Abstract
Transposable elements (TEs) are self-replicating DNA elements that constitute major fractions of eukaryote genomes. Their ability to transpose can modify the genome structure with potentially deleterious effects. To repress TE activity, host cells have developed numerous strategies, including epigenetic pathways, such as DNA methylation or histone modifications. Although TE neo-insertions are mostly deleterious or neutral, they can become advantageous for the host under specific circumstances. The phenomenon leading to the appropriation of TE-derived sequences by the host is known as TE exaptation or co-option. TE exaptation can be of different natures, through the production of coding or non-coding DNA sequences with ultimately an adaptive benefit for the host. In this review, we first give new insights into the silencing pathways controlling TE activity. We then discuss a model to explain how, under specific environmental conditions, TEs are unleashed, leading to a TE burst and neo-insertions, with potential benefits for the host. Finally, we review our current knowledge of coding and non-coding TE exaptation by providing several examples in various organisms and describing a method to identify TE co-option events.
1. Introduction
In eukaryotic cells, the chromatin fiber is precisely packaged and organized in subnuclear compartments that undergo dynamic spatial rearrangements depending on developmental transitions and environmental constraints [1,2,3,4]. Actively transcribed protein-coding genes compose the euchromatin, which is a relaxed form of chromatin, while spatiotemporally regulated genes that are transcriptionally repressed, such as developmental genes, form facultative heterochromatic foci [5]. Finally, the constitutive heterochromatin is highly condensed and enriched in DNA repeats, including silenced transposable elements (TEs) [6,7,8].
Upon their discovery in the 1940s, Barbara McClintock defined TEs as “controlling elements […] operating as an integrated system in the control of gene action” [9]. Subsequently, they received the names of “jumping” genes, as well as selfish, parasitic or junk DNA [10,11], most likely because TEs are highly repeated, self-replicating mobile elements, capable of invading the host genome by a mechanism called transposition. Indeed, TEs constitute a significant fraction of plant genomes, ranging from 15% in Arabidopsis thaliana up to 85% in maize [12,13,14]; in animals, they represent half of the human nuclear DNA content, and one third of the Drosophila melanogaster genome [15]. Altogether, this excessive junk DNA, including TEs and other DNA repeats, can greatly explain the C-value paradox that Freeling and colleagues proposed to solve by hypothesizing that bulk junk DNA would serve as “genome balance” [16].
TEs are classified in two major classes depending on their mode of transposition. Class I TEs (or retrotransposons) mobilize via an RNA intermediate in a “copy-and-paste” strategy, while class II TEs (or DNA transposons) mobilize via a DNA intermediate in a “cut-and-paste” strategy [17]. Each class is composed of several subclasses, superfamilies and families gathering TEs with similar features [17,18,19]. Among class I TEs, the two major subclasses are long terminal repeat (LTR) and non-LTR retrotransposons [13]. Among class II TEs, there are terminal inverted repeat (TIR) and non-TIR DNA transposons and rolling-circle (RC)/Helitrons [12,13,17]. Except for some families such as RC/Helitrons or Spy transposons, most TEs are flanked by target site duplication (TSD) repeats that are a few nucleotides generated by the DNA repair machinery after the TE neo-insertion. TEs subclasses often include autonomous and non-autonomous TEs, the latter using the functional transposition machinery of family-related autonomous TEs to invade the genome. For instance, among non-LTR retrotransposons, short interspersed nuclear elements (SINEs) use the long interspersed nuclear elements (LINEs) machinery to amplify themselves [20]. Similarly, it is now well established that Activator/Dissociation (Ac/Ds) elements, originally described by Barbara McClintock in 1950 [21], are autonomous and non-autonomous TIR DNA transposons, respectively.
Depending on the insertion site, neo-insertions of TEs can differently impact the host genome. They can first be neutral, for instance, if they happen in non-regulatory intergenic regions. They can also be deleterious, by disrupting the sequence of protein-coding genes, or provoking chromosomal rearrangement [18,22,23,24,25]. Because TEs are potentially harmful for the host, they need to be transcriptionally repressed. Several cellular processes, including epigenetic pathways like DNA methylation and histone modifications, serve the purpose of keeping TEs silenced [6,7,8]. Finally, in some cases, TE neo-insertions can turn into a beneficial outcome to the host, by providing an evolutionary advantage [24]. Through these beneficial insertions, TEs can create genetic diversity and innovation, contributing to genome evolution. Thus, despite their potentially deleterious nature, TEs can become beneficial to the host [25,26]. The host-TE interaction relies on a tight and complex relationship, from conflict (or arms race), cooperation (or equilibrium) to co-option (or TE exaptation) [27].
In this review, we will first describe the recent progress made in the field of TE silencing, with a focus on plants. In the second part, we will attempt to explain how harmful, parasitic TEs can turn into beneficial genetic elements that contribute to evolutionary innovation of the host genome. Finally, we will describe different approaches that exist to study host-TE interaction, and to bring to light genuine events of TE exaptation.
2. A Plethora of Sophisticated Epigenetic Pathways Dampen TEs
DNA methylation, histone modifications and histone variants and non-coding RNA (ncRNA), like small interfering RNA (siRNA), chromatin remodelers and other epigenetic factors, play important roles in many chromatin-related processes, including genome organization, DNA replication and repair, regulation of gene expression and TE silencing [7,28,29]. All these epigenetic pathways cooperate to efficiently silence TEs, forming a multilayered lock system that can be depicted as an epigenetic “mille-feuille” [30]. These epigenetic pathways can act synergistically or redundantly, depending on the targeted TEs. In addition, these multifaceted epigenetic pathways are tightly intertwined, and crosstalk is often observed, which makes overall TE silencing a combination of very sophisticated and complex molecular processes.
2.1. DNA Methylation, an Overview
Methylation of the DNA molecule can either occur on adenosines (6mA) or cytosines. Methylation of the 5th carbon of cytosines (5-methylcytosine (5mC)) is a major epigenetic mark conserved in many bacterial, archaeal and most of the eukaryotic genomes, including fungi such as Neurospora crassa, plants and most animals [31,32,33]. In plants, 5mC can occur in three cytosine contexts: mCG, mCHG and mCHH (where H = A, T or C), whereas in mammals, it almost exclusively happens in the CG context [31,33]. Together with the histone H3 lysine 9 dimethylation mark (H3K9me2) in plants and histone H3 lysine 9 trimethylation mark (H3K9me3) in animal and fungi, 5mC is strongly enriched at repetitive DNA elements such as TEs that compose constitutive heterochromatic compartments [7,34] (Figure 1A,B). 5mC is established (or de novo 5mC) and then is maintained by specialized enzymes conserved in eukaryotes. De novo 5mC is catalyzed by DNA methyltransferase 3A (DNMT3A) and DNMT3B in animals. In plants, de novo 5mC in all cytosine contexts requires DOMAINS REARRANGED METHYLATRANSFERASE 2 (DRM2) orthologs. DRM2-mediated de novo 5mC requires many plant factors of the so-called RNA-directed DNA methylation (RdDM) pathway, including RNA-DEPENDENT RNA POLYMERASE 2 (RDR2) and DICER-LIKE 3 (DCL3), producing 24-nt siRNAs, ARGONAUTE 4 (AGO4), as well as the plant-specific RNA polymerase IV (RNA Pol IV) and RNA Pol V proteins, acting in concert in transcriptional gene silencing (TGS) (reviewed in [34,35,36]). Maintenance of mCG is catalyzed by DNMT1 and DNA METHYLTRANSFERASE 1 (MET1) in animals and plants, respectively. In A. thaliana, depending on the genomic locations, CHG methylation is maintained by CHROMOMETHYLASE 2 (CMT2) and CMT3 through a self-reinforcing feedback loop involving H3K9me2, which is catalyzed by SU(VAR)3-9 homologs (SUVH) 4 (or KRYPTONITE (KYP)), SUVH5 and SUVH6 (Figure 1B) [37]. Finally, maintenance of CHH methylation is assured by DRM2 or CMT2 depending on genomic locations [38,39]. It is therefore important to stress that DRM2 acts either at the initial stage of 5mC (de novo in all cytosine contexts) or at the maintenance step, mostly to preserve 5mC in the CHH context (Figure 1A).
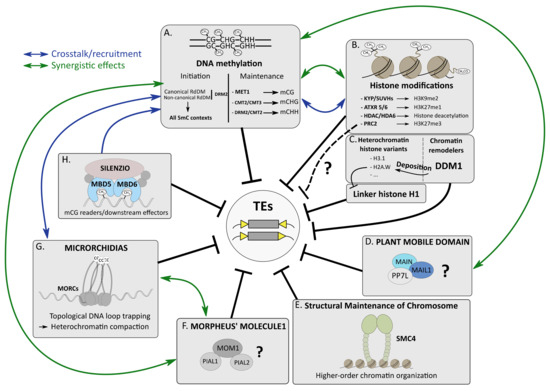
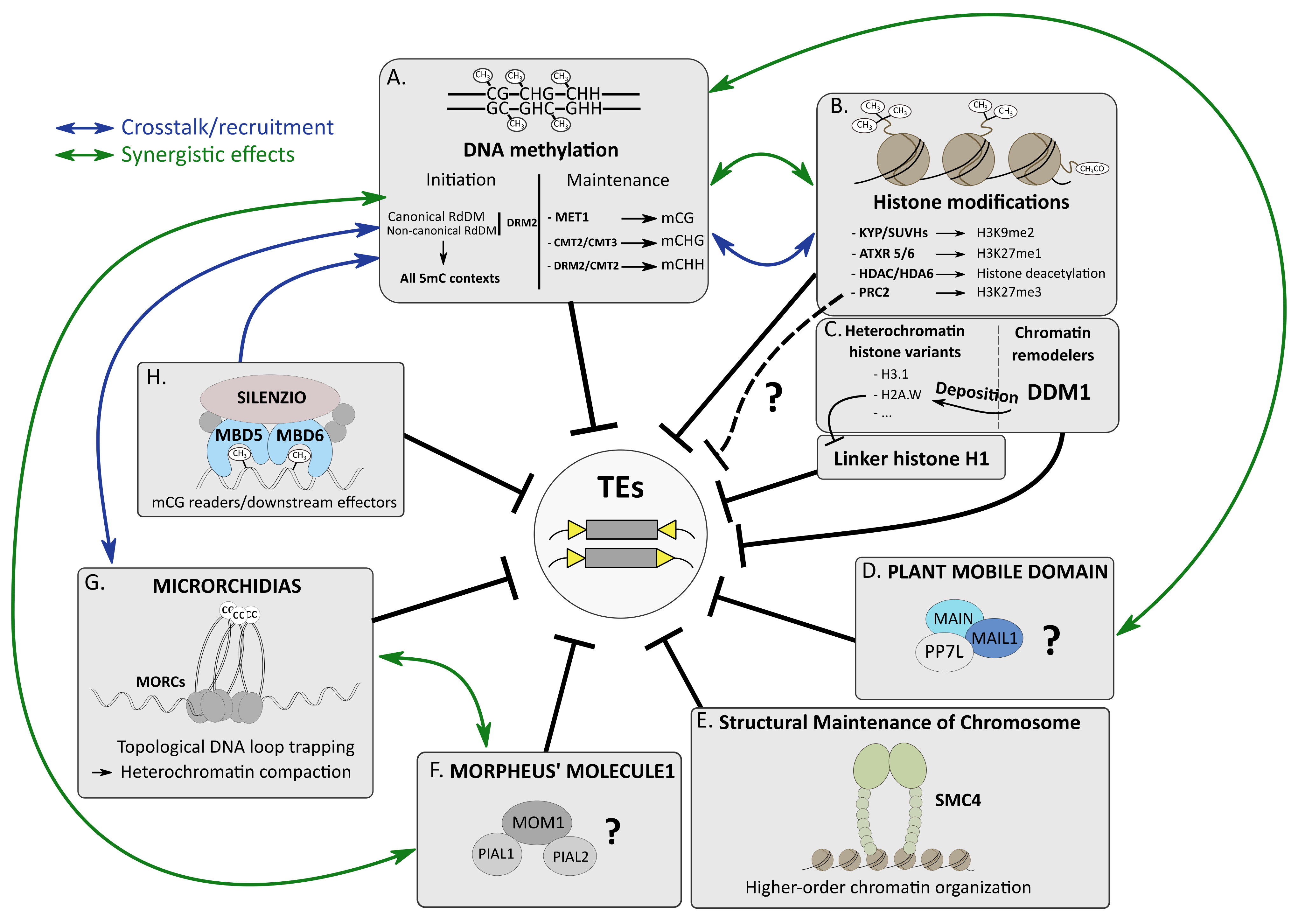
Figure 1.
Several silencing pathways cooperate to silence TEs in A. thaliana. (A). Initiation and maintenance of DNA methylation involve specific DNA methyltransferases to perform 5mC in three cytosine contexts. (B). Histone modifications such as methylation of histone 3 at lysine 9 or lysine 27 involve specialized histone methyltransferases. Deacetylation of histone tails requires HDAC proteins, such as HDA6. (C). DDM1 is involved in the deposition of the heterochromatic histone variant H2A.W, which interferes with linker histone H1 occupancy at constitutive heterochromatin. The connections between DDM1, H3K27me3 and 5mC remain unclear. (D). PMD proteins MAIN and MAIL1 interact with PP7L, forming a complex involved in TE silencing through an unknown mechanism. (E). SMC4 cooperates with other epigenetic pathways to repress TEs, presumably by promoting high-order chromatin organization. (F). MOM1 interacts with PIAL proteins to repress TEs using an elusive process. (G). MORC proteins promote constitutive heterochromatin compaction to ensure TE silencing by topological DNA loop trapping mechanism. CC: coiled-coil domain (H). The SILENZIO factor interacts with MDB5 and MDB6 and HSP factors to read mCG and silence TEs. Crosstalk and synergistic effects have been described between several epigenetic pathways.
2.2. Several DNA Methylation Pathways Cooperate to Silence TEs
The role of 5mC in TE silencing was first demonstrated in maize [40]. More recently, it was confirmed that RdDM and CMT pathways play a crucial role in the silencing of several TE families in this plant species [41]. Studies in A. thaliana also showed that several classes of TEs are reactivated in epigenetic mutants showing DNA hypomethylation [42,43,44,45,46] (Figure 1A). Likewise in mammals, TEs are derepressed in DNMT mutants [47,48,49].
Although de novo and maintenance of 5mC pathways co-exist throughout the plant life cycle, each pathway plays an important role at specific developmental stages or in response to a specific environmental stimulus that can lead to a burst of TEs. Young TEs, capable of transposition, are rapidly targeted by the RNA-DEPENDENT RNA POLYMERASE 6 (RDR6)-dependent RdDM, or non-canonical RdDM [50,51]. The mechanisms by which TEs are targeted by the non-canonical RdDM have been recently described [52]. TEs harbor a low GC content at the third nucleotide position of codons (GC3) when compared with protein-coding genes. This low GC3 correlates with translation inefficiency, caused by ribosome stalling, which triggers the production of epigenetically activated siRNAs (easiRNAs) by the post-transcriptional gene silencing (PTGS) factors RDR6 and DCL2/4 in cytoplasmic siRNA bodies. The formation of these siRNA bodies depends on liquid–liquid phase separation, mediated by the protein SUPPRESSOR OF GENE SILENCING 3 (SGS3), which interacts with RDR6 [52].
Remarkably, a similar scenario was recently described in tomato, in which young TEs undergoing retrotransposition and increasing in copy number are first targeted by PTGS, then by RdDM, and eventually by the CTM3/KYP and MET1 pathways promoting a robust TGS [53]. Then, the authors proposed that over time, as TEs age, undergoing rearrangements and spontaneous mutations impacting their capability of transposition, they would no longer be targeted by CMT3/KYP and MET1, which would relax TGS, allowing basal TE transcription to reestablish a “secondary” RdDM [53]. At the genome level, these old TEs would serve as a siRNA reservoir to protect the host against reinvasion by TEs with similar sequence identity [53].
A major question is how epigenetic factors cooperate to establish the appropriate pattern of 5mC at TE locations. Previous studies reported an intricate relationship between the RdDM and the 5mC maintenance pathways at the heterochromatic regions. In plants with a decreased 5mC in these regions, an activation of RdDM was observed, suggesting an inhibition of RdDM by heterochromatin in A. thaliana [50,51,54,55]. Similar observations were made in maize, tomato and rice, where loss of RdDM was followed by a gain of 5mC in euchromatic regions [56,57,58]. As in A. thaliana, it was suggested that, conversely, 5mC inhibited RdDM in constitutive heterochromatin by blocking RNA Pol IV and Pol V activities. Indeed, loss of this 5mC resulted in the spreading of RdDM across the genome, diluting RdDM factors and then compromising their efficiency, which is defined as the dilution model [56,57,58]. Thus, in plants, the symmetric 5mC, i.e., mCG and mCHG, widely present in constitutive heterochromatin, would act as a barrier against the RdDM pathway, to concentrate RdDM factories at boundary regions between euchromatin and constitutive heterochromatin. Further experiments need to be performed to test whether the dilution model holds true or not.
A study in A. thaliana investigated the reestablishment of 5mC in the progeny of epigenetic mutants impaired in non-CG methylation [59]. The authors showed that recovery of non-CG methylation depended on the TE genomic context. For most of the TEs, the conservation of mCG within their coding region (TE gene) as well as the presence of the histone variant H2A.W were essential to efficiently regain non-CG methylation in an RdDM-independent manner. Conversely, for a subset of TEs defined as gene-like TE (GLT) genes that had lost CG methylation and swapped H2A.W with H2A.Z, the recovery of non-CG methylation was inefficient [59].
Finally, 5mC dynamic at TEs was investigated in A. thaliana shoot apical meristem (SAM), in which TEs are more expressed than in surrounding cells [60]. In SAM stem cells, transient upregulation of TEs correlated with an increase in CHG and a decrease in CHH methylation before flowering. This 5mC signature may reflect some epigenetic reprogramming to initiate the correct 5mC state in male meiocytes, protecting the genome from harmful TE mobilization [60].
2.3. Histone Modifications, a Brief Overview
Post-translational modifications (PTMs) of histones affect nucleosome structure, which impacts DNA accessibility to other epigenetic players and transcription factors (TFs) regulating gene expression [61,62,63]. Histone acetylation is an active mark of transcription. Enriched in euchromatin, it is deposited by histone acetyltransferases (HATs) and removed by histone deacetylases (HDACs) [64,65]. Histone methylation can either correlate with transcriptional activation or repression. Di- or trimethylation at lysine 4 of histone H3 (H3K4me2/me3) is an active mark of transcription, whereas in plants, H3K9me2 and H3K27me1 are hallmarks of constitutive heterochromatin that are deposited by SUVH4/5/6 and ARABIDOPSIS TRITHORAX-RELATED PROTEIN 5/6 (ATRX5/6), respectively [66,67]. Finally, the facultative heterochromatin mark H3K27me3 is deposited by the Polycomb repressive complex 2 (PRC2), and is mostly enriched at developmentally or environmentally responsive genes that are transcriptionally repressed [5] (Figure 1B).
2.4. A Focus on HDA6-Mediated TE Silencing
Among the A. thaliana HDACs, HDA6 is essential for TE silencing, and 5mC hypomethylation was reported in hda6 mutant [68,69,70] (Figure 1B). Previous studies showed that HDA6 interacts with MET1 as well as with SUVH4, SUVH5 and SUVH6, connecting histone deacetylation with CG methylation maintenance and H3K9me2 in TE silencing [71,72,73]. An interaction was also described with the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin-remodeling complex (CRC) subunit SWI3B to repress a subset of TEs [74]. Although these former interactors could not be confirmed, recent HDA6 affinity purification followed by mass spectrometry (AP-MS) analyses highlighted the co-existence of several HDA6 complexes [75,76]. It remains unclear as to what is the composition of the HDA6 complex specifically involved in TE silencing.
2.5. Evidences of H3K27me3-Mediated TE Silencing?
Although it is well established that PRC2-mediated H3K27me3 deposition plays an important role in gene silencing [5,77], a direct connection between H3K27me3 and TE silencing is not as clear (Figure 1B). This is most likely because TEs display a high level of 5mC, and H3K27me3 and 5mC are largely antagonistic [78,79]. Thus, in wild type (wt) A. thaliana, H3K27me3 is usually excluded from TEs. There is, however, at least one exception with EVADE (EVD) showing an enrichment of H3K27me3 in wt plants, despite the presence of mCG. To explain this discrepancy, it was proposed that H3K27me3 accumulation at EVD was due to the low density of mCG at this location, a feature that was also observed in other species or specific conditions ([80] and see below).
