Platelet Distribution Width Is Associated with P-Selectin Dependent Platelet Function: Results from the Moli-Family Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biochemical Measurements
2.3. Cell Functional Assays
2.4. Statistical Analyses
3. Results
3.1. Population Characteristics, Correlations and Demographic Variables
3.2. PDW Is Associated with Platelet Activation and Blood Coagulation
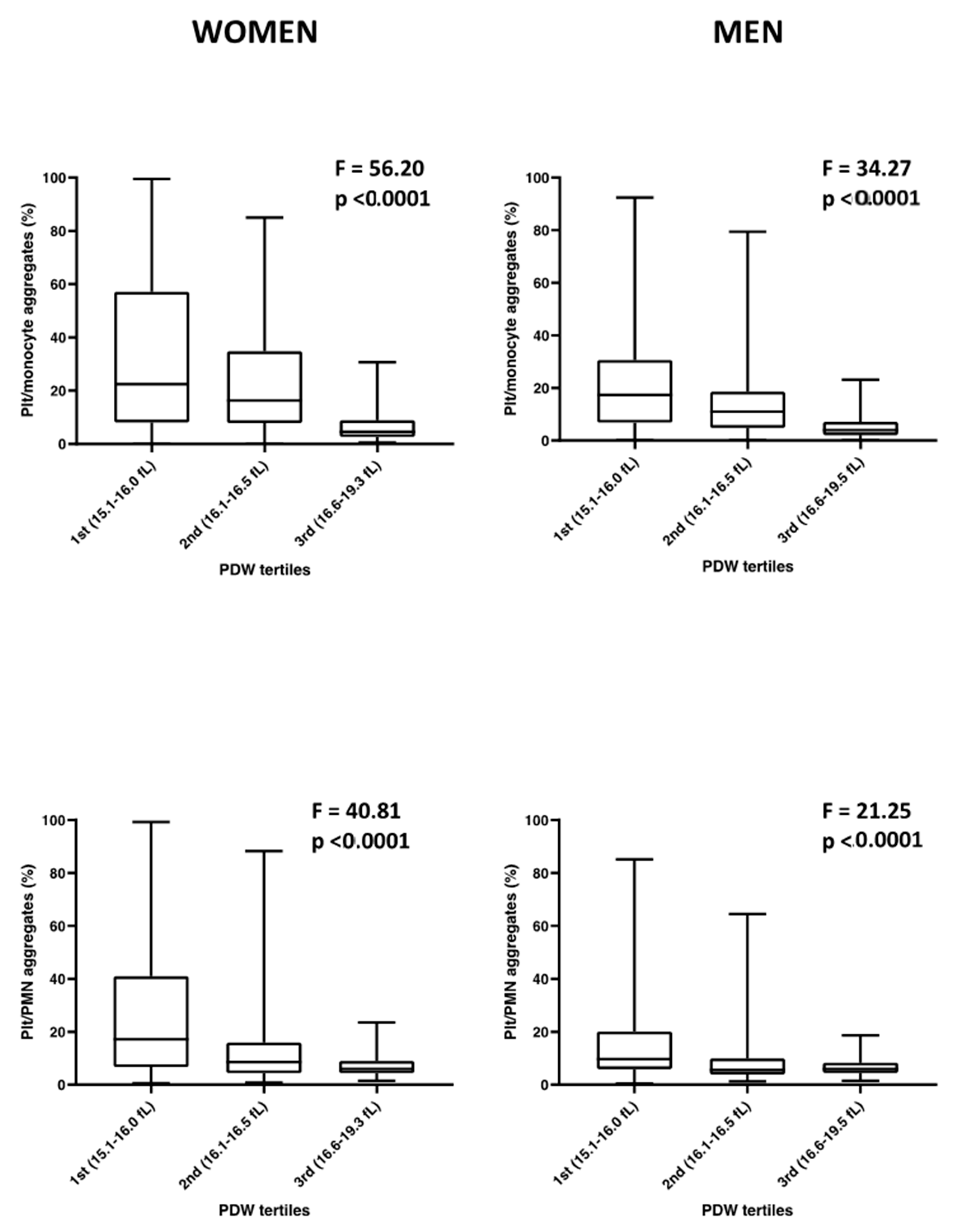
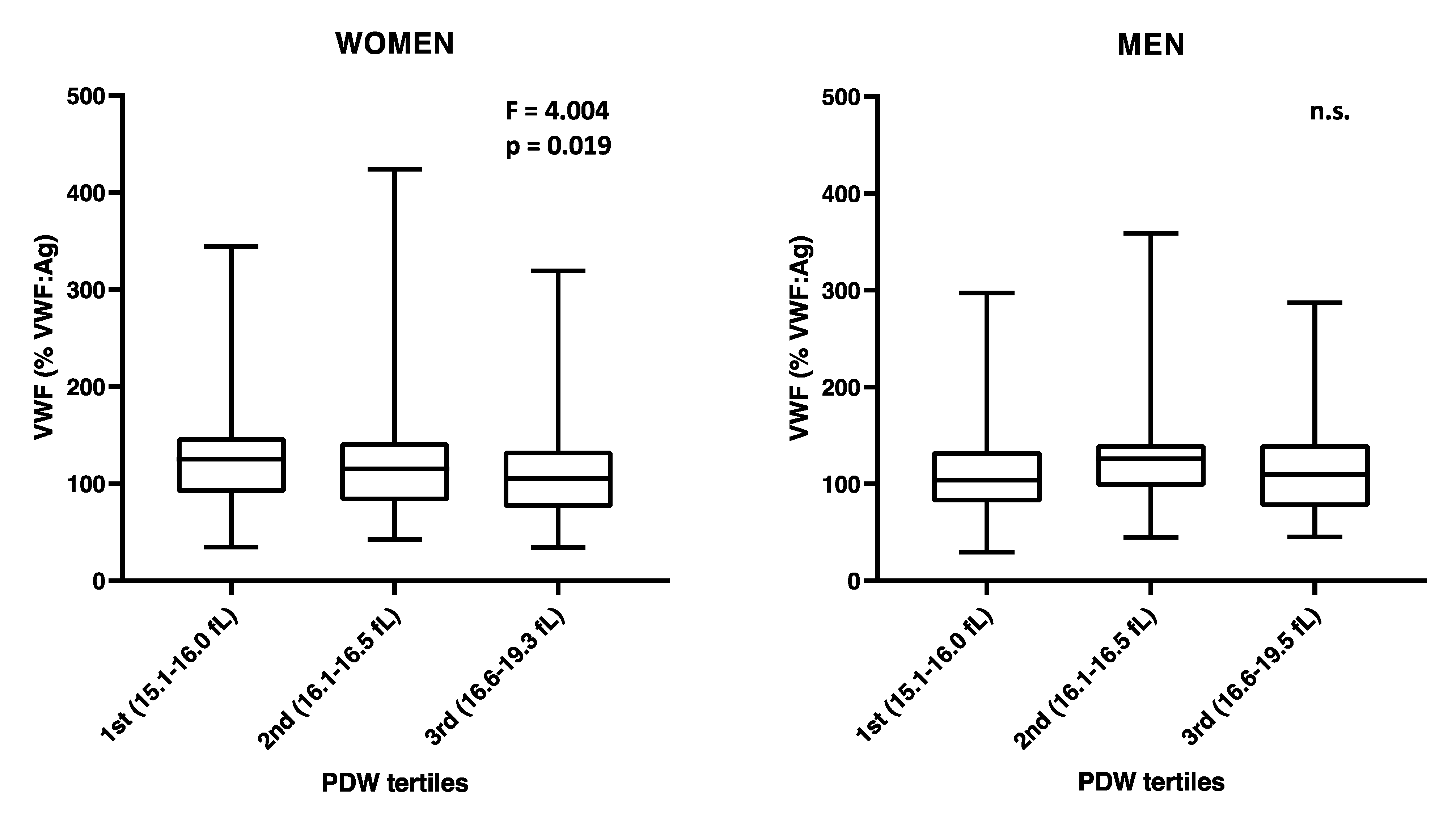
3.3. Sex-Specific Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izzi, B.; Bonaccio, M.; de Gaetano, G.; Cerletti, C. Learning by counting blood platelets in population studies: Survey and perspective a long way after Bizzozero. J. Thromb. Haemost. 2018, 16, 1711–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdev, R.; Tiwari, A.K.; Goel, S.; Raina, V.; Sethi, M. Establishing biological reference intervals for novel platelet parameters (immature platelet fraction, high immature platelet fraction, platelet distribution width, platelet large cell ratio, platelet-X, plateletcrit, and platelet distribution width) and their correlations among each other. Indian J. Pathol. Microbiol. 2014, 57, 231–235. [Google Scholar] [CrossRef]
- Budak, Y.U.; Polat, M.; Huysal, K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: A systematic review. Biochem. Med. 2016, 26, 178–193. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Plateletcrit, mean platelet volume, platelet distribution width: Its expected values and correlation with parallel red blood cell parameters. Clin. Appl. Thromb. Hemost. 2004, 10, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Vagdatli, E.; Gounari, E.; Lazaridou, E.; Katsibourlia, E.; Tsikopoulou, F.; Labrianou, I. Platelet distribution width: A simple, practical and specific marker of activation of coagulation. Hippokratia 2010, 14, 28–32. [Google Scholar] [PubMed]
- Borkataky, S.; Jain, R.; Gupta, R.; Singh, S.; Krishan, G.; Gupta, K.; Kudesia, M. Role of platelet volume indices in the differential diagnosis of thrombocytopenia: A simple and inexpensive method. Hematology 2009, 14, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, M.; Jeon, K.; Lee, J.; Lee, J.S.; Kim, H.S.; Kang, H.J.; Lee, Y.K. Mean Platelet Volume and Platelet Distribution Width Indicate that Platelets Remain Small for Most of Their Lifespans in Patients with Essential Thrombocythemia. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Luzzatto, G.; de Franchis, G.; Fabris, F.; Gerunda, G.E.; Girolami, A. Increased proportion of giant platelets and platelet distribution width are better indicators of altered platelet homeostasis than mean platelet volume in liver cirrhosis. Folia Haematol. 1988, 115, 719–726. [Google Scholar]
