Future Perspectives in Spinal Cord Repair: Brain as Saviour? TSCI with Concurrent TBI: Pathophysiological Interaction and Impact on MSC Treatment
Abstract
1. Introduction
2. Methods
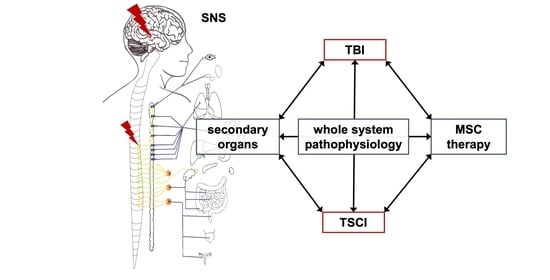
3. Interaction of TSCI and TBI
3.1. A Brief Pathophysiology of Isolated TSCI and TBI
3.2. General Interaction of TSCI and TBI
3.3. Autonomic Dysregulation after TSCI and TBI
3.4. Nociceptive Peptides
3.5. Immune Response and the Blood–Brain/Blood–Spinal Cord Barriers
3.6. Local Perfusion
3.7. Endocrine Dysregulation
3.8. Post-Traumatic Metabolism
3.9. Circadian Rhythm
4. TBI, TSCI and MSCs
4.1. MSC Therapy in Post-Traumatic Neurological Disorders
4.2. The Potential Effect of TBI on MSC-Based TSCI Treatment
- (I)
- Stem cell survival: Following severe TSCI and implantation of stem cells in the acute phase, survival of these cells in TSCI was markedly reduced [84,279]. Regarding concomitant injuries there are currently no data, but further reduced cell survival due to compromised local perfusion and dysregulated systemic and local energy metabolism such as hyperglycaemia is very likely. Concerning secrotomic capacity, it has been shown that inflammatory cytokines inhibit the proangiogenic capacity of the soluble component of the MSC secretome [280], and that the secretomic capacity of BM-MSCs is crucial for the promotion of neuronal survival after TSCI [281].
- (II)
- Cell roaming and differentiation: Interestingly, TSCI and TBI exert differential stimulatory effects on neuronal stem cell niches in the brain, with potential effects on cell recruitment [282]. Regarding MSC chemotaxis to the injured spinal cord, CGRP—which is strongly regulated after TBI—showed a key role in vitro and ex vivo [283]. Antibody blockading of interleukin 6, which is strongly upregulated after concomitant injuries and severe infection in mice with TSCI and MSC treatment, improved MSC survival and locomotor function. The GH/IGF-1 axis, including the parathyroid hormone (PTH) and VitD3, are crucially involved in chondrogenic and osteogenic MSC differentiation as well as MSC-mediated angiogenesis [284,285,286], while local hypoxia enhances MSC proliferation in vitro [287]. As both TSCI and TBI cause heterotopic ossification, positive effects on MSC proliferation as well as negative osteogenic effects on MSC differentiation after severe trauma could be limiting aspects that require further research.
- (III)
- Limiting secondary injury: As severe autonomic dysregulation affects whole-system energy metabolism via distinct effects on gastrointestinal function, glucose and lipid distribution, metabolism, and browning of adipose tissue, direct and indirect effects on MSC treatment in TSCI are very likely. Regarding limiting secondary injury with transplanted MSC, they are directly affected by adrenergic signalling; stimulatory and inhibitory proliferative effects have been described [288,289,290], while increased survival under challenging conditions such as hyperglycaemia and oxidative stress [110,291] were also observed. Overall, the data on SNS impact on MSCs in trauma is limited, while PNS effects on MSCs are little understood at present. Regarding circadian rhythm, melatonin has been shown to be a relevant factor in MSC treatment of TSCI in vivo [292,293]. In MSCs, and derived cell types a significant number of genes show circadian expression, regulating their differentiation and activity [294,295,296,297]. Melatonin preconditioning of these cells could improve their regenerative potential [298,299,300]. As TSCI and TBI both negatively affect circadian rhythm as well as circadian-mediated inflammatory and healing cascades [227,233,301,302], chronotherapeutic aspects in MSC therapy for TSCI should be considered.
- (IV)
- Optimised neural healing: MSC-based therapy was reported to positively affect neural healing in TSCI, specifically through enhanced axonal regeneration and reduced glial scarring via the paracrine effects of secreted cytokines, exosomes, and local mediation of inflammatory response [274]. Specifically, modulation of the local inflammatory micromilieu by an MSC-mediated shift in macrophage polarisation towards M2 [303], as well as an exosome-induced reduction in astrocyte-mediated posttraumatic neurodegradation [304] was observed. As previously stated, TBI induces relevant inflammatory peripheral modulation [94], systemic and peripheral inflammation [305] and disturbed microbiota [89], and has been linked to enhanced bone healing by M2 polarisation in clavicle fractures [306]. In association with TSCI, these effects have not been addressed, although some impact of TBI on MSC-mediated regeneration following TSCI can be expected.