In tissues with low 5mC, like the seed endosperm in plants or during the embryonic development in mammals where DNA demethylation waves are observed, TEs are targeted by H3K27me3 to potentially repress them [81,82]. Furthermore, in DNA hypomethylated A. thaliana mutants such as met1 or decreased in dna methylation 1 (ddm1), an enrichment of H3K27me3 at TEs losing m5C was observed [79,83]. In ddm1, this enrichment was dependent on the PRC2-component SET-domain protein CURLY LEAF (CLF) [83]. Remarkably, the ddm1 clf double mutant did not show a global increase in TE expression, but instead a partial rescue of ddm1 silencing defects that is most likely attributed to DNA hypermethylation. Again, EVD behaved as an outlier, showing increased transcription and transposition rates in ddm1 clf [83]. Therefore, at least for the young TE EVD, CLF-mediated H3K27me3 deposition can act as a backup silencing system in ddm1 mutant plants.
In parallel, a study analyzing A. thaliana plants impaired in the two Jumonji H3K27me3 histone demethylases EARLY FLOWERING 6 (ELF6) and RELATIVE OF EARLY FLOWERING 6 (REF6) showed transgenerational epigenetic defects associated with a loss of 5mC and gain of H3K27me3 at heterochromatic TEs [84]. Of note, this gain of H3K27me3 was not sufficient to efficiently repress TEs. Altogether, these studies demonstrate the complex interaction of epigenetic pathways, including PRC2-mediated H3K27me3 to silence TEs. In the future, it will be important to clarify the molecular processes recruiting PRC2 to promote H3K27me3 deposition at TE locations. In mammals, it was shown that mariner TEs carry silencer elements that are similar to the Polycomb/Trithorax response elements (PRE/TREs), allowing the recruitment of PRC2 to dampen TE expression [85]. Likewise, some A. thaliana TEs carry DNA motifs that are potentially recognized by PRC2 [83]. Furthermore, it was also proposed that H3K27me3 enrichment at TEs could occur by local spreading from nearby genes targeted by PRC2 [80]. To fully unravel the potential role of H3K27me3 in TE silencing, future studies will have to tackle difficult questions such as: what is the connection between H3K27me3 and other epigenetic factors (also see Section 2.6)? Is PRC2-mediated H3K27me3 required for TE silencing? In addition, how is PRC2 recruited to TEs?
In other organisms with a low genomic 5mC level, H3K27me3 enrichment at TEs is also observed: such as in the ciliates Paramecium tetraurelia or Tetrahymena thermophila, in which PRC2 catalyzes the deposition of both H3K9me3 and H3K27me3 on TEs [86,87], or in the red algae Cyanidioschizon merolae, where 50% of TEs are covered by H3K27me3, suggesting that this repressive mark might be involved in TE silencing [88]. Furthermore, H3K27me3 enrichment at TEs was also observed in Marchantia polymorpha, a representative species of liverworts, which diverged from other embryophyte lineages in the early Paleozoic, about 500 Mya [89]. Depending on their location in M. polymorpha genome, TEs are associated with different epigenetic signatures. While a large proportion of TEs that are mostly located on the highly condensed sex chromosome V are predominantly marked by 5mC, H3K9 methylation and H3K27me1, about 20% of TEs, mostly autosomal, are instead targeted by H3K27me3 and display either a significant reduction or a complete depletion in 5mC level [89]. Thus, targeting of TEs by H3K27me3 would be only possible at genomic locations with low 5mC. The authors propose that TE targeting by H3K27me3 could be explained by an inefficient self-reinforcing loop between 5mC and H3K9 methylation in M. polymorpha. Targeting of TEs by H3K27me3 would be an ancient silencing mechanism that would have evolved towards dedicated 5mC- and H3K9-methylation-mediated TE silencing pathways throughout the evolution of other embryophyte lineages (discussed in [90]). In P. tetraurelia or T. thermophila, loss of function of the PRC2 core subunit Enhancer of Zeste E(z)-like1 (EZL1) induces TE silencing defects [86,87]. Nevertheless, as H3K9 and H3K27 methylation are concomitantly impaired in the ezl1 mutant, it is difficult to precisely determine the role of H3K27me3 in TE repression. Similarly, whether PRC2-mediated H3K27me3 is required for TE silencing remains to be tested in liverwort.
2.6. The Chromatin Remodeler DDM1 Deposits the H2A.W Histone Variant to Silence TEs
Chromatin remodelers are protein factors that catalyze a broad range of reactions impacting chromatin organization and structure, like nucleosome sliding across the DNA or changing the conformation of the histone octamer [91]. All chromatin remodelers use ATP hydrolysis to disrupt the contact between histones and DNA, regulating the access of other factors to the chromatin [92].
The A. thaliana AtDDM1 is an SNF2 chromatin remodeler belonging to the mammalian lymphoid-specific helicase (LSH) subfamily that has been studied for decades [93]. This essential epigenetic factor is required for heterochromatin condensation, TE silencing and 5mC maintenance mostly at heterochromatic regions composed of long TEs [39,94,95].
It was initially proposed that DDM1 facilitated DNA methyltransferase activities by evicting the linker histone H1. The h1 mutant shows a complex 5mC pattern: decrease in CHH methylation at short euchromatic TEs and increased 5mC levels at long heterochromatic TEs [39,96,97]. The effect of h1 mutation on TE silencing is modest, but in h1 met1, mutant synergistic effects can be observed at a subset of TEs showing a substantial upregulation in comparison to respective single mutants [98]. Importantly, H1 subgenomic location does not require DNA methylation or DDM1 activity [98,99]. Instead, AtDDM1 is involved in the deposition of the heterochromatin-specific histone variant H2A.W at long TEs to prevent their mobility in a process that can be dissociated from 5mC and H3K9me2 [99] (Figure 1C). Unlike ddm1, h2a.w mutants develop like wt plants, with a minor impact on TE silencing, and a slight decrease of non-CG methylation at pericentromeric TEs [97]. Besides, the H2A.X and replicative H2.A histone variants invade heterochromatin in h2a.w, and heterochromatic H1 level is increased. This correlates with a mild reduction in heterochromatin accessibility in h2a.w compared to wt plants, a molecular phenotype that is abolished in h1 h2a.w double mutant, displaying chromatin accessibility greater than wt plants. It was proposed that H2A.W would regulate H1 occupancy in constitutive heterochromatin by competing with H1 for linker DNA binding in order to optimize heterochromatin accessibility of epigenetic factors such as DNA methyltransferases [97]. Although it is tempting to make connections between these two recent studies [97,99], further analyses will be required to fully apprehend the complex link between these different epigenetic factors. Particularly, the dramatic defects of 5mC or H3K9me2 observed in ddm1 remain obscure, and cannot be attributed to a loss of H2A.W. One hypothesis is that these epigenetic defects would result from major alterations of the chromatin structure occurring in ddm1 that would be inherited upon cell division, with concomitantly an inefficient maintenance of 5mC and H3K9me2, together with an invasion of H3K27me3 at heterochromatic TEs.
It is remarkable to note that in humans, the deposition of the heterochromatic histone variant macroH2A by LSH1 is sufficient to repress transcription in a 5mC- and H3K9me3-independent manner [100]. Thus, AtDDM1- and LSH1-mediated histone H2A variant deposition could reflect mechanisms of convergent evolution.
Several studies have investigated the role of DDM1 orthologs in crops, such as tomato, rice or maize. In tomato, disrupting the two SlDDM1 homologs reallocates the RdDM pathway to heterochromatin, leading to mCHH and siRNA redistribution [57]. In rice, the two OsDDM1 proteins facilitate symmetrical 5mC and H3K9me2 and antagonize RdDM in constitutive heterochromatin [58]. Finally, the maize homologs ZmDDM1a and ZmDDM1b recognize a euchromatic GC-rich DNA sequence, are enriched at transcriptional start site (TSS) of active genes, and by interacting with AGO4 proteins, they would recruit RdDM to promote mCHH in active chromatin [101]. Thus, the connection between DDM1 and RdDM seems to be conserved in crops; whether the euchromatic localization of DDM1 is a general feature in crop species that display a complex genome rich in TEs, remains to be defined. Despite the lack of subgenomic localization or interactome data for A. thaliana DDM1, the fact that DDM1 mediates H2A.Z deposition in this model plant will most likely impose the requirement to investigate this aspect for future DDM1-related studies in crops.
2.7. The Enigmatic Plant Mobile Domain Proteins
The plant mobile domain (PMD) is a protein domain of unknown function only found in angiosperms, and presumably deriving from Ty3/gypsy TEs upon TE gene exaptation [102,103,104] (see Section 3 and Figure 2 for the definition of exaptation). The A. thaliana PMD proteins were first identified as cellular factors required for SAM and root apical meristem (RAM) organization, genome stability and cell division, hence the name MAINTENANCE OF MERISTEMS (MAIN) for the PMD founding member, and MAIN-LIKE 1 (MAIL1) for its closest homolog [105,106]. Later, it was shown that MAIN and MAIL1 were required for the proper expression of a common subset of genes, as well as TE silencing [104] (Figure 1D). MAIN and MAIL1 physically interact together, as well as with a putative serine/threonine phosphoprotein phosphatase (PPP) called PP7-LIKE (PP7L), and the three mutants show similar developmental and molecular phenotypes, including TE silencing defects [107,108]. Genome-wide level of 5mC is mostly unchanged in main and mail1 mutants, with the notable exception of a slight increase in CHG methylation at pericentromeric regions and in chromosome arm TEs [108]. Importantly, this modest non-CG hypermethylation does not correlate with changes in TE expression observed in the mutant [108]. Synergistic effects were described between MAIN and 5mC pathways. The main drm1 drm2 cmt3 quadruple mutant showed exacerbation of TE silencing defects, with a number being specifically derepressed in this mutant background [108]. Altogether, these results suggest that together with MAIL1 and PP7L, MAIN would define a distinct epigenetic pathway that cooperates with DRM2- and CMT3-mediated 5mC to silence TEs. The mode of action of PMD MAIN and MAIL1 complex remains largely unknown. Besides, the role of PP7L in this pathway is obscure. As these proteins are at least partially nuclear, determining whether this protein complex can interact with chromatin will be an important question to address.
2.8. Structural Maintenance of Chromosome Proteins Get Involved in TE Silencing
Structural maintenance of chromosomes (SMC) proteins compose the core unit of condensin and cohesin ATPase complexes that play an essential role in higher-order chromatin organization, chromosome condensation and sister chromatid cohesion by performing topological entrapment of DNA [109]. The role of SMC proteins in TE silencing was first described in Drosophila and Schizosaccharomyces pombe, two species largely depleted in 5mC [110,111,112]. In A. thaliana, the identification of a hypomorphic smc4 mutant revealed that SMC4 was required for TE silencing [113] (Figure 1E). In this mutant, several TEs were upregulated, without significant defect in 5mC or siRNA levels. SMC4 can act on strongly methylated TEs, as well as sparsely methylated TEs, demonstrating that 5mC is not required to recruit SMC4 on silenced loci [113]. The authors proposed that conserved histone modifications or histone variants could serve for condensin recruitment, but further investigations are needed to test this hypothesis.
2.9. The Elusive MORPHEUS’ MOLECULE 1 (MOM1)
MORPHEUS’ MOLECULE 1 (MOM1) is a plant-specific protein identified in a screen for mutants that released transcriptional gene silencing of a transgenic locus [114] (Figure 1F). MOM1 is required for heterochromatic TE silencing and repression of silent 5S rRNA genes, but mom1 mutant is not impaired in m5C maintenance or condensation of chromocenters that are interphasic constitutive heterochromatin foci [94]. The protein carries an incomplete ATPase/helicase domain similar to the SWI2/SNF2 chromatin remodeling domain, as well as three motifs called Conserved MOM1 Motif 1 (CMM1), CMM2 and CMM3; CMM2 being the only motif required for MOM1-mediated silencing activity and for its dimerization [115,116]. CMM2 is also essential for MOM1 interaction with PROTEIN INHIBITOR OF ACTIVATED STAT (PIAS)-type SUMO E3 ligase-like 1 (PIAL1) and PIAL2 [117,118]. Besides, MOM1 interacts with small ubiquitin-like modifier 1 (SUMO1) in a non-covalent manner. Although this interaction is not required for MOM1 silencing activity, it was suggested that SUMO1 would play regulatory functions under certain developmental stages or environmental conditions [117]. Although MOM1 was identified 20 years ago, its mode of action remains largely unknown.
2.10. The MICRORCHIDIA (MORC) ATPases Repress TEs through DNA Loop-Trapping Mechanism
MICRORCHIDIA (MORC) proteins are gyrase, HSP90, histidine kinase, MutL (GHKL)-type ATPases conserved in prokaryotes and eukaryotes [119,120]. A connection between MORC proteins and TE silencing was first described in A. thaliana, through the identification of atmorc1 and atmorc6 mutants showing upregulation of several TEs and decondensation of constitutive heterochromatic chromocenters [121] (Figure 1G). The two AtMORC1 and AtMORC6 proteins form heteromers localizing near chromocenters, and it was suggested that MORC ATPases would be involved in higher-order heterochromatin compaction to maintain TE repression [121,122]. In mouse and nematode, MmMORC1 and CeMorc1 orthologs are required for TE and gene silencing [121,123]. Remarkably, MmMORC1 is essential for male germline development and fertility [124]. The mechanisms involving MORC proteins in heterochromatin compaction remained elusive until Kim and colleagues showed that CeMorc1 multimerization allowed efficient DNA binding in a sequence non-specific manner to highly compact chromatin using a DNA loop-trapping mechanism [125] (Figure 1G). Considering the high similarity between eukaryotic MORC proteins, it was suggested that this mechanism of chromatin compaction was conserved among eukaryotic MORCs. This observation made in nematode is consistent with the fact that MORC proteins act in the silencing of a diverse array of sequences in different organisms. Furthermore, it differentiates MORC from condensing complexes that compact DNA by loop-extrusion mechanism [125]. The mechanisms by which A. thaliana MORC proteins are recruited to constitutive heterochromatin have been extensively studied. AtMORC6 interacts with several RdDM components, and artificial tethering of AtMORC6 to chromatin is sufficient to recruit the RdDM pathway [126,127,128]. Similar observations were recently made with AtMORC7 [129]. AtMORC7 interacts with AtMORC4 as well as with AtMORC1, AtMORC6 and RdDM factors. Like AtMORC4, AtMORC7 is enriched at RdDM subgenomic sites [129]. From these different studies, it was suggested that AtMORCs and RdDM factors would cooperate to mutually recruit each other; AtMORCs act as tethers promoting efficient RdDM subgenomic retargeting (Figure 1G). Transferring this “MORC tethering” model to mammals could provide some clues regarding the mechanisms involving MmMORC1 in de novo 5mC and TE silencing in mouse male germline [123].
2.11. The J-Domain Protein SILENZIO Enters the Game
Lately, a study described the involvement of the two A. thaliana methyl-CpG-binding domain proteins MBD5 and MBD6, which are mCG readers, in the subgenomic recruitment of the J-domain protein called SILENZIO (SLN) that acts as a transcriptional repressor [130] (Figure 1H). Transcriptomic analyses revealed that a common subset of TEs was upregulated in sln and mbd5 mbd6 mutants. Moreover, artificial chromatin tethering of SLN was sufficient to promote gene silencing. Besides, SLN, MBD5 and MBD6 interact with several HEAT SHOCK PROTEIN 70 (HSP70) chaperones [130]. This protein complex might potentially recruit unknown transcriptional repressors or inhibit uncharacterized activator to repress TEs in an undetermined mechanism taking place downstream of 5mC.
2.12. An Evolutionary Arms Race between TEs and Their Host
The interaction between TEs and their host relies on a perpetual conflict [27]. While the host must dampen TEs because of their harmful effects, using, among others, epigenetic pathways, the TEs must find alternatives in order to perpetuate. Thus, some TEs have developed strategies to overcome the host silencing machinery (Figure 2, stage 0). For instance, upon burst, the evolutionary young ATCOPIA93 TE called EVD, which is initially targeted by the PTGS pathway, uses the host alternative splicing machinery to preferentially synthetize a subgenomic RNA that is dedicated to the production of GAG proteins with protective effects on TE RNAs [131,132]. This allows EVD to efficiently multiply in A. thaliana genome, until it reaches approximately 40 genomic copies, triggering canonical RdDM and TGS [132]. Another strategy was described for the class II VANDAL21 TE that encodes the silencing suppressor VANC21, specifically promoting DNA hypomethylation at VANDAL21 TE sequences [133,134]. Finally, the PMD MAIN and MAIL1 TE silencing effectors are phylogenetically related to TE-encoded proteins, suggesting that TEs may produce their own PMD proteins as a counter-silencing strategy [104,108]. Altogether, these studies illustrate the evolutionary arms race between TEs and their host, relying on a fine balance between TE fitness and host genome integrity.
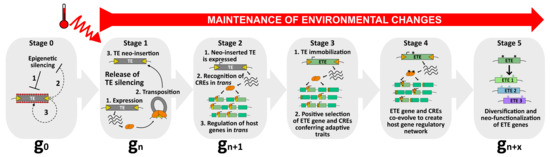
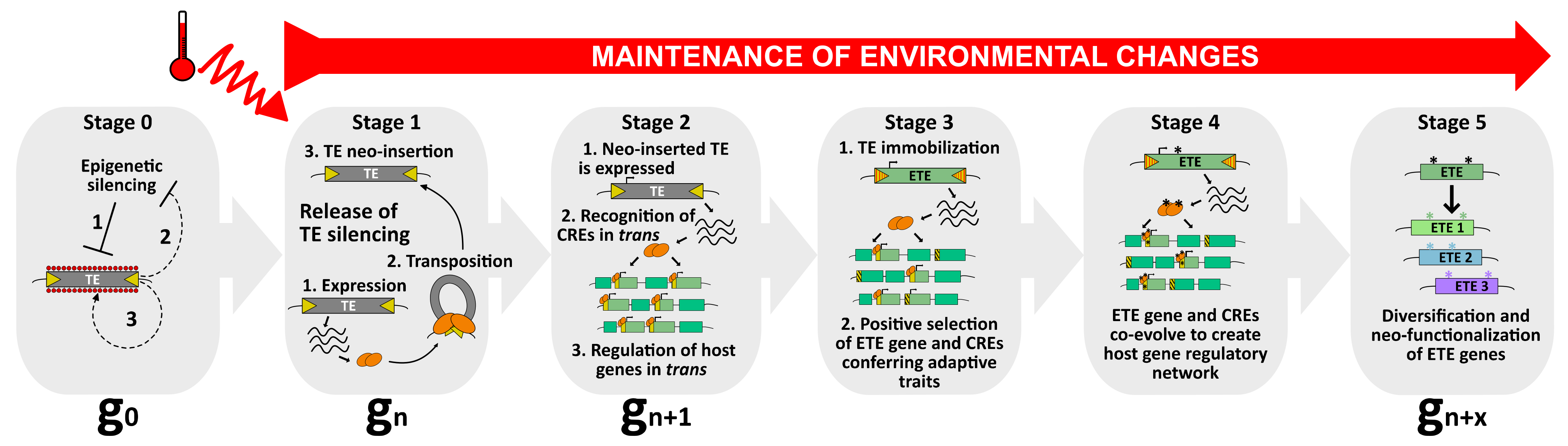
Figure 2.
A model to explain the occurrence of TE exaptation. This hypothetical model describes the exaptation of a class II TE-derived transposase. At stage 0, TEs are repressed by silencing pathways to ensure host genome integrity (1). Nevertheless, as purifying selection and genetic drift negatively impact the transgenerational persistence of TEs within the host genome, these latter might use anti-silencing strategies (2) to maintain some basal level of activity (3), and to be inherited as functional elements. Among the pool of TEs, one of them can be considered as an ETE precursor. At stage 1, upon, for instance, the perception of an environmental stimulus, such as heat, this TE is activated. As a young and functional element, it can transpose and mobilize in new genomic regions (neo-insertions). At stage 2, the neo-inserted TE is expressed, producing transposase proteins that will recognize TE-derived CREs genome-wide and potentially recruit additional chromatin factors to regulate host genes in trans. At this stage, although the neo-inserted TE is still capable of mobilization, it can enter selective pressure when providing positive advantage to the host. However, this feature must be followed by a swift transition to stage 3, in which the neo-inserted TE becomes immobilized. At stage 3, the neo-inserted TE is immobilized by mutations occurring in its TIRs (red stripes), turning into an ETE gene. Undoubtedly, TE immobilization is essential in the process of TE exaptation. Although TE immobilization is an absolute prerequisite for TE exaptation, it is not sufficient. ETE genes must remain stably expressed, most likely because environmental conditions persist. The ETE gene still undergoes positive selection and CREs that are not conferring adaptive traits are negatively selected. At stage 4, ETE gene and CREs co-evolve, accumulating mutations (*) under purifying selections, and together create a new host gene regulatory network that will be perpetuated only if the environmental changes persist. At stage 5, the ETE gene can be considered a genuine host gene, providing phenotypic value. Following relaxed selection pressure, the TE-derived sequence may inherit point mutations (*), or gene duplication may occur. ETE diversification and neo-functionalization ensure “long-term” positive adaptation of ETE sequence to ultimately create genetic variants with new cellular functions.