- Ntaios, G.; Papadopoulos, A.; Chatzinikolaou, A.; Saouli, Z.; Karalazou, P.; Kaiafa, G.; Girtovitis, F.; Kontoninas, Z.; Savopoulos, C.; Hatzitolios, A.; et al. Increased values of mean platelet volume and platelet size deviation width may provide a safe positive diagnosis of idiopathic thrombocytopenic purpura. Acta Haematol. 2008, 119, 173–177. [Google Scholar] [CrossRef]
- Izzi, B.; Gianfagna, F.; Yang, W.Y.; Cludts, K.; De Curtis, A.; Verhamme, P.; Di Castelnuovo, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; et al. Variation of PEAR1 DNA methylation influences platelet and leukocyte function. Clin. Epigenetics 2019, 11, 151. [Google Scholar] [CrossRef] [Green Version]
- Kauskot, A.; Di Michele, M.; Loyen, S.; Freson, K.; Verhamme, P.; Hoylaerts, M.F. A novel mechanism of sustained platelet alphaIIbbeta3 activation via PEAR1. Blood 2012, 119, 4056–4065. [Google Scholar] [CrossRef] [Green Version]
- Eicher, J.D.; Lettre, G.; Johnson, A.D. The genetics of platelet count and volume in humans. Platelets 2018, 29, 125–130. [Google Scholar] [CrossRef]
- Xia, W.; Chen, W.; Tu, J.; Ni, C.; Meng, K. Prognostic Value and Clinicopathologic Features of Platelet Distribution Width in Cancer: A Meta-Analysis. Med. Sci. Monit. 2018, 24, 7130–7136. [Google Scholar] [CrossRef]
- Weymann, A.; Ali-Hasan-Al-Saegh, S.; Sabashnikov, A.; Popov, A.F.; Mirhosseini, S.J.; Nombela-Franco, L.; Testa, L.; Lotfaliani, M.; Zeriouh, M.; Liu, T.; et al. Platelets Cellular and Functional Characteristics in Patients with Atrial Fibrillation: A Comprehensive Meta-Analysis and Systematic Review. Med. Sci. Monit. Basic Res. 2017, 23, 58–86. [Google Scholar] [CrossRef]
- Budzianowski, J.; Pieszko, K.; Burchardt, P.; Rzezniczak, J.; Hiczkiewicz, J. The Role of Hematological Indices in Patients with Acute Coronary Syndrome. Dis. Markers 2017, 2017, 3041565. [Google Scholar] [CrossRef]
- Zaccardi, F.; Rocca, B.; Pitocco, D.; Tanese, L.; Rizzi, A.; Ghirlanda, G. Platelet mean volume, distribution width, and count in type 2 diabetes, impaired fasting glucose, and metabolic syndrome: A meta-analysis. Diabetes Metab. Res. Rev. 2015, 31, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Izzi, B.; Tirozzi, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Hoylaerts, M.F.; Iacoviello, L.; Gialluisi, A. Beyond Haemostasis and Thrombosis: Platelets in Depression and Its Co-Morbidities. Int. J. Mol. Sci. 2020, 21, 8817. [Google Scholar] [CrossRef] [PubMed]
