The Roles of Extracellular Vesicles in Malignant Melanoma
Abstract
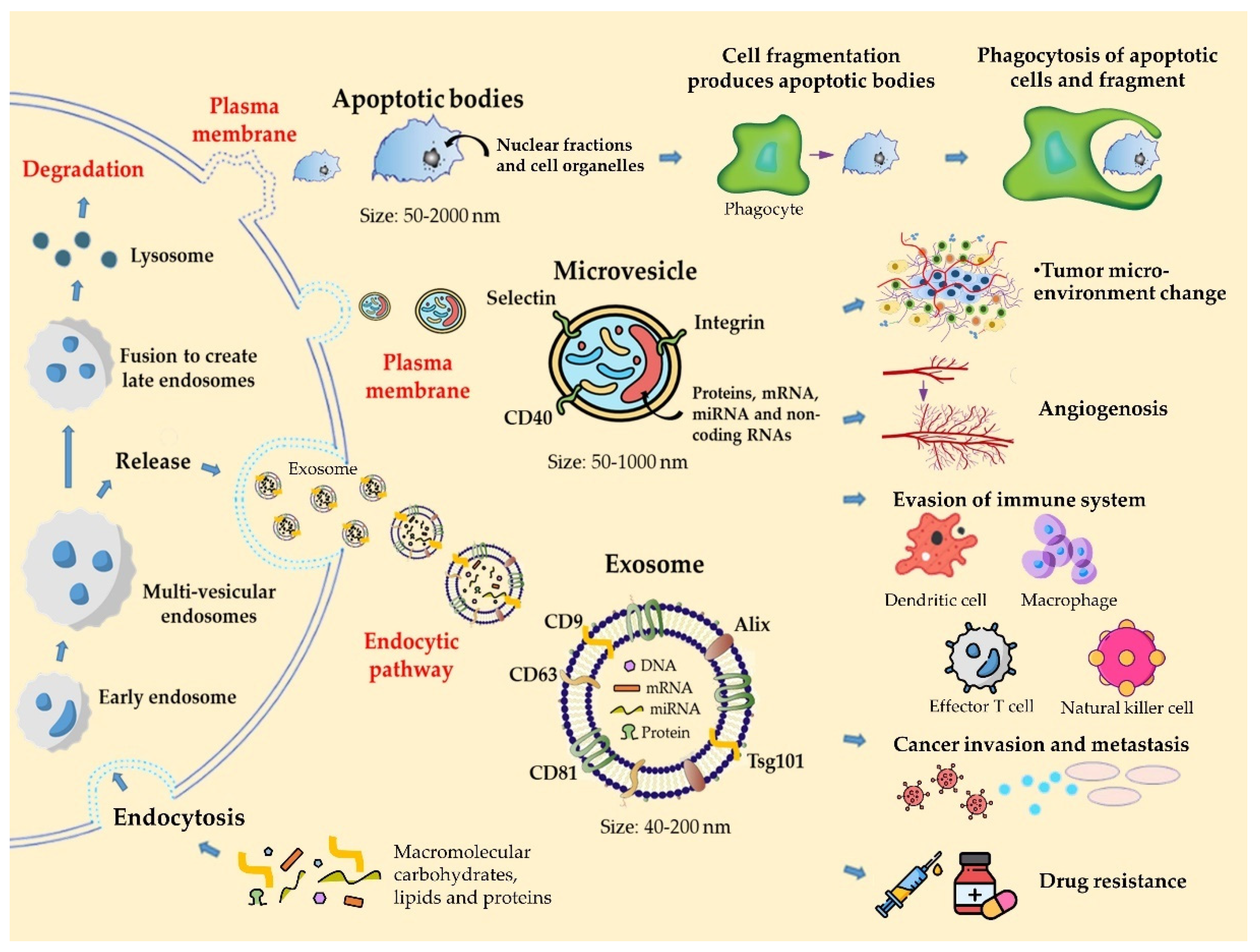
:1. Melanoma
2. Classification and Biology of Extracellular Vesicles
3. EVs Derived from Melanoma and Their Role in Cancer Progression
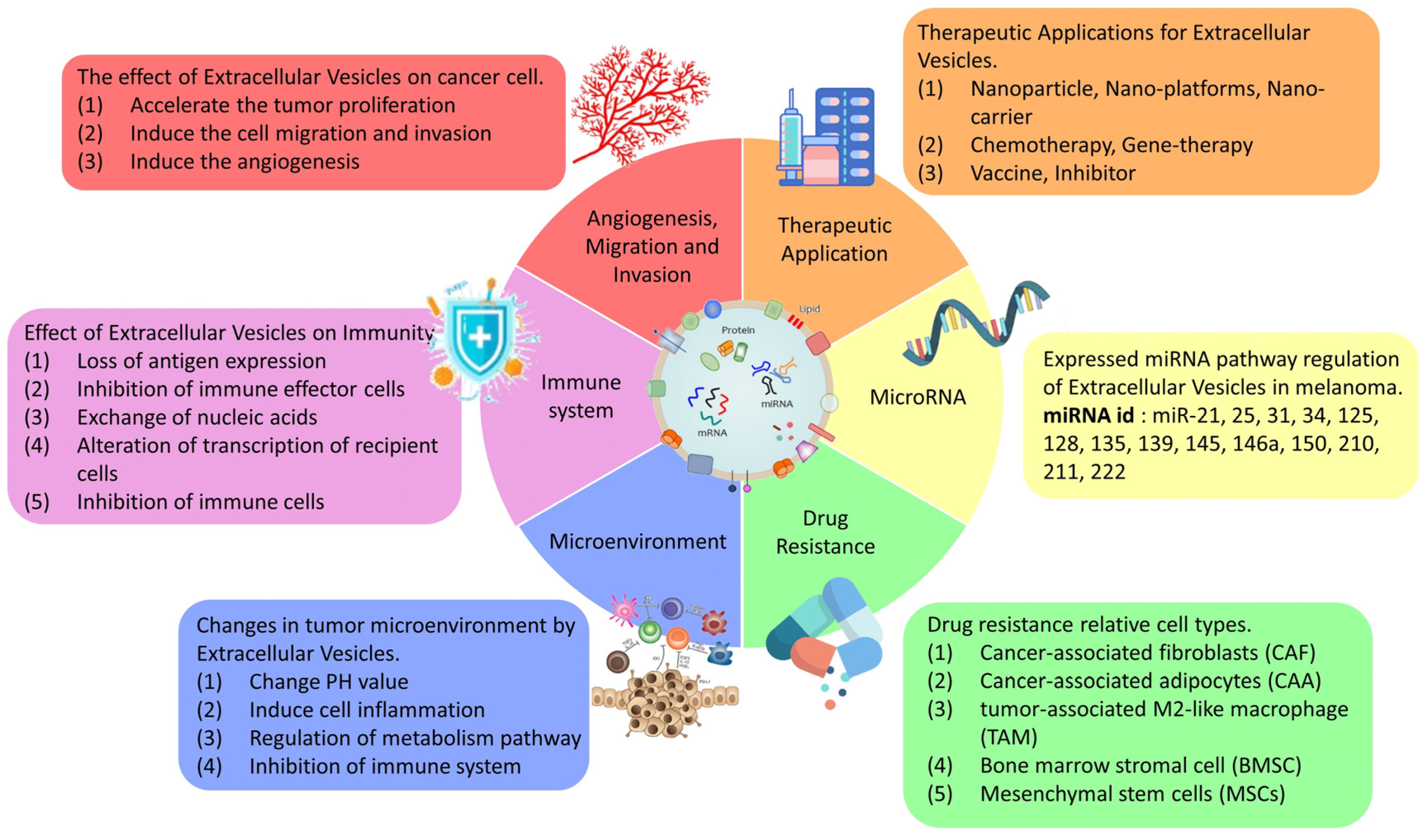
3.1. Growth and Angiogenesis
3.2. Migration and Invasion
3.3. Tumor Microenvironment
3.4. Immune System
3.5. Drug Resistance and Clinical Treatment
3.6. Small RNA (microRNA)
4. Therapeutic Applications of Extracellular Vesicles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hogue, L.; Harvey, V.M. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol. Clin. 2019, 37, 519–526. [Google Scholar] [CrossRef]
- Bandarchi, B.; Ma, L.; Navab, R.; Seth, A.; Rasty, G. From Melanocyte to Metastatic Malignant Melanoma. Dermatol. Res. Pract. 2010, 2010, 583748. [Google Scholar] [CrossRef]
- Volkovova, K.; Bilanicova, D.; Bartonova, A.; Letašiová, S.; Dusinska, M. Associations between environmental factors and incidence of cutaneous melanoma. Review. Env. Health-Glob. 2012, 11, S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: Detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [Green Version]
- Storr, S.J.; Safuan, S.; Mitra, A.; Elliott, F.; Walker, C.; Vasko, M.J.; Ho, B.; Cook, M.; Mohammed, R.A.; Patel, P.M.; et al. Objective assessment of blood and lymphatic vessel invasion and association with macrophage infiltration in cutaneous melanoma. Mod. Pathol. 2012, 25, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I.-I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.; Robertson, B.M.; Iyer, S.; Barry, J.; Dinavahi, S.S.; Robertson, G.P. The role of exosomes in metastasis and progression of melanoma. Cancer Treat Rev. 2020, 85, 101975. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W.; Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- De Broe, M.; Wieme, R.; Roels, F. Letter: Membrane fragments with koinozymic properties released from villous adenoma of the rectum. Lancet 1975, 2, 1214–1215. [Google Scholar] [CrossRef]
- Benz, E.W., Jr.; Moses, H.L. Small, virus-like particles detected in bovine sera by electron microscopy. J. Natl. Cancer Inst. 1974, 52, 1931–1934. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, P.; Almeida, F. Role of Exosomal miRNAs and the Tumor Microenvironment in Drug Resistance. Cells 2020, 9, 1450. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Coscia, C.; Parolini, I.; Sanchez, M.; Biffoni, M.; Boussadia, Z.; Zanetti, C.; Fiani, M.L.; Sargiacomo, M. Generation, Quantification, and Tracing of Metabolically Labeled Fluorescent Exosomes. Methods Mol. Biol. 2016, 1448, 217–235. [Google Scholar]
- Surman, M.; Stępień, E.; Przybyło, M. Melanoma-Derived Extracellular Vesicles: Focus on Their Proteome. Proteomes 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Desrochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 2016, 7, 11958. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, J.L. Natural melanoma-derived extracellular vesicles. Semin. Cancer Biol. 2019, 59, 251–265. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Diamond, M.P.; Al-Hendy, A. The emerging role of extracellular vesicle-derived miRNAs: Implication in cancer progression and stem cell related diseases. J. Clin. Epigenet 2016, 2. [Google Scholar]