Figure 2.
A model to explain the occurrence of TE exaptation. This hypothetical model describes the exaptation of a class II TE-derived transposase. At stage 0, TEs are repressed by silencing pathways to ensure host genome integrity (1). Nevertheless, as purifying selection and genetic drift negatively impact the transgenerational persistence of TEs within the host genome, these latter might use anti-silencing strategies (2) to maintain some basal level of activity (3), and to be inherited as functional elements. Among the pool of TEs, one of them can be considered as an ETE precursor. At stage 1, upon, for instance, the perception of an environmental stimulus, such as heat, this TE is activated. As a young and functional element, it can transpose and mobilize in new genomic regions (neo-insertions). At stage 2, the neo-inserted TE is expressed, producing transposase proteins that will recognize TE-derived CREs genome-wide and potentially recruit additional chromatin factors to regulate host genes in trans. At this stage, although the neo-inserted TE is still capable of mobilization, it can enter selective pressure when providing positive advantage to the host. However, this feature must be followed by a swift transition to stage 3, in which the neo-inserted TE becomes immobilized. At stage 3, the neo-inserted TE is immobilized by mutations occurring in its TIRs (red stripes), turning into an ETE gene. Undoubtedly, TE immobilization is essential in the process of TE exaptation. Although TE immobilization is an absolute prerequisite for TE exaptation, it is not sufficient. ETE genes must remain stably expressed, most likely because environmental conditions persist. The ETE gene still undergoes positive selection and CREs that are not conferring adaptive traits are negatively selected. At stage 4, ETE gene and CREs co-evolve, accumulating mutations (*) under purifying selections, and together create a new host gene regulatory network that will be perpetuated only if the environmental changes persist. At stage 5, the ETE gene can be considered a genuine host gene, providing phenotypic value. Following relaxed selection pressure, the TE-derived sequence may inherit point mutations (*), or gene duplication may occur. ETE diversification and neo-functionalization ensure “long-term” positive adaptation of ETE sequence to ultimately create genetic variants with new cellular functions.
3. From Stress-Induced TE Reactivation to Neo-Insertions with Adaptive Benefits That Fuel Genetic Innovation and Become Exapted
Although primarily considered as junk DNA or parasitic elements, several studies revealed over the years that TEs can be beneficial for their host genome, and therefore are positively selected. There is indeed an accumulation of reports depicting TEs as major drivers of genome adaptation and potential contributors to evolutionary genetic innovation [25,27,135,136].
In natura, environmentally challenged organisms are prone to undergo massive TE reactivation, also known as TE burst, which, upon mobilization, will generate genomic structural variations (SVs) [12,137,138,139,140]. As transcriptional units, TEs carry their own cis-regulatory elements (CREs) that are potentially recognized upon specific stress perceptions. A well-known example is the A. thaliana ONSEN LTR retrotransposon that is transcriptionally activated following heat stress due to a heat response element (HRE) located in its LTR [141,142]. ONSEN activation is subsequently followed by transposition in several heat-stressed RdDM mutants, demonstrating the importance of this epigenetic pathway to regulate TE mobilization [141]. Although often neutral, TE transposition can promote gene mutations with deleterious effects for the host. Conversely, TE-transposition-induced gene mutations could confer, in some instances, a positive advantage to the host. A study showed that ONSEN insertions in two abscisic acid (ABA)-responsive genes improved ABA-related stress tolerance, suggesting TE-driven positive events of adaptation upon environmental changes [143]. Thus, this in-lab study illustrates perfectly how TE-induced SVs can potentially confer a new beneficial adaptive trait (or initial selective advantage) that might engage in natural selection as long as its benefit to the host is maintained. Remarkably, this is fully consistent with the precursory McClintock’s theory foreseeing TEs as potential important drivers of genome reorganization in response to environmental “shocks” [144].
TE-induced SVs have been well documented during the process of plant domestication, in which these events are selected and maintained under high selective pressure by humans, as they provide selective advantages in relation to desired agronomic traits ([23,145,146] and see Section 5.1). TE insertion polymorphisms have been described in several crops, including rice, in which it is an ongoing process still occurring in the field [147]. It is noteworthy to mention that plant domestication is often associated with polyploidization through whole genome duplication (WGD) [148]. Considering that TE mobilization is often observed during WGD [149], that would explain why plant domestication and TE mobilization are genetically connected.
At the macroevolution scale, the phenomenon leading to the appropriation of TE-derived sequences by the host is defined as TE exaptation—also known as co-option or TE domestication [26]. Exaptation is an evolutionary term in the taxonomy of fitness that was initially introduced by Gould and Vbra [150]. The authors claimed that the concept of adaptation, in which natural selection shapes a specific character for its current use, was not sufficient to explain all morphological innovations. They proposed two other processes, together coined exaptation. In the first process, a character whose function was originally shaped by natural selection (an adaptation event) is reallocated to a new function (co-option). In the second, the origin of a character that was subsequently co-opted for its current use cannot be explained by natural selection (a “nonaptation” event) [150]. As a long-term evolutionary process, TE exaptation is the outcome of an initial TE neo-insertion event, most likely occurring swiftly, and conferring a selective advantage to the host at a given time. Under constant environmental constraints, this neo-inserted TE will undergo positive selection to eventually become, in a long timescale, an exapted TE (ETE) gene or exapted TE-derived sequence (Figure 2).
At the molecular level, TE exaptation can be of various natures, through the co-option of coding or non-coding (nc)DNA/RNA sequences (from TE genes or TE-derived regulatory elements) (Figure 3). Nowadays, it is well-accepted that TE exaptation has been a major driver for several genetic and morphological innovations throughout evolution [25,135]. There are many examples depicting TE exaptation events as major contributors to essential evolutionary transitions, such as the elaboration of the vertebrate adaptive immune system [151,152,153], or the development of the embryo-nourishing tissues that are the mammalian placenta and the seed endosperm [154,155].
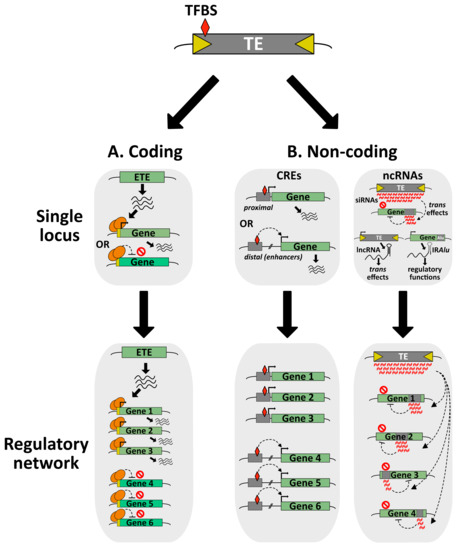
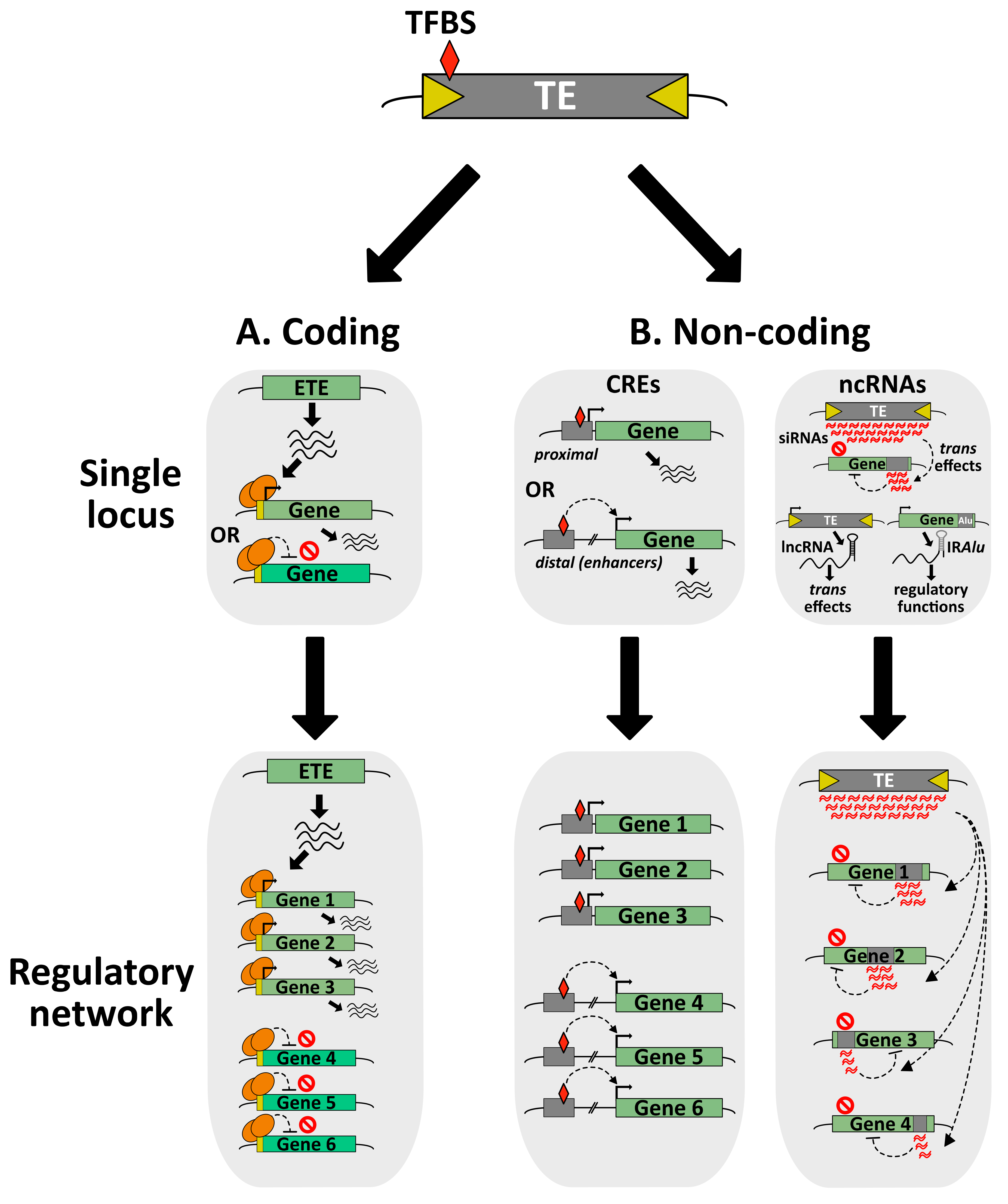
Figure 3.
From single locus positive selection of TE-derived sequences to the elaboration of complex regulatory networks. (A) Co-option of a TE gene can positively impact the host through the regulation of specific gene with the ETE proteins acting as transcriptional activators or repressors, most likely by recruiting other chromatin factors (not represented here). At the genomic scale, ETE proteins can participate in large regulatory networks ensuring the fine tuning of gene expression. (B) Co-option of non-coding TE sequences acting as CREs can regulate the expression of single locus through proximal or distal interactions with the transcriptional machinery. Genome-wide, these TE-derived CREs can be part of interconnected gene regulatory networks. TFBS: transcription factor (TF)-binding sites. Co-option of non-coding TE sequences acting as ncRNAs can either target a discrete host gene as siRNAs or produce lncRNAs with trans regulatory functions. In the case of SINE Alu elements located in the 3′UTR of host genes, they can form IRAlu secondary structure of mRNAs involved in splicing, mRNA subcellular localization and other processes. In a hypothetical model, TE-derived siRNAs can engage in complex regulatory networks repressing several host genes.
4. Exaptation of TE Coding Regions
One of the most striking beneficial contributions of TEs to the host genome is illustrated by the exaptation of TE-derived protein-coding sequences, which is a phenomenon leading to new functional host genes that can be referred to as ETE genes. It is in Drosophila in 1992 that Miller and colleagues identified the first P element-derived ETE genes; the authors suggested that TE exaptation was an evolutionary process potentially favoring genetic innovation and creating new biological functions for the host organism [156]. Since then, ETE genes have been discovered in numerous organisms: from unicellular organisms like prokaryotic archaea and bacteria, yeast and ciliates to multicellular organisms such as insects, metazoans and plants [25]. Considering that TEs compose the genomes of virtually all living organisms, it is tempting to speculate that ETE genes are present in any form of life.
TEs have diverse, attractive and sophisticated molecular tools, making them a dynamic reservoir for potential ETE genes with new cellular functions. Their genomes encode transposases, integrases and reverse transcriptases, as well as structural and envelope proteins that can be co-opted by the host during evolution [25]. Notably, the elaboration of a new ETE gene can occur through the co-option of a whole TE gene or a fragment of it. In addition, ETE gene or fragment can be fused to a preexisting host gene, creating a new chimeric protein. Although there are several reports of class I TE-derived ETE proteins, the majority of ETE proteins are highly similar to class II TE transposases [25,157]. This discrepancy between class I and class II TEs as reservoir of ETE genes can be explained either by an easier identification of class-II-derived ETE genes, or by the fact that there is a real preference for transposase-derived ETEs. Transposases have two essential domains: a specific DNA-binding domain (DBD) and a catalytic DDE or DDD endonuclease domain responsible for DNA cleavage and transposon integration. Although a large number of the ETE genes encode the two domains, several ETE proteins only carry the DBD. This may explain why several ETE proteins are presumably acting as transcription factors (TFs), either activators or repressors of transcription (Figure 3A). Besides, ETE proteins are involved in various chromatin-related processes such as TE silencing, mRNA splicing, DNA repair, telomere integrity, centromere formation, chromosome segregation and recombination, or in other cellular processes regulating translation or nuclear import [15,25,157]. To date, there are more than one hundred and fifty identified eukaryotic ETE genes. For most of them, the biological role and molecular mechanisms remain unknown [15,25,157,158].
4.1. The FAR1/FH3 TF Family: Multitool ETE Proteins Involved in Environment Sensing and Plant Development
In plants, the first and by far the most thoroughly characterized ETE genes are FAR-RED IMPAIRED RESPONSE1 (FAR1) and FAR-RED ELONGATED HYPOCOTYLS3 (FHY3). FAR1, FHY3 and homologs are TFs deriving from Mutator-like (MULE) transposases [159]. In A. thaliana, FAR1 and FHY3 act cooperatively downstream of the photoreceptor phytochrome A to specifically modulate far-red light-responsive gene expression [160]. These proteins are also required for various processes such as chlorophyll biosynthesis, circadian rhythm, shade tolerance, seed germination, flowering, plant immunity and stress responses [161,162,163,164,165,166,167]. Through their DBD, FHY3/FAR1 complexes recognize specific CREs, called FHY3/FAR1-binding site (FBS) that are predominantly located at the transcription start site of promoters [160]. FBS are enriched in the promoters of numerous genes exhibiting diurnal or circadian cycling, like for instance, CIRCADIAN CLOCK ASSOCIATED1 (CCA1), which is a central gene controlling the circadian clock [160,166]. Conversely, Xie and colleagues reported a new function of FHY3 and FAR1, acting as transcriptional repressors. They found that the FHY3/FAR1 complex physically interacts with three SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) TFs. This complex negatively regulates the binding of SPL to the DNA at the promoter of several genes involved in flowering regulation, including FRUITFUL (FUL), LEAFY (LFY) and APETALA1 (AP1). Thus, FHY3/FAR1 complex induces the downregulation of key flowering time master regulators, which ultimately delays flowering [168]. Altogether, these studies demonstrate that FAR1/FHY3 and their homologs are major TFs involved in the perception of environmental changes during plant development.
There are fourteen genes belonging to the FAR1/FHY3 family in A. thaliana [169]. FAR1/FHY3-related TFs are conserved in other eudicot species, as well as in monocots, suggesting that exaptation of these sequences occurred prior to the monocot–dicot split [170]. Comparative genomic analyses have revealed large proportions of FAR1/FHY3 genes in several plant species. A significant expansion of an FAR1/FHY3 gene family would correlate with the prostrate-to-erect growth switch specifically observed in the domesticated Rosa wichuraiana “Basye’s Thornless” (BT) species, with potential links to molecular processes related to light signaling, shade perception and flowering time [171]. The large diversification of FAR1/FHY3 genes observed in the wild species Medicago ruthenica, in comparison to two related species, M. truncatula and M. sativa, could contribute to the higher tolerance of M. ruthenica to various abiotic stresses including drought, making it a valuable alternative as a legume forage crop [172]. In the tea plant (Camellia sinensis), the steady-state level of FAR1/FHY3 mRNAs is highly modulated in response to various abiotic stresses such as high salt, high/low temperature, polyethylene glycol (PEG)-mimicked drought or abscisic acid (ABA) treatment [173]. Altogether, these reports are consistent with a previous study performed in A. thaliana showing that mutant plants knocked out for genes belonging to the FAR1/FHY3 family display changes of their phenotypical traits when challenged by several abiotic stresses [174]. Thus, FAR1/FHY3 TFs can be depicted as multifaceted molecular players involved in plant development and in response to various environmental stimuli.
4.2. The hAT-Derived SLEEPER Genes
DAYSLEEPER, the founding member of the SLEEPER gene family, was first discovered in a yeast one-hybrid screen aimed at identifying Arabidopsis proteins bound to the promoter of Ku70, a gene involved in DNA repair, through the recognition of the DNA Kubox1 motif [175]. DAYSLEEPER encodes a protein carrying the canonical transposase domains of hAT DNA transposons (hobo from Drosophila or hermes from housefly, Activator from maize and Tam3 from snapdragon) that are a multiblock hAT dimerization domain required for self-interaction, and a BED-type ZF domain interacting with DNA [176]. However, the protein lacks the catalytical DDE motif typical of hAT transposases [177]. DAYSLEEPER has also lost the flanking 8-bp genomic target-site duplications (TSDs), hallmarks of hAT integration, and the terminal inverted repeats (TIRs) that are required for transposition [177]. While daysleeper null mutants showed dramatic developmental phenotype, failing to develop normal organs, plants overexpressing DAYSLEEPER were capable of developing despite morphological defects [175]. Gene expression profiling of DAYSLEEPER-overexpressing plants revealed several upregulated genes, although none of them carried a Kubox1 motif in their promoter. DAYSLEEPER is highly expressed in SAM and RAM, reproductive organs and seeds [175]. Subcellular localization experiments confirmed that GFP-tagged DAYSLEEPER localized into the nucleus, which is consistent with the molecular function of the protein and the presence of a nuclear localization signal (NLS) [176]. Besides, overexpressing fluorescent protein (FP)-tagged DAYSLEEPER in Arabidopsis protoplasts revealed a cytoplasmic localization in multi-vesicular bodies and potentially late endosomes [176]. Considering that FP-DAYSLEEPER proteins were overexpressed, further studies will be required to determine the biological significance of its cytoplasmic localization.
In A. thaliana, the DAYSLEEPER closest homolog was called CYTOSLEEPER because the protein is cytoplasmic, which is consistent with the absence of an NLS. Unlike DAYSLEEPER loss-of-function, cytosleeper mutants are undistinguishable from wt plants [178]. Seeking for SLEEPER-like genes using the conserved “sleeperdomains” identified several candidates in the angiosperm sister species Amborella trichopoda as well as in distant species such as the water lily Nuphar variegata. In Oryza sativa, several SLEEPER-like genes were identified and named RICESLEEPERs. Remarkably, functional studies carried out in rice strongly suggested that RICESLEEPER1 and RICESLEEPER2 genes were both crucial for plant growth, as only heterozygote mutant plants could be retrieved [178]. Altogether, these studies demonstrated the pivotal role of DAYSLEEPER and relatives in plants.
4.3. The Angiosperm-Specific MUSTANG Family
Unlike the FAR1/FHY3 or DAYSLEEPER genes that were identified by classical genetic approaches, the MUSTANG (MUG) genes were discovered by in silico analyses seeking for MULEs TE-derived mudrA-like ETE genes conserved in several angiosperms including A. thaliana and O. sativa [179]. The A. thaliana genome encodes eight MUG homologs gathering into two distinct clades: MUGA (MUG1 to MUG4) and MUGB (MUG5 to MUG8) [180]. It has been suggested that MUGA and MUGB would have arisen from two independent exaptation events [170]. MUG genes act redundantly to ensure proper plant development. Although mug1, mug2 and mug3 simple mutants are phenotypically undistinguishable from wt plants, high-order mutant combinations such as mug1 mug2 or mug1 mug2 mug3 can seriously impact plant growth, flower development and fertility [170,180]. Similarly, the mug7 mug8 double mutant displays severe growth delays [180]. Like FAR1, MUG proteins carry the typical MULE transposase domain and a divergent SWIM ZF. They also carry an MuDR domain. In addition, the MUGB MUG7 and MUG8 carry the Phox and Bem1p (PB1) domain of unknown function, most likely acquired through a transduplication event (see Section 4.6). It was proposed that MUG proteins would contact DNA with their MuDR domain and/or SWIM ZF, acting as putative transcriptional regulators of plant development [180]. As observed with far1/fhy3 mutants, phenomic studies showed that the growth of several mug mutants is impacted by phosphate starvation or various abiotic stresses [174].
