroenterol. Motil. 2015, 27, 936–944. [Google Scholar] [CrossRef]
- Woodhams, S.G.; Sagar, D.R.; Burston, J.J.; Chapman, V. The role of the endocannabinoid system in pain. Handb. Exp. Pharmacol. 2015, 227, 119–143. [Google Scholar]
- Pertwee, R.G. Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3353–3363. [Google Scholar] [CrossRef]
- Adamson Barnes, N.S.; Mitchell, V.A.; Kazantzis, N.P.; Vaughan, C.W. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine neuropathic pain model. Br. J. Pharmacol. 2016, 173, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Zubrzycki, M.; Janecka, A.; Liebold, A.; Ziegler, M.; Zubrzycka, M. Effects of centrally administered endocannabinoids and opioids on orofacial pain perception in rats. Br. J. Pharmacol. 2017, 174, 3780–3789. [Google Scholar] [CrossRef] [Green Version]
- Goadsby, P.J.; Charbit, A.R.; Andreou, A.P.; Akerman, S.; Holland, P.R. Neurobiology of migraine. Neuroscience 2009, 161, 327–341. [Google Scholar] [CrossRef]
- Levy, D. Migraine pain, meningeal inflammation, and mast cells. Curr. Pain Headache Rep. 2009, 13, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Holland, P.R.; Lasalandra, M.P.; Goadsby, P.J. Endocannabinoids in the brainstem modulate dural trigeminovascular nociceptive traffic via CB1 and “triptan” receptors: Implications in migraine. J. Neurosci. 2013, 33, 14869–14877. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Grócz, G.; Tar, L.; Bohár, Z.; Fejes-Szabó, A.; Laborc, K.F.; Spekker, E.; Vécsei, L.; Párdutz, Á. The modulatory effect of anandamide on nitroglycerin-induced sensitization in the trigeminal system of the rat. Cephalalgia 2016, 36, 849–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cupini, L.M.; Bari, M.; Battista, N.; Argirò, G.; Finazzi-Agrò, A.; Calabresi, P.; Maccarrone, M. Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia 2006, 26, 277–281. [Google Scholar] [CrossRef]
- Cupini, L.M.; Costa, C.; Sarchielli, P.; Bari, M.; Battista, N.; Eusebi, P.; Calabresi, P.; Maccarrone, M. Degradation of endocannabinoids in chronic migraine and medication overuse headache. Neurobiol. Dis. 2008, 30, 186–189. [Google Scholar] [CrossRef]
- Perrotta, A.; Arce-Leal, N.; Tassorelli, C.; Gasperi, V.; Sances, G.; Blandini, F.; Serrao, M.; Bolla, M.; Pierelli, F.; Nappi, G.; et al. Acute reduction of anandamide-hydrolase (FAAH) activity is coupled with a reduction of nociceptive pathways facilitation in medication-overuse headache subjects after withdrawal treatment. Headache 2012, 52, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Pini, L.A.; Coppola, F.; Rossi, C.; Baldi, A.; Mancini, M.L.; Calabresi, P. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology 2007, 32, 1384–1390. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Piomelli, D.; Tassorelli, C. Endocannabinoid System and Migraine Pain: An Update. Front. Neurosci. 2018, 12, 172. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Tumelero, E.; Icco, R.; Sances, G.; Allena, M.; Tassorelli, C. Peripheral changes of endocannabinoid system components in episodic and chronic migraine patients: A pilot study. Cephalalgia 2021, 41, 185–196. [Google Scholar] [CrossRef]
- Nozaki, C.; Markert, A.; Zimmer, A. Inhibition of FAAH reduces nitroglycerin-induced migraine-like pain and trigeminal neuronal hyperactivity in mice. Eur. Neuropsychopharmacol. 2015, 25, 1388–1396. [Google Scholar] [CrossRef]