- Dong, L.; Zieren, R.C.; Horie, K.; Kim, C.J.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M.D.; Green, J.; Amend, S.R.; et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracell. Vesicles 2020, 10, e12044. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipoor, S.D.; Mortaz, E.; Varahram, M.; Movassaghi, M.; Kraneveld, A.D.; Garssen, J.; Adcock, I.M. The potential biomarkers and immunological effects of tumor-derived exosomes in lung cancer. Front. Immunol. 2018, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Won, J.H.; Lim, G.J.; Han, J.; Lee, J.Y.; Cho, K.O.; Bae, Y.K. A novel population of extracellular vesicles smaller than exosomes promotes cell proliferation. Cell Commun. Signal 2019, 17, 95. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Morishita, M.; Charoenviriyakul, C.; Saji, H.; Takakura, Y. Accelerated growth of B16BL6 tumor in mice through efficient uptake of their own exosomes by B16BL6 cells. Cancer Sci. 2017, 108, 1803–1810. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Lim, Y.; Pecorelli, A. Scavenger receptor class B type I: A multifunctional receptor. Ann. N.Y. Acad. Sci. 2011, 1229, E1–E7. [Google Scholar] [CrossRef]
- Simons, K.; Vaz, W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef]
- Kinslechner, K.; Schütz, B.; Pistek, M.; Rapolter, P.; Weitzenböck, H.P.; Hundsberger, H.; Mikulits, W.; Grillari, J.; Röhrl, C.; Hengstschläger, M.; et al. Loss of SR-BI Down-Regulates MITF and Suppresses Extracellular Vesicle Release in Human Melanoma. Int. J. Mol. Sci. 2019, 20, 1063. [Google Scholar] [CrossRef] [Green Version]
- Ekström, E.J.; Bergenfelz, C.; von Bülow, V.; Serifler, F.; Carlemalm, E.; Jönsson, G.; Andersson, T.; Leandersson, K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer 2014, 13, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, J.L.; Pan, H.; Lanza, G.M.; Wickline, S.A. Consortium for Translational Research in Advanced, I.; Nanomedicine, Paracrine induction of endothelium by tumor exosomes. Lab. Invest. 2009, 89, 1317–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Yan, T.; Huang, C.; Xu, Z.; Wang, L.; Jiang, E.; Wang, H.; Chen, Y.; Liu, K.; Shao, Z.; et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2018, 37, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. Rev. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Gui, J.; Zahedi, F.; Yu, P.; Cho, C.; Bhattacharya, S.; Carbone, C.J.; Yu, Q.; Katlinski, K.V.; Katlinskaya, Y.V.; et al. An Interferon-Driven Oxysterol-Based Defense against Tumor-Derived Extracellular Vesicles. Cancer Cell 2019, 35, 33–45.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, B.; Wu, L.; Hvam, M.L.; Aslan, H.; Dong, M.; Dyrskjøt, L.; Ostenfeld, M.S.; Moghimi, S.M.; Howard, K.A. Tumour exosomes display differential mechanical and complement activation properties dependent on malignant state: Implications in endothelial leakiness. J. Extracell Vesicles 2015, 4, 29685. [Google Scholar] [CrossRef]
- Ghoshal, A.; Rodrigues, L.C.; Gowda, C.P.; Elcheva, I.A.; Liu, Z.; Abraham, T.; Spiegelman, V.S. Extracellular vesicle-dependent effect of RNA-binding protein IGF2BP1 on melanoma metastasis. Oncogene 2019, 38, 4182–4196. [Google Scholar] [CrossRef]
- Xiao, D.; Ohlendorf, J.; Chen, Y.; Taylor, D.D.; Rai, S.N.; Waigel, S.; Zacharias, W.; Hao, H.; McMasters, K.M. Identifying mRNA, MicroRNA and Protein Profiles of Melanoma Exosomes. PLoS ONE 2012, 7, e46874. [Google Scholar] [CrossRef] [Green Version]
