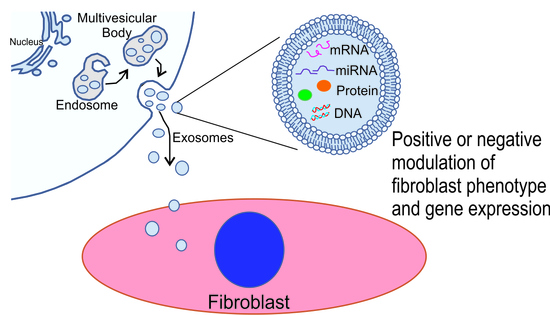
Roles of Exosomes in Cardiac Fibroblast Activation and Fibrosis
Abstract
:1. Introduction
2. Intercellular Communication in the Heart
3. Extracellular Vesicles
4. Exosomes
5. Exosomes and Heart Disease
6. Role of Exosomes in Fibrosis and Fibroblast Activation
7. Therapeutic Utilization and Engineering of Exosomes
8. Diagnostic Value of Exosomes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac fibrosis: The fibroblast awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis, N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Aspects Med. 2019, 65, 70–99. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Zeigler, A.C.; Richardson, W.J.; Holmes, J.W.; Saucerman, J.J. Computational modeling of cardiac fibroblasts and fibrosis. J. Mol. Cell. Cardiol. 2016, 93, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis, N.G. Can myocardial fibrosis be reversed? J. Am. Coll. Cardiol. 2019, 73, 2283–2285. [Google Scholar] [CrossRef]
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and functional characterization of cardiac fibroblasts. Cardio Res. 2005, 1, 40–51. [Google Scholar] [CrossRef]
- Díaz-Araya, G.; Vivar, R.; Humeres, C.; Boza, P.; Bolivar, S.; Muñoz, C. Cardiac fibroblasts as sentinel cells in cardiac tissue: Receptors, signaling pathways and cellular functions. Pharmacol. Res. 2015, 101, 30–40. [Google Scholar] [CrossRef]
- Tallquist, M.D.; Molkentin, J.D. Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 2017, 14, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Kohl, P.; Camelliti, P.; Burton, F.L.; Smith, G.L. Electrical coupling of fibroblasts and myocytes: Relevance for cardiac propagation. J. Electrocardiol. 2005, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Lin, S.C.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reaume, A.G.; de Sousa, P.A.; Kulkarni, S.; Langille, B.L.; Zhu, D.; Davies, T.C.; Juneja, S.C.; Kidder, G.M.; Rossant, J. Cardiac malformation in neonatal mice lacking connexin 43. Science 1995, 267, 1831–1834. [Google Scholar] [CrossRef]
- Ton, Q.V.; Iovine, M.K. Determining how defects in Connexin43 cause skeletal disease. Genesis 2015, 51, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severs, N.J.; Dupont, E.; Coppen, S.R.; Halliday, D.; Inett, E.; Baylis, D.; Rothery, S. Remodeling of gap junctions and connexin expression in heart disease. Biochim. Biophys. Acta 2004, 1662, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, R.; Gorge, P.M.; Gorbe, A.; Ferdinandy, P.; Lampe, P.D.; Leybaert, L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Phamcaol. Ther. 2015, 153, 90–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilton, L.; Giles, W.R.; Smith, G.L. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J. Physiol. 2007, 583, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.S.; Raaijmakers, A.J.; van Doorn, T.; Kok, B.; Nieuwenhuis, S.; van der Nagel, R.; Vos, M.A.; de Boer, T.P.; van Rijen, H.V.; Bierhuizen, M.F. Changes in Cx43 and NaV15 expression precede the occurrence of substantial fibrosis in calcineurin-induced murine cardiac hypertrophy. PLoS ONE 2014, 9, e87226. [Google Scholar] [CrossRef]
- Cao, L.; Chen, Y.; Lu, L.; Liu, Y.; Wang, Y.; Fan, J.; Yin, Y. Angiotensin II upregulates fibroblast-myofibroblast transition through Cx43-dependent CaMKII and TGF-β1 signaling in neonatal rat cardiac fibroblasts. Acta Biochim. Biophys. Sin. 2018, 50, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.A.; van Veen, T.A.B.; de Jong, S.; van der Nagel, R.; van Stuijvenberg, L.; Driessen, H.; Labzowski, R.; Oefner, C.M.; Bosch, A.A.; Nguyen, T.Q.; et al. Reduced Cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ. Arrhythm. Electrophsiol. 2012, 5, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Anderson, H.C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J. Cell Biol. 1969, 41, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Thakur, A.; Ke, X.; Chen, Y.W.; Motallebnejad, P.; Zhang, K.; Lian, Q.; Chen, H.J. The mini player with diverse functions: Extracellular vesicles in cell biology, disease and therapeutics. Protein Cell 2021, 1–24. [Google Scholar] [CrossRef]