- Greco, R.; Bandiera, T.; Mangione, A.S.; Demartini, C.; Siani, F.; Nappi, G.; Sandrini, G.; Guijarro, A.; Armirotti, A.; Piomelli, D.; et al. Effects of peripheral FAAH blockade on NTG-induced hyperalgesia—Evaluation of URB937 in an animal model of migraine. Cephalalgia 2015, 35, 1065–1076. [Google Scholar] [CrossRef] [Green Version]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Laura Berliocchi, L.; Piomelli, D.; Tassorelli, C. Inhibition of monoacylglycerol lipase: Another signalling pathway for potential therapeutic targets in migraine? Cephalalgia 2018, 38, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Tumelero, E.; Reggiani, A.; Misto, A.; Piomelli, D.; Tassorelli, C. FAAH inhibition as a preventive treatment for migraine: A pre-clinical study. Neurobiol. Dis. 2020, 134, 104624. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Casini, I.; De Icco, R.; Reggiani, A.; Misto, A.; Piomelli, D.; Tassorelli, C. Characterization of the peripheral FAAH inhibitor, URB937, in animal models of acute and chronic migraine. Neurobiol. Dis. 2021, 147, 105157. [Google Scholar] [CrossRef]
- Long, J.Z.; Nomura, D.K.; Vann, R.E.; Walentiny, D.M.; Booker, L.; Jin, X.; Burston, J.J.; Sim-Selley, L.J.; Lichtman, A.H.; Wiley, J.L.; et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 20270–20275. [Google Scholar] [CrossRef] [Green Version]
- Manduca, A.; Morena, M.; Campolongo, P.; Servadio, M.; Palmery, M.; Trabace, L.; Hill, M.N.; Vanderschuren, L.J.; Cuomo, V.; Trezza, V. Distinct roles of the endocannabinoid’s anandamide and 2-arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. Eur. Neuropsychopharmacol. 2015, 25, 1362–1374. [Google Scholar] [CrossRef]
- Fegley, D.; Gaetani, S.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; Piomelli, D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 2005, 313, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Seillier, A.; Aguilar, D.D.; Giuffrida, A. The dual FAAH/MAGL inhibitor JZL195 has enhanced effects on endocannabinoid transmission and motor behavior in rats as compared to those of the MAGL inhibitor JZL184. Pharmacol. Biochem. Behav. 2014, 124, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosough, E.M.; Rahimi, V.B.; Masoud, S.A.; Mirkarimi, H.R.; Demneh, M.K.; Abed, A.; Banafshe, H.R.; Askari, V.R. Evaluation of protective effects of non-selective cannabinoid receptor agonist WIN 55,212-2 against the nitroglycerine-induced acute and chronic animal models of migraine: A mechanistic study. Life Sci. 2019, 232, 116670. [Google Scholar] [CrossRef]
- Kaufmann, D.; Brennan, K.C. The Effects of Chronic Stress on Migraine Relevant Phenotypes in Male Mice. Front. Cell Neurosci. 2018, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Vuralli, D.; Wattiez, A.S.; Russo, A.F.; Bolay, H. Behavioral and cognitive animal models in headache research. J. Headache Pain 2019, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, P.; Dallel, R. The orofacial formalin test. Neurosci. Biobehav. Rev. 2004, 28, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Clapper, J.R.; Moreno-Sanz, G.; Russo, R.; Guijarro, A.; Vacondio, F.; Duranti, A.; Tontini, A.; Sanchini, S.; Sciolino, N.R.; Spradley, J.M.; et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat. Neurosci. 2010, 13, 1265–1270. [Google Scholar] [CrossRef]
- Price, T.J.; Helesic, G.; Parghi, D.; Hargreaves, K.M.; Floreset, C.M. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience 2003, 120, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.J.; Messlinger, K. Cannabinoid and vanilloid effects of R(+)-methanandamide in the hemisected meningeal preparation. Cephalalgia 2007, 27, 422–428. [Google Scholar] [CrossRef]
