Arginine Regulates TOR Signaling Pathway through SLC38A9 in Abalone Haliotis discus hannai
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Molecular Cloning, Sequence Analysis and Tissue Distribution of slc38a9 in Abalone
2.2.1. Experimental Animals and Sample Collection
2.2.2. Total RNA Extraction and Reverse Transcription
2.2.3. Molecular Cloning of slc38a9
2.2.4. Sequence Analysis of slc38a9
2.2.5. Tissue Distribution of slc38a9
2.3. Function Analysis of slc38a9
2.3.1. Synthesis and Injection of slc38a9 siRNA
2.3.2. Overexpression Plasmid Construction and Injection
2.3.3. Oral Administration of Arginine after siRNA Injection
2.3.4. siRNA Injection and Feeding
2.4. Arginine Treatment of Abalone In Vitro and In Vivo
2.4.1. Primary Cells Culture and Arginine Treatment
2.4.2. Feeding Trial
2.5. Sample Analysis
2.5.1. Quantitative Real-Time PCR
2.5.2. Western Blot Analysis
2.6. Statistical Analysis
3. Results
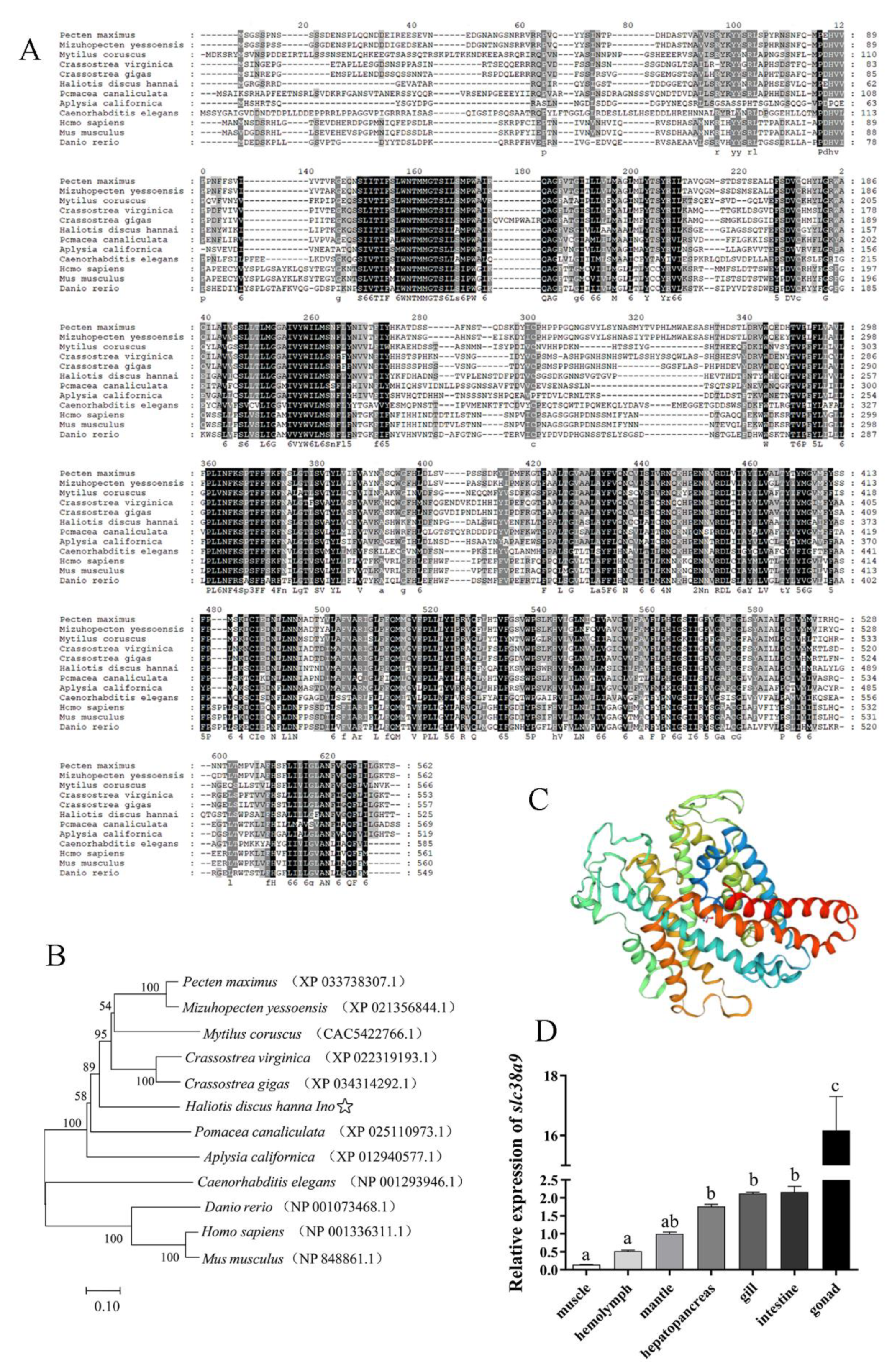
3.1. Molecular Cloning, Sequence Analysis and Tissue Distribution of slc38a9 in Abalone
3.1.1. Characterization and Phylogenetic Analysis of the slc38a9
3.1.2. Expression Analysis of slc38a9 in Different Tissues of Abalone
3.2. Function Analysis of slc38a9
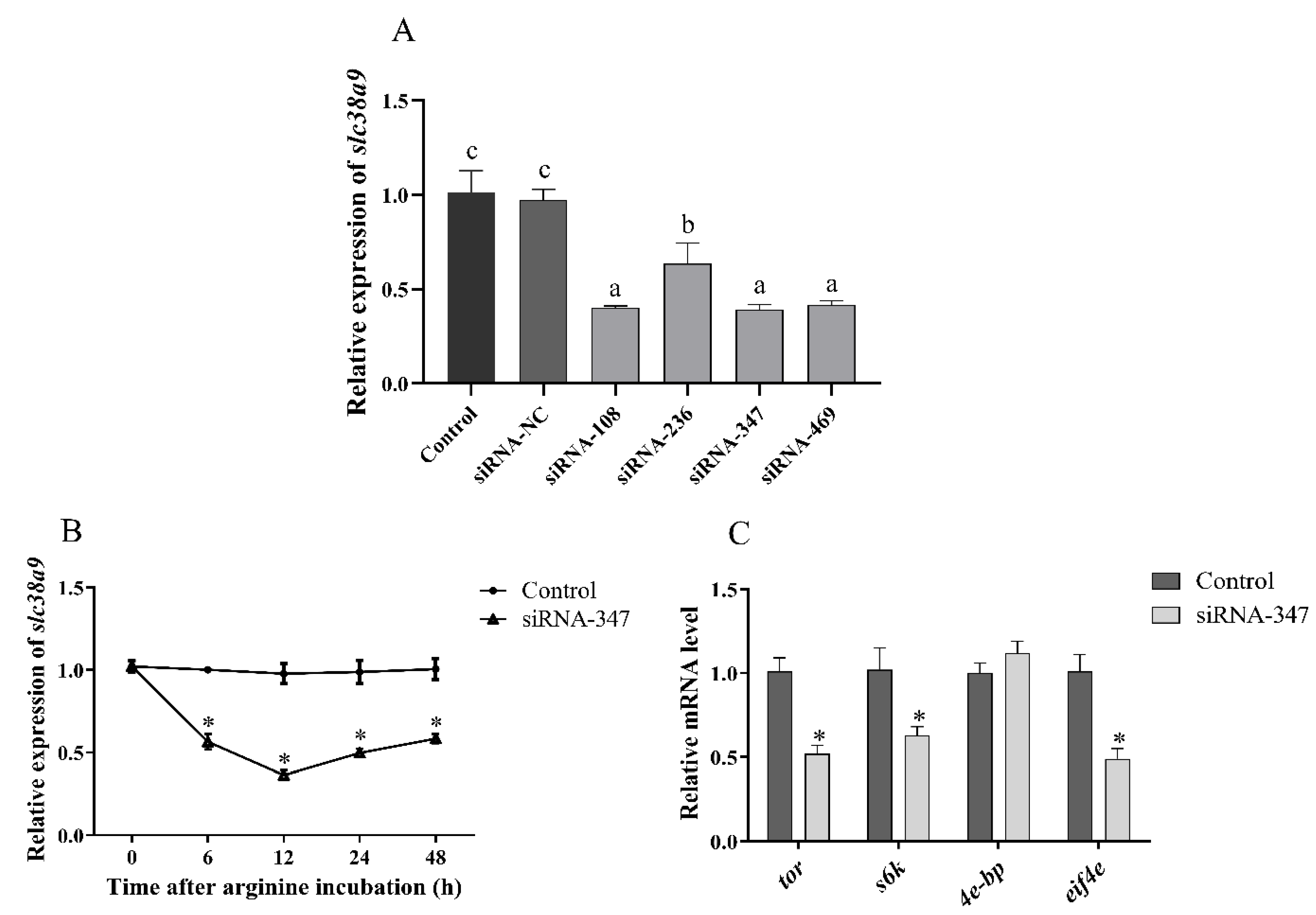
3.2.1. Expressions of TOR Pathway Related Genes after siRNA Injection
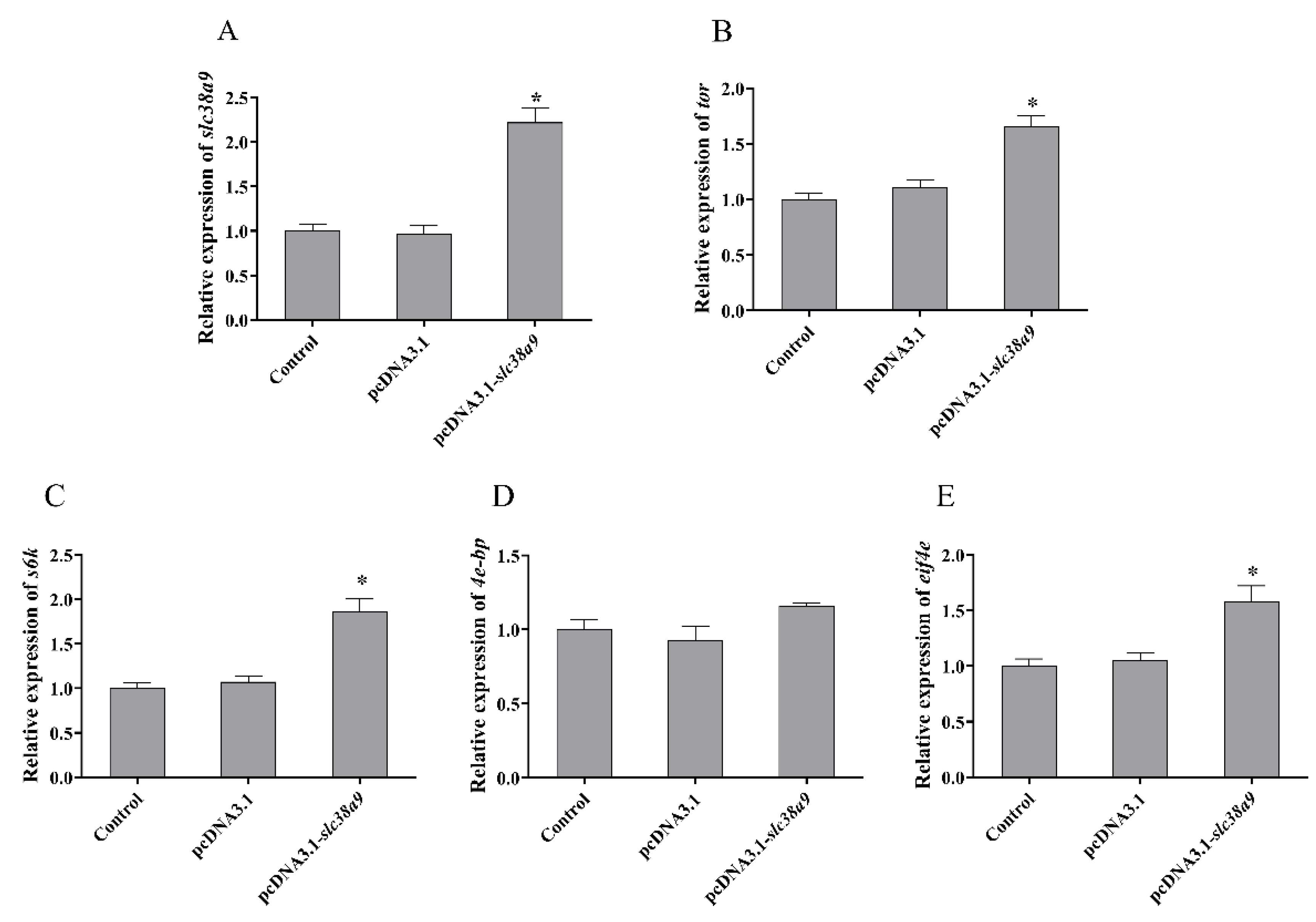
3.2.2. Expressions of TOR Pathway Related Genes after Injection with pcDNA3.1-slc38a9
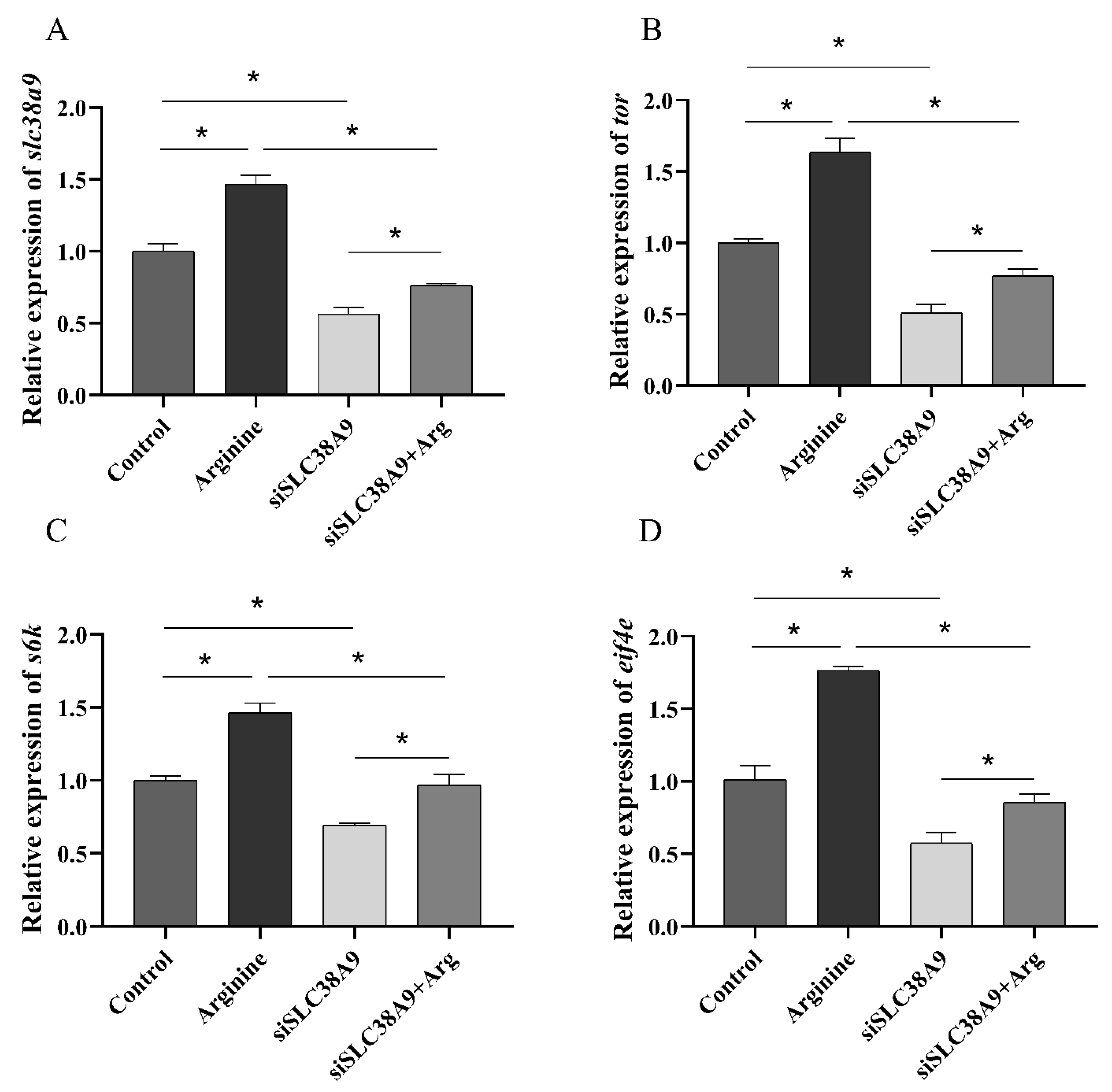
3.2.3. Expressions of TOR Pathway Related Genes after Injection of siRNA and Oral Administration of Arginine
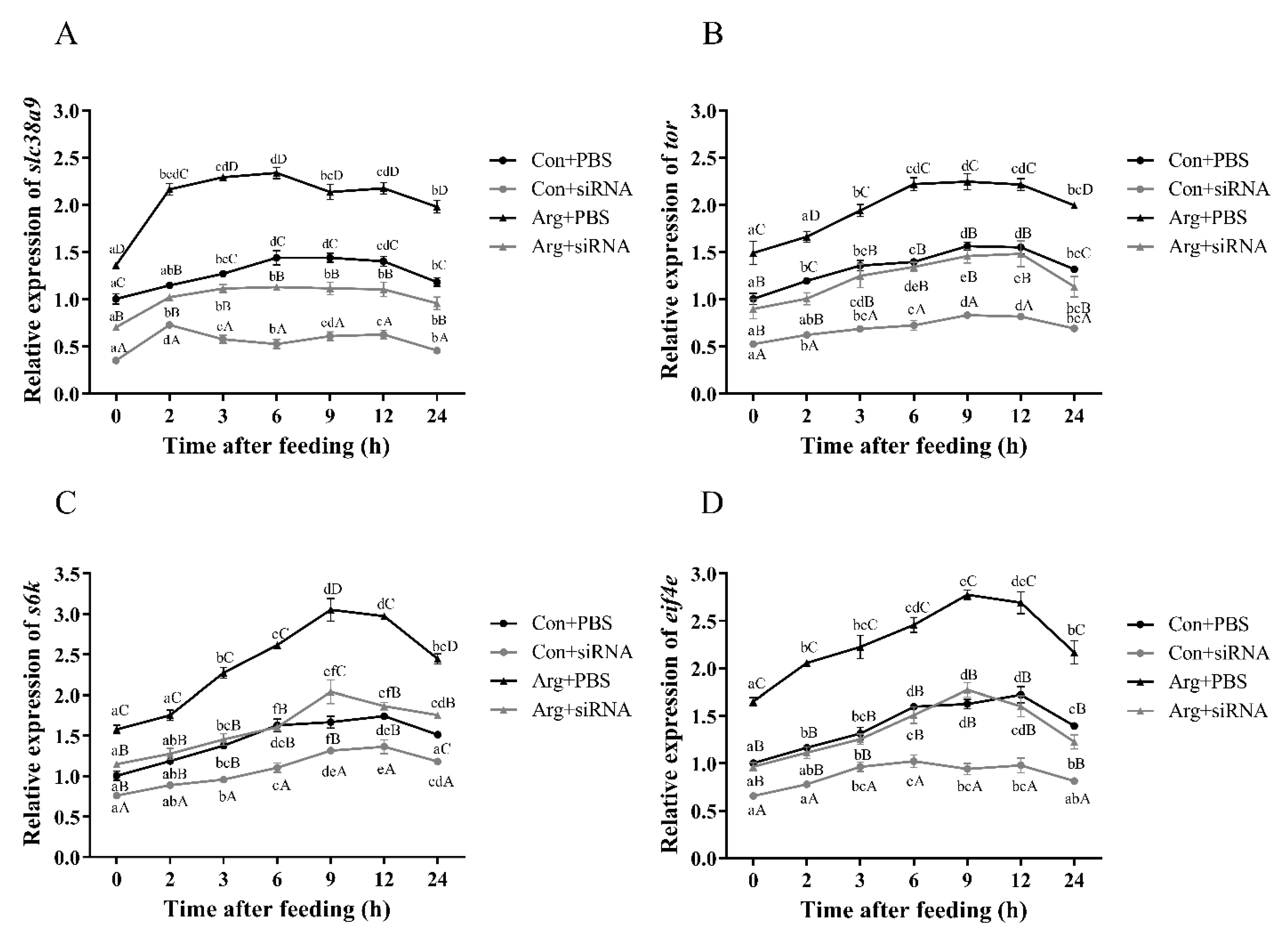
3.2.4. Expressions of TOR Pathway Related Genes after Feeding and siRNA Injection
3.3. Arginine Treatment of Abalone In Vitro and In Vivo
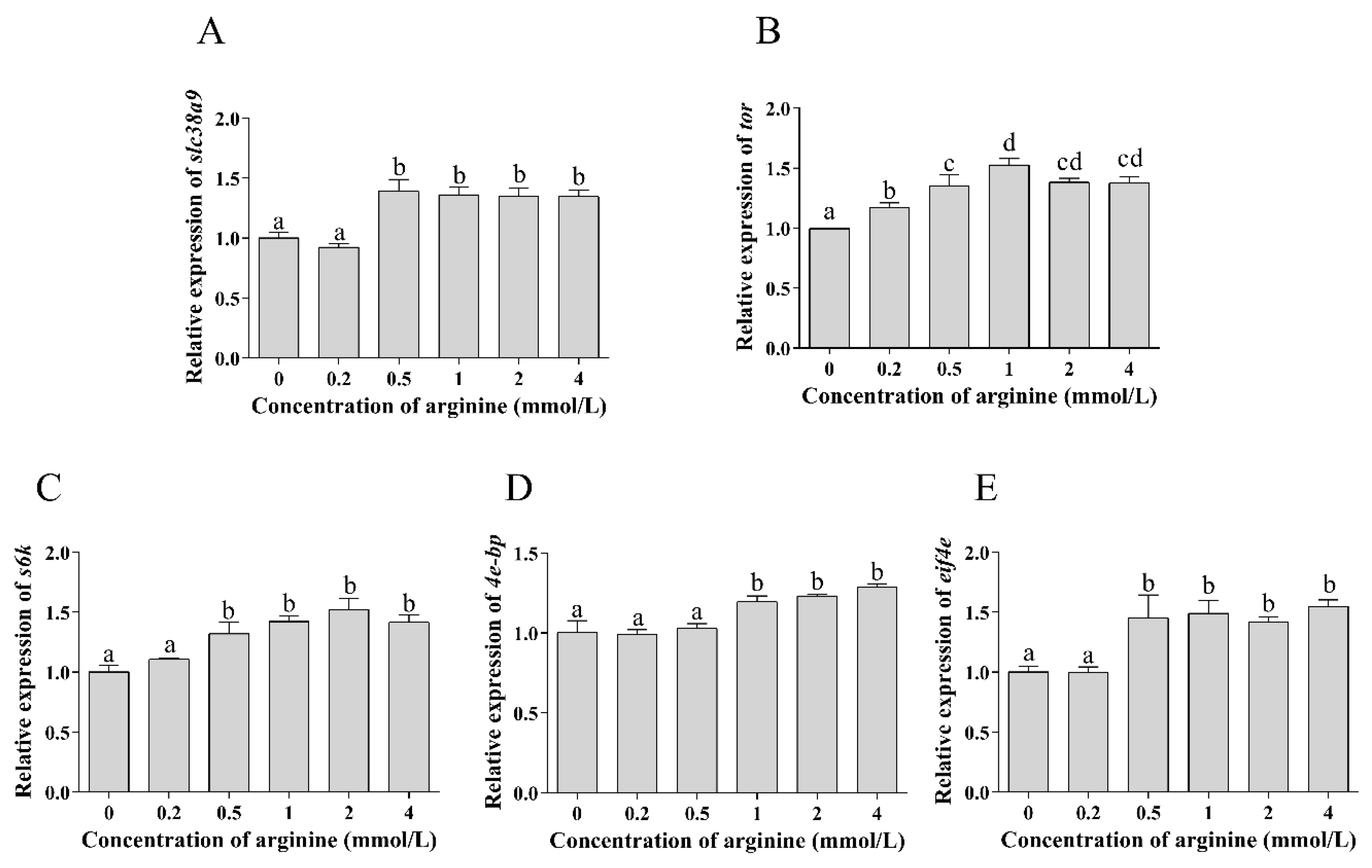
3.3.1. Expressions of TOR Pathway Related Genes in Hemocyte Treated with Different Concentrations of Arginine
3.3.2. Expressions of TOR Pathway Related Genes Affected by Dietary Arginine
3.3.3. Expressions of TOR Pathway Related Proteins Affected by Dietary Arginine
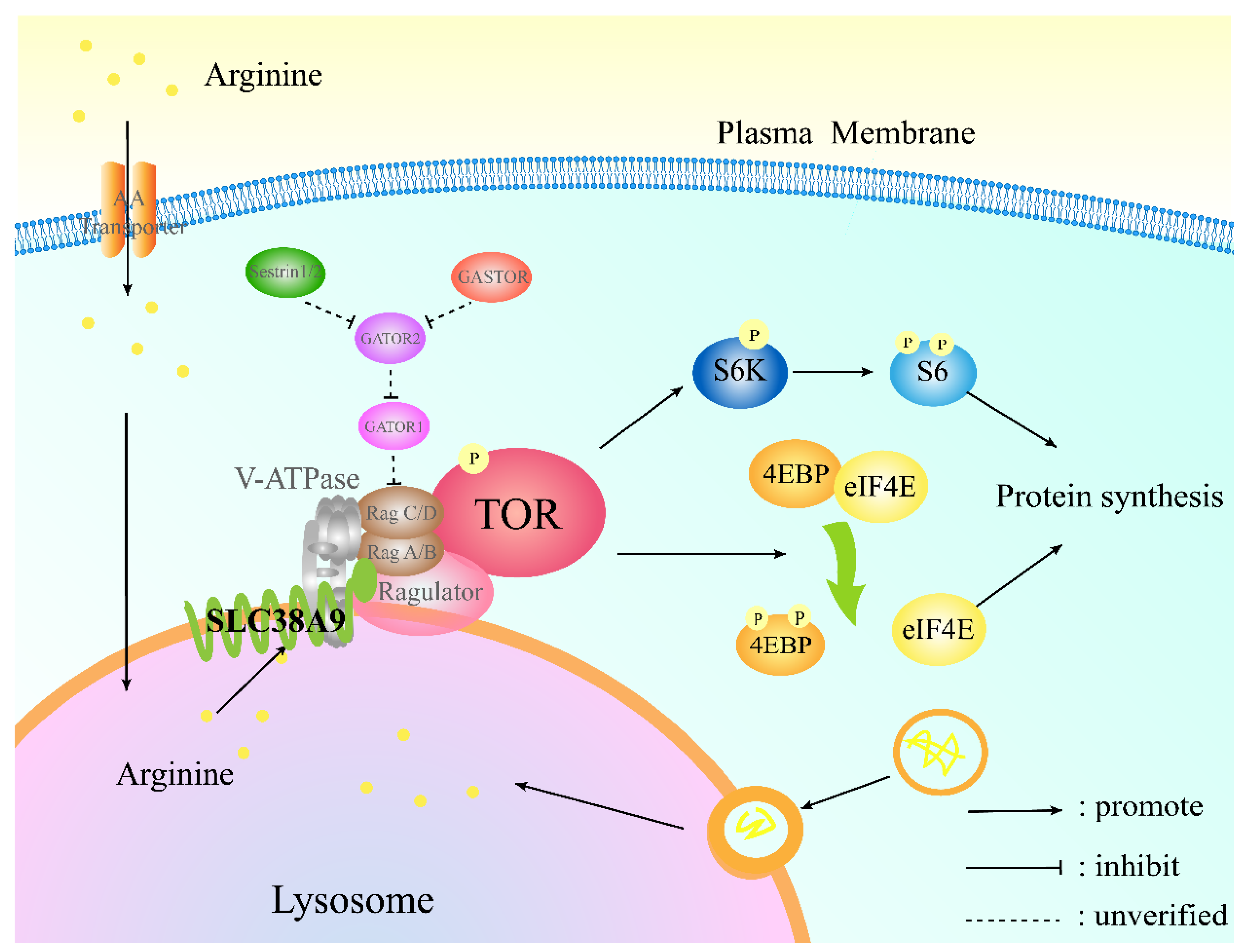
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc, R.J.; Carey, S.M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino. Acids. 2009, 37, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino. Acids. 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef]