- Anand, S.; Samual, M.; Kumar, S.; Mathivanan, S. Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochim. Biophys. Acta 2019, 1867, 140203. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Akbar, N.; Roodselaar, J.; Evans, M.C.; Gardiner, C.; Sargent, I.; Romero, I.A.; Bristow, A.; Buchan, A.M.; Haughey, N.; et al. Circulating endothelial-derived extracellular vesicles mediate the acute phase response and sickness behavior associated with CAN inflammation. Sci. Rep. 2017, 7, 9512–9574. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Rice, S.J.; Wang, J.; Gao, L.; Tsai, J.; Anvari, R.T.; Zhou, F.; Liu, X.; Wang, G.; Tang, Y.; et al. Membrane trafficking and exocytosis are upregulated in port wine stain blood vessels. Histol. Histopathol. 2019, 34, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.C.; Bond, D.T.; McKee, M.; Skog, J.; Paunescu, T.G.; Da Silva, N.; Brown, D.; Russo, L.M. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010, 78, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biological function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signaling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Hope, L.W.; Brasch, M.; Reinhard, C.; Cohen, S.N. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl. Acad Sci. USA 2003, 100, 7626–7631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollert, T.; Wunder, C.; Lippincott-Schwartz, J.; Hurley, J.H. Membrane scission by the ESCRT-III complex. Nature 2009, 12, 172–177. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemler, M.E. Tetrapsanin proteins mediate cellular penetration, invasion and fusion and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef]
- Pols, M.S.; Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome release of β-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef] [Green Version]
- Boker, K.O.; Lemus-Diaz, N.; Ferreira, R.R.; Schiller, L.; Schneider, S.; Gruber, J. The impact of the CD9 tetraspanin on lentivirus infectivity and exosome secretion. Mol. Ther. 2018, 26, 634–647. [Google Scholar] [CrossRef] [Green Version]
- Möbius, W.; Ohno-Iwashita, Y.; van Donselaar, E.G.; Oorschot, V.M.; Shimada, Y.; Fujimoto, T.; Heijnen, H.F.; Geuze, H.J.; Slot, J.W. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J. Histochem. Cytochem. 2002, 50, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Mittelbrunn, M.; Fernández-Delgado, I.; Torralba, D.; Moreno-Gonzalo, O.; Baldanta, S.; Enrich, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016, 7, 13588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.; Hoshino, D.; Hong, N.H.; Kirkbride, K.C.; Grega-Larson, N.E.; Seiki, M.; Tyska, M.J.; Weaver, A.M. Cortactin promotes exosome secretion by controlling branched actin dynamics. J. Cell Biol. 2016, 214, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.T.; Asano, Y.; Stinchcombe, J.C.; Dieckmann, N.M.; Chen, B.C.; Gawden-Bone, C.; van Engelenburg, S.; Legant, W.; Gao, L.; Davidson, M.W.; et al. Actin depletion initiates events leading to granule secretion at the immunological synapse. Immunity 2015, 42, 864–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herranz, G.; Aguilera, P.; Dávila, S.; Sánchez, A.; Stancu, B.; Gómez, J.; Fernández-Moreno, D.; de Martín, R.; Quintanilla, M.; Fernández, T.; et al. Protein kinase C delta regulates the depletion of actin at the immunological synapse required for polarized exosome secretion by T Cells. Front. Immunol. 2019, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2009, 12, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Hedlund, M.; Nagaeva, O.; Kargl, D.; Baranov, V.; Mincheva-Nilsson, L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS ONE 2011, 6, e16899. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M. Platinum nanoparticles enhance exosome release in human lung epithelial adenocarcinoma cancer cells (A549): Oxidative stress and the ceramide pathway are key players. Int. J. Nanomed. 2021, 16, 515–538. [Google Scholar] [CrossRef]
- Pironti, G.; Strachan, R.T.; Abraham, D.; Mon-Wei Yu, S.; Chen, M.; Chen, W.; Hanada, K.; Mao, L.; Watson, L.J.; Rockman, H.A. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 2015, 131, 2120–2130. [Google Scholar] [CrossRef]
- Wang, Z.; Maruyama, K.; Sakisaka, Y.; Suzuki, S.; Tada, H.; Suto, M.; Saito, M.; Yamada, S.; Nemoto, E. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1β production through the inhibition of the NF-κB signaling pathway in macrophages. Front. Immunol. 2019, 10, 1310. [Google Scholar] [CrossRef] [Green Version]
- Koomullil, R.; Tehrani, B.; Goliwas, K.; Wang, Y.; Ponnazhagan, S.; Berry, J.; Deshane, J. Computational simulation of exosome transport in tumor microenvironment. Front. Med. 2021, 8, 643793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Goliwas, K.F.; Severino, P.E.; Hough, K.P.; Van Vessem, D.; Wang, H.; Tousif, S.; Koomullil, R.P.; Frost, A.R.; Ponnazhagan, S.; et al. Mechanical strain induces phenotypic changes in breast cancer cells and promotes immunosuppression in the tumor microenvironment. Lab. Investig. 2020, 100, 1503–1516. [Google Scholar] [CrossRef]