4.4. The PIF/Harbinger-Related ETE Proteins
Originally identified in maize but also present in animals, the P instability factor (PIF)/Harbinger superfamily is composed of class II TIR DNA TEs encoding two distinct proteins: a superfamily-specific transposase with a DDE triad for endonuclease activity, and a DBD protein with a conserved SANT/myb/trihelix motif [152]. The first example of PIF/Harbinger-derived ETE gene was Harbinger Transposase-Derived 1 (HARB1), identified in humans and several mammals, chicken, frogs and fish [152]. Remarkably, exaptation of Harbinger-derived transposase and DBD proteins occurs concomitantly, in pairs. Thus, HARB1 was found to interact with the co-opted DBD protein nuclear apoptosis-inducing factor 1 (NAIF1), and functional homologies in the molecular mechanisms involving Harbinger transposase/DBD proteins and their domesticated counterparts have been proposed [181]. To date, the function of the HARB1/NAIF1 remains unknown.
Exaptation of PIF/Harbinger TEs is frequent in Drosophila [182], and HARB1-like proteins have been identified in plants. In Malus domestica (apple), MdHARB1 transcription is induced by heat stress, and transgenic plants overexpressing MdHARB1 are more resistant to heat [183].
In A. thaliana, several co-opted Harbinger proteins have been related to various epigenetic pathways. The tendency of Harbinger TEs to be inserted in gene-rich regions of the genome, as well as the dual nature of their transposase module could have predisposed them to exaptation as components of chromatin-related processes. Besides, considering that epigenetic pathways repress TEs, it was proposed that co-option of TE genes to be reallocated to these cellular processes could reflect an adaptation to evolutionary conflicts in the never-ending fight between the host and invasive elements [25].
The transposase ANTAGONIST OF LIKE HETEROCHROMATIN PROTEIN 1 (ALP1) and the divergent DBD protein ALP2 interact together to antagonize PRC2-mediated H3K27me3 deposition and gene silencing [184,185]. Importantly, ALP2 is required for the interaction between ALP1 and MULTICOPY SUPPRESSOR OF IRA1 (MSI1), a core component of PRC2 [185]. ALP1/ALP2 subgenomic locations are unknown; more experiments and structural approaches of higher resolution are needed to unravel the significance of the various protein domains in the ALP1-ALP2-MSI1 interaction. Besides, the effect of alp1 and alp2 mutants on TE silencing is unknown. Nevertheless, these functional studies clearly showed the importance of ALP1 and ALP2, most likely by controlling PRC2 activities during important developmental transitions.
Another example of dual exaptation involves the transposase Harbinger-derived protein 1 (HDP1) and the DBD protein HDP2. The two proteins interact together as well as with several components of the increased DNA methylation 1 (IDM1) histone acetyltransferase complex, including the methyl-DNA-binding protein MBD7, to prevent DNA hypermethylation and TE silencing [186]. HDP2 DBD is capable of binding DNA in vitro, and at the genomic level, the protein is significantly enriched at MBD7 locations [186]. Thus, the co-opted HDP1 and HDP2 are recruited to histone acetyltransferase complex to promote basal TE expression and antagonize DNA methylation-mediated gene silencing. It will be important in the future to precisely delineate the biological significance of this phenomenon.
Finally, the co-opted transposase HDA6-associated Harbinger transposon-derived protein 1 (HHP1) was recently identified as a new HDA6 partner, forming a complex together with the four Harbinger-derived SANT-domain-containing proteins SANT1, SANT2, SANT3 and SANT4, and the MBD1, MBD2 and MBD4 [76]. Then, split luciferase assays suggested that HHP1 and MBD1 would act as bridges between HDA6 and the SANTs. Although the hhp1 or mbd1/2/4 mutants did not show abnormal growth, the sant quadruple mutant displayed a late flowering phenotype similar to the hda6 mutant. Further analyses revealed that SANT and HDA6 co-regulate histone acetylation and are required for the proper expression of a common subset of genes, such as the floral repressor FLOWERING LOCUS C. It is unknown whether HDA6 targeting at these misregulated genes required the DBD of SANT proteins. Besides, it will be important to precisely delineate the role of HHP1 and MBDs in this pathway. Considering that these proteins are most likely required for the indirect interaction of HDA6 and SANT proteins, it would be relevant to test whether the hhp1 mbd1/2/4 quadruple mutant displays a late flowering phenotype.
4.5. TE Exonization and Exon Shuffling to Create New Chimeric Proteins (Host Gene-TE Fusion)
TE exonization is a molecular process leading to the creation of new exons from mutated introns composed of TE sequences [158]. TE exonization is especially prevalent in animals, where alternative splicing is largely observed [187]. Exon shuffling promotes the rearrangement of exons from different genes to elaborate new chimeric genes, a process that can be sourced from TEs [188]. Importantly, TE-mediated exon shuffling can solely involve host gene sequences (also see Section 4.6), or integrate TE sequences in the process. Together with TE exonization, TE-mediated exon shuffling is an important molecular process contributing to innovations of the host genome by creating new cellular functions through genetic rearrangement [15,157,158].
A striking example of host gene-transposase fusion (HTF) is the primate gene SETMAR which is composed of a Tcl-Mariner transposase inserted downstream of a SET histone methyl transferase gene [189,190]. The SETMAR DBD has kept its specificity for a DNA motif derived from the ancestral Tcl-Mariner terminal sequence (Hsmar1 ITRs) that is still present in thousands of copies in the human genome. A modest overexpression of SETMAR leads to the misregulation of 1500 genes, with an enrichment for the Hsmar1 ITRs in upregulated loci. Furthermore, these genes may be involved in cancer [191]. The exaptation of SETMAR gene occurred in the anthropoid primate lineage. The three SETMAR protein domains, i.e., its SET, catalytic transposase and DNA-binding domains are under a strong purifying selection [192]. All these results suggest that the fusion of the ancestral transposase and SET genes in anthropoid primates would have contributed to the emergence of new gene regulatory networks.
Recently, an elegant analysis using comparative genomics surveyed the occurrence of HTFs in available tetrapod genomes [193]. The authors identified 106 HTFs deriving from 94 independent fusion events over the course of ~300 million years of evolution. Analysis of HTF gene structure revealed that transposase capture occurred through alternative splicing events. All known eukaryotic DNA transposon superfamilies contribute to HTF formation, with a predominance of Tc1/mariner-, hAT- and P element/Kolobok-derived transposases fused to host domains involved in transcriptional regulation. While host gene/transposase-derived DBD fusions are prevalent, HTFs can also involve whole transposase sequences. For instance, the vesper bats KRABINER protein derives from an HTF event between the host transcriptional repressor Krüppel- associated box (KRAB) and a full-length Mlmar1 mariner transposase. KRABINER is involved in transcriptional repression in a DNA-sequence-specific manner, and binds genome-wide hundreds of cognate TE-derived CREs, controlling a large network of genes [193]. The fact that several HTFs are composed of host domains connected to chromatin, such as KRAB, SCAN zinc finger (ZF) or SET domains, suggests that most of the HTF proteins would play a role in chromatin-related processes, possibly acting as activator or repressor TFs [193].
In humans, intronic SINE Alu elements are particularly prone to TE exonization, through the use of cryptic splice sites residing within their sequences [194,195]. Thousands of human gene transcripts may contain TE-derived sequences [196,197,198]. Recently, it was shown that an alternative spliced transcript (CD274-L2A) was generated by exaptation of an intronic LINE endogenous retroelement and created a new variant of PD-L1 (a membrane-bound protein) without the transmembrane domain and the regulatory sequence in the 3′UTR [199].
Advances in sequencing technologies have made it possible to investigate the transcriptional landscape of TEs at the genomic scale. Five prime cap (5ʹ-Cap) capture coupled to nanopore-based long-read sequencing of mRNA was carried out in locust that has a complex genome highly enriched in TEs, to characterize full-length transcripts in their native form and evaluate the propensity for TE exonization. Among the 60,908 representative RNA transcripts, 51.88% of them contained at least one TE-derived sequence, mostly in the first or last exons, but to a lesser extent, in internal exons as well [200]. It will be relevant to develop similar analyses in A. thaliana and plants with more complex genomes.
In angiosperms, there are a substantial proportion of genes carrying TE fragments. For example, in rice, more than 10% of transcripts were reported to contain TEs or TE-derived sequences, and TEs contribute to about 2% of rice protein coding regions [201]. Similarly, in A. thaliana, 7.8% of expressed genes were found to contain a region with close similarity to a known TE sequence [202]. Recently, in mulberry, it was shown that the proportion of MITE-associated genes was about 1,5%, and the proportion of MITE-related alternative splicing was 2,5% to 5% compared to the number of all alternative splicing events, according to the tissues tested, bud and flower, respectively [203]. This illustrates the great potential of TE-driven genetic diversification through alternative splicing events.
4.6. Transduction and Transduplication
As mentioned above, TEs have a propensity to capture and transpose entire host genes or fragments through the process of TE-mediated exon shuffling, which can result in gene duplication or rearrangement. This phenomenon is named transduction or transduplication, depending on the involvement of class I or class II TEs, respectively [204]. Although the molecular mechanisms and evolutionary consequences of these phenomena for the host as well as for TE fitness are not well understood, there is an increasing amount of evidence proving the importance of these processes in the elaboration of genetic diversity.
Class I TE-mediated transduction involves transcriptional readthrough from the retrotransposon promoter to the adjacent host gene sequence and its subsequent incorporation into the TE sequence during reverse transcription [205]. There are few cases of transduction in plants. In maize, transduction involving the LTR retrotransposon Bs1 created a novel chimeric gene composed of three host gene fragments fused to the Bs1 gag domain. This chimeric gene is expressed at the protein level, and its function could be involved in reproductive development [206]. Another example is Katydid-At1, an element of the terminal-repeat retrotransposons in miniature (TRIM) family of non-autonomous LTR retrotransposons that was involved in the transduction of a gene potentially involved in nonsense-mediated mRNA decay (NMD) [207].
In humans, LINE-1 L1 and SVA (SINE/VNTR/Alu) elements also contribute to transduction events of adjacent host sequences [208,209]. It was proposed that mammalian L1 TEs would be involved in the generation of ten thousand genes through the process of transduction, many of which are transcribed and some of them acquired new cellular functions [210].
In plants, Class II TE-mediated transduplication involves Pack-TYPE TEs that are predominantly derived from MULE, CACTA and Harbinger TEs in A. thaliana. Pack-TEs share many features with host genes. They are usually not highly repeated, and enriched in euchromatic instead of pericentromeric regions. Pack-TE-mediated transduplication events generate genes that are under purifying selection [211,212]. This implies that transduplication events may have a direct influence on genome evolution, generating new gene functions or regulatory activities by shuffling fragments of various genes across the genome. Zhao and colleagues reviewed very well the features of transduplicated genes in comparison with the original versions or donor genes [164]. The first feature is the size of transduplicated genes, which is usually smaller than primary genes. It is relatively rare for a Pack-TE to transduplicate a whole gene. The second feature is that, unlike regular duplicated genes, the alignment of transduplicated gene fragments with their original counterpart reveals a sharp boundary at the breakpoint of duplication. The third feature is that transduplicated gene fragments are often flanked by inverted or direct repeats. The presence of TSD flanking the TE DNA repeats is the unambiguous evidence that the entire structure belongs to a single TE. Furthermore, each transduplication event should be associated with specific flanking TSD. However, TSD are not always trivial to spot, making the identification of this phenomenon difficult. Thus, it is probable that the number of transduplicated genes may be strongly underestimated, as it is only recently that transduplication events may have been identified with confidence. As a consequence, it remains a great challenge to identify this type of gene [164].
As mentioned above, the MUGB MUG7 and MUG8 proteins carry a PB1 domain of unknown function, usually not associated with TEs, that was likely acquired via transduplication before the exaptation of MUGB TE precursor [170,180]. The PB1 domain belongs to an additional short 5′-exon, which is consistent with the general pattern of Pack-MULE transduplication. This phenomenon is interesting because it suggests that the MUGB PB1 domain may have undergone a complete co-evolutionary cycle.
Recently, the first report of a real-time mobilization of non-autonomous Pack-CACTA has been highlighted [213]. In this study, new transposed copies were found mostly within euchromatic regions (65%), which is consistent with previous observations [214]. The authors proposed a model where acquisition of host DNA was tightly linked to transposition, and importantly, DNA methylation played a direct role in controlling the transposition of Pack-TYPE TEs [213]. When two Pack-CACTA are close together, frequent aberrant excisions of one of the two TIRs in each pair allow the remaining termini of the neighboring transposons to encompass a new single element incorporating the host DNA [213].
Finally, the epigenetic state of over a thousand transduplicated genes was analyzed in the maize genome [215]. Remarkably, the level of DNA methylation and siRNAs was higher at donor genes than at genes with no evidence of transduplication capture. Moreover, transduplicated genes were highly DNA methylated and less expressed than donor genes. Furthermore, Pack-TEs mapped fewer siRNAs and were slightly less methylated than related TEs without captured fragments. All together, these observations could reflect some epigenetic conflicts between Pack-TEs and their relative, as well as between donor and transduplicated genes [215]. This undoubtedly depicts the complex interaction between TEs and host genomes.
5. Exaptation of TE-Derived Non-Coding Regions
5.1. From Positive Selection to Exaptation of TE-Derived Non-Coding Regions
TE mobilization can potentially lead to the production of TE-derived non-coding (nc)DNA sequences with positive selection signatures for host adaptation. They can be TE-promoter-derived CREs used as alternative promoters, as well as enhancers, insulators or repressor DNA elements regulating the transcription of host genes by the recognition of specific TFs [139]. Because of their self-replicative properties, TE-derived CREs can colonize the host genome, making them a powerful driving force to create transcriptional regulatory networks, which under constant environmental constraints, might become exapted (Figure 2). Nevertheless, it is important to mention that defining TE-derived ncDNAs as a genuine exaptation event should always be cautiously assessed [216].
TEs do not mobilize randomly in the genomes, and genomic distribution varies according to TE families, as exemplified in A. thaliana [13,217]. Furthermore, some specific TE families tend to preferentially mobilize upstream of genes, in the promoter regions, which is an ideal location to be potentially positively selected as new CREs of adjacent genes [23]. In humans, 25% of promoters and CREs may derive from TEs [218,219]. Comparative analyses of A. thaliana and A. lyrata, two sister species that diverged 10 million years ago, showed that 16% and 24% of genes harbor a TE insertion in a 500 bp window of the coding region [220]. This difference could be explained by a higher TE content in A. lyrata genome [220]. In rice and in maize, the MITE miniature mPing and MuDr TEs, respectively, preferentially insert into the promoters of genes, which can potentially upregulate adjacent genes [221,222]. In humans, statistical analyses of class I TE sequences for their propensity to harbor TF binding sites (TFBS) revealed that LTRs are most likely to contain almost all known TFBS in comparison to randomly generated sequences [223]. These results clearly indicate that TEs are well equipped to contribute to host gene regulation [224]. It is important to mention that following insertion, class I TEs often undergo ectopic recombination between their LTRs, resulting in the removal of their coding regions, with remnant solo-LTRs. This process allows the dissemination of several hundred thousand solo-LTRs throughout the human genome [225]. Likewise, in rice, 14,7% of LTR-retrotransposons are in the form of solo-LTR [226]. As these solo-LTRs have potentially kept their TF-specific CREs, they can influence the expression of neighbor genes.
Specific epigenetic chromatin states are most likely important factors influencing TE neo-insertions. In A. thaliana, while the neo-insertions of VANDAL21, ATENSPM3 and ATCOPIA93 (EVD) TEs occur preferentially in or nearby genes, only VANDAL21 TEs are significantly more often inserted in the promoters or 5′UTRs of transcriptionally active genes, enriched in H3K4me3 and H3K36me3 active marks. Conversely, ATENSPM3 and ATCOPIA93 TEs are more often inserted throughout the whole body of genes that are transcriptionally repressed by H3K27me3 deposition, and enriched in H2A.Z variant [227]. According to Wells and Feschotte, the propensity of some TE families to specifically target promoters of transcriptionally active genes could be a strategy favoring subsequent TE expression and transposition [228]. It is unclear whether the expression levels of neo-inserted TEs correlate with the chromatin state of insertion sites. For instance, in A. thaliana, it would be interesting to determine if VANDAL21 neo-inserted TEs harbor active epigenetic mark and tend to be more expressed in comparison to their ATENSPM3 and ATCOPIA93 counterparts, which would display a developmental-specific expression pattern, as they are inserted in or nearby PcG-repressed genes. In a similar approach, Zhang and colleagues analyzed the genome-wide distribution and chromatin landscape of TE neo-insertions in several plant and animal genomes [229]. They noticed that in addition to passive mobilization into open chromatin, several TE families tend to mobilize at TSS and/or transcriptional termination sites (TTS) of actively transcribed genes, suggesting a process of convergent evolution among eukaryotic TE families [229].
As TE promoters often carry stress-responsive elements, TE-derived ncDNAs might to some extent be positively selected to temporally regulate the expression of adjacent genes upon environmental stimuli or stresses [230]. In plants, there are several examples of TE-derived ncDNAs acting as cryptic promoters of nearby genes. One of the most striking examples is in Citrus sinensis (orange), where the LTR-retrotransposon Rider controls the expression of Ruby, a gene encoding a MYB TF required for anthocyanin production. Remarkably, different fruit pigmentations among orange varieties correlate with allelic variation of the cold-responsive Ruby locus carrying or not Rider in its promoter [231]. As this Rider insertion is recent, it should not be considered a TE exaptation. It could, however, be seen as a preliminary step to a future TE exaptation event. Similar cases have been highlighted in other crops, as in maize and apple [232,233]. In A. thaliana as well, it was suggested that an unmethylated ATCOPIA93-derived solo-LTR had been evolutionarily co-opted to cis-regulate the disease resistance gene RPP4, conferring plant immunity advantage [80]. In addition, a study described a LINE TE called EPCOT3 that was positively selected in A. thaliana, but not in A. lyrata, to regulate the expression of CYP82C2 through the binding of WRKY33, a pathogen-responsive TF [234]. The authors showed that CYP82C2, which is a gene involved in the production of metabolites for pathogen defense, originated from the duplication of the iron-stress response gene CYP82C4 in the two Arabidopsis species. They proposed that CYP82C2 underwent neo-functionalization upon EPCOT3 insertion in its promoter, and subsequent rewiring of WRKY33 regulon, leading to a positive effect on fitness [234]. Although the molecular mechanisms regulating A. lyrata CYP82C2 transcription remain elusive, purifying selection analyses suggest that it might be involved in pathogen defense.
5.2. TE-Derived CREs Shape Transcriptional Regulatory Networks
During transposition bursts and subsequent genomic rearrangements, TEs can spread their own promoter-borne CREs, as well as host CREs through the processes of transduction or transduplication (as described in Section 4.6). When inserted near genes, these TE neo-insertions can ultimately create new transcriptional regulatory networks or rewire existing networks, giving rise to new phenotypes and creating diversity. Furthermore, TEs are sources of lineage-specific regulatory elements, since TEs evolve rapidly, both in sequences and genomic distribution [220,235]. Here, we present examples of transcriptional regulatory networks shaped by TE-derived CREs in several evolutionary lineages that coordinate the expression of host genes involved in complex biological processes (reviewed in [18,139,155,224]).
One of the most outstanding examples of TE-derived CRE network is the elaboration of the mammalian placenta, involving MER20 DNA transposons in an evolutionary process that occurred more than 100 million years ago [236]. More recently, in the past 15–25 million years, the RLTR13 endogenous retroviruses (ERVs) rewired hundreds of placenta-specific enhancers in mice, suggesting a role for ERVs in the swift evolution of placenta morphology between mammal lineages [237]. It is noteworthy to mention that ERVs can be structurally indistinguishable from retrotransposons and may represent evolutionary intermediates between these latter and classical retroviruses [238]. Other studies reported TE-based rewiring of cis-regulatory networks in several developmental and cellular processes such as stem cell pluripotency, neo-cortex development, innate immunity and mammary gland evolution [136,239]. The regulatory functions of TEs may differ between cell types and developmental stages. Indeed, TEs can function in host gene regulation as alternative promoters, enhancers or boundary elements [235].