5. Outlook: Brain as Saviour?
| Proposed Advantages | Proposed Limitations | Clinical Trials TSCI (Total/Completed/Published) | Clinical Trials TBI (Total/Completed/Published) | Recent Reviews Cell Therapy And TSCI | Recent Reviews Cell Therapy and TBI | |
|---|---|---|---|---|---|---|
| Cell Therapies | ||||||
| Omnipotent Cells | ||||||
| Embryonal stem cells (ESCs) |
|
| Systematic: [275,328,329] Narrative: [84,330,331,332,333,334] | Narrative: [245,264] | ||
| Induced pluripotent stem-cells (iPSCs) |
|
| ||||
| Multipotent cells & differentiated cells | 73/36/25 | 14/6/3 | ||||
| Cells of (Neuro-) Ectodermal Lineage | 13/9/7 | 0/0/0 | ||||
| Neural stem cells (NSCs) |
|
| 6/5/5 [335,336,337,338] | [339] | ||
| Neural precursor cells (NPCs) |
| 1/0/0 | ||||
| Schwann cells (SCs) |
|
| 2/2/2 [340,341] | |||
| Olfactory ensheathing cells (OECs) |
|
| 2/0/0 | [326] | ||
| oligodendrocyte precursor cell (OPC) | - secretomic activity (e.g., trophic factors)- remyelinisation- local immunomodulation- stimulation of angiogenesis | - ESC or iPSC as source mostly needed- immunosuppression regiments in allogenous strategies | 2/2/0 | |||
| Cells of Mesodermal Lineage | 60/27/18 | 14/6/3 | ||||
| Bone marrow derived cells/aspirate (BMCs) |
|
| 1/1/1 [342] | |||
| Bone marrow derived stem cells (BM-SCs) |
| 6/3/1 [343] | 2/0/0 | [344] | ||
| Bone marrow derived mononuclear cells (BM-MNCs) |
| 7/0/0 2 × withdrawn | 5/4/3 [265,345,346,347] | [348] | [264] | |
| Bone marrow derived mesenchymal stem-cells (BM-MSCs) |
|
| 17/10/9 1 × suspended [271,349,350,351,352,353,354,355,356] | 1/1/0 1 × interim data published [357] | [19,268,272,273,358] | [359,360,361,362] |
| Adipose tissue derived mesenchymal stem cells (AD-MSCs) |
| 14/4/2 [363,364] 1× publication of interim data [365] 4x individual patient expand access | 3/0/0 1 × withdrawn | |||
| Umbilical cord-derived mesenchymal stem cells (UC-MSCs) |
|
| 11/5/2 1×withdrawn [366,367] | 2/0/0 1× withdrawn | ||
| Umbilical cord derived cells (UC-MNCs & UC-MSCs) | 3/2/2 [368] | |||||
| further and undefined MSCs | 3/2/1 [369] | 1/1/0 | ||||
| Macrophages |
|
| 1/0/1 [370] (suspended) | |||
| Sum | 73/36/25 | 14/6/3 | ||||
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekhon, L.H.; Fehlings, M. Epidemiology, Demographics, and Pathophysiology of Acute Spinal Cord Injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-T.; Lin, H.-C.; Lam, C.; Chu, S.-F.; Chiang, Y.-H.; Tsai, S.-H. Review Paper: Epidemiology of Traumatic Spinal Cord Injury: Comparisons Between Developed and Developing Countries. Asia Pac. J. Public Health 2009, 22, 9–18. [Google Scholar] [CrossRef]
- Rogers, W.K.; Todd, M. Acute spinal cord injury. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Frotzler, A.; Cheikh-Sarraf, B.; Pourtehrani, M.; Krebs, J.S.; Lippuner, K. Long-bone fractures in persons with spinal cord injury. Spinal Cord 2015, 53, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Azarhomayoun, A.; Aghasi, M.; Mousavi, N.; Shokraneh, F.; Vaccaro, A.R.; Mirzaian, A.H.; Derakhshan, P.; Rahimi-Movaghar, V. Mortality Rate and Predicting Factors of Traumatic Thoracolumbar Spinal Cord Injury; A Systematic Review and Meta-Analysis. Bull. Emerg. Trauma 2018, 6, 181–194. [Google Scholar] [CrossRef]
- Chu, D.; Lee, Y.-H.; Lin, C.-H.; Chou, P.; Yang, N.-P. Prevalence of associated injuries of spinal trauma and their effect on medical utilization among hospitalized adult subjects—A nationwide data-based study. BMC Health Serv. Res. 2009, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.L.; Witkiewicz, P.M. The therapeutic challenges of dual diagnosis: TBI/SCI. Brain Inj. 2004, 18, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Conti, A.; Clari, M.; Nolan, M.; Wallace, E.; Tommasini, M.; Mozzone, S.; Campagna, S. The Relationship Between Psychological and Physical Secondary Conditions and Family Caregiver Burden in Spinal Cord Injury: A Correlational Study. Top. Spinal Cord Inj. Rehabil. 2019, 25, 271–280. [Google Scholar] [CrossRef]
- Cao, Y.; Krause, J.S. Estimation of indirect costs based on employment and earnings changes after spinal cord injury: An observational study. Spinal Cord 2020, 58, 908–913. [Google Scholar] [CrossRef]
- Lo, J.; Chan, L.; Flynn, S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131. [Google Scholar] [CrossRef]
- Lien, W.-C.; Wang, W.-M.; Wang, F.; Wang, J.-D. Savings of loss-of-life expectancy and lifetime medical costs from prevention of spinal cord injuries: Analysis of nationwide data followed for 17 years. Inj. Prev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Tetreault, L.A.; Kwon, B.K.; Arnold, P.M.; Mroz, T.E.; Shaffrey, C.; Harrop, J.S.; Chapman, J.R.; Casha, S.; Skelly, A.C.; et al. Timing of Decompression in Patients with Acute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7, 95S–115S. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Y.; He, L.; Pang, M.; Luo, C.; Liu, B.; Rong, L. High-dose methylprednisolone for acute traumatic spinal cord injury. Neurol. 2019, 93, e841–e850. [Google Scholar] [CrossRef]