- Chen, Q.; Takada, R.; Noda, C.; Kobayashi, S.; Takada, S. Different populations of Wnt-containing vesicles are individually released from polarized epithelial cells. Sci. Rep. 2016, 6, 35562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, T.; Osaki, F.; Hiragi, S.; Sakamaki, Y.; Fukuda, M. ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Rep. 2021, 22, e51475. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Jadli, A.S.; Ballasy, N.; Edalat, P.; Patel, V.B. Inside(sight) of tiny communicator: Exosome biogenesis, secretion, and uptake. Mol. Cell. Biochem. 2020, 467, 77–94. [Google Scholar] [CrossRef]
- Li, X.; Chen, R.; Kemper, S.; Brigstock, D.R. Structural and functional characterization of fibronectin in extracellular vesicles from hepatocytes. Front. Cell Dev. Biol. 2021, 9, 640667. [Google Scholar] [CrossRef]
- Li, M.; Jiang, M.; Meng, J.; Tao, L. Exosomes: Carriers of pro-fibrotic signals and therapeutic targets in fibrosis. Curr. Pharm. Des. 2019, 25, 4496–4509. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Lin, C.; An, Z.; Yu, J.; Cao, H.; Fan, Y.; Liang, X. Exosomal Malat1 derived from Ox-Ldl-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol. Chem. 2020, 401, 367–376. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Xue, F.; Li, Y.; Liu, W.; Zhang, S. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am. J. Transl. Res. 2018, 10, 4350–4366. [Google Scholar]
- Guo, D.; Xu, Y.; Ding, J.; Dong, J.; Jia, N.; Li, Y.; Zhang, M. Roles and clinical applications of exosomes in cardiovascular disease. BioMed Res. Int. 2020, 2020, 5424281. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.; Shoja-Taheri, F.; Davis, M.E.; Kishore, R. Extracellular vesicles and the application of system biology and computational modeling in cardiac repair. Circ. Res. 2018, 123, 188–204. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, H.; Li, B.; Qin, Q.; Qi, L.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; Wang, X.L.; Cui, T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 89, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Bang, C.; Batkai, S.; Dangwal, S.; Gupta, S.K.; Foinquinos, A.; Holzmann, A.; Just, A.; Remke, J.; Zimmer, K.; Zeug, A.; et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Investig. 2014, 124, 2136–2146. [Google Scholar] [CrossRef]
- Gao, Y.; Pu, J. Differentiation and application of human pluripotent stem cells derived cardiovascular cells for treatment of heart diseases: Promises and challenges. Front. Cell Dev. Biol. 2021, 9, 658088. [Google Scholar] [CrossRef]
- Barile, L.; Cervio, E.; Lionetti, V.; Milano, G.; Ciullo, A.; Biemmi, V.; Bolis, S.; Altomare, C.; Matteucci, M.; Di Silvestre, D.; et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: Role of pregnancy-associated plasma protein-A. Cardiovasc. Res. 2018, 114, 992–1005. [Google Scholar] [CrossRef] [Green Version]
- Luther, K.M.; Haar, L.; McGuinness, M.; Wang, Y.; Lynch, T.L., IV; Phan, A.; Song, Y.; Shen, Z.; Gardner, G.; Kuffel, G.; et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell. Cardiol. 2018, 119, 125–137. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Li, Q.; Xu, J.; Xu, J.; Xiong, Y.; Chen, G.; Qian, H.; Jin, C.; Yu, Y.; et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res. Ther. 2019, 10, 300. [Google Scholar] [CrossRef] [Green Version]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 655, 1525–1536. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, H.; Chen, S.; Zhang, W.; Chen, Y.; Yang, Y. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp. Neurol. 2020, 330, 113325. [Google Scholar] [CrossRef] [PubMed]
- Dutka, M.; Bobiński, R.; Korbecki, J. The relevance of microRNA in post-infarction left ventricular remodelling and heart failure. Heart Fail. Rev. 2019, 24, 575–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, M.B.; Shu, J.; Sardu, C.; Matarese, A.; Santulli, G. Cardiosomal microRNAs are essential in post-infarction myofibroblast phenoconversion. Int. J. Mol. Sci. 2019, 21, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, X.; Fan, J.; Li, H.; Yin, Z.; Zhao, Y.; Dai, B.; Dong, N.; Chen, C.; Wang, D.W. miR-217 promotes cardiac hypertrophy and dysfunction by targeting PTEN. Mol. Ther. Nucleic Acids 2018, S12, 254–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, P.; Kalani, A.; Medina, I.; Familtseva, A.; Tyagi, S.C. Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. J. Cell. Mol. Med. 2015, 19, 2153–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Liu, H.; Gao, W.; Zhang, L.; Ye, Y.; Yuan, L.; Ding, Z.; Wu, J.; Kang, L.; Zhang, X.; et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics 2018, 8, 2565–2582. [Google Scholar] [CrossRef]