- Izzi, B.; Pampuch, A.; Costanzo, S.; Vohnout, B.; Iacoviello, L.; Cerletti, C.; de Gaetano, G. Determinants of platelet conjugate formation with polymorphonuclear leukocytes or monocytes in whole blood. Thromb. Haemost. 2007, 98, 1276–1284. [Google Scholar] [PubMed]
- Di Castelnuovo, A.; de Curtis, A.; Costanzo, S.; Persichillo, M.; Olivieri, M.; Zito, F.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Investigators, M.-S.P. Association of D-dimer levels with all-cause mortality in a healthy adult population: Findings from the MOLI-SANI study. Haematologica 2013, 98, 1476–1480. [Google Scholar] [CrossRef]
- Gianfagna, F.; Tamburrelli, C.; Vohnout, B.; Crescente, M.; Izzi, B.; Pampuch, A.; De Curtis, A.; Di Castelnuovo, A.; Cutrone, A.; Napoleone, E.; et al. Heritability, genetic correlation and linkage to the 9p21.3 region of mixed platelet-leukocyte conjugates in families with and without early myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 684–692. [Google Scholar] [CrossRef]
- Noro, F.; Gianfagna, F.; Gialluisi, A.; De Curtis, A.; Di Castelnuovo, A.; Napoleone, E.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Hoylaerts, M.F.; et al. ZBTB12 DNA methylation is associated with coagulation- and inflammation-related blood cell parameters: Findings from the Moli-family cohort. Clin. Epigenetics 2019, 11, 74. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Pounis, G.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. A score of low-grade inflammation and risk of mortality: Prospective findings from the Moli-sani study. Haematologica 2016, 101, 1434–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, C.P.; Harrison, P.; Cattaneo, M.; Ortel, T.L.; Rao, A.K.; Platelet Physiology Subcommittee of the Scientific; Standardization Committee of the International Society on Thrombosis and Haemostasis. Platelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet function. J. Thromb. Haemost. 2006, 4, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Santimone, I.; Di Castelnuovo, A.; De Curtis, A.; Spinelli, M.; Cugino, D.; Gianfagna, F.; Zito, F.; Donati, M.B.; Cerletti, C.; de Gaetano, G.; et al. White blood cell count, sex and age are major determinants of heterogeneity of platelet indices in an adult general population: Results from the MOLI-SANI project. Haematologica 2011, 96, 1180–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwaginga, J.J.; Nash, G.; King, M.R.; Heemskerk, J.W.; Frojmovic, M.; Hoylaerts, M.F.; Sakariassen, K.S.; Biorheology Subcommittee of the, S.S.C.o.t.I. Flow-based assays for global assessment of hemostasis. Part 1: Biorheologic considerations. J. Thromb. Haemost. 2006, 4, 2486–2487. [Google Scholar] [CrossRef] [PubMed]