- Joaquim, A.F.; Daniel, J.W.; Schroeder, G.D.; Vaccaro, A.R. Neuroprotective Agents as an Adjuvant Treatment in Patients with Acute Spinal Cord Injuries. Clin. Spine Surg. 2020, 33, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.L.; Looi, Q.H.; Chia, W.C.; Subramaniam, T.; Ng, M.H.; Law, J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, F.; Sallì, M.; Grasso, G. Emerging Therapeutic Strategies for Traumatic Spinal Cord Injury. World Neurosurg. 2020, 140, 591–601. [Google Scholar] [CrossRef]
- Muthu, S.; Jeyaraman, M.; Gulati, A.; Arora, A. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: Systematic review and meta-analysis. Cytotherapy 2021, 23, 186–197. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Chang, P.-Y.; Zhu, Q.-S.; Zhu, Y.-H.; Saijilafu. Decoding epigenetic codes: New frontiers in exploring recovery from spinal cord injury. Neural Regen. Res. 2020, 15, 1613. [Google Scholar] [CrossRef]
- Uyeda, A.; Muramatsu, R. Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review. Int. J. Mol. Sci. 2020, 21, 8116. [Google Scholar] [CrossRef]
- Ziegler, A.; Antes, G.; König, I.; Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Bevorzugte Report Items für systematische Übersichten und Meta-Analysen: Das PRISMA-Statement. DMW - Dtsch. Med. Wochenschr. 2011, 136, e9–e15. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Lambris, J.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Schwartz, M.; Baruch, K. The resolution of neuroinflammation in neurodegeneration: Leukocyte recruitment via the choroid plexus. EMBO J. 2014, 33, 7–22. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Dumont, D.J.; Gradwohl, G.J.; Fong, G.H.; Auerbach, R.; Breitman, M.L. The endothelial-specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene 1993, 8, 1293–1301. [Google Scholar]
- Guha, A.; Tator, C.H. Acute Cardiovascular Effects of Experimental Spinal Cord Injury. J. Trauma Inj. Infect. Crit. Care 1988, 28, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Senter, H.J.; Venes, J.L. Altered blood flow and secondary injury in experimental spinal cord trauma. J. Neurosurg. 1978, 49, 569–578. [Google Scholar] [CrossRef]
- Kwon, B.K. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004, 4, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of Vascular Disruption and Blood–Spinal Cord Barrier Permeability following Traumatic Spinal Cord Injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef]
- Loy, D.N.; Crawford, C.H.; Darnall, J.B.; Burke, D.A.; Onifer, S.M.; Whittemore, S.R. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J. Comp. Neurol. 2002, 445, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Dray, C.; Rougon, G.; Debarbieux, F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc. Natl. Acad. Sci. USA 2009, 106, 9459–9464. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.T.; Marcillo, A.; Bunge, M.B.; Wood, P.M. New Vascular Tissue Rapidly Replaces Neural Parenchyma and Vessels Destroyed by a Contusion Injury to the Rat Spinal Cord. Exp. Neurol. 2002, 173, 63–76. [Google Scholar] [CrossRef]
- Imperato-Kalmar, E.L.; McKinney, R.; Schnell, L.; Rubin, B.P.; Schwab, M.E. Local Changes in Vascular Architecture Following Partial Spinal Cord Lesion in the Rat. Exp. Neurol. 1997, 145, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Göritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. A Pericyte Origin of Spinal Cord Scar Tissue. Science 2011, 333, 238–242. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Alexander, S.W.; Miller, F.D.; A Bisby, M. Response of facial and rubrospinal neurons to axotomy: Changes in mRNA expression for cytoskeletal proteins and GAP-43. J. Neurosci. 1991, 11, 2528–2544. [Google Scholar] [CrossRef]
- Oudega, M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012, 349, 269–288. [Google Scholar] [CrossRef]
- Norenberg, M.D.; Smith, J.; Marcillo, A. The Pathology of Human Spinal Cord Injury: Defining the Problems. J. Neurotrauma 2004, 21, 429–440. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Onifer, S.M.; Smith, G.M.; Fouad, K. Plasticity After Spinal Cord Injury: Relevance to Recovery and Approaches to Facilitate It. Neurotherapeutics 2011, 8, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Falone, A.E.; Frank, E. Sensory axon regeneration: Rebuilding functional connections in the spinal cord. Trends Neurosci. 2012, 35, 156–163. [Google Scholar] [CrossRef]
- Cregg, J.; DePaul, M.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Duhaime, A.-C.; Bullock, R.; Maas, A.I.; Valadka, A.; Manley, G.T. Classification of Traumatic Brain Injury for Targeted Therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.K.; Smith, D.H.; Meaney, D.; Kotapka, M.J.; A Gennarelli, T.; I Graham, D. Neuropathological sequelae of traumatic brain injury: Relationship to neurochemical and biomechanical mechanisms. Lab. Investig. 1996, 74, 315–342. [Google Scholar]