- Wu, X.; Gao, Y.; Xu, L.; Dang, W.; Yan, H.; Zou, D.; Zhu, Z.; Luo, L.; Tian, N.; Wang, X.; et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci. Rep. 2017, 7, 9371. [Google Scholar] [CrossRef] [Green Version]
- de Jong, O.G.; van Balkom, B.W.; Gremmels, H.; Verhaar, M.C. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J. Cell. Mol. Med. 2016, 20, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Gollmann-Tepeköylü, C.; Pölzl, L.; Graber, M.; Hirsch, J.; Nägele, F.; Lobenwein, D.; Hess, M.W.; Blumer, M.J.; Kirchmair, E.; Zipperle, J.; et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc. Res. 2020, 116, 1226–1236. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Mai, J.; Chen, Z.; Huang, T.; Wang, S.; Chen, Y.; Wang, J. Distinct Anti-Fibrotic Effects of Exosomes Derived from Endothelial Colony-Forming Cells Cultured Under Normoxia and Hypoxia. Med. Sci. Monit. 2018, 24, 6187–6199. [Google Scholar] [CrossRef]
- Chen, J.; Cui, C.; Yang, X.; Xu, J.; Venkat, P.; Zacharek, A.; Yu, P.; Chopp, M. MiR-126 Affects Brain-Heart Interaction after Cerebral Ischemic Stroke. Transl. Stroke Res. 2017, 8, 374–385. [Google Scholar] [CrossRef]
- Lin, F.; Zeng, Z.; Song, Y.; Li, L.; Wu, Z.; Zhang, X.; Li, Z.; Ke, X.; Hu, X. YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Res. Ther. 2019, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Chen, L.; Feng, Z.; Chen, W.; Yan, S.; Yang, R.; Xiao, J.; Gao, J.; Zhang, D.; Ke, X. EPC-derived exosomal miR-1246 and miR-1290 regulate phenotypic changes of fibroblasts to endothelial cells to exert protective effects on myocardial infarction by targeting ELF5 and SP1. Front. Cell Dev. Biol. 2021, 9, 647763. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; He, Y.; Chen, S.; Zhang, D.; Yu, Y.; Fan, C. Macrophage-Derived miRNA-Containing Exosomes Induce Peritendinous Fibrosis after Tendon Injury through the miR-21-5p/Smad7 Pathway. Mol. Ther. Nucleic Acids 2019, 14, 114–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhang, C.; Liu, L.A.X.; Chen, B.; Li, Y.; Du, J. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol. Ther. 2017, 25, 192–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindappa, P.K.; Patil, M.; Garikipati, V.N.S.; Verma, S.K.; Saheera, S.; Narasimhan, G.; Zhu, W.; Kishore, R.; Zhang, J. Krishnamurthy, P. Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. FASEB J. 2020, 34, 2238–2251. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhou, R.; Liang, Y.; Fu, X.; Wang, D.; Wang, C. Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. FASEB J. 2019, 33, 12200–12212. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Yin, S.; Ren, R.; Liu, S.; Yong, L.; Liu, Y.; Li, Y.; Zheng, M.H.; Kunos, G.; Gao, B.; et al. Myeloid-cell-specific IL-6 signaling promotes microRNA-223-enriched exosome production to attenuate NAFLD-associated fibrosis. Hepatology 2021, 74, 116–132. [Google Scholar] [CrossRef]
- Guiot, J.; Cambier, M.; Boeckx, A.; Henket, M.; Nivelles, O.; Gester, F.; Louis, E.; Malaise, M.; Dequiedt, F.; Louis, R.; et al. Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p. Thorax 2020, 75, 870–881. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Liu, T.; Jiang, W.; Hu, X. miR-19a/19b-loaded exosomes in combination with mesenchymal stem cell transplantation in a preclinical model of myocardial infarction. Regen. Med. 2020, 15, 1749–1759. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, A.; Wang, H.; Klein, J.D.; Tan, L.; Wang, Z.M.; Du, J.; Naqvi, N.; Liu, B.C.; Wang, X.H. miR-26a limits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics 2019, 9, 1864–1877. [Google Scholar] [CrossRef]
- Hirai, K.; Ousaka, D.; Fukushima, Y.; Kondo, M.; Eitoku, T.; Shigemitsu, Y.; Hara, M.; Baba, K.; Iwasaki, T.; Kasahara, S.; et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci. Transl. Med. 2020, 12, eabb3336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.; Benedict, C.; et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhu, Y.; Wu, C.; Liu, W.; He, Y.; Yang, Q. Adipose-derived mesenchymal stem cells-derived exosomes carry microRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation 2021, 44, 1815–1830. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Kumari, R.; Verma, S.K. Cardiac fibroblasts and cardiac fibrosis: Precise role of exosomes. Front. Cell Dev. Biol. 2019, 7, 318. [Google Scholar] [CrossRef]