- Pohlenz, C.; Buentello, A.; Miller, T.; Small, B.C.; MacKenzie, D.S.; Gatlin, D.M. Effects of dietary arginine on endocrine growth factors of channel catfish, Ictalurus punctatus. Comp. Biochem. Physiol. A Mol Integr. Physiol. 2013, 166, 215–221. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Wang, C.; Li, J.; Zhao, Z.; Luo, L.; Du, X.; Xu, Q. Dietary arginine requirement of juvenile hybrid sturgeon (Acipenser schrenckii♀ × Acipenser baerii♂). Aquac. Res. 2017, 48, 5193–5201. [Google Scholar] [CrossRef]
- Andoh, T. Stress inhibits insulin release induced by feeding and arginine injection in barfin flounder Verasper moseri. Fish. Sci. 2014, 80, 311–316. [Google Scholar] [CrossRef]
- Sink, T.D.; Lochmann, R.T. Insulin Response of Largemouth Bass to Glucose, Amino Acid, and Diet Stimulation. N. Am. J. Aquac. 2007, 69, 429–434. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, H.; Huang, Y.; Cao, J.; Wang, G.; Sun, Y.; Li, Y. Effects of dietary arginine levels on growth performance, body composition, serum biochemical indices and resistance ability against ammonia-nitrogen stress in juvenile yellow catfish (Pelteobagrus fulvidraco). Anim. Nutr. 2016, 2, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, I.; Thorsen, A.; Finn, R.N. Fish larval nutrition: A review of recent advances in the roles of amino acids. Aquaculture 1999, 177, 201–216. [Google Scholar] [CrossRef]
- Liang, H.; Ren, M.; Habte-Tsion, H.M.; Ge, X.; Xie, J.; Mi, H.; Xi, B.; Miao, L.; Liu, B.; Zhou, Q.; et al. Dietary arginine affects growth performance, plasma amino acid contents and gene expressions of the TOR signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2016, 461, 1–8. [Google Scholar] [CrossRef]
- Scot, R.K.; Leonard, S.J. Control of protein synthesis by amino acid availability. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 63–67. [Google Scholar]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilberg, M.S.; Pan, Y.X.; Chen, H.; Leung-Pineda, V. Nutritional control of gene expression: How mammalian cells respond to amino acid limitation. Annu. Rev. Nutr. 2005, 25, 59–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotechuang, N.; Azzout-Marniche, D.; Bos, C.; Chaumontet, C.; Gausseres, N.; Steiler, T.; Gaudichon, C.; Tome, D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1313–E1323. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Burghardt, R.C.; Wu, G.; Johnson, G.A.; Spencer, T.E.; Bazer, F.W. Select nutrients in the ovine uterine lumen. VIII. Arginine stimulates proliferation of ovine trophectoderm cells through MTOR-RPS6K-RPS6 signaling cascade and synthesis of nitric oxide and polyamines. Biol. Reprod. 2011, 84, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Tan, B.; Yin, Y.; Gao, H.; Li, X.; Jaeger, L.A.; Bazer, F.W.; Wu, G. L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J. Nutr. Biochem. 2012, 23, 1178–1183. [Google Scholar] [CrossRef]
- Ma, X.; Han, M.; Li, D.; Hu, S.; Gilbreath, K.R.; Bazer, F.W.; Wu, G. L-Arginine promotes protein synthesis and cell growth in brown adipocyte precursor cells via the mTOR signal pathway. Amino. Acids. 2017, 49, 957–964. [Google Scholar] [CrossRef]
- Wang, M.; Xu, B.; Wang, H.; Bu, D.; Wang, J.; Loor, J.J. Effects of Arginine concentration on the in vitro expression of Casein and mTOR pathway related genes in mammary epithelial cells from dairy cattle. PLoS ONE 2014, 9, e95985. [Google Scholar] [CrossRef]
- Yao, K.; Yin, Y.; Chu, W.; Liu, Z.; Deng, D.; Li, T.; Huang, R.; Zhang, J.; Tan, B.; Wang, W.; et al. Dietary Arginine Supplementation Increases mTOR Signaling Activity in Skeletal Muscle of Neonatal Pigs. J. Nutr. 2008, 138, 867–872. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Tan, Q.; Liu, M.; Hu, P. Arginine affects growth and integrity of grass carp enterocytes by regulating TOR signaling pathway and tight junction proteins. Fish Physiol. Biochem. 2019, 45, 539–549. [Google Scholar] [CrossRef]
- Wu, M.; Wu, X.; Lu, S.; Gao, Y.; Yao, W.; Li, X.; Dong, Y.; Jin, Z. Dietary arginine affects growth, gut morphology, oxidation resistance and immunity of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Br. J. Nutr. 2018, 120, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Xie, S.; Han, D.; Yang, Y.; Jin, J.; Zhu, X. Dietary arginine requirement for gibel carp (Carassis auratus gibelio var. CAS ⅠⅠⅠ ) reduces with fish size from 50g to 150g associated with modulation of genes involved in TOR signaling pathway. Aquaculture 2015, 449, 37–47. [Google Scholar] [CrossRef]