- McGinn, M.J.; Povlishock, J.T. Pathophysiology of Traumatic Brain Injury. Neurosurg. Clin. North Am. 2016, 27, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Hume, J.A.; Graham, D. An Introduction to Neuropathology; Churchill Livingstone: Edinburgh, UK, 1994. [Google Scholar]
- Greve, M.W.; Zink, B.J. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. A J. Transl. Pers. Med. 2009, 76, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Hayes, J.P.; Bigler, E.D.; Verfaellie, M. Traumatic Brain Injury as a Disorder of Brain Connectivity. J. Int. Neuropsychol. Soc. 2016, 22, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, H.; Guo, X.; Pluimer, B.; Zhao, Z. Blood–Brain Barrier Dysfunction in Mild Traumatic Brain Injury: Evidence from Preclinical Murine Models. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Brody, D.L.; Kochanek, P.M.; Levin, H.; McKee, A.; Ribbers, G.M.; Yaffe, K.; Zetterberg, H. Traumatic brain injuries. Nat. Rev. Dis. Prim. 2016, 2, 16084. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, D.W.S.H.B.R.S.B.; Loane, D.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Morganti-Kossmann, M.C.; Rancan, M.; Stahel, P.F.; Kossmann, T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr. Opin. Crit. Care 2002, 8, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; Schwartz, M. CNS sterile injury: Just another wound healing? Trends Mol. Med. 2013, 19, 135–143. [Google Scholar] [CrossRef]
- Putatunda, R.; Bethea, J.R.; Hu, W.-H. Potential immunotherapies for traumatic brain and spinal cord injury. Chin. J. Traumatol. 2018, 21, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Heled, E.; Tal, K.; Zeilig, G. Does lack of brain injury mean lack of cognitive impairment in traumatic spinal cord injury? J. Spinal Cord Med. 2020, 1–8. [Google Scholar] [CrossRef]
- Distel, D.F.; Amodeo, M.; Joshi, S.; Abramoff, B.A. Cognitive Dysfunction in Persons with Chronic Spinal Cord Injuries. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 345–368. [Google Scholar] [CrossRef]
- Algethamy, H. Baseline Predictors of Survival, Neurological Recovery, Cognitive Function, Neuropsychiatric Outcomes, and Return to Work in Patients after a Severe Traumatic Brain Injury: An Updated Review. Mater. Socio Medica 2020, 32, 148–157. [Google Scholar] [CrossRef]
- Varma, A.; Hill, E.G.; Nicholas, J.; Selassie, A. Predictors of Early Mortality After Traumatic Spinal Cord Injury. Spine 2010, 35, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; De Sire, A.; Carda, S.; Venetis, K.; Renò, F.; Cisari, C.; Fusco, N. Bone Muscle Crosstalk in Spinal Cord Injuries: Pathophysiology and Implications for Patients’ Quality of Life. Curr. Osteoporos. Rep. 2020, 18, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Findlay, D.M. Musculoskeletal Health in the Context of Spinal Cord Injury. Curr. Osteoporos. Rep. 2017, 15, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.D.; Shultz, S.R.; McDonald, S.; O’Brien, T. Neurological heterotopic ossification: Current understanding and future directions. Bone 2018, 109, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, N.M.; Kesavan, C.; Mohan, S. Long-term Consequences of Traumatic Brain Injury in Bone Metabolism. Front. Neurol. 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Suto, Y.; Nagata, K.; Ahmed, S.M.; Jacovides, C.L.; Browne, K.D.; Cognetti, J.; Johnson, V.E.; Leone, R.; Kaplan, L.J.; Smith, D.H.; et al. Cerebral Edema and Neurological Recovery after Traumatic Brain Injury Are Worsened if Accompanied by a Concomitant Long Bone Fracture. J. Neurotrauma 2019, 36, 609–618. [Google Scholar] [CrossRef]
- Suto, Y.; Nagata, K.; Ahmed, S.M.; Jacovides, C.; Browne, K.D.; Cognetti, J.; Weber, M.T.; Johnson, V.E.; Leone, R.; Kaplan, L.J.; et al. A concomitant bone fracture delays cognitive recovery from traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Otto, E.; Knapstein, P.-R.; Jahn, D.; Appelt, J.; Frosch, K.-H.; Tsitsilonis, S.; Keller, J. Crosstalk of Brain and Bone—Clinical Observations and Their Molecular Bases. Int. J. Mol. Sci. 2020, 21, 4946. [Google Scholar] [CrossRef] [PubMed]
- Obri, A.; Khrimian, L.; Karsenty, G.; Oury, F. Osteocalcin in the brain: From embryonic development to age-related decline in cognition. Nat. Rev. Endocrinol. 2018, 14, 174–182. [Google Scholar] [CrossRef]
- Hofman, M.; Koopmans, G.; Kobbe, P.; Poeze, M.; Andruszkow, H.; Brink, P.R.G.; Pape, H.-C. Improved Fracture Healing in Patients with Concomitant Traumatic Brain Injury: Proven or Not? Mediat. Inflamm. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Yao, X.; Xiao, L.; Tang, X.; Ding, H.; Zhang, H.; Yuan, J. The effects of spinal cord injury on bone healing in patients with femoral fractures. J. Spinal Cord Med. 2013, 37, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Morioka, K.; Marmor, Y.; Sacramento, J.A.; Lin, A.; Shao, T.; Miclau, K.R.; Clark, D.; Beattie, M.S.; Marcucio, R.S.; Miclau, T.; et al. Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Tsitsilonis, S.; Seemann, R.; Misch, M.; Wichlas, F.; Haas, N.P.; Schmidt-Bleek, K.; Kleber, C.; Schaser, K.-D. The effect of traumatic brain injury on bone healing: An experimental study in a novel in vivo animal model. Injury 2015, 46, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Js, Y.; Hx, Z.; Ding, H.; Lei, W.; Ji-Shan, Y.; Zhang, H.-X.; Hua, D.; Tang, X.-G.; Wei, Y.-Z. Effect of leptin on bone metabolism in rat model of trau- matic brain injury and femoral fracture. Chin. J. Traumatol. 2011, 14, 7–137. [Google Scholar] [CrossRef]