In plants as well, several examples of TE-driven CRE networks promoting TFBS rewiring have been described [155]. Among the most striking examples, TEs-derived ncDNAs have been involved in the rewiring of regulatory networks modulating flower development during rosid evolution, the C3 to C4 photosynthesis transition and seed endosperm development [155,240,241,242]. Endosperm formation involved RC/Helitron-derived CREs that rewired the genomic binding sites of MADS-box TF PHERES1 (PHE1) to ensure the proper expression of genes essential for seed development [242]. As the endosperm is the nourishing tissue feeding the embryo, this is reminiscent of the mammalian placenta formation, which suggests convergent evolution events.
5.3. TE-Derived RNAs Matter, but Is It Exaptation?
TEs are a major source of long non-coding (lnc)RNAs and small RNAs with cis and trans regulatory functions. TE-derived lncRNAs and small RNAs acting in cis have been well documented, especially in the RdDM or PTGS processes that were discussed above. TE-derived ncRNAs can also act in trans to regulate the expression of host genes at the transcriptional or post-transcriptional level. Besides, when embedded in a transcribed host mRNA, they can contribute to a plethora of mechanisms governing the mRNA outcome [158,243,244,245]. Although all these processes might not reflect genuine exaptation events, they clearly demonstrate that interaction between TEs and their host also occurs at the level of the RNA molecule (Figure 3B).
5.3.1. SINE RNA
In mammals, the RNA Pol-III-transcribed SINE are well-studied TEs producing ncRNAs that have been involved in virtually all molecular processes regulating gene expression and organizing chromatin and nucleolus structure [20,246,247]. Although widely distributed in the human genome, SINE TEs, such as Alu elements (Alus), are predominantly found in introns and 3′UTR of RNA Pol-II-transcribed genes [247]. Embedded in introns, SINE sequences can promote back-splicing events to form exonic circular RNAs [247]. In the 3′UTR of genes, Alus can form inverted-repeat (IRAlu) structures on mRNAs with regulatory functions. These 3′UTR IRAlus can influence host mRNA metabolism by controlling its subcellular localization, translation and stability through the recruitment of specific RNA-binding proteins [246,247]. Besides, 3′UTR Alus may be involved in alternative polyadenylation (APA) site usage to produce various mRNA isoforms [246], a process that is widely observed at the genomic level [248]. Remarkably, a similar process involving the Drosophila pogo DNA transposon was observed; pogo insertion in the 3′ UTR of the CG11699 gene results in a shorter 3′UTR, elevated transcript levels and increased resistance to xenobiotic stress, suggesting an adaptive effect of the insertion [249].
5.3.2. TE-Derived lncRNAs
There is compelling evidence, both in animals and plants, that TEs are a major source of lncRNAs, which are transcripts with a size greater than 200 bp and low protein-coding potential. In vertebrates, TEs account for up to 80% of lncRNA compositions. Comparative analyses performed in 40 plant species revealed more than 14,000 overlaps between TE and lncRNAs. While vertebrate lncRNAs appear to be lineage-specific, plant lncRNAs are generally poorly conserved between the same family species [158,244]. There is, however, a substantial number of TE-derived lncRNAs that are conserved among Brassicaceae species, suggesting conserved biological functions between these lncRNAs [250]. It is most likely that by nature, TE mobilization events have made the creation of lncRNAs a dynamic and fast-evolving process [251]. Thus, it seems fair to propose that TEs are an important source of lncRNA variability in genomes [252].
5.3.3. TE-Derived Small RNAs
As mentioned above, TE-derived small RNAs have been widely described in plants, especially in the process of siRNA-mediated heterochromatinization (for recent reviews see also [158,244,245]). Different evolutionary scenarios depict how TE-derived small RNAs control host gene expression. For instance, transduplication or transduction processes might capture gene fragments, which will be potentially converted into small RNAs together with TE-associated sequences. Alternatively, upon TE transposition and subsequent immobilization, a TE or its fragment can become part of host gene. In both scenarios, small RNAs that are complementary to the host genes might be produced, which in turn will regulate its expression. We will focus here on a few examples of TE-derived small RNAs in complex plant genomes.
The interaction between TE-derived small RNAs and host genome is well documented in rice. First, the TE-related microRNA280 (miRNA820) downregulates in trans the host gene OsDRM2, which is involved in TE silencing, invoking the evolutionary arms race phenomenon between the TE and its host [253]. This is reminiscent of a previous report in A. thaliana describing Athila Ty3/Gypsy TE-derived siRNAs targeting UBP1b, which encodes a factor repressing Athila translation [254]. A second example described the two rice WRKY45 allelic variants, WRKY45-1 and WRKY45-2, which differ from each other by the presence or absence of two intronic WANDERER-type MITE TEs in opposite orientation, respectively [255]. The expression of WRKY45-1 allele correlates with the accumulation of MITE-derived TE-siR815, which downregulates SL1 expression by RdDM, conferring higher sensitivity to Xanthomonas oryzae (Xoo) infection. Conversely, WRKY45-2 expression confers improved resistance. The negative effect of TE-siR815 was confirmed by creating transgenic plants overexpressing WRKY45-1 with deleted TE-siR815 sequence that showed enhanced resistance, similar to WRKY45-2-overexpressing plants [255]. Third, the stowaway-like MITE (sMITE) TE is integrated in the 3’-UTR of the Ghd2 gene, which promotes Ghd2 translational repression in a siRNA-dependent manner interfering with panicle development and grain yield [256]. Ghd2 is important for agronomical purposes, as it controls grain number, plant height and heading date. Comparative analyses revealed that sMITE is present in O. rufipogon, a wild relative of cultivated Asian rice [256], which suggests a positive selection event. Moreover, Shen and colleagues showed that several rice genes display MITEs in their 3′UTR, implying that MITE-driven translation repression could be a widespread mechanism [256].
In maize, captures of gene fragments by TEs can lead to evolutionary conflicts [215]. SiRNA crosstalk can occur between donor genes and TEs carrying the gene fragments, making donor genes more prone to accumulating siRNAs, to be DNA methylated and transcriptionally repressed. While this would be particularly true for translocated donor genes that are under low selective pressure, it is not the case for donor genes with important function that maintain high expression level with lower DNA methylation/siRNA levels, suggesting that capture of gene fragments could provide selective advantage to TEs [215]. Finally, in bread wheat (Triticum aestivum L.), a recent study showed that class II MITE and Mariner TEs are important sources of miRNAs with a potential role in plant immunity [257]. Particularly, the authors identified Tae_miR1436-1, which downregulates the accumulation of the Metallothionein-3-like (TaeMt3) protein to induce cell death as an efficient immune response against the powdery mildew fungus Blumeria graminis f.sp tritici. Tae_miR1436-1 is conserved in the triticum lineage, but not in other plant species [257]. Whether this case is a genuine event of exaptation or not is still a matter of debate, but it undoubtedly demonstrates the major evolutionary force of TEs to rewire host gene expression at the RNA level.
5.3.4. Biological Function of TE-Derived ncRNAs
Briefly, in animals, TE-derived lncRNAs have been related to several developmental processes. The maintenance of pluripotent embryonic stem cell program involves ERV-lncRNAs and LINE RNAs in humans. The mouse and human SINEUP lncRNAs have been involved in brain development by positively regulating the translation of factors essential for brain development. Finally, the LINE- and SINE-derived Xist is most likely the most-studied lncRNA, with a major role in the process of X-chromosome inactivation in female eutherian mammals [158,243]. In plants, the transcription of several TE-derived lncRNAs and small RNAs is induced by environmental stresses or phytohormone treatments. TE-derived small RNAs play a role in abiotic and biotic stress responses, plant development, hybridization barrier and of course, TE silencing [244,245].
Considering that overall, plant lncRNAs are involved in many biological processes, such as chromatin topology, transcriptional regulation, alternative splicing and small RNA buffering, all together regulating fundamental processes of plant development such as flowering time or stress perception [244,245], it is most likely that plant-TE-derived lncRNAs greatly contribute to these mechanisms as well.
6. How to Identify TE-Derived Sequences?
A stepwise approach must be implemented to define true exapted TE events. Some steps, such as identification, functional validation and conservation among close relative species are requisite and common to both types of exapted sequences (coding and non-coding ETE). Others, for instance, expression analyses when referring to ETE genes or exaptation of TE-derived RNAs, are more specific to one type of event [155,258].
6.1. Identification of TE-Derived Sequences
Constant improvements in sequencing technologies together with drastic reduction of sequencing costs have allowed large-scale genomic projects aimed at determining the genome of hundreds of species. It is nevertheless important to mention that careful genomic annotations, with a special attention to DNA repeats, are a prerequisite for the identification of TE-derived sequences. Indeed, identifying an exapted TE among a pool of “regular” TEs can be seen as looking for a needle in a haystack. Thus, meticulous computational analyses must be undertaken.
For the identification of ETE genes, one can integrate a suite of common properties including loss of mobility—absence of flanking terminal repeats such as TIRs or LTRs, sequence similarity with functional proteins (like transposases), low copy number, a conserved genomic location among sister/cousin species (synteny), evidence of active transcription and purifying selection of key codons [25]. Using similar approaches, several putative ETE genes were identified in A. thaliana [259]. A particularly challenging aspect is to establish the evolutionary history of an ETE gene family in order to determine the number and timing of exaptation events, and the identity of the ancestral genomes in which they arose. Such an approach was designed to resolve the phylogenetic context and timing of exaptation events and subsequent patterns of MUSTANG and FAR1/FHY3 diversification [170].
To identify ETE non-coding elements acting as CREs, it is important to first annotate TE-derived sequences in the genome. A recent study described a new approach to find old and degenerated TEs in A. thaliana. Based on a k-mer strategy, the authors suggested that half of the genome would originate from TEs, which is significantly more than previously anticipated [260]. Then, the identification of TE-derived sequences localized near genes, for instance in their promoter, needs to be realized. Bioinformatic tools can be used to predict the presence of binding motifs in these sequences [261]. Baud and colleagues found that among the degenerated TE sequences, a significant number corresponded to TFBS [260]. Wet lab approaches based on chromatin immunoprecipitation followed by sequencing (ChIP-seq) can be achieved to identify TFBS in TE-derived sequences at the genomic scale [235,262,263]. Importantly, some TE-derived CREs can also act as distal regulators such as enhancers (Figure 3B). Moreover, ETE non-coding DNA elements can be involved in other chromatin-related processes, such as, for instance, genome organization and compartmentalization.
6.2. Functional Validation
It is subsequently important to perform functional experiments when evaluating gene regulatory roles of TEs. For this purpose, in vivo enhancer assays, such as luciferase-based reporter experiments, can validate candidates [193]. However, these experiments are limited by the fact that they dissociate the exapted TE from its native chromosomal context, which can render the establishment of a direct causal link between the cis-regulatory activity of the TE and the endogenous gene expression difficult. Another approach to validate the role of a specific non-coding ETE sequence on host gene regulation is to carry out a loss of function experiment. Recent development of precise genome editing technologies such as CRISPR-Cas9 system allows one to answer this question [264,265,266].
6.2.1. Conservation among Closely Related Species
Microsynteny analyses are an essential step to define true ETE events. They rely on analyzing the genomic environment of TE-derived sequences among sister and more distant cousin species of a specific lineage to highlight evolutionary conservation. However, these analyses can be tricky when dealing with old events. Indeed, synteny can be interrupted by genomic rearrangements, making it difficult to determine the conservation of exapted events among distantly related species. This demonstrates the difficulty in drawing a conclusion based on a single feature. Furthermore, evidence of purifying selection events for conserved key codons within orthologous ETE genes is another essential feature reinforcing the confidence in exaptation events. Nevertheless, it is likely that many ETE sequences have escaped detection due to lack of solid evidence of conservation among closely related species.
6.2.2. Expression of ETE Genes or TE-Derived RNAs
Although not sufficient by itself, evidence of active transcription is a good clue of TE exaptation if combined with other features described above. Nevertheless, in some cases, it might be difficult to detect signs of expression. Indeed, it is important to bear in mind that the ETE sequences could be expressed at a low level or display spatiotemporal expression patterns, as they are often cell-lineage- or tissue-specific [267]. The new method CELLO-seq, which is based on long-read RNA sequencing, allows the detection of TE expression at single cell resolution, considering allelic and isoform variants [268]. In addition, the detection of TE-host fusion mRNAs must rely on robust detection methods integrating alternative splicing events. An excellent review synthetizes the different technologies and computational approaches that can be undertaken to detect TE expression [269].
6.2.3. Combining Computational and Wet Lab Approaches
The best way to highlight the impact of ETE events on host genome is to combine different approaches, such as computational genomics, transcriptomics (RNA-seq), genome editing and multiple chromatin-related analyses allowing the genome-wide mapping of TE-derived CREs or TF-like ETE proteins (ChIP-seq, DAP-seq, ATAC-seq, CAGE-seq…). Besides, cell biology and biochemical studies are essential complementary approaches to fully picture the functional role of ETE sequences. Although they might not be genuine ETEs, it is most likely that TEs and other DNA repeats overall contribute to 3D genome organization. Chromosome conformation capture (3C) analysis, such as Hi-C, studies subnuclear chromatin organization and compartmentalization, which can give new insights into the role of TEs in these processes. Mammalian class I TEs contribute to the species-specific subgenomic binding of the insulator protein CCCTC-binding factor (CTCF), which differentially impacts DNA looping and gene expression at the species level [270,271]. Likewise, 3C-based 4Tran experiments in human and mice captured long-range interactions between ERVs and host genomes, with a potential role in regulation of gene expression [272].
6.2.4. Long-Read-Based New Sequencing Technologies
As already mentioned, the fact that ETE sequences derive from highly repeated TEs can make their identification strenuous. For instance, upon a recent exaptation event, it might be difficult to differentiate the functional ETE gene from homologous copies that did not enter positive selection. Long read sequencing technologies, such as Pacific Biosciences (PacBio) or Oxford Nanopore sequencing, can identify whole TE or TE-derived sequences in complex genomes, as described recently in a genomically instable A thaliana line depicting chimeric TE/gene fusion event [273]. Such technologies can also be used to identify ETE genes. Besides, by performing long read nanopore RNA sequencing, it is now possible to capture full-length TE transcripts [274], giving important information about TE—and potentially ETE—expression in specific genetic background or environmental conditions.
7. Conclusions
In the future, the advent of large-scale genome sequencing combined with long read sequencing technologies will undoubtedly contribute to the identification of new TE-derived sequences with potential selective advantage for the host. Besides, pursuing our understanding of molecular processes involved in the regulation of epigenetic pathways and TE silencing will also be crucial. Altogether, these studies will undoubtedly contribute to unravelling the complex interplay between TEs and their host.