- Wang, Q.G.; Cheng, B.C.; He, Y.Z.; Li, L.J.; Ling, Y.; Luo, G.; Wang, L.; Liang, S.; Zhang, Y. miR-320a in serum exosomes promotes myocardial fibroblast proliferation via regulating the PIK3CA/Akt/mTOR signaling pathway in HEH2 cells. Exp. Ther. Med. 2021, 22, 873. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Mu, Y.; Olveda, R.M.; Ross, A.G.; Olveda, D.U.; McManus, D.P. Serum exosomal miRNAs for grading hepatic fibrosis due to Schistosomiasis. Int. J. Mol. Sci. 2020, 21, 3560. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Yin, Z.; Wang, F.; Chen, C.; Wang, D.W. Identification of cardiac-related circulating microRNA profile in human chronic heart failure. OncoTarget 2016, 7, 33–45. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 18255–18260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, B.A.; Levick, S.P.; Carver, W.E. Alterations in cardiac structure and function in a murine model of chronic alcohol consumption. Microsc. Microanal. 2012, 18, 453–461. [Google Scholar] [CrossRef]
- Loyer, X.; Zlatanova, I.; Devue, C.; Yin, M.; Howangyin, K.Y.; Klaihmon, P.; Guerin, C.L.; Kheloufi, M.; Vilar, J.; Zannis, K.; et al. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction. Circ. Res. 2018, 123, 100–106. [Google Scholar] [CrossRef]
- Yu, H.; Qin, L.; Peng, Y.; Bai, W.; Wang, Z. Exosomes derived from hypertrophic cardiomyocytes induce inflammation in macrophages via miR-155 mediated MAPK pathway. Front. Immunol. 2021, 11, 606045. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lin, Z.Q.; Long, B.; Li, J.H.; Zhou, J.; Li, P.F. Cardiac hypertrophy is positively regulated by MicroRNA miR-23a. J. Biol. Chem. 2012, 287, 589–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, Q.; Jiao, H. LncRNA NRON promotes M2 macrophage polarization and alleviates atrial fibrosis through suppressing exosomal miR-23a derived from atrial myocytes. J. Formosan Med. Assoc. 2020, 120, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.S.; Ismahil, M.A.; Goel, M.; Patel, B.; Hamid, T.; Rokosh, G.; Prabhu, S.D. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ. Heart Fail. 2017, 10, e003688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Hao, C.; Zhang, L.; Zhang, L.; Liu, S.; Li, Y.; Qi, Y.; Zhao, Y.; Huang, R.; Wei, J.; et al. Exosomal miR-125a-5p derived from silica-exposed macrophages induces fibroblast transdifferentiation. Ecotoxicol. Environ. Saf. 2020, 192, 110253. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.J.; Yu, X.Y.; et al. miRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanisms to enhance cardiac repair. BioMed Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cheng, S.; Wang, S.; Li, W.; Liu, J. BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Int. Immunopharmacol. 2021, 93, 107389. [Google Scholar] [CrossRef]
- Malgulwar, P.B.; Pathak, P.; Singh, M.; Kale, S.S.; Suri, V.; Sarkar, C.; Sharma, M.C. Downregulation of SMARCB1/INI1 expression in pediatric chordomas correlates with upregulation of miR-671-5p and miR-193a-5p expressions. Brain Tumor Pathol. 2017, 34, 155–159. [Google Scholar] [CrossRef]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The miR-29 family: Genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. Genom. 2012, 44, 237–244. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, X.R.; Wei, L.H.; Chung, A.C.; Yu, C.M.; Lan, H.Y. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol. Ther. 2014, 22, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, J.; He, W.; Zhao, Y.; Zhang, A.; Liu, Y.; Hassounah, F.; Ma, F.; Klein, J.D.; Wang, X.H.; et al. Exogenous miR-29a attenuates muscle atrophy and kidney fibrosis in unilateral ureteral obstruction mice. Hum. Gene Ther. 2020, 31, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.T.; Melo, S.A.; Özdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H., 2nd; LeBleu, V.S.; Kalluri, R. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 385–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, J. Exosomal lncRNA AK139128 derived from hypoxic cardiomyocytes promotes apoptosis and inhibits cell proliferation in cardiac fibroblasts. Int. J. Nanonmed. 2020, 15, 3363–3376. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Xia, T.; Du, Y.; Liu, J.; Xie, Y.; Zhang, Y.; Guan, F.; Wu, J.; Wang, X.; Shi, C. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: A role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J. 2019, 33, 8530–8542. [Google Scholar] [CrossRef] [PubMed]