- Suero, M.C.E.M.; Meindl, R.; Schildhauer, T.; Citak, M. Clinical Prediction Rule for Heterotopic Ossification of the Hip in Patients with Spinal Cord Injury. Spine 2018, 43, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Yolcu, Y.U.; Wahood, W.; Goyal, A.; Alvi, M.A.; Reeves, R.K.; Qu, W.; Gerberi, D.J.; Bydon, M. Pharmacologic prophylaxis for heterotopic ossification following spinal cord injury: A systematic review and meta-analysi. Clin. Neurol. Neurosurg. 2020, 193, 105737. [Google Scholar] [CrossRef]
- Wong, K.R.; Mychasiuk, R.; O’Brien, T.J.; Shultz, S.R.; McDonald, S.J.; Brady, R.D. Neurological heterotopic ossification: Novel mechanisms, prognostic biomarkers and prophylactic therapies. Bone Res. 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Lin, A.; Ma, X.; McKenna, S.L.; Creasey, G.H.; Manley, G.T.; Ferguson, A.R.; Bresnahan, J.C.; Beattie, M.S. Combined SCI and TBI: Recovery of forelimb function after unilateral cervical spinal cord injury (SCI) is retarded by contralateral traumatic brain injury (TBI), and ipsilateral TBI balances the effects of SCI on paw placement. Exp. Neurol. 2013, 248, 136–147. [Google Scholar] [CrossRef]
- Zdunczyk, A.; Schwarzer, V.; Mikhailov, M.; Bagley, B.; Rosenstock, T.; Picht, T.; Vajkoczy, P. The Corticospinal Reserve Capacity: Reorganization of Motor Area and Excitability as a Novel Pathophysiological Concept in Cervical Myelopathy. Neurosurgury 2018, 83, 810–818. [Google Scholar] [CrossRef]
- Kan, C.; Chen, L.; Hu, Y.; Lu, H.; Li, Y.; A Kessler, J.; Kan, L. Microenvironmental factors that regulate mesenchymal stem cells: Lessons learned from the study of heterotopic ossification. Histol. Histopathol. 2017, 32, 977–985. [Google Scholar] [CrossRef]
- Kan, C.; Ding, N.; Yang, J.; Tan, Z.; McGuire, T.L.; Lu, H.; Zhang, K.; Berger, D.M.P.; Kessler, J.A.; Kan, L. BMP-dependent, injury-induced stem cell niche as a mechanism of heterotopic ossification. Stem Cell Res. Ther. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Popovich, P.G.; Kopp, M.A.; Schwab, J.M. The neuroanatomical–functional paradox in spinal cord injury. Nat. Rev. Neurol. 2021, 17, 53–62. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; Van Der Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.H. Basics of autonomic nervous system function. In The Frontal Lobes; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 407–418. [Google Scholar]
- Espinosa-Medina, I.; Saha, O.; Boismoreau, F.; Brunet, J.-F. The “sacral parasympathetic”: Ontogeny and anatomy of a myth. Clin. Auton. Res. 2017, 28, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Medina, I.; Saha, O.; Boismoreau, F.; Chettouh, Z.; Rossi, F.; Richardson, W.D.; Brunet, J.-F. The sacral autonomic outflow is sympathetic. Science 2016, 354, 893–897. [Google Scholar] [CrossRef]
- Dupont, G.; Tubbs, R.S. Autonomics of the Abdomen. In Surgical Anatomy of the Lateral Transpsoas Approach to the Lumbar Spine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–183. [Google Scholar]
- Besecker, E.M.; Blanke, E.N.; Holmes, G.M. Altered physiology of gastrointestinal vagal afferents following neurotrauma. Neural Regen. Res. 2021, 16, 254–263. [Google Scholar] [CrossRef]
- Rabchevsky, A.G. Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. In Advances in Vasopressin and Oxytocin from Genes to Behaviour to Disease; Elsevier: Amsterdam, The Netherlands, 2006; Volume 152, pp. 265–274. [Google Scholar]
- Chen, M.; Tang, H.; Shan, J. Anatomic relationship between the cervical sympathetic trunk and cervical fascia and its application in the anterolateral cervical spine surgical approach. Eur. Spine J. 2021, 30, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Chen, C.; Kong, C.; Su, X.; Zhang, X.; Bai, W.; He, X. Acute Traumatic Brain Injury Induces CD4+ and CD8+ T Cell Functional Impairment by Upregulating the Expression of PD-1 via the Activated Sympathetic Nervous System. Neuroimmunomodulation 2019, 26, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Meyfroidt, G.; Baguley, I.J.; Menon, D.K. Paroxysmal sympathetic hyperactivity: The storm after acute brain injury. Lancet Neurol. 2017, 16, 721–729. [Google Scholar] [CrossRef]
- Kox, M.; Pompe, J.C.; Pickkers, P.; Hoedemaekers, C.W.; Van Vugt, A.B.; Van Der Hoeven, J.G. Increased vagal tone accounts for the observed immune paralysis in patients with traumatic brain injury. Neurology 2008, 70, 480–485. [Google Scholar] [CrossRef]