- Chen, G.; Feng, L.; Kuang, S.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.; Li, S.; Tang, L.; Zhou, X. Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Br. J. Nutr. 2012, 108, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zhang, Q.; Jia, L.; Xu, H.; Liang, M. Effects of dietary arginine levels on growth, intestinal peptide and amino acid transporters, and gene expressions of the TOR signaling pathway in tiger puffer, Takifugu rubripes. Aquaculture 2021, 532, 736086. [Google Scholar] [CrossRef]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Peled, L.; Schweitzer, L.D.; Zoncu, R.; Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012, 150, 1196–1208. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Genau, H.M.; Behrends, C. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol. Cell Biol. 2015, 35, 2479–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebsamen, M.; Pochini, L.; Stasyk, T.; de Araujo, M.E.; Galluccio, M.; Kandasamy, R.K.; Snijder, B.; Fauster, A.; Rudashevskaya, E.L.; Bruckner, M.; et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015, 519, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhang, W.; Mai, K.; Xu, W.; Wang, X.; Ma, H.; Liufu, Z. Transcriptional up-regulation of a novel ferritin homolog in abalone Haliotis discus hannai Ino by dietary iron. Comp. Biochem. Physiol. C Toxicol. Pharm. 2010, 152, 424–432. [Google Scholar] [CrossRef]
- Ma, S.; Guo, Y.; Sun, L.; Fan, W.; Liu, Y.; Liu, D.; Huang, D.; Li, X.; Zhang, W.; Mai, K. Over high or low dietary protein levels depressed the growth, TOR signaling, apoptosis, immune and anti-stress of abalone Haliotis discus hannai. Fish Shellfish Immunol. 2020, 106, 241–251. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, D.; Chen, F.; Ma, S.; Zhou, W.; Zhang, W.; Mai, K. Lipid deposition in abalone Haliotis discus hannai affected by dietary lipid levels through AMPKα2/PPARα and JNK/mTOR/SREBP-1c pathway. Aquaculture 2021, 532, 736040. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Y.; Pan, M.; Li, X.; Huang, D.; Liu, Y.; Wu, C.; Zhang, W.; Mai, K. Functions of Forkhead Box O on Glucose Metabolism in Abalone Haliotis discus hannai and Its Responses to High Levels of Dietary Lipid. Genes 2021, 12, 297. [Google Scholar] [CrossRef]
- Liu, J.; Pan, M.; Huang, D.; Guo, Y.; Yang, M.; Zhang, W.; Mai, K. Myostatin-1 Inhibits Cell Proliferation by Inhibiting the mTOR Signal Pathway and MRFs, and Activating the Ubiquitin-Proteasomal System in Skeletal Muscle Cells of Japanese Flounder Paralichthys olivaceus. Cells 2020, 9, 2376. [Google Scholar] [CrossRef]