- Mannavola, F.; Tucci, M.; Felici, C.; Passarelli, A.; D’Oronzo, S. Tumor-derived exosomes promote the in vitro osteotropism of melanoma cells by activating the SDF-1/CXCR4/CXCR7 axis. J. Transl. Med. 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plebanek, M.P.; Angeloni, N.L.; Vinokour, E.; Li, J.; Henkin, A.; Martinez-Marin, D.; Filleur, S.; Bhowmick, R.; Henkin, J.; Miller, S.D.; et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat. Commun. 2017, 8, 1319. [Google Scholar] [CrossRef]
- Tung, K.H.; Ernstoff, M.S.; Allen, C.; Shu, S. A Review of Exosomes and their Role in The Tumor Microenvironment and Host-Tumor "Macroenvironment". J. Immunol. Sci. 2019, 3, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Logozzi, M.; Mizzoni, D.; Angelini, D.F.; Di Raimo, R.; Falchi, M.; Battistini, L.; Fais, S. Microenvironmental pH and Exosome Levels Interplay in Human Cancer Cell Lines of Different Histotypes. Cancers 2018, 10, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lee, S.; Shin, E.; Seong, K.M.; Jin, Y.W.; Youn, H.; Youn, B. The Emerging Roles of Exosomes as EMT Regulators in Cancer. Cells 2020, 9, 861. [Google Scholar] [CrossRef]
- Gener Lahav, T.; Adler, O.; Zait, Y.; Shani, O.; Amer, M.; Doron, H.; Abramovitz, L.; Yofe, I.; Cohen, N.; Erez, N. Melanoma-derived extracellular vesicles instigate proinflammatory signaling in the metastatic microenvironment. Int. J. Cancer 2019, 145, 2521–2534. [Google Scholar] [CrossRef]
- Shu, S.L.; Yang, Y.; Allen, C.L.; Maguire, O.; Minderman, H.; Sen, A.; Ciesielski, M.J.; Collins, K.A.; Bush, P.J.; Singh, P.; et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci. Rep. 2018, 8, 12905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siveen, K.S.; Raza, A.; Ahmed, E.I.; Khan, A.Q.; Prabhu, K.S.; Kuttikrishnan, S.; Mateo, J.M.; Zayed, H.; Rasul, K.; Azizi, F.; et al. The Role of Extracellular Vesicles as Modulators of the Tumor Microenvironment, Metastasis and Drug Resistance in Colorectal Cancer. Cancers 2019, 11, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Shen, H.; Li, Z.; Wang, T.; Wang, S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J. Hematol. Oncol. 2019, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucci, M.; Mannavola, F.; Passarelli, A.; Stucci, L.S.; Cives, M.; Silvestris, F. Exosomes in melanoma: A role in tumor progression, metastasis and impaired immune system activity. Oncotarget 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Düchler, M.; Czernek, L.; Peczek, L.; Cypryk, W.; Sztiller-Sikorska, M.; Czyz, M. Melanoma-Derived Extracellular Vesicles Bear the Potential for the Induction of Antigen-Specific Tolerance. Cells 2019, 8, 665. [Google Scholar] [CrossRef] [Green Version]
- Isola, L.A.; Chen, S. Exosomes: The messengers of health and disease. Curr. Neuropharmacol. 2017, 15, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef]
- Ventola, C.L. Cancer Immunotherapy, Part 1: Current Strategies and Agents. Pharm. Ther. 2017, 42, 375–383. [Google Scholar]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yang, Y.; Wang, W.; Zhang, Y.; Chen, Z.; Hao, C.; Zhang, J. Melanoma-released exosomes directly activate the mitochondrial apoptotic pathway of CD4+ T cells through their microRNA cargo. Exp. Cell Res. 2018, 371, 364–371. [Google Scholar] [CrossRef]
- Yin, Y.; Cai, X.; Chen, X.; Liang, H.; Zhang, Y.; Li, J.; Wang, Z.; Chen, X.; Zhang, W.; Yokoyama, S.; et al. Tumor-secreted miR-214 induces regulatory T cells: A major link between immune evasion and tumor growth. Cell Res. 2014, 24, 1164–1180. [Google Scholar] [CrossRef]