- Theilmeier, G.; Lenaerts, T.; Remacle, C.; Collen, D.; Vermylen, J.; Hoylaerts, M.F. Circulating activated platelets assist THP-1 monocytoid/endothelial cell interaction under shear stress. Blood 1999, 94, 2725–2734. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, F.; Yu, Z.; Bai, X.; Yang, C.; Zhuang, F.; Ruan, C. Effects of von Willebrand factor concentration and platelet collision on shear-induced platelet activation. Thromb. Haemost. 2008, 100, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Xu, Y.; Chen, W.; Paul, D.S.; Syed, A.K.; Dragovich, M.A.; Liang, X.; Zakas, P.; Berndt, M.C.; Di Paola, J.; et al. Platelet clearance via shear-induced unfolding of a membrane mechanoreceptor. Nat. Commun. 2016, 7, 12863. [Google Scholar] [CrossRef]
- Denorme, F.; Martinod, K.; Vandenbulcke, A.; Denis, C.V.; Lenting, P.J.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. The von Willebrand Factor A1 domain mediates thromboinflammation, aggravating ischemic stroke outcome in mice. Haematologica 2021, 106, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Sporn, L.A.; Chavin, S.I.; Marder, V.J.; Wagner, D.D. Biosynthesis of von Willebrand protein by human megakaryocytes. J. Clin. Investig. 1985, 76, 1102–1106. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105 (Suppl. 1), S13–S33. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, J.M.; Gambaryan, S.; Watson, S.P.; Jurk, K.; Walter, U.; Sickmann, A.; Heemskerk, J.W.; Zahedi, R.P. What can proteomics tell us about platelets? Circ. Res. 2014, 114, 1204–1219. [Google Scholar] [CrossRef] [Green Version]
- De Gaetano, G.; Vermylen, J.; Verstraete, M. Platelet factor 3—Properties and clinical significance. Adv. Exp. Med. Biol. 1972, 34, 159–172. [Google Scholar] [CrossRef]
- Celi, A.; Pellegrini, G.; Lorenzet, R.; De Blasi, A.; Ready, N.; Furie, B.C.; Furie, B. P-selectin induces the expression of tissue factor on monocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 8767–8771. [Google Scholar] [CrossRef] [Green Version]
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 2003, 197, 1585–1598. [Google Scholar] [CrossRef]
- Marcus, A.J.; Spaet, T.H. Platelet phosphatides: Their separation, identification, and clotting activity. J. Clin. Investig. 1958, 37, 1836–1847. [Google Scholar] [CrossRef]