- Wirtz, M.R.; Moekotte, J.; Balvers, K.; Admiraal, M.M.; Pittet, J.-F.; Colombo, J.; Wagener, B.M.; Goslings, J.C.; Juffermans, N. Autonomic nervous system activity and the risk of nosocomial infection in critically ill patients with brain injury. Intensiv. Care Med. Exp. 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Kumaria, A.; Belli, A. The role of vagus nerve overactivity in the increased incidence of pneumonia following traumatic brain injury. Br. J. Neurosurg. 2013, 28, 181–186. [Google Scholar] [CrossRef]
- La Fountaine, M.F. An anatomical and physiological basis for the cardiovascular autonomic nervous system consequences of sport-related brain injury. Int. J. Psychophysiol. 2018, 132, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Lavinio, A.; Ene-Iordache, B.; Nodari, I.; Girardini, A.; Cagnazzi, E.; Rasulo, F.; Smielewski, P.; Czosnyka, M.; Latronico, N. Cerebrovascular reactivity and autonomic drive following traumatic brain injury. Cerebral Hemorrhage 2008, 102, 3–7. [Google Scholar] [CrossRef]
- Andrade, M.J.; Quintas, F.L.; Silva, A.M.; Cruz, P. Is autonomic dysreflexia a cause of respiratory dysfunction after spinal cord injury? Spinal Cord Ser. Cases 2021, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sharif, H.; Wainman, L.; O’Leary, D.; Ditor, D. Cardiac parasympathetic activity and ventricular diastolic interactions in individuals with spinal cord injury. Spinal Cord 2018, 57, 419–426. [Google Scholar] [CrossRef]
- White, A.R.; Holmes, G.M. Investigating neurogenic bowel in experimental spinal cord injury: Where to begin? Neural Regen. Res. 2019, 14, 222–226. [Google Scholar] [CrossRef]
- Prüss, H.; Tedeschi, A.; Thiriot, A.; Lynch, L.; Loughhead, S.M.; Stutte, S.; Mazo, I.B.; A Kopp, M.; Brommer, B.; Blex, C.; et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat. Neurosci. 2017, 20, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.A.; Puskas, F.; Bell, M.T.; Mares, J.M.; Foley, L.S.; Weyant, M.J.; Cleveland, J.C.; Fullerton, D.A.; Meng, X.; Herson, P.S.; et al. Alpha-2 agonist attenuates ischemic injury in spinal cord neurons. J. Surg. Res. 2015, 195, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gok, H.B.; Solaroglu, I.; Okutan, O.; Cimen, B.; Kaptanoglu, E.; Palaoglu, S. Metoprolol treatment decreases tissue myeloperoxidase activity after spinal cord injury in rats. J. Clin. Neurosci. 2007, 14, 138–142. [Google Scholar] [CrossRef]
- Longchamp, A.; Tao, M.; Bartelt, A.; Ding, K.; Lynch, L.; Hine, C.; Corpataux, J.-M.; Kristal, B.S.; Mitchell, J.R.; Ozaki, C.K. Surgical injury induces local and distant adipose tissue browning. Adipocyte 2016, 5, 163–174. [Google Scholar] [CrossRef]
- Sidossis, L.S.; Porter, C.; Saraf, M.K.; Børsheim, E.; Radhakrishnan, R.S.; Chao, T.; Ali, A.; Chondronikola, M.; Mlcak, R.; Finnerty, C.C.; et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015, 22, 219–227. [Google Scholar] [CrossRef]
- Kiefer, F.W. Browning and thermogenic programing of adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 479–485. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, F.J.; Alastrue-Agudo, A.; Erceg, S.; Stojkovic, M.; Moreno-Manzano, V. FM19G11 Favors Spinal Cord Injury Regeneration and Stem Cell Self-Renewal by Mitochondrial Uncoupling and Glucose Metabolism Induction. Stem Cells 2012, 30, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Mattiasson, G.; Shamloo, M.; Gido, G.; Mathi, K.; Tomasevic, G.; Yi, S.; Warden, C.H.; Castilho, R.; Melcher, T.; Gonzalez-Zulueta, M.; et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 2003, 9, 1062–1068. [Google Scholar] [CrossRef]
- Kong, Y.; Cheng, L.; Ma, L.; Li, H.; Cheng, B.; Zhao, Y. Norepinephrine protects against apoptosis of mesenchymal stem cells induced by high glucose. J. Cell. Physiol. 2019, 234, 20801–20815. [Google Scholar] [CrossRef]
- Maytalman, E.; Yegani, A.A.; Kozanoglu, I.; Aksu, F. Adrenergic receptor behaviors of mesenchymal stem cells obtained from different tissue sources and the effect of the receptor blockade on differentiation. J. Recept. Signal Transduct. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chung, A. Calcitonin gene-related peptide (CGRP): Role in peripheral nerve regeneration. Rev. Neurosci. 2017, 29, 369–376. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kawabori, M.; Seki, T.; Takamiya, S.; Tateno, T.; Konno, K.; Watanabe, M.; Houkin, K. FTY720 Attenuates Neuropathic Pain after Spinal Cord Injury by Decreasing Systemic and Local Inflammation in a Rat Spinal Cord Compression Model. J. Neurotrauma 2020, 37, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Song, Y.; Bi, L.; Zhang, Z.; Huang, Z.; Hou, W.; Lu, X.; Sun, P. Increased levels of calcitonin gene-related peptide in serum accelerate fracture healing following traumatic brain injury. Mol. Med. Rep. 2011, 5, 432–438. [Google Scholar] [CrossRef]
- He, X.-Y.; Dan, Q.-Q.; Wang, F.; Li, Y.-K.; Fu, S.-J.; Zhao, N.; Wang, T.-H. Protein Network Analysis of the Serum and Their Functional Implication in Patients Subjected to Traumatic Brain Injury. Front. Neurosci. 2019, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.L.; Mendes, J.; Leitão, R.; Silva, A.P.; Pinto, A.M. A multi-staged neuropeptide response to traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2020, 1–11. [Google Scholar] [CrossRef]