- Hiltbrunner, S.; Larssen, P.; Eldh, M.; Martinez-Bravo, M.J.; Wagner, A.K.; Karlsson, M.C.; Gabrielsson, S. Exosomal cancer immunotherapy is independent of MHC molecules on exosomes. Oncotarget 2016, 7, 38707–38717. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, W.; McGinley, E.C.; Klinke, D.J., 2nd. Melanoma exosomes deliver a complex biological payload that upregulates PTPN11 to suppress T lymphocyte function. J. Extracell Vesicles 2017, 30, 203–218. [Google Scholar]
- Cheng, L.; Wang, Y.; Huang, L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node. Mol. Ther. 2017, 25, 1665–1675. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Diergaarde, B.; Ferrone, S.; Kirkwood, J.M.; Whiteside, T.L. Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Sci. Rep.-UK 2020, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.J.; Behren, A.; Coleman, B.; Greening, D.W.; Hill, A.F.; Cebon, J. Intercellular Resistance to BRAF Inhibition Can Be Mediated by Extracellular Vesicle–Associated PDGFRβ. Neoplasia 2017, 19, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Cesi, G.; Philippidou, D.; Kozar, I.; Kim, Y.J.; Bernardin, F.; Van Niel, G.; Wienecke-Baldacchino, A.; Felten, P.; Letellier, E.; Dengler, S.; et al. A new ALK isoform transported by extracellular vesicles confers drug resistance to melanoma cells. Mol. Cancer 2018, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.T.; Gong, J.; Pham, T.T.; Kim, Y.; Le, M.T.N. microRNA exchange via extracellular vesicles in cancer. Cell Proliferat. 2020, 53, e12877. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.; Hershkovitz, L.; Itzhaki, O.; Hajdu, S.; Nemlich, Y.; Ortenberg, R.; Gefen, N.; Edry, L.; Modai, S.; Keisari, Y.; et al. Regulation of cancer aggressive features in melanoma cells by microRNAs. PLoS ONE 2011, 6, e18936. [Google Scholar] [CrossRef]
- Gajos-Michniewicz, A.; Czyz, M. Role of miRNAs in melanoma metastasis. Cancers 2019, 11, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajos-Michniewicz, A.; Duechler, M.; Czyz, M. MiRNA in melanoma-derived exosomes. Cancer Lett. 2014, 347, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Svedman, F.C.; Lohcharoenkal, W.; Bottai, M.; Brage, S.E.; Sonkoly, E.; Hansson, J.; Pivarcsi, A.; Eriksson, H. Extracellular microvesicle microRNAs as predictive biomarkers for targeted therapy in metastastic cutaneous malignant melanoma. PLoS ONE 2018, 13, e0206942. [Google Scholar] [CrossRef]
- Lee, J.H.; Dindorf, J.; Eberhardt, M.; Lai, X.; Ostalecki, C.; Koliha, N.; Gross, S.; Blume, K.; Bruns, H.; Wild, S.; et al. Innate extracellular vesicles from melanoma patients suppress β-catenin in tumor cells by miRNA-34a. Life Sci. Alliance 2019, 2, e201800205. [Google Scholar] [CrossRef]
- Dror, S.; Sander, L.; Schwartz, H.; Sheinboim, D.; Barzilai, A.; Dishon, Y.; Apcher, S.; Golan, T.; Greenberger, S.; Barshack, I.; et al. Melanoma miRNA trafficking controls tumour primary niche formation. Nat. Cell Biol. 2016, 18, 1006–1017. [Google Scholar] [CrossRef]
- Felicetti, F.; De Feo, A.; Coscia, C.; Puglisi, R.; Pedini, F.; Pasquini, L.; Bellenghi, M.; Errico, M.C.; Pagani, E.; Carè, A. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J. Transl. Med. 2016, 14, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- House, I.G.; Petley, E.V.; Beavis, P.A. Tumor-derived exosomes modulate T cell function through transfer of RNA. FEBS J. 2018, 285, 1030–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, J.; Wang, S.; Li, P.; Zheng, C.; Zhou, X.; Tao, Y.; Chen, X.; Sun, L.; Wang, A.; et al. Blockage of transferred exosome-shuttled miR-494 inhibits melanoma growth and metastasis. J. Cell Physiol. 2019, 234, 15763–15774. [Google Scholar] [CrossRef]