- Agbani, E.O.; van den Bosch, M.T.; Brown, E.; Williams, C.M.; Mattheij, N.J.; Cosemans, J.M.; Collins, P.W.; Heemskerk, J.W.; Hers, I.; Poole, A.W. Coordinated Membrane Ballooning and Procoagulant Spreading in Human Platelets. Circulation 2015, 132, 1414–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangalpally, K.K.; Siqueiros-Garcia, A.; Vaduganathan, M.; Dong, J.F.; Kleiman, N.S.; Guthikonda, S. Platelet activation patterns in platelet size sub-populations: Differential responses to aspirin in vitro. J. Thromb. Thrombolysis 2010, 30, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, D.; Aranda, E.; Foradori, A. Comparative study of size, total protein, fibrinogen and 5-HT content of human and canine platelet density subpopulations. Thromb. Haemost. 1986, 56, 288–292. [Google Scholar] [CrossRef]
- Polanowska-Grabowska, R.; Raha, S.; Gear, A.R. Adhesion efficiency, platelet density and size. Br. J. Haematol. 1992, 82, 715–720. [Google Scholar] [CrossRef]
- Frojmovic, M.; Wong, T. Dynamic measurements of the platelet membrane glycoprotein IIb-IIIa receptor for fibrinogen by flow cytometry. II. Platelet size-dependent subpopulations. Biophys. J. 1991, 59, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Handtke, S.; Steil, L.; Palankar, R.; Conrad, J.; Cauhan, S.; Kraus, L.; Ferrara, M.; Dhople, V.; Wesche, J.; Volker, U.; et al. Role of Platelet Size Revisited-Function and Protein Composition of Large and Small Platelets. Thromb. Haemost. 2019, 119, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Karpatkin, S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. J. Clin. Investig. 1969, 48, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, K.G.; Penington, D.G. Monoamine oxidase and other mitochondrial enzymes in density subpopulations of human platelets. Thromb. Haemost. 1988, 59, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Clancy, L.; Beaulieu, L.M.; Tanriverdi, K.; Freedman, J.E. The role of RNA uptake in platelet heterogeneity. Thromb. Haemost. 2017, 117, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B.; Jakubowski, J.A.; Quinn, P.G.; Deykin, D.; Valeri, C.R. Platelet size as a determinant of platelet function. J. Lab. Clin. Med. 1983, 101, 205–213. [Google Scholar]
- Li, B.Y.; He, S.Z.; Li, W.H. Heterogeneity of human platelet density subpopulations in aggregation, secretion of ATP, and cytosolic-free calcium concentration. Zhongguo Yao Li Xue Bao 1996, 17, 152–155. [Google Scholar]
- Selvadurai, M.V.; Hamilton, J.R. Structure and function of the open canalicular system—The platelet’s specialized internal membrane network. Platelets 2018, 29, 319–325. [Google Scholar] [CrossRef]
- Briggs, C. Quality counts: New parameters in blood cell counting. Int. J. Lab. Hematol. 2009, 31, 277–297. [Google Scholar] [CrossRef]