- Kitamura, T.; Harada, N.; Goto, E.; Tanaka, K.; Arai, M.; Shimada, S.; Okajima, K. Activation of sensory neurons contributes to reduce spinal cord injury in rats. Neuropharmacology 2007, 52, 506–514. [Google Scholar] [CrossRef]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71. [Google Scholar]
- Blennow, K.; Hardy, J.; Zetterberg, H. The Neuropathology and Neurobiology of Traumatic Brain Injury. Neuron 2012, 76, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Horner, P.J.; Mullin, B.B.; Stokes, B.T. A Quantitative Spatial Analysis of the Blood–Spinal Cord Barrier: I. Permeability Changes after Experimental Spinal Contusion Injury. Exp. Neurol. 1996, 142, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Dinet, V.; Petry, K.G.; Badaut, J. Brain–Immune Interactions and Neuroinflammation After Traumatic Brain Injury. Front. Neurosci. 2019, 13, 1178. [Google Scholar] [CrossRef]
- Ankeny, D.; Popovich, P. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neurosci. 2009, 158, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Mohapatra, S.; Mohapatra, S.S. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J. Neuroinflammation 2012, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Schnell, L.; Fearn, S.; Klassen, H.; Schwab, M.E.; Perry, V.H. Acute inflammatory responses to mechanical lesions in the CNS: Differences between brain and spinal cord. Eur. J. Neurosci. 1999, 11, 3648–3658. [Google Scholar] [CrossRef]
- Schnell, L.; Fearn, S.; Schwab, M.; Perry, V.; Anthony, D. Cytokine-induced Acute Inflammation in the Brain and Spinal Cord. J. Neuropathol. Exp. Neurol. 1999, 58, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Anthony, D.C.; Couch, Y. The systemic response to CNS injury. Exp. Neurol. 2014, 258, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gensel, J. Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord. Exp. Neurol. 2014, 258, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R.; Kerschensteiner, M.; Meinl, E. Dual role of inflammation in CNS disease. Neurology 2007, 68, S58–S63. [Google Scholar] [CrossRef]
- Balogh, Z.J.; Reumann, M.K.; Gruen, R.L.; Mayer-Kuckuk, P.; A Schuetz, M.; A Harris, I.; Gabbe, B.; Bhandari, M. Advances and future directions for management of trauma patients with musculoskeletal injuries. Lancet 2012, 380, 1109–1119. [Google Scholar] [CrossRef]
- Gruen, R.L.; Brohi, K.; Schreiber, M.; Balogh, Z.J.; Pitt, V.; Narayan, M.; Maier, R.V. Haemorrhage control in severely injured patients. Lancet 2012, 380, 1099–1108. [Google Scholar] [CrossRef]
- McDonald, S.J.; Sun, M.; Agoston, D.V.; Shultz, S.R. The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J. Neuroinflammation 2016, 13, 1–14. [Google Scholar] [CrossRef]
- Stahel, P.F.; Smith, W.R.; Moore, E.E. Role of biological modifiers regulating the immune response after trauma. Injury 2007, 38, 1409–1422. [Google Scholar] [CrossRef]
- Keel, M.; Trentz, O. Pathophysiology of polytrauma. Injury 2005, 36, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Wafaisade, A.; Lefering, R.; Bouillon, B.; Sakka, S.G.; Thamm, O.C.; Paffrath, T.; Neugebauer, E.; Maegele, M. Epidemiology and risk factors of sepsis after multiple trauma: An analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery*. Crit. Care Med. 2011, 39, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Strbian, D.; Durukan, A.; Pitkonen, M.; Marinkovic, I.; Tatlisumak, E.; Pedrono, E.; Abo-Ramadan, U. The blood–brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience 2008, 153, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Zhang, Y.; Kopp, M.; Brommer, B.; Popovich, P.G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 2014, 258, 121–129. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Brommer, B.; Engel, O.; Kopp, M.A.; Watzlawick, R.; Müller, S.; Prüss, H.; Chen, Y.; DeVivo, M.J.; Finkenstaedt, F.W.; Dirnagl, U.; et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain 2016, 139, 692–707. [Google Scholar] [CrossRef] [PubMed]
- Sezer, N. Chronic complications of spinal cord injury. World J. Orthop. 2015, 6, 24–33. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Sharkey, J.M.; Sun, M.; Kaukas, L.M.; Shultz, S.R.; Turner, R.J.; Leonard, A.V.; Brady, R.D.; Corrigan, F. Beyond the Brain: Peripheral Interactions after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, R.; Schlachetzki, J.C.; Glass, C.K.; Mazmanian, S.K. Microbiome–microglia connections via the gut–brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jogia, T.; Ruitenberg, M.J. Traumatic Spinal Cord Injury and the Gut Microbiota: Current Insights and Future Challenges. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Koyanagi, I. Vascular Mechanisms in the Pathophysiology of Human Spinal Cord Injury. Available online: https://pubmed.ncbi.nlm.nih.gov/9046306/ (accessed on 7 February 2021).