- Huber, V.; Vallacchi, V.; Fleming, V.; Hu, X.; Cova, A.; Dugo, M.; Shahaj, E.; Sulsenti, R.; Vergani, E.; Filipazzi, P.; et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J. Clin. Invest. 2018, 128, 5505–5516. [Google Scholar] [CrossRef] [Green Version]
- Luan, W.; Ding, Y.; Xi, H.; Ruan, H.; Lu, F.; Ma, S.; Wang, J. Exosomal miR-106b-5p derived from melanoma cell promotes primary melanocytes epithelial-mesenchymal transition through targeting EphA4. J.Exp. Clin. Cancer Res. 2021, 40, 107. [Google Scholar] [CrossRef]
- Dar, A.A.; Majid, S.; de Semir, D.; Nosrati, M.; Bezrookove, V.; Kashani-Sabet, M. miRNA-205 Suppresses Melanoma Cell Proliferation and Induces Senescence via Regulation of E2F1 Protein*. J. Biol. Chem. 2011, 286, 16606–16614. [Google Scholar] [CrossRef] [Green Version]
- Segura, M.F.; Hanniford, D.; Menendez, S.; Reavie, L.; Zou, X.; Alvarez-Diaz, S.; Zakrzewski, J.; Blochin, E.; Rose, A.; Bogunovic, D.; et al. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. P. Natl. A. Sci. Bel. Agr. 2009, 106, 1814–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shull, A.Y.; Latham-Schwark, A.; Ramasamy, P.; Leskoske, K.; Oroian, D.; Birtwistle, M.R.; Buckhaults, P.J. Novel Somatic Mutations to PI3K Pathway Genes in Metastatic Melanoma. PLoS ONE 2012, 7, e43369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; He, Q.; Wei, J. MicroRNA-342 prohibits proliferation and invasion of melanoma cells by directly targeting Zinc-finger E-box binding homeobox 1. Oncol. Res. 2018, 26, 1447. [Google Scholar] [CrossRef]
- Wang, J.; Wuethrich, A.; Sina, A.A.; Lane, R.E.; Lin, L.L.; Wang, Y.; Cebon, J.; Behren, A.; Trau, M. Tracking extracellular vesicle phenotypic changes enables treatment monitoring in melanoma. Sci. Adv. 2020, 6, eaax3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melling, G.E.; Carollo, E.; Conlon, R.; Simpson, J.C.; Carter, D.R.F. The Challenges and Possibilities of Extracellular Vesicles as Therapeutic Vehicles. Eur. J. Pharm. Biopharm. 2019, 144, 50–56. [Google Scholar] [CrossRef]
- Willis, G.R.; Kourembanas, S.; Mitsialis, S.A. Therapeutic Applications of Extracellular Vesicles: Perspectives from Newborn Medicine. Methods Mol. Biol. 2017, 1660, 409–432. [Google Scholar]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; EL Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdouh, M.; Floris, M.; Gao, Z.H.; Arena, V.; Arena, M.; Arena, G.O. Colorectal cancer-derived extracellular vesicles induce transformation of fibroblasts into colon carcinoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 257. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S.; et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Macklin, R.; Wang, H.; Loo, D.; Martin, S.; Cumming, A.; Cai, N.; Lane, R.; Ponce, N.S.; Topkas, E.; Inder, K.; et al. Extracellular vesicles secreted by highly metastatic clonal variants of osteosarcoma preferentially localize to the lungs and induce metastatic behaviour in poorly metastatic clones. Oncotarget 2016, 7, 43570–43587. [Google Scholar] [CrossRef] [Green Version]
- Serpe, C.; Monaco, L.; Relucenti, M.; Iovino, L.; Familiari, P.; Scavizzi, F.; Raspa, M.; Familiari, G.; Civiero, L.; D’Agnano, I.; et al. Microglia-Derived Small Extracellular Vesicles Reduce Glioma Growth by Modifying Tumor Cell Metabolism and Enhancing Glutamate Clearance through miR-124. Cells 2021, 10, 2066. [Google Scholar] [CrossRef]