| PDW 1st (15.1–16.0 fL) | PDW 2nd (16.1–16.5 fL) | PDW 3rd (16.6–19.5 fL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Continuous variables | N | Mean | SD | N | Mean | SD | N | Mean | SD | Pr > F |
| Age (years) | 221 | 39.94 | 17.63 | 268 | 41.71 | 19.17 | 238 | 46.02 | 18.73 | 0.001 |
| BMI (kg/m2) | 219 | 26.11 | 4.74 | 265 | 26.41 | 5.82 | 238 | 26.77 | 5.18 | 0.405 |
| Energy intake (Kcal/d) | 203 | 1655.19 | 561.05 | 239 | 1728.83 | 526.26 | 213 | 1671.63 | 587.51 | 0.339 |
| Categorical variables | N | % | N | % | N | % | ||||
| Males | 77 | 34.84 | 125 | 46.64 | 123 | 51.68 | 0.001 | |||
| Smoker status (smokers) | 0.018 | |||||||||
| Ever | 123 | 55.66 | 146 | 54.48 | 129 | 54.2 | ||||
| Current | 73 | 33.03 | 82 | 30.6 | 57 | 23.95 | ||||
| Former | 25 | 11.31 | 40 | 14.93 | 52 | 21.85 | ||||
| Physical activity | 0.029 | |||||||||
| Sedentary | 76 | 35.02 | 92 | 35.11 | 62 | 26.61 | ||||
| Light physical activity | 45 | 20.47 | 59 | 22.52 | 71 | 30.47 | ||||
| Moderate physical activity | 72 | 33.18 | 65 | 24.81 | 63 | 27.04 | ||||
| Intense physical activity | 24 | 11.06 | 46 | 17.56 | 36 | 15.45 | ||||
| Alcohol consumption | 0.092 | |||||||||
| 0 g/day (Former/ever drinker) | 78 | 37.5 | 82 | 33.2 | 64 | 29.49 | ||||
| ≤12 g/day | 78 | 37.5 | 107 | 43.32 | 79 | 36.41 | ||||
| 12.1–24 g/day | 32 | 15.38 | 28 | 11.34 | 42 | 19.35 | ||||
| >24 g/day | 20 | 9.62 | 30 | 12.15 | 32 | 14.75 | ||||
| Variable | PDW | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALL @ | WOMEN # | MEN # | |||||||||||||
| β | SE | Probt | Lower | Upper | β | SE | Probt | Lower | Upper | β | SE | Probt | Lower | Upper | |
| Platelet P-selectin basal | −0.140 | 0.037 | 0.00018 * | −0.213 | −0.067 | −0.127 | 0.048 | 0.009 | −0.221 | −0.032 | −0.156 | 0.059 | 0.008 | −0.271 | −0.041 |
| Platelet P-selectin ADP/Collagen | 0.002 | 0.038 | 0.960 | −0.074 | 0.078 | −0.017 | 0.052 | 0.748 | −0.120 | 0.086 | 0.033 | 0.056 | 0.562 | −0.078 | 0.143 |
| Platelet/monocyte aggregates basal | −0.147 | 0.036 | 0.00006 * | −0.219 | −0.076 | −0.152 | 0.049 | 0.002 * | −0.248 | −0.057 | −0.134 | 0.055 | 0.016 | −0.242 | −0.026 |
| Platelet/monocyte aggregates ADP/Collagen | −0.090 | 0.038 | 0.019 | −0.165 | −0.015 | −0.132 | 0.046 | 0.005 * | −0.223 | −0.041 | 0.007 | 0.068 | 0.917 | −0.127 | 0.142 |
| Platelet/PMN aggregates basal | −0.132 | 0.036 | 0.00024 * | −0.202 | −0.062 | −0.117 | 0.046 | 0.011 | −0.206 | −0.027 | −0.175 | 0.060 | 0.004 * | −0.293 | −0.057 |
| Platelet/PMN aggregates ADP/Collagen | −0.051 | 0.037 | 0.169 | −0.124 | 0.022 | −0.106 | 0.045 | 0.019 | −0.194 | −0.017 | 0.062 | 0.068 | 0.362 | −0.071 | 0.195 |