Author Contributions
Writing—original draft preparation, M.N. and N.P.; writing—review and editing, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study is set within the framework of “Laboratoires d’Excellences (LABEX)” TULIP (ANR-10-LABX-41) and “École Universitaire de Recherche (EUR)” TULIP-GS (ANR-18-EURE-0019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the reviewers for their constructive comments, Frederic Pontvianne for critical reading of the manuscript and lab members for fruitful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rowley, M.J.; Corces, V.G. The three-dimensional genome: Principles and roles of long-distance interactions. Curr. Opin. Cell Biol. 2016, 40, 8–14. [Google Scholar] [CrossRef]
- Bonev, B.; Cavalli, G. Organization and function of the 3D genome. Nat. Rev. Genet. 2016, 17, 661–678. [Google Scholar] [CrossRef]
- Doğan, E.S.; Liu, C. Three-dimensional chromatin packing and positioning of plant genomes. Nat. Plants 2018, 4, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.P.; Meyer, B.J. Spatial organization of chromatin: Emergence of chromatin structure during development. Annu. Rev. Cell Dev. Biol. 2021, 37, 199–232. [Google Scholar] [CrossRef]
- Mozgova, I.; Köhler, C.; Hennig, L. Keeping the gate closed: Functions of the polycomb repressive complex PRC2 in development. Plant J. 2015, 83, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Grewal, S.I.S.; Jia, S. Heterochromatin revisited. Nat. Rev. Genet. 2007, 8, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef]
- Deniz, Ö.; Frost, J.M.; Branco, M.R. Regulation of transposable elements by DNA modifications. Nat. Rev. Genet. 2019, 20, 417–431. [Google Scholar] [CrossRef]
- McClintock, B. Controlling elements and the gene. Cold Spring Harb. Symp. Quant. Biol. 1956, 21, 197–216. [Google Scholar] [CrossRef]
- Ohno, S. So much “junk” DNA in our genome. Brookhaven Symp. Biol. 1972, 23, 366–370. [Google Scholar]
- Orgel, L.E.; Crick, F.H.C. Selfish DNA: The ultimate parasite. Nature 1980, 284, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.; Rai, A.N.; Suprasanna, P. Moving through the stressed genome: Emerging regulatory roles for transposons in plant stress response. Front. Plant Sci. 2016, 7, 1448. [Google Scholar] [CrossRef] [PubMed]
- Quesneville, H. Twenty years of transposable element analysis in the Arabidopsis thaliana genome. Mob. DNA 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- El Baidouri, M.; Panaud, O. Comparative genomic paleontology across plant kingdom reveals the dynamics of TE-driven genome evolution. Genome Biol. Evol. 2013, 5, 954–965. [Google Scholar] [CrossRef]
- Feschotte, C.; Pritham, E.J. DNA transposons and the evolution of eukaryotic genomes. Annu. Rev. Genet. 2007, 41, 331–368. [Google Scholar] [CrossRef]
- Freeling, M.; Xu, J.; Woodhouse, M.; Lisch, D. A solution to the C-value paradox and the function of junk DNA: The genome balance hypothesis. Mol. Plant 2015, 8, 899–910. [Google Scholar] [CrossRef][Green Version]
- Bourque, G.; Burns, K.H.; Gehring, M.; Gorbunova, V.; Seluanov, A.; Hammell, M.; Imbeault, M.; Izsvák, Z.; Levin, H.L.; Macfarlan, T.S.; et al. Ten things you should know about transposable elements. Genome Biol. 2018, 19, 1–12. [Google Scholar] [CrossRef]
- Wicker, T.; Sabot, F.; Hua-van, A.; Bennetzen, J.L.; Capy, P.; Chalhoub, B.; Flavell, A.; Leroy, P.; Morgante, M.; Panaud, O.; et al. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 2007, 8, 973–982. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Jurka, J. A universal classification of eukaryotic transposable elements implemented in Repbase. Nat. Rev. Genet. 2008, 9, 411–412. [Google Scholar] [CrossRef]
- Deragon, J.-M. SINE Exaptation as cellular regulators occurred numerous times during eukaryote evolution. Top. Curr. Genet. 2012, 24, 253–271. [Google Scholar] [CrossRef]
- McClintock, B. The origin and behavior of mutable loci in maize. Genetics 1950, 36, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.R.L.; Burns, K.H.; Boeke, J.D. Active transposition in genomes. Annu. Rev. Genet. 2012, 46, 651–675. [Google Scholar] [CrossRef] [PubMed]
- Lisch, D. How important are transposons for plant evolution? Nat. Rev. Genet. 2013, 14, 49–61. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; van Wijnen, A.J.; Rice, D.; Rafii, M.Y.; Azizi, P.; Osman, M.; Taheri, S.; Bakar, M.F.A.; Isa, M.N.M.; et al. Contribution of transposable elements in the plant’s genome. Gene 2018, 665, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Jangam, D.; Feschotte, C.; Betrán, E. Transposable element domestication as an adaptation to evolutionary conflicts. Trends Genet. 2017, 33, 139–148. [Google Scholar] [CrossRef]
- Joly-Lopez, Z.; Bureau, T.E. Exaptation of transposable element coding sequences. Curr. Opin. Genet. Dev. 2018, 49, 34–42. [Google Scholar] [CrossRef]
- Cosby, R.L.; Chang, N.C.; Feschotte, C. Host–transposon interactions: Conflict, cooperation, and cooption. Genes Dev. 2019, 33, 1098–1116. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef]
- Rigal, M.; Mathieu, O. A “ mille-feuille ” of silencing: Epigenetic control of transposable elements. BBA Gene Regul. Mech. 2011, 1809, 452–458. [Google Scholar] [CrossRef]
- Lister, R.; O’Malley, R.C.; Tonti-Filippini, J.; Gregory, B.D.; Berry, C.C.; Millar, A.H.; Ecker, J.R. Highly integrated single-base resolution maps of the epigenome in arabidopsis. Cell 2008, 113, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Cokus, S.J.; Feng, S.; Zhang, X.; Chen, Z.; Merriman, B.; Haudenschild, C.D.; Pradhan, S.; Nelson, S.F.; Pellegrini, M.; Jacobsen, S.E. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 2008, 452, 215–219. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Matzke, M.A.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Wendte, J.M.; Pikaard, C.S. Targeting Argonaute to chromatin. Genes Dev. 2016, 30, 2649–2650. [Google Scholar] [CrossRef]
- Du, J.; Johnson, L.M.; Jacobsen, S.E.; Patel, D.J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 2015, 16, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Stroud, H.; Do, T.; Du, J.; Zhong, X.; Feng, S.; Johnson, L.; Patel, D.J.; Jacobsen, S.E. Non-CG methylation patterns shape the epigenetic landscape in Arabidopsis. Nat. Struct. Mol. Biol. 2014, 21, 64–72. [Google Scholar] [CrossRef]
- Zemach, A.; Kim, M.Y.; Hsieh, P.-H.; Coleman-Derr, D.; Williams, L.E.-; Thao, K.; Harmer, S.L.; Zilberman, D. The nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell 2013, 153, 193–205. [Google Scholar] [CrossRef]
- Chomet, P.S.; Wessler, S.; Dellaporta, S.L. Inactivation of the maize transposable element Activator (Ac) is associated with its DNA modification. EMBO J. 1987, 6, 295–302. [Google Scholar] [CrossRef]
- Anderson, S.N.; Zynda, G.J.; Song, J.; Han, Z.; Vaughn, M.W.; Li, Q.; Springer, N.M. Subtle perturbations of the maize methylome reveal genes and transposons silenced by chromomethylase or RNA-directed DNA methylation pathways. G3 Genes Genomes Genet. 2018, 8, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Yordan, C.; Martienssen, R.A. Robertson’s Mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1). Genes Dev. 2001, 15, 591–602. [Google Scholar] [CrossRef]
- Miura, A.; Yonebayashi, S.; Watanabe, K.; Toyama, T.; Shimada, H.; Kakutani, T. Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature 2001, 411, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Miura, A.; Bender, J.; Jacobsen, S.E.; Kakutani, T. Role of CG and non-CG methylation in immobilization of transposons in Arabidopsis. Curr. Biol. 2003, 13, 421–426. [Google Scholar] [CrossRef]
- Tsukahara, S.; Kobayashi, A.; Kawabe, A.; Mathieu, O.; Miura, A.; Kakutani, T. Bursts of retrotransposition reproduced in Arabidopsis. Nature 2009, 461, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Mirouze, M.; Reinders, J.; Bucher, E.; Nishimura, T.; Schneeberger, K.; Ossowski, S.; Cao, J.; Weigel, D.; Paszkowski, J.; Mathieu, O. Selective epigenetic control of retrotransposition in Arabidopsis. Nature 2009, 461, 427–430. [Google Scholar] [CrossRef]
- Walsh, C.P.; Chaillet, J.R.; Bestor, T.H. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat. Genet. 1998, 20, 116–117. [Google Scholar] [CrossRef]
- Bourc’his, D.; Bestor, T.H. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 2004, 431, 96–99. [Google Scholar] [CrossRef]
- Barau, J.; Teissandier, A.; Zamudio, N.; Roy, S.; Nalesso, V.; Hérault, Y.; Guillou, F.; Bourc’his, D. The DNA methyltransferase DNMT3C protects male germ cells from transposon activity. Science 2016, 354, 909–912. [Google Scholar] [CrossRef]
- McCue, A.D.; Panda, K.; Nuthikattu, S.; Choudury, S.G.; Thomas, E.N.; Slotkin, R.K. ARGONAUTE 6 bridges transposable element m RNA -derived si RNA s to the establishment of DNA methylation. EMBO J. 2015, 34, 20–35. [Google Scholar] [CrossRef]
- Panda, K.; Ji, L.; Neumann, D.A.; Daron, J.; Schmitz, R.J.; Slotkin, R.K. Full-length autonomous transposable elements are preferentially targeted by expression-dependent forms of RNA-directed DNA methylation. Genome Biol. 2016, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Wang, L.; Lei, Z.; Li, H.; Fan, W.; Cho, J. Ribosome stalling and SGS3 phase separation prime the epigenetic silencing of transposons. Nat. Plants 2021, 7, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Baulcombe, D.C. Transposon age and non-CG methylation. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nuthikattu, S.; McCue, A.D.; Panda, K.; Fultz, D.; DeFraia, C.; Thomas, E.N.; Keith Slotkin, R. The initiation of epigenetic silencing of active transposable elements is triggered by RDR6 and 21-22 nucleotide small interfering RNAs. Plant Physiol. 2013, 162, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Creasey, K.M.; Zhai, J.; Borges, F.; Van Ex, F.; Regulski, M.; Meyers, B.C.; Martienssen, R.A. miRNAs trigger widespread epigenetically-activated siRNAs from transposons in Arabidopsis. Nature 2014, 508, 411–415. [Google Scholar] [CrossRef]
- Fu, F.F.; Dawe, R.K.; Gent, J.I. Loss of RNA-directed DNA methylation in maize chromomethylase and DDM1-type nucleosome remodeler mutants. Plant Cell 2018, 30, 1617–1627. [Google Scholar] [CrossRef]
- Corem, S.; Doron-Faigenboim, A.; Jouffroy, O.; Maumus, F.; Arazi, T.; Bouché, N. Redistribution of CHH methylation and small interfering RNAs across the genome of tomato ddm1 mutants. Plant Cell 2018, 30, 1628–1644. [Google Scholar] [CrossRef]
- Tan, F.; Lu, Y.; Jiang, W.; Wu, T.; Zhang, R.; Zhao, Y.; Zhou, D.X. DDM1 represses noncoding RNA expression and RNA-directed DNA methylation in heterochromatin. Plant Physiol. 2018, 177, 1187–1197. [Google Scholar] [CrossRef]
- To, T.K.; Nishizawa, Y.; Inagaki, S.; Tarutani, Y.; Tominaga, S.; Toyoda, A.; Fujiyama, A.; Berger, F.; Kakutani, T. RNA interference-independent reprogramming of DNA methylation in Arabidopsis. Nat. Plants 2020, 6, 1455–1467. [Google Scholar] [CrossRef]
- Gutzat, R.; Rembart, K.; Nussbaumer, T.; Hofmann, F.; Pisupati, R.; Bradamante, G.; Daubel, N.; Gaidora, A.; Lettner, N.; Donà, M.; et al. Arabidopsis shoot stem cells display dynamic transcription and DNA methylation patterns. EMBO J. 2020, 39, e103667. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, A.B.; Liu, F.; Zhong, X. Linking signaling pathways to histone acetylation dynamics in plants. J. Exp. Bot. 2020, 71, 5179–5190. [Google Scholar] [CrossRef] [PubMed]
- Banaszynski, L.A.; Allis, C.D.; Lewis, P.W. Histone Variants in metazoan development. Dev. Cell 2010, 19, 662–674. [Google Scholar] [CrossRef]
- Probst, A.V.; Desvoyes, B.; Gutierrez, C. Similar yet critically different: The distribution, dynamics and function of Histone Variants. J. Exp. Bot. 2020, 71, 5191–5204. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.; Avramova, Z. ATX1/AtCOMPASS and the H3K4me3 marks: How do they activate arabidopsis genes? Curr. Opin. Plant Biol. 2014, 21, 75–82. [Google Scholar] [CrossRef]
- Jacob, Y.; Feng, S.; LeBlanc, C.A.; Bernatavichute, Y.V.; Stroud, H.; Cokus, S.; Johnson, L.M.; Pellegrini, M.; Jacobsen, S.E.; Michaels, S.D. ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat. Struct. Mol. Biol. 2009, 16, 763–768. [Google Scholar] [CrossRef]
- Aufsatz, W.; Stoiber, T.; Rakic, B.; Naumann, K. Arabidopsis histone deacetylase 6: A green link to RNA silencing. Oncogene 2007, 26, 5477–5488. [Google Scholar] [CrossRef][Green Version]
- Probst, A.V.; Fagard, M.; Proux, F.; Mourrain, P.; Boutet, S.; Earley, K.; Lawrence, R.J.; Pikaard, C.S.; Murfett, J.; Furner, I.; et al. Arabidopsis histone deacetylase HDA6 is required for maintenance of transcriptional gene silencing and determines nuclear organization of rDNA repeats. Plant Cell 2004, 16, 1021–1034. [Google Scholar] [CrossRef]
- Lippman, Z.; May, B.; Yordan, C.; Singer, T.; Martienssen, R. Distinct mechanisms determine transposon inheritance and methylation via small interfering RNA and Histone modification. PLoS Biol. 2003, 1, e67. [Google Scholar] [CrossRef]
- Liu, X.; Yu, C.W.; Duan, J.; Luo, M.; Wang, K.; Tian, G.; Cui, Y.; Wu, K. HDA6 directly interacts with DNA methyltransferase MET1 and maintains transposable element silencing in Arabidopsis. Plant Physiol. 2012, 158, 119–129. [Google Scholar] [CrossRef]
- To, T.K.; Kim, J.M.; Matsui, A.; Kurihara, Y.; Morosawa, T.; Ishida, J.; Tanaka, M.; Endo, T.; Kakutani, T.; Toyoda, T.; et al. Arabidopsis hda6 regulates locus-directed heterochromatin silencing in cooperation with met1. PLoS Genet. 2011, 7, e1002055. [Google Scholar] [CrossRef]
- Yu, M.; Ren, B. The three-dimensional organization of mammalian genomes. Annu. Rev. Cell Dev. Biol. 2017, 33, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yuan, L.; Yen, M.; Zheng, F.; Ji, R.; Peng, T.; Gu, D.; Yang, S.; Cui, Y.; Chen, P.; et al. SWI3B and HDA6 interact and are required for transposon silencing in Arabidopsis. Plant J. 2020, 102, 809–822. [Google Scholar] [CrossRef]
- Feng, C.; Cai, X.W.; Su, Y.N.; Li, L.; Chen, S.; He, X.J. Arabidopsis RPD3-like histone deacetylases form multiple complexes involved in stress response. J. Genet. Genom. 2021, 48, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, J.; Velanis, C.N.; Zhu, Y.; He, Y.; Tang, K.; Zhu, M.; Graser, L.; de Leau, E.; Wang, X.; et al. A domesticated Harbinger transposase forms a complex with HDA6 and promotes histone H3 deacetylation at genes but not TEs in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, S.; Wang, G.G. Polycomb gene silencing mechanisms: PRC2 chromatin targeting, H3K27me3 “Readout”, and phase separation-based compaction. Trends Genet. 2021, 37, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, A.M.; Park, Y.J.; McLean, C.M.; Dokshin, G.A.; Persson, J.M.; Herman, H.; Pasini3, D.; Miro, X.; Donohoe, M.E.; Lee, J.T.; et al. Antagonism between DNA and H3K27 methylation at the imprinted Rasgrf1 locus. PLoS Genet. 2008, 4, e1000145. [Google Scholar] [CrossRef]
- Deleris, A.; Stroud, H.; Bernatavichute, Y.; Johnson, E.; Klein, G.; Schubert, D.; Jacobsen, S.E. Loss of the DNA methyltransferase MET1 Induces H3K9 hypermethylation at PcG target genes and redistribution of H3K27 trimethylation to transposons in Arabidopsis thaliana. PLoS Genet. 2012, 8, e1003062. [Google Scholar] [CrossRef]
- Zervudacki, J.; Yu, A.; Amesefe, D.; Wang, J.; Drouaud, J.; Navarro, L.; Deleris, A. Transcriptional control and exploitation of an immune-responsive family of plant retrotransposons. EMBO J. 2018, 37, e98482. [Google Scholar] [CrossRef]
- Weinhofer, I.; Hehenberger, E.; Roszak, P.; Hennig, L.; Köhler, C. H3K27me3 profiling of the endosperm implies exclusion of polycomb group protein targeting by DNA methylation. PLoS Genet. 2010, 6, e1001152. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Teissandier, A.; Pérez-Palacios, R.; Bourc’his, D. An epigenetic switch ensures transposon repression upon dynamic loss of DNA methylation in embryonic stem cells. Elife 2016, 5, e11418. [Google Scholar] [CrossRef]
- Rougée, M.; Quadrana, L.; Zervudacki, J.; Hure, V.; Colot, V.; Navarro, L.; Deleris, A. Polycomb mutant partially suppresses DNA hypomethylation–associated phenotypes in Arabidopsis. Life Sci. Alliance 2020, 4, e202000848. [Google Scholar] [CrossRef]
- Antunez-Sanchez, J.; Naish, M.; Ramirez-Prado, J.S.; Ohno, S.; Huang, Y.; Dawson, A.; Manza-Mianza, D.; Ariel, F.; Raynaud, C.; Wilbowo, A.; et al. A new role for histone demethylases in the maintenance of plant genome integrity. Elife 2020, 9, e58533. [Google Scholar] [CrossRef] [PubMed]
- Bire, S.; Casteret, S.; Piégu, B.; Beauclair, L.; Moiré, N.; Arensbuger, P.; Bigot, Y. Mariner transposons contain a silencer: Possible role of the polycomb repressive Complex 2. PLoS Genet. 2016, 12, e1005902. [Google Scholar] [CrossRef] [PubMed]
- Frapporti, A.; Miró Pina, C.; Arnaiz, O.; Holoch, D.; Kawaguchi, T.; Humbert, A.; Eleftheriou, E.; Lombard, B.; Loew, D.; Sperling, L.; et al. The Polycomb protein Ezl1 mediates H3K9 and H3K27 methylation to repress transposable elements in Paramecium. Nat. Commun. 2019, 10, 2710. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, X.; Mao, F.; Basrur, V.; Ueberheide, B.; Chait, B.T.; Allis, C.D.; Taverna, S.D.; Gao, S.; Wang, W.; et al. A Polycomb repressive complex is required for RNAi-mediated heterochromatin formation and dynamic distribution of nuclear bodies. Nucleic Acids Res. 2021, 49, 5407–5425. [Google Scholar] [CrossRef]
- Mikulski, P.; Komarynets, O.; Fachinelli, F.; Weber, A.P.M.; Schubert, D. Characterization of the polycomb-group mark H3K27me3 in unicellular algae. Front. Plant Sci. 2017, 8, 607. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Tanizawa, Y.; Galik, B.; Wang, N.; Ito, T.; Mochizuki, T.; Akimcheva, S.; Bowman, J.L.; Cognat, V.; Maréchal-Drouard, L.; et al. Chromatin organization in early land plants reveals an ancestral association between H3K27me3, transposons, and constitutive heterochromatin. Curr. Biol. 2020, 30, 573–588. [Google Scholar] [CrossRef]
- Déléris, A.; Berger, F.; Duharcourt, S. Role of Polycomb in the control of transposable elements. Trends Genet. 2021, 37, 882–889. [Google Scholar] [CrossRef]
- Rippe, K.; Schrader, A.; Riede, P.; Strohner, R.; Lehmann, E.; Längst, G. DNA sequence- and conformation-directed positioning of nucleosomes by chromatin-remodeling complexes. Proc. Natl. Acad. Sci. USA 2007, 104, 15635–15640. [Google Scholar] [CrossRef] [PubMed]
- Clapier, C.R.; Iwasa, J.; Cairns, B.R.; Peterson, C.L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 2017, 18, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Vongs, A.; Kakutani, T.; Martienssen, R.; Richards, E. Arabidopsis thaliana DNA methylation mutants. Science 1993, 260, 1926–1928. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.V.; Fransz, P.F.; Paszkowski, J.; Mittelsten Scheid, O. Two means of transcriptional reactivation within heterochromatin. Plant J. 2003, 33, 743–749. [Google Scholar] [CrossRef]
- Stroud, H.; Greenberg, M.V.C.C.; Feng, S.; Bernatavichute, Y.V.; Jacobsen, S.E. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 2013, 152, 352–364. [Google Scholar] [CrossRef]
- Rutowicz, K.; Puzio, M.; Halibart-Puzio, J.; Lirski, M.; Kotliński, M.; Kroteń, M.A.; Knizewski, L.; Lange, B.; Muszewska, A.; Śniegowska-Świerk, K.; et al. A specialized Histone H1 Variant is required for adaptive responses to complex abiotic stress and related DNA methylation in Arabidopsis. Plant Physiol. 2015, 169, 2080–2101. [Google Scholar] [CrossRef]
- Bourguet, P.; Picard, C.L.; Yelagandula, R.; Pélissier, T.; Lorković, Z.J.; Feng, S.; Pouch-Pélissier, M.-N.; Schmücker, A.; Jacobsen, S.E.; Berger, F.; et al. The Histone variant H2A.W and linker histone H1 co-regulate heterochromatin accessibility and DNA methylation. Nat. Commun. 2021, 12, 2683. [Google Scholar] [CrossRef]
- Choi, J.; Lyons, D.B.; Kim, M.Y.; Moore, J.D.; Zilberman, D. DNA methylation and Histone H1 jointly repress transposable elements and aberrant intragenic transcripts. Mol. Cell 2020, 77, 310–323.e7. [Google Scholar] [CrossRef]
