Prompt Graft Cooling Enhances Cardioprotection during Heart Transplantation Procedures through the Regulation of Mitophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials and Instruments
2.3. Anesthesia
2.4. Surgery
2.5. Warm Ischemia Time Record during Procurement
2.6. Ultrastructure Measurement
2.7. Histological Staining
2.8. Western Blot and Band Quantitation
2.9. LDH and CK Activity Assay
2.10. Rebeating Time and Beating Score
2.11. Left Ventricle Function Measurements
2.12. Rapamycin Treatment and Survival Analysis
2.13. Statistical Analysis
3. Results
3.1. Warm Ischemic Time Is Reduced by Immediate Perfusion with Cold Cardioplegic Solution
3.2. Graft Mitochondrial Integrity Is Maintained by Prompt Cardiac Cooling
3.3. Prompt Cardiac Cooling Protects Graft from Ischemic Injury during Preservation
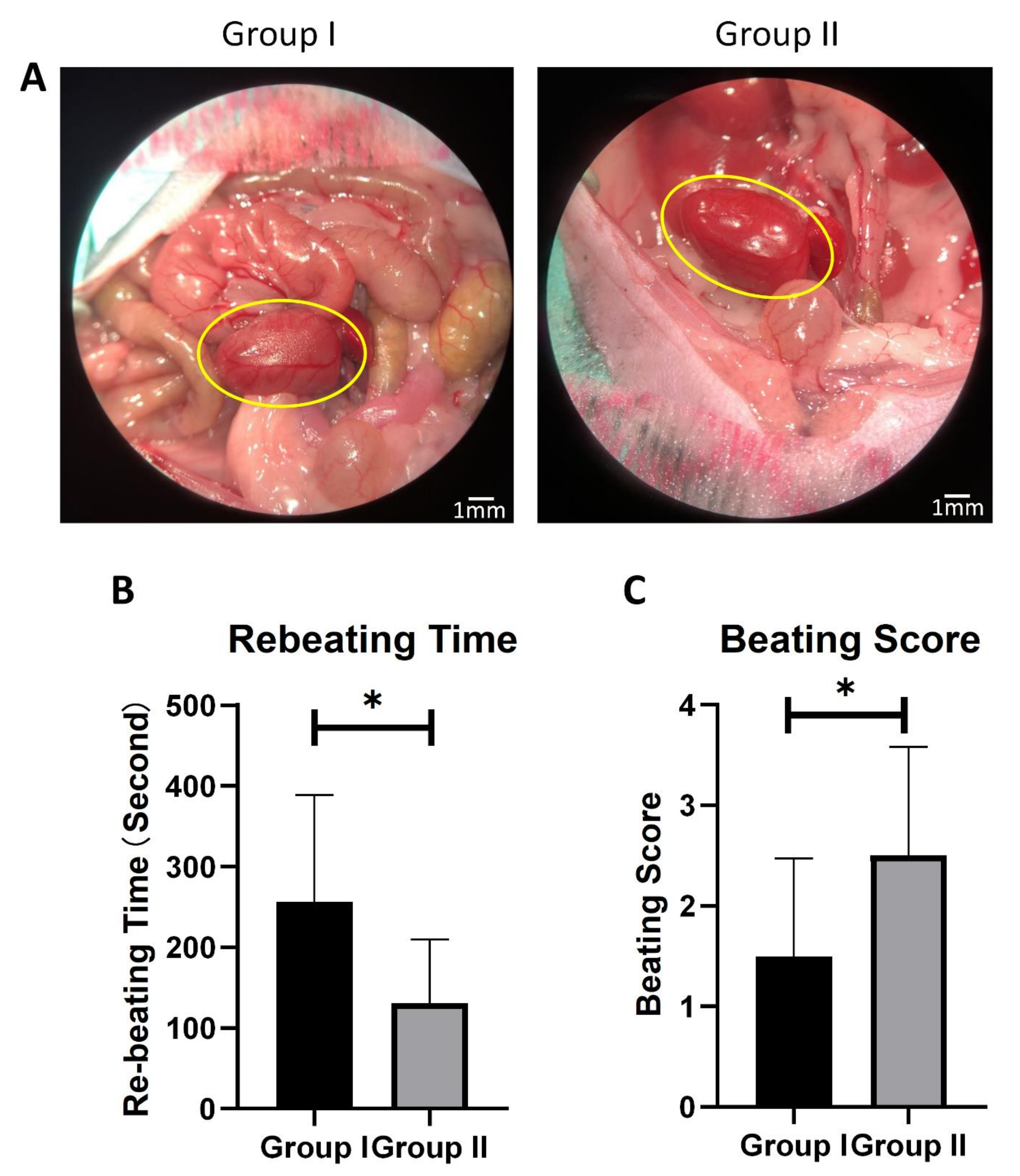
3.4. Prompt Cardiac Cooling Enhances Graft-Beating Recovery after Transplantation
3.5. Prompt Cardiac Cooling Brings Stronger Cardiac Contractility
3.6. Mitophagy Is Enhanced with Prompt Perfusion of Cold Cardioplegic Solution
3.7. Activation of Mitophagy Prolongs Isolated Heart Preservation Time
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X. Cold and Warm Ischemia Result in Different Histological Characteristics of Reperfusion Injury After Lung Transplantation in Mice. J. Heart Lung Transplant. 2018, 37, S224–S225. [Google Scholar] [CrossRef]
- Lutterova, M.; Szatmary, Z.; Kukan, M.; Kuba, D.; Vajdova, K. Marked difference in tumor necrosis factor-alpha expression in warm ischemia- and cold ischemia-reperfusion of the rat liver. Cryobiology 2000, 41, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Krüger, B.; Krick, S.; Dhillon, N.; Lerner, S.M.; Ames, S.; Bromberg, J.S.; Lin, M.; Walsh, L.; Vella, J.; Fischereder, M.; et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 3390–3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskender, I.; Cypel, M.; Martinu, T.; Chen, M.; Sakamoto, J.; Kim, H.; Yu, K.; Lin, H.; Guan, Z.; Hashimoto, K.; et al. Effects of Warm Versus Cold Ischemic Donor Lung Preservation on the Underlying Mechanisms of Injuries During Ischemia and Reperfusion. Transplantation 2018, 102, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, R.; Krigman, J.; McAdams, A.; Ozgen, S.; Sun, N. A Healthy Heart and a Healthy Brain: Looking at Mitophagy. Front. Cell Dev. Biol. 2020, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Mita, Y.; Okawa, Y.; Ariyoshi, M.; Iwai-Kanai, E.; Ueyama, T.; Ikeda, K.; Ogata, T.; Matoba, S. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 2013, 4, 2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubli, D.A.; Zhang, X.; Lee, Y.; Hanna, R.A.; Quinsay, M.N.; Nguyen, C.K.; Jimenez, R.; Petrosyan, S.; Murphy, A.N.; Gustafsson, Å.B. Parkin Protein Deficiency Exacerbates Cardiac Injury and Reduces Survival following Myocardial Infarction. J. Biol. Chem. 2013, 288, 915–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Saito, T.; Nah, J.; Oka, S.-i.; Mukai, R.; Monden, Y.; Maejima, Y.; Ikeda, Y.; Sciarretta, S.; Liu, T.; Li, H.; et al. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J. Clin. Investig. 2019, 129, 802–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, K.; Lindsey, E.S. Improved technique of heart transplantation in rats. J. Thorac. Cardiovasc. Surg. 1969, 57, 225–229. [Google Scholar] [CrossRef]