- Akerman, S.; Kaube, H.; Goadsby, P.J. Anandamide acts as a vasodilator of dural blood vessels in vivo by activating TRPV1 receptors. Br. J. Pharmacol. 2004, 142, 1354–1360. [Google Scholar] [CrossRef] [Green Version]
- Greco, R.; Mangione, A.S.; Sandrini, G.; Maccarrone, M.; Nappi, G.; Tassorelli, C. Effects of anandamide in migraine: Data from an animal model. J. Headache Pain 2011, 12, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Greco, R.; Mangione, A.S.; Sandrini, G.; Nappi, G.; Tassorelli, C. Activation of CB2 receptors as a potential therapeutic target for migraine: Evaluation in an animal model. J. Headache Pain 2014, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Kilinc, E.; Ankarali, S.; Torun, I.E.; Dagistan, Y. Receptor mechanisms mediating the anti-neuroinflammatory effects of endocannabinoid system modulation in a rat model of migraine. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Greco, R.; Gasperi, V.; Sandrini, G.; Bagetta, G.; Nappi, G.; Maccarrone, M.; Tassorelli, C. Alterations of the endocannabinoid system in an animal model of migraine: Evaluation in cerebral areas of rat. Cephalalgia 2010, 30, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Limebeer, C.L.; Abdullah, R.A.; Rock, E.M.; Imhof, E.; Wang, K.; Lichtman, A.H.; Parker, L.A. Attenuation of anticipatory nausea in a rat model of contextually elicited conditioned gaping by enhancement of the endocannabinoid system. Psychopharmacology 2014, 231, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.A.; Izydorczyk, I.; Mueller-Tribbensee, S.M.; Becker, C.; Neurath, M.F.; Reeh, P.W. Inhibitory CB1 and activating/desensitizing TRPV1-mediated cannabinoid actions on CGRP release in rodent skin. Neuropeptides 2011, 45, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Stella, N. Cannabinoid signaling in glial cells. Glia 2004, 48, 267–277. [Google Scholar] [CrossRef]
- Bradshaw, H.B.; Walker, J.M. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 2005, 144, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [Green Version]
- McKenna, M.; McDougall, J.J. Cannabinoid control of neurogenic inflammation. Br. J. Pharmacol. 2020, 177, 4386–4399. [Google Scholar]
- Ortega-Gutiérrez, S.; Molina-Holgado, E.; Guaza, C. Effect of anandamide uptake inhibition in the production of nitric oxide and in the release of cytokines in astrocyte cultures. Glia 2005, 52, 163–168. [Google Scholar] [CrossRef]
- Alhouayek, M.; Masquelier, J.; Cani, P.D.; Lambert, D.M.; Muccioli, G.G. Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc. Natl. Acad. Sci. USA 2013, 110, 17558–17563. [Google Scholar] [CrossRef] [Green Version]
- Reuter, U.; Bolay, H.; Jansen-Olesen, I.; Chiarugi, A.; Sanchez del Rio, M.; Letourneau, R.; Theoharides, T.C.; Waeber, C.; Moskowitz, M.A. Delayed inflammation in rat meninges: Implications for migraine pathophysiology. Brain 2001, 124 Pt 12, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Comelli, F.; Giagnoni, G.; Bettoni, I.; Colleoni, M.; Costa, B. The inhibition of monoacylglycerol lipase by URB602 showed an anti-inflammatory and anti-nociceptive effect in a murine model of acute inflammation. Br. J. Pharmacol. 2007, 152, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, D.M.; Harhen, B.; Okine, B.N.; Egan, L.J.; Finn, D.P.; Roche, M. The monoacylglycerol lipase inhibitor JZL184 attenuates LPS-induced increases in cytokine expression in the rat frontal cortex and plasma: Differential mechanisms of action. Br. J. Pharmacol. 2013, 169, 808–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernangómez, M.; Carrillo-Salinas, F.J.; Mecha, M.; Correa, F.; Mestre, L.; Loría, F.; Feliú, A.; Docagne, F.; Guaza, C. Brain innate immunity in the regulation of neuroinflammation: Therapeutic strategies by modulating CD200-CD200R interaction involve the cannabinoid system. Curr. Pharm. Des. 2014, 20, 4707–4722. [Google Scholar] [CrossRef] [Green Version]