- Iessi, E.; Logozzi, M.; Lugini, L.; Azzarito, T.; Federici, C.; Spugnini, E.P.; Mizzoni, D.; Di Raimo, R.; Angelini, D.F.; Battistini, L.; et al. Acridine Orange/exosomes increase the delivery and the effectiveness of Acridine Orange in human melanoma cells: A new prototype for theranostics of tumors. J. Enzyme Inhib. Med. Chem. 2017, 32, 648–657. [Google Scholar] [CrossRef]
- Lin, Q.; Qu, M.; Zhou, B.; Patra, H.K.; Sun, Z.; Luo, Q.; Yang, W.; Wu, Y.; Zhang, Y.; Li, L.; et al. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J. Control. Release. 2019, 311-312, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular vesicles in cancer nanomedicine. Semin. Cancer Biol. 2019, 69. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Koyama, Y.; Ito, T.; Hasegawa, A.; Eriguchi, M.; Inaba, T.; Ushigusa, T.; Sugiura, K. Exosomes derived from tumor cells genetically modified to express Mycobacterium tuberculosis antigen: A novel vaccine for cancer therapy. Biotechnol. Lett. 2016, 38, 1857–1866. [Google Scholar] [CrossRef]
- Erkan, E.P.; Saydam, N.; Chen, C.C.; Saydam, O. Extracellular vesicles as carriers of suicide mRNA and/or protein in cancer therapy. Methods Mol. Biol. 2019, 1895, 87–96. [Google Scholar] [PubMed]
- Chao, O.S.; Chang, T.C.; Di Bella, M.A.; Alessandro, R.; Anzanello, F.; Rappa, G.; Goodman, O.B.; Lorico, A. The HDAC6 Inhibitor Tubacin Induces Release of CD133(+) Extracellular Vesicles From Cancer Cells. J. Cell Biochem. 2017, 118, 4414–4424. [Google Scholar] [CrossRef]
| Method | Mechanism | Reference |
|---|---|---|
| pH | Extracellular acidity may increase the ability of cancer cells to release EVs. The pH of the environment can be used to regulate the release of EVs, affecting the development of the tumor or the control of drug resistance. | [43] |
| EMT pathway | During EV-mediated epithelial–mesenchymal transition (EMT)-like processes, the mitogen-activated protein kinase (MAPK) signaling pathway is activated and promotes metastasis. It was demonstrated that melanoma-cell-derived EVs promote the EMT in the tumor microenvironment. | [44] |
| Inflammatory | EVs secreted by metastatic melanoma cells spontaneously metastasize to the lungs and brain and activate proinflammatory signals that induce cell inflammation to promote tumor metastasis. | [45] |
| Metabolism | miRNA inhibitors of melanoma-derived EVs regulate stromal cell metabolism, inhibit the activity of miR-155 and miR-210, and may contribute to the promotion of metastasis. | [46] |
| Immune system | The lipid, protein, DNA, mRNA, and miRNA components in EVs are transferred to recipient tumor cells, affecting many immune-related pathways, leading to the activation, differentiation, and expression of the immune cells and the regulation of the tumor microenvironment, thus affecting tumor development, metastasis, and drug resistance. EVs are regulated and released by the TME and regulate the cell biology of myeloid-derived suppressor cells (MDSCs), including promoting their activation and amplification and enhancing their immunosuppressive functions. | [47,48] |
| Target | Mechanism | Reference |
|---|---|---|
| CD8(+) effector T cells | Melanoma-derived EVs induce immune suppression by promoting T regulatory cell expansion and destroying antitumor CD8(+) effector T cells, thus leading to tumor escape. | [56] |