- Graham, L.; Orenstein, J.M. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat. Protoc. 2007, 2, 2439–2450. [Google Scholar] [CrossRef]
- Flameng, W.; Borgers, M.; Daenen, W.; Stalpaert, G. Ultrastructural and cytochemical correlates of myocardial protection by cardiac hypothermia in man. J. Biomed. Sci. 1980, 79, 413–424. [Google Scholar] [CrossRef]
- Tanaka, M.; Sydow, K.; Gunawan, F.; Jacobi, J.; Tsao Phil, S.; Robbins Robert, C.; Cooke John, P. Dimethylarginine Dimethylaminohydrolase Overexpression Suppresses Graft Coronary Artery Disease. CirculationCirculation 2005, 112, 1549–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Nagayama, T.; Mukhopadhyay, P.; Bátkai, S.; Kass, D.A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 2008, 3, 1422–1434. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.C.; Yanos, M.E.; Kayser, E.-B.; Quintana, A.; Sangesland, M.; Castanza, A.; Uhde, L.; Hui, J.; Wall, V.Z.; Gagnidze, A.; et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 2013, 342, 1524–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padman, B.S.; Nguyen, T.N.; Uoselis, L.; Skulsuppaisarn, M.; Nguyen, L.K.; Lazarou, M. LC3/GABARAPs drive ubiquitin-independent recruitment of Optineurin and NDP52 to amplify mitophagy. Nat. Commun. 2019, 10, 408. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Dai, C.; Fan, Y.; Guo, B.; Ren, K.; Sun, T.; Wang, W. From autophagy to mitophagy: The roles of P62 in neurodegenerative diseases. J. Bioenerg. Biomembr. 2017, 49, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, J.; Xu, X.; Yang, Z.; Zhang, T. Rapamycin Activates Mitophagy and Alleviates Cognitive and Synaptic Plasticity Deficits in a Mouse Model of Alzheimer’s Disease. J. Gerontol. Ser. A 2021, 76, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.M.; Barandiaran Cornejo, J.F. Organ preservation review: History of organ preservation. Curr. Opin. Organ Transplant. 2015, 20, 146–151. [Google Scholar] [CrossRef]
- Minasian, S.M.; Galagudza, M.M.; Dmitriev, Y.V.; Karpov, A.A.; Vlasov, T.D. Preservation of the donor heart: From basic science to clinical studies. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.N.; Haworth, R.A.; Southard, J.H. Warm and cold ischemia result in different mechanisms of injury to the coronary vasculature during reperfusion of rat hearts. Transplant. Proc. 2000, 32, 15–18. [Google Scholar] [CrossRef]