- Vincent, S.R. Nitric oxide: A radical neurotransmitter in the central nervous system. Prog. Neurobiol. 1994, 42, 129–160. [Google Scholar] [CrossRef]
- Liao, C.C.; Li, J.M.; Chen, C.H.; Lin, C.L.; Hsieh, C.L. Effect of Paeonia lactiflora, a traditional Chinese herb, on migraines based on clinical application and animal behavior analyses. Biomed. Pharmacother. 2019, 118, 109276. [Google Scholar] [CrossRef]
- Taheri, P.; Mohammadi, F.; Nazeri, M.; Zarei, M.R.; Chamani, G.; Esfahlani, M.A.; Taheri, F.; Shabani, M. Nitric oxide role in anxiety-like behavior, memory and cognitive impairments in animal model of chronic migraine. Heliyon 2020, 6, e05654. [Google Scholar] [CrossRef]
- Sufka, K.J.; Staszko, S.M.; Johnson, A.P.; Davis, M.E.; Davis, R.E.; Smitherman, T.A. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. J. Headache Pain 2016, 17, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, L.E.; Long, K.A.; Abdullah, R.A.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS Chem. Neurosci. 2012, 3, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Santos, A.F.; Gobira, P.H.; Rosa, L.C.; Guimaraes, F.S.; Moreira, F.A.; Aguiar, D.C. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behav. Brain Res. 2013, 252, 10–17. [Google Scholar] [CrossRef]
- Kinsey, S.G.; O’Neal, S.T.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol. Biochem. Behav. 2011, 98, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciolino, N.R.; Zhou, W.; Hohmann, A.G. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol. Res. 2011, 64, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.; Johnson, D.S.; Mileni, M.; Beidler, D.; Long, J.Z.; McKinney, M.K.; Weerapana, E.; Sadagopan, N.; Liimatta, M.; Smith, S.E.; et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem. Biol. 2009, 16, 411–420. [Google Scholar] [CrossRef] [Green Version]







| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | AACCTGCCAAGTATGATGAC | GGAGTTGCTGTTGAAGTCA |
| CGRP | CAGTCTCAGCTCCAAGTCATC | TTCCAAGGTTGACCTCAAAG |
| IL-6 | TTCTCTCCGCAAGAGACTTC | GGTCTGTTGTGGGTGGTATC |
| TNF-alpha | CCTCACACTCAGATCATCTTCTC | CGCTTGGTGGTTTGCTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, R.; Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Dual Inhibition of FAAH and MAGL Counteracts Migraine-like Pain and Behavior in an Animal Model of Migraine. Cells 2021, 10, 2543. https://doi.org/10.3390/cells10102543
Greco R, Demartini C, Francavilla M, Zanaboni AM, Tassorelli C. Dual Inhibition of FAAH and MAGL Counteracts Migraine-like Pain and Behavior in an Animal Model of Migraine. Cells. 2021; 10(10):2543. https://doi.org/10.3390/cells10102543
Chicago/Turabian StyleGreco, Rosaria, Chiara Demartini, Miriam Francavilla, Anna Maria Zanaboni, and Cristina Tassorelli. 2021. "Dual Inhibition of FAAH and MAGL Counteracts Migraine-like Pain and Behavior in an Animal Model of Migraine" Cells 10, no. 10: 2543. https://doi.org/10.3390/cells10102543
APA StyleGreco, R., Demartini, C., Francavilla, M., Zanaboni, A. M., & Tassorelli, C. (2021). Dual Inhibition of FAAH and MAGL Counteracts Migraine-like Pain and Behavior in an Animal Model of Migraine. Cells, 10(10), 2543. https://doi.org/10.3390/cells10102543