| CD4+ T cells | Melanoma-derived EVs may directly activate the mitochondrial apoptotic pathway of CD4+ T cells through the microRNA in the EVs. | [57] |
| PTEN | Tumor-secreted miR-214 is sufficiently delivered to recipient T cells by EVs specifically targeting mouse peripheral CD4+ T cells. miR-214 downregulates phosphatase and tensin homolog (PTEN) and promotes Treg expansion. Tumor-derived EVs enhance immune suppression and tumor implantation/growth in mice. | [58] |
| MHC | The major histocompatibility complex (MHC) class I molecules and EVs have an important correlation with the induction of antigen-specific T cell responses and the stable development of tumors. | [59] |
| PD-L1 | Increased tumor surface expression of programmed death-ligand 1 (PD-L1) facilitates tumor cell escape from immune surveillance. PD-L1 interacts with the programmed death-1 (PD-1) receptor on T cells to elicit the immune checkpoint response. Metastatic melanomas release EVs that carry PD-L1 on their surface, which suppresses the function of CD8(+) T cells and facilitates tumor growth. | [60] |
| PTPN11 | Melanoma-derived EVs provide a complex biological load, and the upregulation of tumor tyrosine-protein phosphatase nonreceptor type 11 (PTPN11) expression by B16F0 EVs suppresses T lymphocyte function. | [61] |
| M1 and M2 macrophages | EVs derived from melanoma in premetastatic lymph nodes trigger angiogenesis in tumors by inducing classically activated (M1) and alternatively activate (M2) macrophage-mediated angiogenesis by inducing endothelial cell proliferation. | [62] |
| NKG2D | Melanoma-cell-derived EVs downregulate NKG2D expression in natural killer cells to induce immune suppression. | [63] |
| Gene ID | Mechanisms | Reference |
|---|---|---|
| ALK | ALK activates the MAPK signaling pathway to target cancer. Combined treatment with the inhibitor of ALK and BRAF can significantly reduce tumor growth and induce apoptosis in melanoma. | [65] |
| PDGFRβ | PDGFRβ is a resistance driver transferred to recipient melanoma cells via EVs, resulting in the activation of phosphoinositide 3-kinases (PI3K)/protein kinase B (PKB) signaling and escape from the MAPK pathway in BRAF-inhibitor-sensitive cells, thus influencing drug sensitivity in the recipient melanoma cells. | [64] |
| miRNA ID | EV Origin | Effect | Target Site | Reference |
|---|---|---|---|---|
| let-7g-5p | Patient’s plasma | Increases levels of let-7g-5p in EVs, which is associated with better disease control | MAPK | [70] |
| miR-34a | Patient’s plasma | Prevents tumor relapse and blocks tumor cell proliferation | β-catenin | [71] |
| miR-211 | Melanosome | Targets IGF2R and leads to activation of MAPK signaling, which promotes melanoma growth | IGF2R | [72] |
| miR-222 | Melanoma EVs | Increases tumor malignancy | PI3K/AKT | [73] |
| miR-155, miR-210 | Melanoma EVs | Modulate stromal cell metabolism, which promotes the development of metastasis | OXPHOS | [46] |
| miR-709, miR-2137 | Melanoma EVs | Modulate T cell function | PD-L1 | [74] |
| miR-494 | Melanoma EVs | Suppresses tumor growth and metastasis when levels are increased | none | [75] |
| miR-146a, miR-155, miR-125b, miR-100, miR-125a, miR-146b, miR-99b | Melanoma EVs | Convert myeloid cells into myeloid-derived suppressor cells | CTLA-4, PD-1 | [76] |
| miR-106b-5p | Melanoma EVs | Activates the ERK pathway | EphA4 | [77] |