- Moyzis, A.G.; Sadoshima, J.; Gustafsson, Å.B. Mending a broken heart: The role of mitophagy in cardioprotection. Am. J. Physiol. -Heart Circ. Physiol. 2014, 308, H183–H192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morciano, G.; Patergnani, S.; Bonora, M.; Pedriali, G.; Tarocco, A.; Bouhamida, E.; Marchi, S.; Ancora, G.; Anania, G.; Wieckowski, M.R.; et al. Mitophagy in Cardiovascular Diseases. J. Clin. Med. 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, M.; Yu, J.D.; Kono, M.; Tan, V.P.; Smith, J.M.; Miyamoto, S. RhoA induces mitophagy through PINK1 stabilization to confer cardioprotection. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Petit, P.X.; Goubern, M.; Diolez, P.; Susin, S.A.; Zamzami, N.; Kroemer, G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: The impact of irreversible permeability transition. FEBS Lett. 1998, 426, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Petit, P.X.; Lecoeur, H.; Zorn, E.; Dauguet, C.; Mignotte, B.; Gougeon, M.L. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell Biol. 1995, 130, 157–167. [Google Scholar] [CrossRef]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Zanin, C.; Vayssière, J.L.; Petit, P.X.; Kroemer, G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 1995, 181, 1661–1672. [Google Scholar] [CrossRef] [Green Version]
- Chipuk, J.E.; Bouchier-Hayes, L.; Green, D.R. Mitochondrial outer membrane permeabilization during apoptosis: The innocent bystander scenario. Cell Death Differ. 2006, 13, 1396–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonora, M.; Wieckowski, M.R.; Sinclair, D.A.; Kroemer, G.; Pinton, P.; Galluzzi, L. Targeting mitochondria for cardiovascular disorders: Therapeutic potential and obstacles. Nat. Rev. Cardiol. 2019, 16, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Marasco, S.F.; Kras, A.; Schulberg, E.; Vale, M.; Lee, G.A. Impact of Warm Ischemia Time on Survival after Heart Transplantation. Transplant. Proc. 2012, 44, 1385–1389. [Google Scholar] [CrossRef]
- Rossard, L.; Favreau, F.; Giraud, S.; Thuillier, R.; Le Pape, S.; Goujon, J.M.; Valagier, A.; Hauet, T. Role of warm ischemia on innate and adaptive responses in a preclinical renal auto-transplanted porcine model. J. Transl. Med. 2013, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, Y.; Sugimoto, S.; Yamamoto, S.; Okada, M.; Otani, S.; Ohara, T.; Yamane, M.; Matsukawa, A.; Oto, T.; Toyooka, S. Prolonged warm ischemia exacerbated acute rejection after lung transplantation from donation after cardiac death in a mouse. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 57–62. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Liang, J.; Huang, W.; Jiang, L.; Paul, C.; Lin, B.; Zheng, J.; Wang, Y. Prompt Graft Cooling Enhances Cardioprotection during Heart Transplantation Procedures through the Regulation of Mitophagy. Cells 2021, 10, 2912. https://doi.org/10.3390/cells10112912
Wu Z, Liang J, Huang W, Jiang L, Paul C, Lin B, Zheng J, Wang Y. Prompt Graft Cooling Enhances Cardioprotection during Heart Transplantation Procedures through the Regulation of Mitophagy. Cells. 2021; 10(11):2912. https://doi.org/10.3390/cells10112912
Chicago/Turabian StyleWu, Zhichao, Jialiang Liang, Wei Huang, Lin Jiang, Christian Paul, Bonnie Lin, Junmeng Zheng, and Yigang Wang. 2021. "Prompt Graft Cooling Enhances Cardioprotection during Heart Transplantation Procedures through the Regulation of Mitophagy" Cells 10, no. 11: 2912. https://doi.org/10.3390/cells10112912