| PFA-100 CT | 0.005 | 0.001 | 0.00012 * | 0.002 | 0.007 | 0.005 | 0.002 | 0.001 * | 0.002 | 0.009 | 0.005 | 0.002 | 0.019 | 0.001 | 0.009 |
| Coagulation time | 0.358 | 0.200 | 0.074 | −0.034 | 0.751 | 0.217 | 0.265 | 0.414 | −0.305 | 0.739 | 0.715 | 0.293 | 0.016 | 0.137 | 1.293 |
| LPS stimulated coagulation time | 0.653 | 0.174 | 0.00018 * | 0.312 | 0.995 | 0.607 | 0.238 | 0.011 | 0.139 | 1.075 | 0.916 | 0.250 | 0.00031 * | 0.423 | 1.410 |
| TNF-α stimulated coagulation time | 0.578 | 0.184 | 0.002 * | 0.217 | 0.940 | 0.573 | 0.240 | 0.018 | 0.100 | 1.045 | 0.720 | 0.285 | 0.012 | 0.158 | 1.283 |
| VWF | −0.298 | 0.098 | 0.002 * | −0.490 | −0.106 | −0.452 | 0.131 | 0.001 * | −0.710 | −0.193 | −0.116 | 0.149 | 0.436 | −0.409 | 0.177 |
| Soluble P-selectin | −0.093 | 0.050 | 0.064 | −0.190 | 0.005 | −0.082 | 0.073 | 0.258 | −0.226 | 0.061 | −0.129 | 0.066 | 0.053 | −0.260 | 0.001 |
| CRP | −0.004 | 0.037 | 0.910 | −0.078 | 0.069 | −0.077 | 0.052 | 0.141 | −0.180 | 0.026 | 0.087 | 0.055 | 0.113 | −0.021 | 0.195 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzi, B.; Gialluisi, A.; Gianfagna, F.; Orlandi, S.; De Curtis, A.; Magnacca, S.; Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; de Gaetano, G.; et al. Platelet Distribution Width Is Associated with P-Selectin Dependent Platelet Function: Results from the Moli-Family Cohort Study. Cells 2021, 10, 2737. https://doi.org/10.3390/cells10102737
Izzi B, Gialluisi A, Gianfagna F, Orlandi S, De Curtis A, Magnacca S, Costanzo S, Di Castelnuovo A, Donati MB, de Gaetano G, et al. Platelet Distribution Width Is Associated with P-Selectin Dependent Platelet Function: Results from the Moli-Family Cohort Study. Cells. 2021; 10(10):2737. https://doi.org/10.3390/cells10102737
Chicago/Turabian StyleIzzi, Benedetta, Alessandro Gialluisi, Francesco Gianfagna, Sabatino Orlandi, Amalia De Curtis, Sara Magnacca, Simona Costanzo, Augusto Di Castelnuovo, Maria Benedetta Donati, Giovanni de Gaetano, and et al. 2021. "Platelet Distribution Width Is Associated with P-Selectin Dependent Platelet Function: Results from the Moli-Family Cohort Study" Cells 10, no. 10: 2737. https://doi.org/10.3390/cells10102737
APA StyleIzzi, B., Gialluisi, A., Gianfagna, F., Orlandi, S., De Curtis, A., Magnacca, S., Costanzo, S., Di Castelnuovo, A., Donati, M. B., de Gaetano, G., Hoylaerts, M. F., Cerletti, C., Iacoviello, L., & on behalf of the Moli-family Study Investigators. (2021). Platelet Distribution Width Is Associated with P-Selectin Dependent Platelet Function: Results from the Moli-Family Cohort Study. Cells, 10(10), 2737. https://doi.org/10.3390/cells10102737