- Scalise, M.; Galluccio, M.; Pochini, L.; Cosco, J.; Trotta, M.; Rebsamen, M.; Superti-Furga, G.; Indiveri, C. Insights into the transport side of the human SLC38A9 transceptor. Biochim Biophys Acta. Biomembr. 2019, 1861, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. The SLC38 family of sodium-amino acid co-transporters. Pflug. Arch. 2014, 466, 155–172. [Google Scholar] [CrossRef]
- Hellsten, S.V.; Eriksson, M.M.; Lekholm, E.; Arapi, V.; Perland, E.; Fredriksson, R. The gene expression of the neuronal protein, SLC38A9, changes in mouse brain after in vivo starvation and high-fat diet. PLoS ONE 2017, 12, e0172917. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Srivastava, S.; Phadke, R.S.; Govil, G. Arginine Activates Glycolysis of Goat Epididymal Spermatozoa: An NMR Study. Biophys. J. 1998, 75, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Adnan; Mroueh, Effect of Arginine on Oligospermia. Fertil. Steril. 1970, 21, 217–219. [CrossRef]
- Goud, P.T.; Goud, A.P.; Diamond, M.P.; Gonik, B.; Abu-Soud, H.M. Nitric oxide extends the oocyte temporal window for optimal fertilization. Free Radic. Biol. Med. 2008, 45, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Stein, E.D.; Chang, S.D.; Diamond, J.M. Comparison of different dietary amino acids as inducers of intestinal amino acid transport. Am. J. Physiol. 1987, 252, G626–G635. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Barrera, M.A.; Araiza, A.B.; Zijlstra, R.T.; Bernal, H.; Cervantes, M. Effect of excess levels of lysine and leucine in wheat-based, amino acid-fortified diets on the mRNA expression of two selected cationic amino acid transporters in pigs. J Anim. Physiol. Anim. Nutr. 2013, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Goraksha-Hicks, P.; Li, L.; Neufeld, T.P.; Guan, K.L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 2008, 10, 935–945. [Google Scholar] [CrossRef] [Green Version]
- Holz, M.K.; Ballif, B.A.; Gygi, S.P.; Blenis, J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 2005, 123, 569–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [Green Version]