- Osakabe, A.; Jamge, B.; Axelsson, E.; Montgomery, S.A.; Akimcheva, S.; Kuehn, A.L.; Pisupati, R.; Lorković, Z.J.; Yelagandula, R.; Kakutani, T.; et al. The chromatin remodeler DDM1 prevents transposon mobility through deposition of histone variant H2A.W. Nat. Cell Biol. 2021, 23, 391–400. [Google Scholar] [CrossRef]
- Ni, K.; Ren, J.; Xu, X.; He, Y.; Finney, R.; Braun, S.M.G.; Hathaway, N.A.; Crabtree, G.R.; Muegge, K. LSH mediates gene repression through macroH2A deposition. Nat. Commun. 2020, 11, 5647. [Google Scholar] [CrossRef]
- Long, J.; Liu, J.; Xia, A.; Springer, N.M.; He, Y. Maize decrease in DNA methylation 1 targets RNA-directed DNA methylation on active chromatin. Plant Cell 2021, 33, 2183–2196. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.M.; Iyer, L.; Balaji, S.; Aravind, L. The natural history of the WRKY-GCM1 zinc fingers and the relationship between transcription factors and transposons. Nucleic Acids Res. 2006, 34, 6505–6520. [Google Scholar] [CrossRef]
- Steinbauerová, V.; Neumann, P.; Novák, P.; Macas, J. A widespread occurrence of extra open reading frames in plant Ty3/gypsy retrotransposons. Genetica 2011, 139, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Pelissier, T.; Bourguet, P.; Becker, C.; Pouch-Pelissier, M.-N.; Pogorelcnik, R.; Weingartner, M.; Weigel, D.; Deragon, J.; Mathieu, O. Arabidopsis proteins with a transposon-related domain act in gene silencing. Nat. Commun. 2017, 8, 15122. [Google Scholar] [CrossRef]
- Wenig, U.; Meyer, S.; Stadler, R.; Fischer, S.; Werner, D.; Lauter, A.; Melzer, M.; Hoth, S.; Weingartner, M.; Sauer, N. Identification of MAIN, a factor involved in genome stability in the meristems of Arabidopsis thaliana. Plant J. 2013, 75, 469–483. [Google Scholar] [CrossRef]
- Ühlken, C.; Horvath, B.; Stadler, R.; Sauer, N.; Weingartner, M. MAIN-LIKE1 is a crucial factor for correct cell division and differentiation in Arabidopsis thaliana. Plant J. 2014, 78, 107–120. [Google Scholar] [CrossRef]
- De Luxán-Hernández, C.; Lohmann, J.; Hellmeyer, W.; Seanpong, S.; Wöltje, K.; Magyar, Z.; Pettkó-Szandtner, A.; Pélissier, T.; De Jaeger, G.; Hoth, S.; et al. PP7L is essential for MAIL1-mediated transposable element silencing and primary root growth. Plant J. 2020, 102, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, M.; Picault, N.; Descombin, J.; Jami-alahmadi, Y.; Feng, S.; Bucher, E.; Jacobsen, S.E.; Deragon, J.; Wohlschlegel, J.; Moissiard, G. The plant mobile domain proteins MAIN and MAIL1 interact with the phosphatase PP7L to regulate gene expression and silence transposable elements in Arabidopsis thaliana. PLoS Genet. 2020, 16, e1008324. [Google Scholar] [CrossRef]
- Yatskevich, S.; Rhodes, J.; Nasmyth, K. Organization of chromosomal DNA by SMC complexes. Annu. Rev. Genet. 2019, 53, 445–482. [Google Scholar] [CrossRef]
- Gerasimova, T.I.; Byrd, K.; Corces, V.G. A chromatin insulator determines the nuclear localization of DNA. Mol. Cell 2000, 6, 1025–1035. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanizawa, H.; Sriswasdi, S.; Iwasaki, O.; Chatterjee, A.G.; Speicher, D.W.; Levin, H.L.; Noguchi, E.; Noma, K.I. Epigenetic regulation of condensin-mediated genome organization during the cell cycle and upon DNA damage through Histone H3 Lysine 56 Acetylation. Mol. Cell 2012, 48, 532–546. [Google Scholar] [CrossRef]
- Murton, H.E.; Grady, P.J.R.; Chan, T.H.; Cam, H.P.; Whitehall, S.K. Restriction of retrotransposon mobilization in Schizosaccharomyces pombe by transcriptional silencing and higher-order chromatin organization. Genetics 2016, 203, 1669–1678. [Google Scholar] [CrossRef]
- Wang, J.; Blevins, T.; Podicheti, R.; Haag, J.R.; Tan, E.H.; Wang, F.; Pikaard, C.S. Mutation of Arabidopsis SMC4 identifies condensin as a corepressor of pericentromeric transposons and conditionally expressed genes. Genes Dev. 2017, 31, 1601–1614. [Google Scholar] [CrossRef]
- Amedeo, P.; Habu, Y.; Afsar, K.; Mittelsten Scheid, O.; Paszkowski, J. Disruption of the plant gene MOM releases transcriptional silencing of methylated genes. Nature 2000, 405, 203–206. [Google Scholar] [CrossRef]
- Čaikovski, M.; Yokthongwattana, C.; Habu, Y.; Nishimura, T.; Mathieu, O.; Paszkowski, J. Divergent evolution of CHD3 proteins resulted in MOM1 refining epigenetic control in vascular plants. PLoS Genet. 2008, 4, e1000165. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Molinard, G.; Petty, T.J.; Broger, L.; Gabus, C.; Halazonetis, T.D.; Thore, S.; Paszkowski, J. Structural basis of transcriptional gene silencing mediated by Arabidopsis MOM1. PLoS Genet. 2012, 8, e1002484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Y.; He, X.J. Exploring potential roles for the interaction of MOM1 with SUMO and the SUMO E3 ligase-like protein PIAL2 in transcriptional silencing. PLoS ONE 2018, 13, e0202137. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-F.; Zhao, Q.-Y.; Dang, L.-L.; Luo, Y.-X.; Chen, S.-S.; Shao, C.-R.; Huang, H.-W.; Li, Y.-Q.; Li, L.; Cai, T.; et al. The SUMO E3 ligase-like proteins PIAL1 and PIAL2 interact with MOM1 and form a novel complex required for transcriptional silencing. Plant Cell 2016, 28, 1215–1229. [Google Scholar] [CrossRef]
- Iyer, L.M.; Abhiman, S.; Aravind, L. MutL homologs in restriction-modification systems and the origin of eukaryotic MORC ATPases. Biol. Direct 2008, 3, 1–9. [Google Scholar] [CrossRef]
- Dong, W.; Vannozzi, A.; Chen, F.; Hu, Y.; Chen, Z.; Zhang, L. MORC domain definition and evolutionary analysis of the MORC gene family in green plants. Genome Biol. Evol. 2018, 10, 1730–1744. [Google Scholar] [CrossRef]
- Moissiard, G.; Cokus, S.J.; Cary, J.; Feng, S.; Allison, C.; Stroud, H.; Husmann, D.; Zhan, Y.; Lajoie, B.R.; Mccord, P.; et al. MORC family ATPases required for heterochromatin condensation and gene silencing. Science 2012, 336, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Moissiard, G.; Bischof, S.; Husmann, D.; Pastor, W.A.; Hale, C.J.; Yen, L.; Stroud, H.; Papikian, A.; Vashisht, A.A.; Wohlschlegel, J.A.; et al. Transcriptional gene silencing by Arabidopsis microrchidia homologues involves the formation of heteromers. Proc. Natl. Acad. Sci. USA 2014, 111, 7474–7479. [Google Scholar] [CrossRef] [PubMed]
- Pastor, W.A.; Stroud, H.; Nee, K.; Liu, W.; Pezic, D.; Manakov, S.; Lee, S.A.; Moissiard, G.; Zamudio, N.; Bourc’his, D.; et al. MORC1 represses transposable elements in the mouse male germline. Nat. Commun. 2014, 5, 5795. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Hess, K.D.; Moreadith, R.W.; Richardson, L.L.; Handel, M.A.; Watson, M.L.; Zinn, A.R. New gene family defined by MORC, a nuclear protein required for mouse spermatogenesis. Hum. Mol. Genet. 1999, 8, 1201–1207. [Google Scholar] [CrossRef]
- Kim, H.; Yen, L.; Wongpalee, S.P.; Kirshner, J.A.; Mehta, N.; Xue, Y.; Johnston, J.B.; Burlingame, A.L.; Kim, J.K.; Loparo, J.J.; et al. The gene-silencing protein MORC-1 topologically entraps DNA and forms multimeric assemblies to cause DNA compaction. Mol. Cell 2019, 75, 700–710.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.W.; Shao, C.R.; Zhang, C.J.; Zhou, J.X.; Zhang, S.W.; Li, L.; Chen, S.; Huang, H.W.; Cai, T.; He, X.J. The SET domain proteins SUVH2 and SUVH9 are required for Pol V occupancy at RNA-directed DNA methylation loci. PLoS Genet. 2014, 10, e1003948. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhou, J.X.; Huang, H.W.; Li, Y.Q.; Shao, C.R.; Li, L.; Cai, T.; Chen, S.; He, X.J. Two components of the RNA-directed DNA methylation pathway associate with MORC6 and silence loci targeted by MORC6 in Arabidopsis. PLoS Genet. 2016, 12, e1006026. [Google Scholar] [CrossRef]
- Gallego-Bartolomé, J.; Liu, W.; Kuo, P.H.; Feng, S.; Ghoshal, B.; Gardiner, J.; Zhao, J.M.C.; Park, S.Y.; Chory, J.; Jacobsen, S.E. Co-targeting RNA polymerases IV and V promotes efficient de novo DNA methylation in Arabidopsis. Cell 2019, 176, 1068–1082.e19. [Google Scholar] [CrossRef]
- Xue, Y.; Zhong, Z.; Harris, C.J.; Gallego-Bartolomé, J.; Wang, M.; Picard, C.; Cao, X.; Hua, S.; Kwok, I.; Feng, S.; et al. Arabidopsis MORC proteins function in the efficient establishment of RNA directed DNA methylation. Nat. Commun. 2021, 12, 4292. [Google Scholar] [CrossRef]
- Ichino, L.; Boone, B.A.; Strauskulage, L.; Harris, C.J.; Kaur, G.; Gladstone, M.A.; Tan, M.; Feng, S.; Jami-Alahmadi, Y.; Duttke, S.H.; et al. MBD5 and MBD6 couple DNA methylation to gene silencing through the J-domain protein SILENZIO. Science 2021, 372, 1434–1439. [Google Scholar] [CrossRef]
- Oberlin, S.; Sarazin, A.; Chevalier, C.; Voinnet, O.; Marí-Ordóñez, A. A genome-wide transcriptome and translatome analysis of Arabidopsis transposons identifies a unique and conserved genome expression strategy for Ty1/Copia retroelements. Genome Res. 2017, 27, 1549–1562. [Google Scholar] [CrossRef]
- Marí-Ordóñez, A.; Marchais, A.; Etcheverry, M.; Martin, A.; Colot, V.; Voinnet, O. Reconstructing de novo silencing of an active plant retrotransposon. Nat. Genet. 2013, 45, 1029–1039. [Google Scholar] [CrossRef]
- Fu, Y.; Kawabe, A.; Etcheverry, M.; Ito, T.; Toyoda, A.; Fujiyama, A.; Colot, V.; Tarutani, Y.; Kakutani, T. Mobilization of a plant transposon by expression of the transposon-encoded anti-silencing factor. EMBO J. 2013, 32, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, A.; Saito, R.; Takashima, K.; Sasaki, T.; Fu, Y.; Kawabe, A.; Ito, T.; Toyoda, A.; Fujiyama, A.; Tarutani, Y.; et al. Evolution of sequence-specific anti-silencing systems in Arabidopsis. Nat. Commun. 2017, 8, 2161. [Google Scholar] [CrossRef]
- Schrader, L.; Schmitz, J. The impact of transposable elements in adaptive evolution. Mol. Ecol. 2018, 28, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H. Retrotransposons spread potential cis-regulatory elements during mammary gland evolution. Nucleic Acids Res. 2019, 47, 11551–11562. [Google Scholar] [CrossRef] [PubMed]
- Horváth, V.; Merenciano, M.; González, J. Revisiting the relationship between transposable elements and the eukaryotic stress response. Trends Genet. 2017, 33, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Lanciano, S.; Mirouze, M. Transposable elements: All mobile, all different, some stress responsive, some adaptive? Curr. Opin. Genet. Dev. 2018, 49, 106–114. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory activities of transposable elements: From conflicts to benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef]
- Morgante, M.; De Paoli, E.; Radovic, S. Transposable elements and the plant pan-genomes. Curr. Opin. Plant Biol. 2007, 10, 149–155. [Google Scholar] [CrossRef]
- Ito, H.; Gaubert, H.; Bucher, E.; Mirouze, M.; Vaillant, I.; Paszkowski, J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature 2011, 472, 115–120. [Google Scholar] [CrossRef]
- Cavrak, V.V.; Lettner, N.; Jamge, S.; Kosarewicz, A.; Bayer, L.M.; Mittelsten Scheid, O. How a retrotransposon exploits the plant’s heat stress response for its activation. PLoS Genet. 2014, 10, e1004115. [Google Scholar] [CrossRef]
- Ito, H.; Kim, J.M.; Matsunaga, W.; Saze, H.; Matsui, A.; Endo, T.A.; Harukawa, Y.; Takagi, H.; Yaegashi, H.; Masuta, Y.; et al. A stress-activated transposon in Arabidopsis induces transgenerational abscisic acid insensitivity. Sci. Rep. 2016, 6, 23181. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. The significance of responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef]
- Dubin, M.J.; Mittelsten Scheid, O.; Becker, C. Transposons: A blessing curse. Curr. Opin. Plant Biol. 2018, 42, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Paszkowski, J. Controlled activation of retrotransposition for plant breeding. Curr. Opin. Biotechnol. 2015, 32, 200–206. [Google Scholar] [CrossRef]
- Carpentier, M.C.; Manfroi, E.; Wei, F.J.; Wu, H.P.; Lasserre, E.; Llauro, C.; Debladis, E.; Akakpo, R.; Hsing, Y.I.; Panaud, O. Retrotranspositional landscape of Asian rice revealed by 3000 genomes. Nat. Commun. 2019, 10, 24. [Google Scholar] [CrossRef]
- Salman-Minkov, A.; Sabath, N.; Mayrose, I. Whole-genome duplication as a key factor in crop domestication. Nat. Plants 2016, 2, 16115. [Google Scholar] [CrossRef]
- Casacuberta, E.; González, J. The impact of transposable elements in environmental adaptation. Mol. Ecol. 2013, 22, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.S.; Vbra, E.S. Exaptation—A missing word in the science of form. Paleobiology 1982, 8, 4–15. [Google Scholar] [CrossRef]
- Agrawal, A.; Eastman, Q.M.; Schatz, D.G. Implications of transposition mediated by V (D) J-recombination proteins RAG1 and RAG2 for origins of antigen-specific immunity. Nature 1998, 394, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, V.V.; Jurka, J. Harbinger transposons and an ancient HARBI1 gene derived from a transposase. DNA Cell Biol. 2004, 23, 311–324. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Jurka, J. RAG1 core and V(D)J recombination signal sequences were derived from Transib transposons. PLoS Biol. 2005, 3, 0998–1011. [Google Scholar] [CrossRef]
- Rawn, S.M.; Cross, J.C. The evolution, regulation, and function of placenta-specific genes. Annu. Rev. Cell Dev. Biol. 2008, 24, 159–181. [Google Scholar] [CrossRef]
- Qiu, Y.; Köhler, C. Mobility connects: Transposable elements wire new transcriptional networks by transferring transcription factor binding motifs. Biochem. Soc. Trans. 2020, 48, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.J.; Hagemannt, S.; Reitert, E.; Pinskertt, W. P-element homologous sequences are tandemly repeated in the genome of Drosophila guanche. Proc. Natl. Acad. Sci. USA 1992, 89, 4018–4022. [Google Scholar] [CrossRef] [PubMed]
- Sinzelle, L.; Izsvák, Z.; Ivics, Z. Molecular domestication of transposable elements: From detrimental parasites to useful host genes. Cell. Mol. Life Sci. 2009, 66, 1073–1093. [Google Scholar] [CrossRef]
- Etchegaray, E.; Naville, M.; Volff, J.N.; Haftek-Terreau, Z. Transposable element-derived sequences in vertebrate development. Mob. DNA 2021, 12, 1–24. [Google Scholar] [CrossRef]
- Hudson, M.E.; Lisch, D.R.; Quail, P.H. The FHY3 and FAR1 genes encode transposase-related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway. Plant J. 2003, 34, 453–471. [Google Scholar] [CrossRef]
- Lin, R.; Ding, L.; Casola, C.; Ripoll, D.R.; Feschotte, C.; Wang, H. Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 2007, 318, 1302–1305. [Google Scholar] [CrossRef]
- Nagy, F.; Kircher, S.; Schäfer, E. Nucleo-cytoplasmic partitioning of the plant photoreceptors phytochromes. Semin. Cell Dev. Biol. 2000, 11, 505–510. [Google Scholar] [CrossRef]
- Allen, T.; Koustenis, A.; Theodorou, G.; Somers, D.E.; Kay, S.A.; Whitelam, G.C.; Devlin, P.F. Arabidopsis FHY3 specifically gates phytochrome signaling to the circadian clock. Plant Cell 2006, 18, 2506–2516. [Google Scholar] [CrossRef]
- Tang, W.; Wang, W.; Chen, D.; Ji, Q.; Jing, Y.; Wang, H.; Lin, R. Transposase-derived proteins FHY3/FAR1 interact with PHYTOCHROME-INTERACTING FACTOR1 to regulate chlorophyll biosynthesis by modulating HEMB1 during deetiolation in Arabidopsis. Plant Cell 2012, 24, 1984–2000. [Google Scholar] [CrossRef]
- Zhao, D.; Ferguson, A.A.; Jiang, N. What makes up plant genomes: The vanishing line between transposable elements and genes. Biochim. Biophys. Acta Gene Regul. Mech. 2016, 1859, 366–380. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, J.; Qin, Q.; Zhang, J.; Chen, Y.; Zhao, L.; He, K.; Hou, S. Type one protein phosphatases (TOPPs) contribute to the plant defense response in Arabidopsis. J. Integr. Plant Biol. 2019, 62, 360–377. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, M.; Li, G.; Yuan, L.; Xie, Y.; Wei, H.; Ma, X.; Li, Q.; Devlin, P.F.; Xu, X.; et al. Transcription factors FHY3 and FAR1 regulate light-induced circadian clock AsSOCIATED1 gene expression in Arabidopsis. Plant Cell 2020, 32, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calvo, P.; Iñigo, S.; Glauser, G.; Vanden Bossche, R.; Tang, M.; Li, B.; De Clercq, R.; Nagels Durand, A.; Eeckhout, D.; Gevaert, K.; et al. FRS7 and FRS12 recruit NINJA to regulate expression of glucosinolate biosynthesis genes. New Phytol. 2020, 227, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, Q.; Zhao, Y.; Li, Q.; Liu, Y.; Ma, M.; Wang, B.; Shen, R.; Zheng, Z.; Wang, H. FHY3 and FAR1 integrate light signals with the miR156-SPL module-mediated aging pathway to regulate Arabidopsis flowering. Mol. Plant 2020, 13, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, H. Arabidopsis FHY3/FAR1 gene family and distinct roles of its members in light control of arabidopsis development. Plant Physiol. 2004, 136, 4010–4022. [Google Scholar] [CrossRef]
- Joly-Lopez, Z.; Hoen, D.R.; Blanchette, M.; Bureau, T.E. Phylogenetic and genomic analyses resolve the origin of important plant genes derived from transposable elements. Mol. Biol. Evol. 2016, 33, 1937–1956. [Google Scholar] [CrossRef][Green Version]
- Zhong, M.C.; Jiang, X.D.; Cui, W.H.; Hu, J.Y. Expansion and expression diversity of FAR1/FRS-like genes provides insights into flowering time regulation in roses. Plant Divers. 2021, 43, 173–179. [Google Scholar] [CrossRef]
- Wang, T.; Ren, L.; Li, C.; Zhang, D.; Zhang, X.; Zhou, G.; Gao, D.; Chen, R.; Chen, Y.; Wang, Z.; et al. The genome of a wild Medicago species provides insights into the tolerant mechanisms of legume forage to environmental stress. BMC Biol. 2021, 19, 96. [Google Scholar] [CrossRef]
- Liu, Z.; An, C.; Zhao, Y.; Xiao, Y.; Bao, L.; Gong, C.; Gao, Y. Genome-wide identification and characterization of the csfhy3/far1 gene family and expression analysis under biotic and abiotic stresses in tea plants (Camellia sinensis). Plants 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Joly-Lopez, Z.; Forczek, E.; Vello, E.; Hoen, D.R.; Tomita, A.; Bureau, T.E. Abiotic stress phenotypes are associated with conserved genes derived from transposable elements. Front. Plant Sci. 2017, 8, 2027. [Google Scholar] [CrossRef] [PubMed]
- Bundock, P.; Hooykaas, P. An Arabidopsis hAT-like transposase is essential for plant development. Nature 2005, 436, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Hiemstra, S.; Sietsma, A.; Castelein, M.; de Pater, S.; Hooykaas, P. DAYSLEEPER: A nuclear and vesicular-localized protein that is expressed in proliferating tissues. BMC Plant Biol. 2013, 13, 211. [Google Scholar] [CrossRef]
- Arensburger, P.; Hice, R.H.; Zhou, L.; Smith, R.C.; Tom, A.C.; Wright, J.A.; Knapp, J.; O’Brochta, D.A.; Craig, N.L.; Atkinson, P.W. Phylogenetic and functional characterization of the hAT transposon superfamily. Genetics 2011, 188, 45–57. [Google Scholar] [CrossRef]
- Knip, M.; de Pater, S.; Hooykaas, P.J. The SLEEPER genes: A transposase-derived angiosperm-specific gene family. BMC Plant Biol. 2012, 12, 192. [Google Scholar] [CrossRef]
- Cowan, R.K.; Hoen, D.R.; Schoen, D.J.; Bureau, T.E. MUSTANG is a novel family of domesticated transposase genes found in diverse angiosperms. Mol. Biol. Evol. 2005, 22, 2084–2089. [Google Scholar] [CrossRef]
- Joly-Lopez, Z.; Forczek, E.; Hoen, D.R.; Juretic, N.; Bureau, T.E. A gene family derived from transposable elements during early angiosperm evolution has reproductive fitness benefits in Arabidopsis thaliana. PLoS Genet. 2012, 8, e1002931. [Google Scholar] [CrossRef]
- Sinzelle, L.; Kapitonov, V.V.; Grzela, D.P.; Jursch, T.; Jurka, J.; Izsvák, Z.; Ivics, Z. Transposition of a reconstructed Harbinger element in human cells and functional homology with two transposon-derived cellular genes. Proc. Natl. Acad. Sci. USA 2007, 105, 4715–4720. [Google Scholar] [CrossRef] [PubMed]