- Dzialo, E.; Rudnik, M.; Koning, R.I.; Czepiel, M.; Tkacz, K.; Baj-Krzyworzeka, M.; Distler, O.; Siedler, M.; Kania, G.; Blyszczuk, P. WNT3a and WNT5a transported by exosomes activate WNT signaling in human cardiac fibroblasts. Int. J. Mol. Sci. 2019, 20, 1436. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Miao, J.; Wang, C.; Zhou, S.; Chen, S.; Ren, Q.; Hong, X.; Wang, Y.; Hou, F.F.; Zhou, L.; et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 2020, 96, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Roda, M.A.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN exosomes: Pathogenic entities causing matrix destruction and disease in the lung. Cell 2019, 176, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef]
- Shen, D.; He, Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann. Transl. Med. 2021, 9, 1323. [Google Scholar] [CrossRef]
- Liao, Z.; Chen, Y.; Duan, C.; Zhu, K.; Huang, R.; Zhao, H.; Hintze, M.; Pu, Q.; Yuan, Z.; Lv, L.; et al. Cardiac telocytes inhibit cardiac microvascular endothelial cell apoptosis through exosomal miRNA-21-5p-targeted cdip1 silencing to improve angiogenesis following myocardial infarction. Theranostics 2021, 11, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhu, W.; Wan, D. Downregulation of microRNA-21-5p from macrophages-derived exosomes represses ventricular remodeling after myocardial infarction via inhibiting tissue inhibitors of metalloproteinase 3. Int. Immunopharmacol. 2021, 96, 107611. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Kong, X.; Li, H.; He, X. Exosomes released from mesenchymal stem cells overexpressing microRNA-30e ameliorate heart failure in rats with myocardial infarction. Am. J. Transl. Res. 2021, 13, 4007–4025. [Google Scholar] [PubMed]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from miR-126-overexpressing adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell. Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Cui, C.; Chen, Z.; Chopp, M.; Zacharek, A.; Landschoot-Ward, J.; Culmone, L.; Yang, X.P.; Xu, J.; Chen, J. CD133+Exosome Treatment Improves Cardiac Function after Stroke in Type 2 Diabetic Mice. Transl. Stroke Res. 2021, 12, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Alimujiang, M.; Chen, Q.; Shi, H.; Luo, X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J. Cell. Biochem. 2019, 120, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Yang, D.; Liang, J.; Wang, X.; Wu, S.; Wang, X.; Hu, C. Human Endothelial Progenitor Cell-Derived Exosomes Increase Proliferation and Angiogenesis in Cardiac Fibroblasts by Promoting the Mesenchymal-Endothelial Transition and Reducing High Mobility Group Box 1 Protein B1 Expression. DNA Cell Biol. 2017, 36, 1018–1028. [Google Scholar] [CrossRef]
- Fu, D.L.; Jiang, H.; Li, C.Y.; Gao, T.; Liu, M.R.; Li, H.W. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10107–10117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Wang, Q. Constitutive activation of the NEAT1/miR-22-3p/Ltb4r1 signaling pathway in mice with myocardial injury following acute myocardial infarction. Aging 2021, 13, 15307–15319. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, X.F.; Li, Y.Y.; Xue, F.T.; Zhang, S. Decreased HSP70 expression on serum exosomes contributes to cardiac fibrosis during senescence. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, F.M.; Hossain, A.; Gumin, J.; Momin, E.N.; Shimizu, Y.; Ledbetter, D.; Shahar, T.; Yamashita, S.; Parker Kerrigan, B.; Fueyo, J.; et al. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-Oncology 2018, 20, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered exosomes with ischemic myocardium targeting peptide for targeted therapy in myocardial infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Chen, X.; Bao, L.; Liu, T.; Yuan, P.; Yang, X.; Qiu, X.; Gooding, J.J.; Bai, Y.; Xiao, J.; et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat. Biomed. Eng. 2020, 4, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Bjørnetrø, T.; Redalen, K.R.; Meltzer, S.; Thusyanthan, N.S.; Samiappan, R.; Jegerschöld, C.; Handeland, K.R.; Ree, A.H. An experimental strategy unveiling exosomal microRNAs 486-5p, 181a-5p and 30d-5p from hypoxic tumor cells as circulating indicators of high-risk rectal cancer. J. Extracell. Vesicles 2019, 8, 1567219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Hou, L.; Ma, Y.; Zhou, L.; Wang, F.; Cheng, B.; Wang, W.; Lu, B.; Liu, P.; Lu, W.; et al. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol. Cancer 2019, 18, 76. [Google Scholar] [CrossRef]