- Hara, K.; Yonezawa, K.; Weng, Q.P.; Kozlowski, M.T.; Avruch, J. Amino acid Sufficiency and mTOR Regulate p70 S6 Kinase and eIF-4E BP1 through a Common Effector Mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habte-Tsion, H.M. A review on fish immuno-nutritional response to indispensable amino acids in relation to TOR, NF-kappaB and Nrf2 signaling pathways: Trends and prospects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 241, 110389. [Google Scholar] [CrossRef]
- Carroll, B.; Maetzel, D.; Maddocks, O.D.; Otten, G.; Ratcliff, M.; Smith, G.R.; Dunlop, E.A.; Passos, J.F.; Davies, O.R.; Jaenisch, R.; et al. Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity. Elife 2016, 5, e11058. [Google Scholar] [CrossRef]
- Taylor, P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014, 99, 223S–230S. [Google Scholar] [CrossRef] [Green Version]
- Ruvinsky, I.; Meyuhas, O. Ribosomal protein S6 phosphorylation: From protein synthesis to cell size. Trends Biochem. Sci. 2006, 31, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, G.; Wu, G.; Gao, H.; Johnson, G.A.; Bazer, F.W. Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol. Reprod. 2013, 88, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellsten, S.V.; Tripathi, R.; Ceder, M.M.; Fredriksson, R. Nutritional Stress Induced by Amino Acid Starvation Results in Changes for Slc38 Transporters in Immortalized Hypothalamic Neuronal Cells and Primary Cortex Cells. Front Mol. Biosci. 2018, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Graber, T.G.; Borack, M.S.; Reidy, P.T.; Volpi, E.; Rasmussen, B.B. Essential amino acid ingestion alters expression of genes associated with amino acid sensing, transport, and mTORC1 regulation in human skeletal muscle. Nutr. Metab. 2017, 14, 35. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Z.; Li, W.; Zhang, C.; Sun, K.; Ji, Y.; Wang, B.; Jiao, N.; He, B.; Wang, W.; et al. Dietary L-leucine supplementation enhances intestinal development in suckling piglets. Amino. Acids. 2015, 47, 1517–1525. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, B.; Yu, C.; Li, J.; Zhang, L.; Sun, H.; Gao, F.; Zhou, G. L-Glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PLoS ONE 2014, 9, e111950. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Han, F.; Chi, S.; Tan, B.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S. Molecular cloning of the amino acid transporter b0,+AT cDNA from the orange-spotted grouper (Epinephelus coioides) and the effect of arginine on its expression. Aquac. Nutr. 2020, 26, 876–884. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Huang, S. mTOR Signaling in Metabolism and Cancer. Cells 2020, 9, 2278. [Google Scholar] [CrossRef]