| miR-205 | Melanoma | Regulates E2F-regulated AKT phosphorylation to inhibit the proliferative capacity of melanoma cells | E2F1, E2F5 | [78] |
| miR-182 | Melanoma | Suppresses the expression of MITF and FOXO3 and stimulates migration of melanoma cells | MITF and FOXO3 | [79] |
| miR-21 | Melanoma | Upon upregulation in melanocytes, increases the proliferation rate and decreases the apoptosis rate | PTEN | [80] |
| miRNA-342 | Melanoma | Targets zinc-finger E-box-binding homeobox 1 (ZEB1) and decreases the proliferation and invasion rates of melanoma cells. | ZEB1 | [81] |
| Method | Mechanisms | Reference |
|---|---|---|
| Nanoparticle | Acridine orange (AO) is an eosinophilic dye that is coated onto a system with EVs as nanocarriers for molecular therapy. AO not only extends the time of drug delivery but also attenuates the toxicity induced in normal cells. Exo-AO treatment has great potential and can be used as a new method for treating tumors by delivering Exo-AO. Nanoplatforms, such as EVs modified with targeting ligands, can improve the anticancer and anti-inflammatory effects of imperialin. The system not only significantly improves the release of the drug in the tumor but also is more biocompatible, showing extremely low systemic toxicity both in vitro and in vivo. This platform provides a new method for more efficient use of EVs for drug delivery and targeting. EV biomimetic porous sputum nanoparticles (PSiNPs) secreted by biocompatible tumor cells were developed as drug carriers for targeting cancer chemotherapy. After intravenous administration, the drug is delivered with specificity. | [91,92,93] |
| Chemotherapy | EVs can act as carriers for chemotherapeutic/chemopreventive agents to suppress tumor proliferation. | [94] |
| Vaccine | EVs loaded with tumor antigens and Mycobacterium tuberculosis antigens have great potential to be used as vaccines to overcome the immune escape of tumor cells after genetic modification. | [95] |
| Gene therapy | The suicide fusion gene construct was loaded into EVs derived from nontumorigenic cell lines. Delivery to glioblastoma cell lines and spheres effectively induced apoptosis of glioblastoma cells and thus inhibited tumor growth in vivo. | [96] |
| Inhibitor | CD133 (Prominin-1) is a stem cell marker that is involved in the development of tumors, differentiation, and anticancer treatment. The use of histone deacetylase 6 (HDAC6) inhibitors to induce CD133 + release in cancer cell EVs has potential as an antitumor mechanism. | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-C.; Chang, Y.-A.; Chen, Y.-J.; Sung, H.-M.; Bogeski, I.; Su, H.-L.; Hsu, Y.-L.; Wang, H.-M.D. The Roles of Extracellular Vesicles in Malignant Melanoma. Cells 2021, 10, 2740. https://doi.org/10.3390/cells10102740
Cheng Y-C, Chang Y-A, Chen Y-J, Sung H-M, Bogeski I, Su H-L, Hsu Y-L, Wang H-MD. The Roles of Extracellular Vesicles in Malignant Melanoma. Cells. 2021; 10(10):2740. https://doi.org/10.3390/cells10102740
Chicago/Turabian StyleCheng, Ying-Chen, Yu-An Chang, Yi-Jen Chen, Hsu-Min Sung, Ivan Bogeski, Hong-Lin Su, Ya-Ling Hsu, and Hui-Min David Wang. 2021. "The Roles of Extracellular Vesicles in Malignant Melanoma" Cells 10, no. 10: 2740. https://doi.org/10.3390/cells10102740
APA StyleCheng, Y.-C., Chang, Y.-A., Chen, Y.-J., Sung, H.-M., Bogeski, I., Su, H.-L., Hsu, Y.-L., & Wang, H.-M. D. (2021). The Roles of Extracellular Vesicles in Malignant Melanoma. Cells, 10(10), 2740. https://doi.org/10.3390/cells10102740