- Sheridan, C. Exosome cancer diagnostic reaches market. Nat. Biotechnol. 2016, 34, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; de Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position paper from the working group on cellular biology of the heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2021, 4, 446–454. [Google Scholar] [CrossRef]
- Olivieri, F.; Antonicelli, R.; Lorenzi, M.; D’Alessandra, Y.; Lazzarini, R.; Santini, G.; Spazzafumo, L.; Lisa, R.; La Sala, L.; Galeazzi, R.; et al. Diagnostic potential of circulating miR-499-5p in elderly patients with acutenon ST-elevation myocardial infarction. Int J. Cardiol. 2013, 167, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Kinetics of plasma microRNA-499 expression in acute myocardial infarction. J. Thorac. Dis. 2015, 7, 890–896. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Yang, J.; Duan, X.; Yao, Y.; Shi, X.; Chen, Z.; Fan, Z.; Liu, X.; Qin, S.; et al. A translational study of urine miRNAs in acute myocardial infarction. J. Mol. Cell. Cardiol. 2012, 53, 668–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, S.; Wang, C.; Jin, Y.; Lu, Z.; Xing, X.; Lu, Q. Correlation between serum exosome derived miR-208a and acute coronary syndrome. Int J. Clin. Exp. Med. 2015, 8, 4275–4280. [Google Scholar] [PubMed]
- Crouser, E.D.; Julian, M.W.; Bicer, S.; Ghai, V.; Kim, T.K.; Maier, L.A.; Gillespie, M.; Hamzeh, N.Y.; Wang, K. Circulating exosomal microRNA expression patterns distinguish cardiac sarcoidosis from myocardial ischemia. PLoS ONE 2021, 16, e0246083. [Google Scholar] [CrossRef]
- Minnis, P.; Kane, R.; Anglin, R.; Walsh, S.M.; Worrel, J.; Khan, F.; Lumsden, R.V.; Whity, S.; Keane, M. Serum exosomes from IPF patients display fibrotic miRNA profile that correlates to clinical measures of disease severity. Eur. Respir. J. 2015, 46, PA3845. [Google Scholar]
- Makiguchi, T.; Yamada, M.; Yoshioka, Y.; Sugiura, H.; Koarai, A.; Chiba, S.; Fujino, N.; Tojo, Y.; Ota, C.; Kubo, H. Serum extracellular vesiclular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saejong, S.; Townamchai, N.; Somparn, P.; Tangtanatakul, P.; Ondee, T.; Hirankarn, N.; Leelahavanichkul, A. MicroRNA-21 in plasma exosome, but not from whole plasma, as a biomarker for the severe interstitial fibrosis and tubular atrophy (IF/TA) in post-renal transplantation. Asian Pac. J. Allergy Immunol. 2020, 1–9. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Z.; Chen, D.; Wang, Z.; Miao, J.; Li, Q.; Zhang, D.; Song, J.; Cui, D. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 2020, 16, e1903916. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liao, X.; Tian, Y.; Li, G. Exosome separation using microfluidic systems: Size-based, immunoaffinity-based and dynamic methodologies. Biotechnol. J. 2017, 12, 1600699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Luo, S.H.; Lin, X.M.; Hu, X.M.; Zhang, Y.; Zhang, X.H.; Wu, C.M.; Zheng, L.; Wang, Q. A novel electrochemical biosensor for exosomal microRNA-181 detection based on a catalytic hairpin assembly circuit. Anal. Chim. Acta 2021, 1157, 338396. [Google Scholar] [CrossRef]
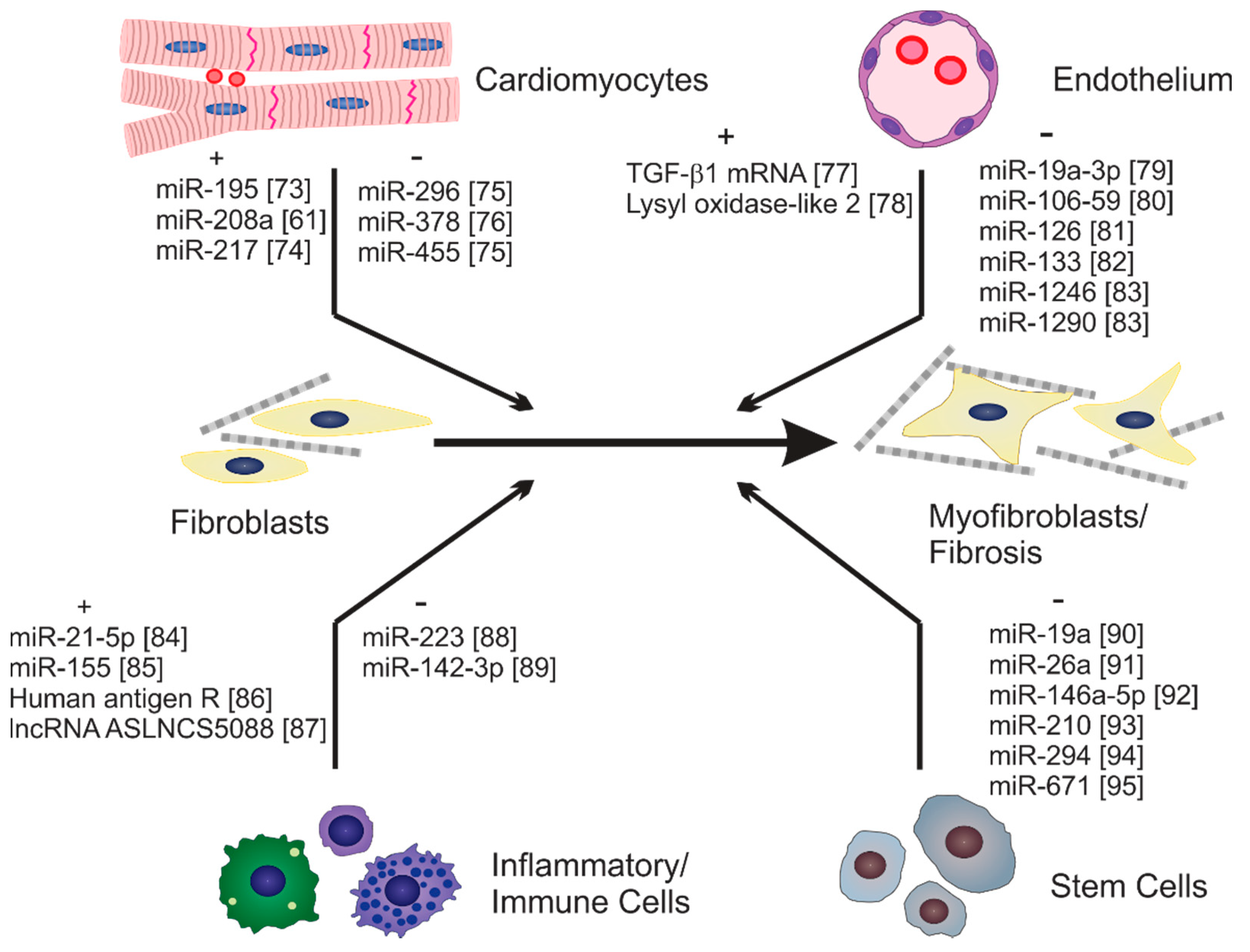

| Exosomal Cargo | Exosome Source | Pathophysiological Effects | Molecular Mechanisms | Reference |
|---|---|---|---|---|
| miR-19a-3p | Endothelial cells | Enhanced cardiac function and angiogenesis and reduced myocardial fibrosis in a mouse MI model. | Downregulation of thrombospondin 1 and increased expression of VEGFR2 | [79] |
| miR-21 | Stem cells | Preserved cardiac function and reduced apoptosis and fibrosis in rat MI model. | Modulation of PTEN-Akt pathway. | [121] |
| miR-21-5p | Mesenchymal stem cells | Improved cardiac function and reduced infarct size in mouse MI model. Enhanced macrophage polarization to the M2 phenotype. Reduced H9c2 apoptosis in response to oxygen glucose deprivation. | Reduced expression of pro-apoptotic genes. | [68,122] |
| miR-21-5p | Cardiac telocytes | Improved cardiac function, reduced infarct size and fibrosis and enhanced angiogenesis in a rat MI model. Promoted cardiac microvascular endothelial cell survival and tube formation in vitro. | Silencing of Cdip1 and downregulation of Caspase-3 | [123] |
| miR-21-5p | Macrophages | Further reduction of cardiac function, increased cardiomyocyte apoptosis and expansion of fibrosis in mouse MI model. | Downregulation of TIMP 3 | [124] |
| miR-22 | Mesenchymal stem cells | Improve cardiac function and reduce myocardial apoptosis and fibrosis in a mouse MI model. | Suppression of Mecp2 expression | [125] |