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Vander Heiden, M.G.; Sabatini, D.M. mTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell 2017, 171, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Vander Heiden, M.G.; McCormick, F. The Metabolic Landscape of RAS-Driven Cancers from biology to therapy. Nat. Cancer 2021, 2, 271–283. [Google Scholar] [CrossRef]
- Rebsamen, M.; Superti-Furga, G. Antagonists of slc38a9 and their use in therapy. WO2015173398, 19 November 2015. [Google Scholar]
- Saqcena, M.; Menon, D.; Patel, D.; Mukhopadhyay, S.; Chow, V.; Foster, D.A. Amino acids and mTOR mediate distinct metabolic checkpoints in mammalian G1 cell cycle. PLoS ONE 2013, 8, e74157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, S.; Saqcena, M.; Foster, D.A. Synthetic lethality in KRas-driven cancer cells created by glutamine deprivation. Oncoscience 2015, 2, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Bansal, S. Arginine depriving enzymes: Applications as emerging therapeutics in cancer treatment. Cancer Chemother Pharm. 2021, 88, 565–594. [Google Scholar] [CrossRef]
- Du, T.; Han, J. Arginine Metabolism and Its Potential in Treatment of Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 658861. [Google Scholar] [CrossRef]


| Name | Primer Sequence (5′ to 3′) | Accession No. |
|---|---|---|
| Gene Cloning | ||
| slc38a9 | F: GAGGCGGCTATGGGTCAAT R: CTGAGAACAGGTCCGAGGT | MW390888 |
| Real-Time PCR | ||
| tor | F: AGATTCCCTTCCGATTGACGA R: GTACGGCCATCAGACTGTCC | MT473702 |
| s6k | F: GCCCCTGCTTTACTCGATG R: CAGCTCTTCACACCCGGTA | MT497737 |
| 4e-bp | F: ATCGGTCTTTCTTACTGGAATGTCG R: AGGCTGTTCTTCAGGGTGGTC | MT497738 |
| eif4e | F: AGAATCAGCGTTGTATCACCT R: TGCGAGAATCTTCCCATGCC | MW183129 |
| slc38a9 | F: CGCCATGTCCTGATGCTC R: TGGCATACGAGAACCCACA | MW390888 |
| β-actin | F: ACTCCATCATGAAGTGCGAT R: TTCTGCATACGGTCAGCGAT | AY380809.1 |
| Site | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| siRNA-108 | GCTACTCAGTCGCTACAAA | TTTGTAGCGACTGAGTAGC |
| siRNA-236 | GCAGCATCATCACCATCTT | AAGATGGTGATGATGCTGC |
| siRNA-347 | CCAGCTACAGAATACTCAA | TTGAGTATTCTGTAGCTGG |
| siRNA-469 | GCAGAAATAGGAGCAGTTA | TAACTGCTCCTATTTCTGC |
| Ingredients | Dietary Arginine Levels (%) | |
|---|---|---|
| 1.23 | 1.72 | |
| Casein | 22 | 22 |