- Casola, C.; Lawing, A.M.; Betrán, E.; Feschotte, C. PIF-like transposons are common in Drosophila and have been repeatedly domesticated to generate new host genes. Mol. Biol. Evol. 2007, 24, 1872–1888. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Guo, Z.; Wang, P.; Sun, X.; Xu, K.; Ma, F. MdHARBI1, a MdATG8i-interacting protein, plays a positive role in plant thermotolerance. Plant Sci. 2021, 306, 110850. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Hartwig, B.; Perera, P.; Mora-García, S.; de Leau, E.; Thornton, H.; de Alves, F.L.; Rapsilber, J.; Yang, S.; James, G.V.; et al. Kicking against the PRCs—A domesticated transposase antagonises silencing mediated by polycomb group proteins and is an accessory component of Polycomb Repressive Complex 2. PLoS Genet. 2015, 11, e1005660. [Google Scholar] [CrossRef] [PubMed]
- Velanis, C.N.; Perera, P.; Thomson, B.; de Leau, E.; Liang, S.C.; Hartwig, B.; Förderer, A.; Thornton, H.; Arede, P.; Chen, J.; et al. The domesticated transposase ALP2 mediates formation of a novel Polycomb protein complex by direct interaction with MSI1, a core subunit of Polycomb Repressive Complex 2 (PRC2). PLoS Genet. 2020, 16, e1008681. [Google Scholar] [CrossRef]
- Duan, C.G.; Wang, X.; Xie, S.; Pan, L.; Miki, D.; Tang, K.; Hsu, C.C.; Lei, M.; Zhong, Y.; Hou, Y.J.; et al. A pair of transposon-derived proteins function in a histone acetyltransferase complex for active DNA demethylation. Cell Res. 2017, 27, 226–240. [Google Scholar] [CrossRef]
- Cordaux, R.; Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef]
- Long, M.; Vankuren, N.W.; Chen, S.; Vibranovski, M.D. New gene evolution: Little did we know. Annu. Rev. Genet. 2013, 47, 307–333. [Google Scholar] [CrossRef]
- Cordaux, R.; Udit, S.; Batzer, M.A.; Feschotte, C. Birth of a chimeric primate gene by capture of the transposase gene from a mobile element. Proc. Natl. Acad. Sci. USA 2006, 103, 8101–8106. [Google Scholar] [CrossRef]
- Shaheen, M.; Williamson, E.; Nickoloff, J.; Lee, S.H.; Hromas, R. Metnase/SETMAR: A domesticated primate transposase that enhances DNA repair, replication, and decatenation. Genetica 2010, 138, 559–566. [Google Scholar] [CrossRef]
- Tellier, M.; Chalmers, R. Human SETMAR is a DNA sequence-specific histone-methylase with a broad effect on the transcriptome. Nucleic Acids Res. 2019, 47, 122–133. [Google Scholar] [CrossRef]
- Antoine-Lorquin, A.; Arensburger, P.; Arnaoty, A.; Asgari, S.; Batailler, M.; Beauclair, L.; Belleannée, C.; Buisine, N.; Coustham, V.; Guyetant, S.; et al. Two repeated motifs enriched within some enhancers and origins of replication are bound by SETMAR isoforms in human colon cells. Genomics 2021, 113, 1589–1604. [Google Scholar] [CrossRef]
- Cosby, R.L.; Judd, J.; Zhang, R.; Zhong, A.; Garry, N.; Pritham, E.J.; Feschotte, C. Recurrent evolution of vertebrate transcription factors by transposase capture. Science 2021, 371, eabc6405. [Google Scholar] [CrossRef]
- Lev-Maor, G.; Ram, O.; Kim, E.; Sela, N.; Goren, A.; Levanon, E.Y.; Ast, G. Intronic Alus influence alternative splicing. PLoS Genet. 2008, 4, e1000204. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Brosius, J. Exonization of transposed elements: A challenge and opportunity for evolution. Biochimie 2011, 93, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Sela, N.; Ast, G. TranspoGene and microTranspoGene: Transposed elements influence on the transcriptome of seven vertebrates and invertebrates. Nucleic Acids Res. 2008, 36, 47–52. [Google Scholar] [CrossRef][Green Version]
- Sela, N.; Mersch, B.; Hotz-Wagenblatt, A.; Ast, G. Characteristics of transposable element exonization within human and mouse. PLoS ONE 2010, 5, e10907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.-Y.; Zeng, L.; Tanaka, K.; Zhang, C.-J.; Ma, J.; Bai, G.; Wang, P.; Zhang, S.-W.; Liu, Z.-W.; et al. DTF1 is a core component of RNA-directed DNA methylation and may assist in the recruitment of Pol IV. Proc. Natl. Acad. Sci. USA 2013, 110, 8290–8295. [Google Scholar] [CrossRef]
- Ng, K.W.; Attig, J.; Young, G.R.; Ottina, E.; Papamichos, S.I.; Kotsianidis, I.; Kassiotis, G. Soluble PD-L1 generated by endogenous retroelement exaptation is a receptor antagonist. Elife 2019, 8, e50256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, J.; Liu, Q.; Liu, X.; Wang, H.; He, J.; Kang, L. Long-read direct RNA sequencing by 5’-Cap capturing reveals the impact of Piwi on the widespread exonization of transposable elements in locusts. RNA Biol. 2019, 16, 950–959. [Google Scholar] [CrossRef]
- Sakai, H.; Tanaka, T.; Itoh, T. Birth and death of genes promoted by transposable elements in Oryza sativa. Gene 2007, 392, 59–63. [Google Scholar] [CrossRef]
- Lockton, S.; Gaut, B.S. The contribution of transposable elements to expressed coding sequence in Arabidopsis thaliana. J. Mol. Evol. 2009, 68, 80–89. [Google Scholar] [CrossRef]
- Xin, Y.; Ma, B.; Xiang, Z.; He, N. Amplification of miniature inverted-repeat transposable elements and the associated impact on gene regulation and alternative splicing in mulberry (Morus notabilis). Mob. DNA 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Joly-Lopez, Z.; Bureau, T.E. Diversity and evolution of transposable elements in Arabidopsis. Chromosom. Res. 2014, 22, 203–216. [Google Scholar] [CrossRef]
- Kazazian, H.H. Mobile elements: Drivers of genome evolution. Science 2004, 303, 1626–1632. [Google Scholar] [CrossRef]
- Elrouby, N.; Bureau, T.E. Bs1, a new chimeric gene formed by retrotransposon-mediated exon shuffling in maize. Plant Physiol. 2010, 153, 1413–1424. [Google Scholar] [CrossRef]
- Witte, C.P.; Le, Q.H.; Bureau, T.; Kumar, A. Terminal-repeat retrotransposons in miniature (TRIM) are involved in restructuring plant genomes. Proc. Natl. Acad. Sci. USA 2001, 98, 13778–13783. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.R.; Doucet, A.J.; Kopera, H.C.; Moldovan, J.B.; Garcia-Perez, J.L.; Moran, J.V. The Influence of LINE-1 and SINE Retrotransposons on Mammalian Genomes. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, H.; Belancio, V.P.; Cordaux, R.; Deininger, P.L.; Batzer, M.A. Emergence of primate genes by retrotransposon-mediated sequence transduction. Proc. Natl. Acad. Sci. USA 2006, 103, 17608–17613. [Google Scholar] [CrossRef] [PubMed]
- Carelli, F.N.; Hayakawa, T.; Go, Y.; Imai, H.; Warnefors, M.; Kaessmann, H. The life history of retrocopies illuminates the evolution of new mammalian genes. Genome Res. 2016, 26, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Vallejo, V.; Nobuta, K.; Slotkin, R.K.; Lisch, D.; Meyers, B.C.; Shiu, S.H.; Jiang, N. The functional role of pack-MULEs in rice inferred from purifying selection and expression profile. Plant Cell 2009, 21, 25–38. [Google Scholar] [CrossRef][Green Version]
- Dhillon, B.; Kema, G.H.J.; Hamelin, R.C.; Bluhm, B.H.; Goodwin, S.B. Variable genome evolution in fungi after transposon-mediated amplification of a housekeeping gene. Mob. DNA 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Catoni, M.; Jonesman, T.; Cerruti, E.; Paszkowski, J. Mobilization of Pack-CACTA transposons in Arabidopsis suggests the mechanism of gene shuffling. Nucleic Acids Res. 2019, 47, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Bao, Z.; Zhang, X.; Eddy, S.R.; Wessler, S.R. Pack-MULE transposable elements mediate gene evolution in plants. Nature 2004, 431, 569–573. [Google Scholar] [CrossRef]
- Muyle, A.; Seymour, D.; Darzentas, N.; Primetis, E.; Gaut, B.S.; Bousios, A. Gene capture by transposable elements leads to epigenetic conflict in maize. Mol. Plant 2021, 14, 237–252. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.S.J.; Franchini, L.F.; Rubinstein, M. Exaptation of transposable elements into novel Cis-regulatory elements: Is the evidence always strong? Mol. Biol. Evol. 2013, 30, 1239–1251. [Google Scholar] [CrossRef]
- Underwood, C.J.; Henderson, I.R.; Martienssen, R.A. Genetic and epigenetic variation of transposable elements in Arabidopsis. Curr. Opin. Plant Biol. 2017, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bourque, G.; Leong, B.; Vega, V.B.; Chen, X.; Yen, L.L.; Srinivasan, K.G.; Chew, J.L.; Ruan, Y.; Wei, C.L.; Huck, H.N.; et al. Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Res. 2008, 18, 1752–1762. [Google Scholar] [CrossRef]
- Kunarso, G.; Chia, N.Y.; Jeyakani, J.; Hwang, C.; Lu, X.; Chan, Y.S.; Ng, H.H.; Bourque, G. Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat. Genet. 2010, 42, 631–634. [Google Scholar] [CrossRef]
- Hollister, J.D.; Smith, L.M.; Guo, Y.L.; Ott, F.; Weigel, D.; Gaut, B.S. Transposable elements and small RNAs contribute to gene expression divergence between Arabidopsis thaliana and Arabidopsis lyrata. Proc. Natl. Acad. Sci. USA 2011, 108, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yeh, C.T.; Ji, T.; Ying, K.; Wu, H.; Tang, H.M.; Fu, Y.; Nettleton, D.; Schnable, P.S. Mu transposon insertion sites and meiotic recombination events co-localize with epigenetic marks for open chromatin across the maize genome. PLoS Genet. 2009, 5, e1000733. [Google Scholar] [CrossRef]
- Naito, K.; Zhang, F.; Tsukiyama, T.; Saito, H.; Hancock, C.N.; Richardson, A.O.; Okumoto, Y.; Tanisaka, T.; Wessler, S.R. Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature 2009, 461, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, B.G.; Gotea, V.; Makałowski, W. Transposable elements as a significant source of transcription regulating signals. Gene 2006, 365, 104–110. [Google Scholar] [CrossRef]
- Rebollo, R.; Romanish, M.T.; Mager, D.L. Transposable elements: An abundant and natural source of regulatory sequences for host genes. Annu. Rev. Genet. 2012, 46, 21–42. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; Fitzhugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, E.M.; Ganko, E.W.; McDonald, J.F. Evolutionary history of Oryza sativa LTR retrotransposons: A preliminary survey of the rice genome sequences. BMC Genom. 2004, 5, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Quadrana, L.; Etcheverry, M.; Gilly, A.; Caillieux, E.; Madoui, M.A.; Guy, J.; Bortolini Silveira, A.; Engelen, S.; Baillet, V.; Wincker, P.; et al. Transposition favors the generation of large effect mutations that may facilitate rapid adaption. Nat. Commun. 2019, 10, 3421. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.N.; Feschotte, C. A field guide to eukaryotic transposable elements. Annu. Rev. Genet. 2020, 54, 539–561. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, M.; McCarty, D.R.; Lisch, D. Transposable elements employ distinct integration strategies with respect to transcriptional landscapes in eukaryotic genomes. Nucleic Acids Res. 2020, 48, 6685–6698. [Google Scholar] [CrossRef]
- Hirsch, C.D.; Springer, N.M. Transposable element influences on gene expression in plants. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef]
- Mao, H.; Wang, H.; Liu, S.; Li, Z.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.P.; Qin, F. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 2015, 6, 8326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Han, X.; Li, J.; Gao, Y.; Richards, C.M.; Zhang, C.; Tian, Y.; Liu, G.; Gul, H.; et al. A high-quality apple genome assembly reveals the association of a retrotransposon and red fruit colour. Nat. Commun. 2019, 10, 149. [Google Scholar] [CrossRef]
- Barco, B.; Kim, Y.; Clay, N.K. Expansion of a core regulon by transposable elements promotes Arabidopsis chemical diversity and pathogen defense. Nat. Commun. 2019, 10, 3444. [Google Scholar] [CrossRef]
- Sundaram, V.; Wysocka, J. Transposable elements as a potent source of diverse cis-regulatory sequences in mammalian genomes. Philos. Trans. R. Soc. B Biol. Sci. 2019, 375, 20190347. [Google Scholar] [CrossRef]
- Lynch, V.J.; Leclerc, R.D.; May, G.; Wagner, G.P. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat. Genet. 2011, 43, 1154–1159. [Google Scholar] [CrossRef]
- Chuong, E.B.; Rumi, M.A.K.; Soares, M.J.; Baker, J.C. Endogenous retroviruses function as species-specific enhancer elements in the placenta Edward. Nat. Genet. 2013, 45, 325–329. [Google Scholar] [CrossRef]
- Bannert, N.; Kurth, R. The evolutionary dynamics of human endogenous retroviral families. Annu. Rev. Genom. Hum. Genet. 2006, 7, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Goudot, C.; Hoyler, T.; Lemoine, B.; Amigorena, S.; Zueva, E. Specific subfamilies of transposable elements contribute to different domains of T lymphocyte enhancers. Proc. Natl. Acad. Sci. USA 2020, 117, 7905–7916. [Google Scholar] [CrossRef] [PubMed]
- Muino, J.M.; De Bruijn, S.; Pajoro, A.; Geuten, K.; Vingron, M.; Angenent, G.C.; Kaufmann, K. Evolution of DNA-binding sites of a floral master regulatory transcription factor. Mol. Biol. Evol. 2016, 33, 185–200. [Google Scholar] [CrossRef]
- Cao, C.; Xu, J.; Zheng, G.; Zhu, X.G. Evidence for the role of transposons in the recruitment of cis-regulatory motifs during the evolution of C4 photosynthesis. BMC Genom. 2016, 17, 201. [Google Scholar] [CrossRef]
- Batista, R.A.; Moreno-Romero, J.; Qiu, Y.; van Boven, J.; Santos-González, J.; Figueiredo, D.D.; Köhler, C. The mads-box transcription factor pheres1 controls imprinting in the endosperm by binding to domesticated transposons. Elife 2019, 8, e50541. [Google Scholar] [CrossRef] [PubMed]
- Fort, V.; Khelifi, G.; Hussein, S.M.I. Long non-coding RNAs and transposable elements: A functional relationship. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118837. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.D.; Manavella, P.A. When junk DNA turns functional: Transposon-derived non-coding RNAs in plants. J. Exp. Bot. 2021, 72, 4132–4143. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Lu, D.; Mason, A.S.; Li, B.; Xiao, M.; An, S.; Fu, D. Non-coding RNAs and transposable elements in plant genomes: Emergence, regulatory mechanisms and roles in plant development and stress responses. Planta 2019, 250, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Maquat, L.E. Short interspersed nuclear element (SINE)-mediated post-transcriptional effects on human and mouse gene expression: SINE-UP for active duty. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190344. [Google Scholar] [CrossRef]
- Zhang, X.-O.; Pratt, H.; Weng, Z. Investigating the potential roles of SINEs in the human genome. Annu. Rev. Genom. Hum. Genet. 2021, 22, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Lavi, E.; Carmel, L. Alu exaptation enriches the human transcriptome by introducing new gene ends. RNA Biol. 2018, 15, 715–725. [Google Scholar] [CrossRef]
- Mateo, L.; Ullastres, A.; González, J. A transposable element insertion confers xenobiotic resistance in Drosophila. PLoS Genet. 2014, 10, e1004560. [Google Scholar] [CrossRef]
- Deforges, J.; Reis, R.S.; Jacquet, P.; Vuarambon, D.J.; Poirier, Y. Prediction of regulatory long intergenic non-coding RNAs acting in trans through base-pairing interactions. BMC Genom. 2019, 20, 601. [Google Scholar] [CrossRef]
- Kapusta, A.; Feschotte, C. Volatile evolution of long noncoding RNA repertoires: Mechanisms and biological implications. Trends Genet. 2014, 30, 439–452. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Koonin, E.V. Functional long non-coding RNAs evolve from junk transcripts. Cell 2020, 183, 1151–1161. [Google Scholar] [CrossRef]
- Nosaka, M.; Itoh, J.I.; Nagato, Y.; Ono, A.; Ishiwata, A.; Sato, Y. Role of transposon-derived small RNAs in the interplay between genomes and parasitic DNA in rice. PLoS Genet. 2012, 8, e1002953. [Google Scholar] [CrossRef]
- McCue, A.D.; Nuthikattu, S.; Slotkin, R.K. Genome-wide identification of genes regulated in trans by transposable element small interfering RNAs. RNA Biol. 2013, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tao, Z.; Hong, H.; Chen, Z.; Wu, C.; Li, X.; Xiao, J.; Wang, S. Transposon-derived small RNA is responsible for modified function of WRKY45 locus. Nat. Plants 2016, 2, 16016. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Xie, K.; Xing, F.; Xiong, F.; Xiao, J.; Li, X.; Xiong, L. Translational repression by a miniature inverted-repeat transposable element in the 3′ untranslated region. Nat. Commun. 2017, 8, 14651. [Google Scholar] [CrossRef] [PubMed]
- Poretti, M.; Praz, C.R.; Meile, L.; Kälin, C.; Schaefer, L.K.; Schläfli, M.; Widrig, V.; Sanchez-Vallet, A.; Wicker, T.; Bourras, S. Domestication of high-copy transposons underlays the wheat small RNA response to an obligate pathogen. Mol. Biol. Evol. 2020, 37, 839–848. [Google Scholar] [CrossRef]
- Nishihara, H. Transposable elements as genetic accelerators of evolution: Contribution to genome size, gene regulatory network rewiring and morphological innovation. Genes Genet. Syst. 2020, 94, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Hoen, D.R.; Bureau, T.E. Discovery of novel genes derived from transposable elements using integrative genomic analysis. Mol. Biol. Evol. 2015, 32, 1487–1506. [Google Scholar] [CrossRef]
- Baud, A.; Wan, M.; Nouaud, D.; Francillonne, N.; Anxolabéhère, D.; Quesneville, H. Traces of transposable elements in genome dark matter co-opted by flowering gene regulation networks. BioRxiv 2021, 547877. [Google Scholar] [CrossRef]
- Ramsay, L.A.; Bourque, G. In silico methods to identify exapted transposable element families. Methods Mol. Biol. 2016, 1400, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Trizzino, M.; Park, Y.; Holsbach-Beltrame, M.; Aracena, K.; Mika, K.; Caliskan, M.; Perry, G.; Lynch, V.; Brown, C. Transposable element exaptation is the primary source of novelty in the primate gene regulatory landscape. Genome Res. 2017, 27, 1623–1633. [Google Scholar] [CrossRef]
- Trizzino, M.; Kapusta, A.; Brown, C.D. Transposable elements generate regulatory novelty in a tissue-specific fashion. BMC Genom. 2018, 19, 468. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 2016, 351, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, D.R.; Swigut, T.; Wysocka, J. Systematic perturbation of retroviral LTRs reveals widespread long-range effects on human gene regulation. Elife 2018, 7, e35989. [Google Scholar] [CrossRef]
- Todd, C.D.; Deniz, Ö.; Taylor, D.; Branco, M.R. Functional evaluation of transposable elements as enhancers in mouse embryonic and trophoblast stem cells. Elife 2019, 8, e44344. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Maehara, K.; Handa, T.; Arimura, Y.; Nogami, J.; Hayashi-Takanaka, Y.; Shirahige, K.; Kurumizaka, H.; Kimura, H.; Ohkawa, Y. A chromatin integration labelling method enables epigenomic profiling with lower input. Nat. Cell Biol. 2019, 21, 287–296. [Google Scholar] [CrossRef]
- Berrens, R.V.; Yang, A.; Laumer, C.E.; Lun, A.T.; Bieberich, F.; Law, C.-T.; Lan, G.; Imaz, M.; Gaffney, D.; Marioni, J.C. Transposable element expression at unique loci in single cells with CELLO-seq. BioRxiv 2020, 10.02.322073. [Google Scholar] [CrossRef]
- Lanciano, S.; Cristofari, G. Measuring and interpreting transposable element expression. Nat. Rev. Genet. 2020, 21, 721–736. [Google Scholar] [CrossRef]
- Schmidt, D.; Schwalie, P.C.; Wilson, M.D.; Ballester, B.; Gonalves, Â.; Kutter, C.; Brown, G.D.; Marshall, A.; Flicek, P.; Odom, D.T. Waves of retrotransposon expansion remodel genome organization and CTCF binding in multiple mammalian lineages. Cell 2012, 148, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.G.; Ouyang, N.; Boyle, A.P. Transposable elements contribute to cell and species-specific chromatin looping and gene regulation in mammalian genomes. Nat. Commun. 2020, 11, 1796. [Google Scholar] [CrossRef] [PubMed]
- Raviram, R.; Rocha, P.P.; Luo, V.M.; Swanzey, E.; Miraldi, E.R.; Chuong, E.B.; Feschotte, C.; Bonneau, R.; Skok, J.A. Analysis of 3D genomic interactions identifies candidate host genes that transposable elements potentially regulate. Genome Biol. 2018, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Picart-Picolo, A.; Grob, S.; Picault, N.; Franek, M.; Llauro, C.; Halter, T.; Maier, T.R.; Jobet, E.; Descombin, J.; Zhang, P.; et al. Large tandem duplications affect gene expression, 3D organization, and plant-pathogen response. Genome Res. 2020, 30, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Panda, K.; Slotkin, R.K. Long-read cDNA sequencing enables a “gene-like” transcript annotation of transposable elements. Plant Cell 2020, 32, 2687–2698. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).