| miR-30e | Bone marrow-derived mesenchymal stem cells | Reduced infarct size and myocardial fibrosis in rat MI model. Attenuated oxygen glucose deprivation-induced H9c2 apoptosis. | Reduction in expression of LOX1 and NF-kB p65/Caspase-9 signaling. | [126] |
| miR-126 | Adipose-derived mesenchymal stem cells Umbilical cord blood-derived cells | Reduced infarct size and fibrosis in rat model of MI. Improved cardiac function, reduced myocardial hypertrophy and fibrosis in mouse model of diabetes (db/db). | Silencing of miR-126 target genes including Spred-1, VCAM and MCP1 | [127,128] |
| miR-142-3p | CD4+ T cells | Aggravated cardiac dysfunction, infarct size and fibrosis in mouse MI model. Enhanced fibroblast proliferation, activation and pro-fibrotic gene expression in isolated fibroblasts. | Enhanced Wnt signaling via downregulation of Adenomatous Polyposis Coli (APC) expression | [106] |
| miR-146 | Adipose-derived stem cells | Reduced inflammation and fibrosis in MI model. | Inhibition of early growth response factor 1 expression and attenuation of TLR4/NF-kB signaling. | [129] |
| miR-146a-5p | Cardiosphere-derived cells | Improved cardiac function and reduced myocardial fibrosis in porcine dilated cardiomyopathy model. | Unknown | [92] |
| miR-150-5p | Bone marrow-derived stem cells | Preserves cardiac function and inhibits cardiomyocyte apoptosis in MI model. | Downregulation of Bax. | [109] |
| miR-208a | Cardiomyocytes | Promotes fibroblast activation and fibrosis. | Decreased expression of Dyrk2. | [61] |
| miR-210 | Hypoxia-treated mesenchymal stem cells | Improved cardiac function and reduced myocardial fibrosis in a mouse MI model. Enhanced tube formation by endothelial cells and reduced cardiomyocyte apoptosis in vitro. | Unknown | [93] |
| miR-217 | Cardiomyocytes | Enhanced fibroblast proliferation and cardiac hypertrophy. | Modulation of PTEN/Akt pathway. | [74] |
| miR-218-5p miR-363-3p | Endothelial progenitor cells | Enhanced fibroblast mesenchymal to endothelial transition and angiogenesis in vitro. Improved cardiac function and reduced fibrosis in rat MI model. | Regulation of p53 and junction-mediating regulatory protein (JMY) expression | [130] |
| miR-290–295 cluster | Embryonic stem cells | Enhanced cardiomyocyte survival and neovascularization and reduced fibrosis in a mouse MI model. | Unknown | [94] |
| miR-320a | Serum | Promoted fibroblast activation and proliferation. | Regulation of PIK3CA/Akt/mTOR signaling. | [97] |
| miR-338 | Bone marrow-derived mesenchymal stem cells | Improved cardiac function and inhibited cardiomyocyte apoptosis in rat MI model and in H9c2 cells. | Reduced MAP3K2 and JNK expression. | [131] |
| miR-671 | Adipose-derived stem cells | Reduced cardiomyocyte apoptosis, inflammation and fibrosis in MI model. | Inactivation of TGFBR2/Smad2 axis. | [110] |
| miR-1246 miR-1290 | Endothelial progenitor cells | Improved cardiac function and reduced infarct size and fibrosis in rat MI model. Enhanced fibroblast mesenchymal to endothelial conversion and angiogenesis in vitro. | Enhanced expression of ELF5, SP1 and CD31 in cardiac fibroblasts. | [83] |
| lncRNA ZFAS1 | Human cardiac myocytes | Exaggerated decrease in cardiac function and increase in myocardial fibrosis in chronic kidney disease mouse model. Promoted fibroblast activation and pro-fibrotic gene expression in isolated fibroblasts. | Modulation of Wnt4/β-catenin signaling via miR-4711-5p regulation. | [132] |
| Human antigen R (HuR) | Macrophages | Increased fibrosis in angiotensin II infusion mouse model. Enhanced pro-inflammatory and pro-fibrotic gene expression in isolated fibroblasts. | Unknown | [86] |
| Decreased HSP70 | Serum of aged rats | Stimulation of fibroblast activation, proliferation and pro-fibrotic gene expression in vitro. | Unknown | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohn, J.; Tan, W.; Carver, A.; Barrett, H.; Carver, W. Roles of Exosomes in Cardiac Fibroblast Activation and Fibrosis. Cells 2021, 10, 2933. https://doi.org/10.3390/cells10112933
Hohn J, Tan W, Carver A, Barrett H, Carver W. Roles of Exosomes in Cardiac Fibroblast Activation and Fibrosis. Cells. 2021; 10(11):2933. https://doi.org/10.3390/cells10112933
Chicago/Turabian StyleHohn, Julia, Wenbin Tan, Amanda Carver, Hayden Barrett, and Wayne Carver. 2021. "Roles of Exosomes in Cardiac Fibroblast Activation and Fibrosis" Cells 10, no. 11: 2933. https://doi.org/10.3390/cells10112933
APA StyleHohn, J., Tan, W., Carver, A., Barrett, H., & Carver, W. (2021). Roles of Exosomes in Cardiac Fibroblast Activation and Fibrosis. Cells, 10(11), 2933. https://doi.org/10.3390/cells10112933