| Gelatin | 5 | 5 |
| Dextrin | 35 | 35 |
| Fish oil + Soybean oil a | 3.5 | 3.5 |
| CM-cellulose | 6 | 6 |
| Microcrystalline cellulose | 1.5 | 1 |
| Sodium alginate | 20 | 20 |
| Mineral mix b | 4.5 | 4.5 |
| Vitamin mix c | 2 | 2 |
| Choline chloride | 0.5 | 0.5 |
| Arginine | 0 | 0.5 |
| Proximate analysis (% dry matter) | ||
| Moisture | 28.8 | 28.4 |
| Crude protein | 28.7 | 29.4 |
| Crude lipid | 3.84 | 3.67 |
| Arginine | 1.23 | 1.72 |
| Ingredients | Dietary Arginine Levels (%) | ||
|---|---|---|---|
| 1.17 | 1.68 | 3.43 | |
| Fish meal | 3 | 3 | 3 |
| Soy protein concentrate | 3 | 3 | 3 |
| Corn gluten meal | 24 | 24 | 24 |
| Wheat gluten | 5 | 5 | 5 |
| High gluten flour | 25 | 25.2 | 25.2 |
| Fish oil + Soybean oil a | 1.6 | 1.6 | 1.6 |
| Calcium dihydrogen phosphate | 2 | 2 | 2 |
| Choline chloride | 0.2 | 0.2 | 0.2 |
| Mineral mixture b | 1 | 1 | 1 |
| Vitamin mixture c | 1 | 1 | 1 |
| Ethoxyquinoline | 0.1 | 0.1 | 0.1 |
| Calcium propionate | 0.1 | 0.1 | 0.1 |
| Vitamin C | 0.4 | 0.4 | 0.4 |
| Kelp powder | 31 | 31 | 31 |
| L-arginine | 0 | 0.8 | 2.4 |
| L-glycine | 2.4 | 1.6 | 0 |
| Proximate Analysis (% dry matter) | |||
| Moisture | 4.44 | 5.32 | 4.52 |
| Crude protein | 32.2 | 31.9 | 32.5 |
| Crude lipid | 2.98 | 2.95 | 3.02 |
| Ash | 17.6 | 16.8 | 17.6 |
| Arginine | 1.17 | 1.68 | 3.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yu, H.; Guo, Y.; Huang, D.; Liu, J.; Pan, M.; Wang, L.; Zhang, W.; Mai, K. Arginine Regulates TOR Signaling Pathway through SLC38A9 in Abalone Haliotis discus hannai. Cells 2021, 10, 2552. https://doi.org/10.3390/cells10102552
Liu Y, Yu H, Guo Y, Huang D, Liu J, Pan M, Wang L, Zhang W, Mai K. Arginine Regulates TOR Signaling Pathway through SLC38A9 in Abalone Haliotis discus hannai. Cells. 2021; 10(10):2552. https://doi.org/10.3390/cells10102552
Chicago/Turabian StyleLiu, Yue, Haixia Yu, Yanlin Guo, Dong Huang, Jiahuan Liu, Mingzhu Pan, Liu Wang, Wenbing Zhang, and Kangsen Mai. 2021. "Arginine Regulates TOR Signaling Pathway through SLC38A9 in Abalone Haliotis discus hannai" Cells 10, no. 10: 2552. https://doi.org/10.3390/cells10102552
APA StyleLiu, Y., Yu, H., Guo, Y., Huang, D., Liu, J., Pan, M., Wang, L., Zhang, W., & Mai, K. (2021). Arginine Regulates TOR Signaling Pathway through SLC38A9 in Abalone Haliotis discus hannai. Cells, 10(10), 2552. https://doi.org/10.3390/cells10102552