- Anjum, A.; Yazid, M.D.; Daud, M.F.; Idris, J.; Ng, A.M.H.; Naicker, A.S.; Ismail, O.H.R.; Kumar, R.K.A.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Obenaus, A.; Ng, M.; Orantes, A.M.; Kinney-Lang, E.; Rashid, F.; Hamer, M.; DeFazio, R.A.; Tang, J.; Zhang, J.H.; Pearce, W. Traumatic brain injury results in acute rarefication of the vascular network. Sci. Rep. 2017, 7, 239. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C. Targeted Perfusion Therapy in Spinal Cord Trauma. Neurotherapy 2020, 17, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Varsos, G.V.; Budohoski, K.P.; Kolias, A.G.; Liu, X.; Smielewski, P.; Varsos, V.G.; Hutchinson, P.J.; Pickard, J.D.; Czosnyka, M. Relationship of Vascular Wall Tension and Autoregulation Following Traumatic Brain Injury. Neurocritical Care 2014, 21, 266–274. [Google Scholar] [CrossRef]
- Hay, J.R.; Johnson, V.E.; Young, A.M.; Smith, D.H.; Stewart, W. Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [CrossRef]
- Woolf, P.D.; Hamill, R.W.; Lee, L.A.; Cox, C.; McDonald, J.V. The predictive value of catecholamines in assessing outcome in traumatic brain injury. J. Neurosurg. 1987, 66, 875–882. [Google Scholar] [CrossRef]
- Altaf, F.; E Griesdale, D.; Belanger, L.; Ritchie, L.; Markez, J.; Ailon, T.; Boyd, M.C.; Paquette, S.; Fisher, C.G.; Street, J.; et al. The differential effects of norepinephrine and dopamine on cerebrospinal fluid pressure and spinal cord perfusion pressure after acute human spinal cord injury. Spinal Cord 2016, 55, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Streijger, F.; So, K.; Manouchehri, N.; Gheorghe, A.; Okon, E.B.; Chan, R.M.; Ng, B.; Shortt, K.; Sekhon, M.S.; Griesdale, D.E.; et al. A Direct Comparison between Norepinephrine and Phenylephrine for Augmenting Spinal Cord Perfusion in a Porcine Model of Spinal Cord Injury. J. Neurotrauma 2018, 35, 1345–1357. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Bieler, L.; Vogl, M. Pathophysiology of Traumatic Spinal Cord Injury. In Neurological Aspects of Spinal Cord Injury; Springer: Berlin/Heidelberg, Germany, 2017; pp. 503–528. [Google Scholar]
- Weinberg, J.A.; Farber, S.H.; Kalamchi, L.D.; Brigeman, S.T.; Bohl, M.A.; Varda, B.M.; Sioda, N.A.; Radosevich, J.J.; Chapple, K.M.; Snyder, L.A. Mean arterial pressure maintenance following spinal cord injury: Does meeting the target matter? J. Trauma Acute Care Surg. 2021, 90, 97–106. [Google Scholar] [CrossRef]
- Saadeh, Y.S.; Smith, B.W.; Joseph, J.R.; Jaffer, S.Y.; Buckingham, M.J.; Oppenlander, M.E.; Szerlip, N.J.; Park, P. The impact of blood pressure management after spinal cord injury: A systematic review of the literature. Neurosurg. Focus 2017, 43, E20. [Google Scholar] [CrossRef] [PubMed]
- Grocott, M.P.W. Human physiology in extreme environments: Lessons from life at the limits? Postgrad. Med. J. 2008, 84, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.M.; Thiele, I. Applying systems biology methods to the study of human physiology in extreme environments. Extreme Physiol. Med. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Shaughness, M.; Acs, D.; Brabazon, F.; Hockenbury, N.; Byrnes, K.R. Role of Insulin in Neurotrauma and Neurodegeneration: A Review. Front. Neurosci. 2020, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ntali, G.; Tsagarakis, S. Pituitary dysfunction after traumatic brain injury: Prevalence and screening strategies. Expert Rev. Endocrinol. Metab. 2020, 15, 341–354. [Google Scholar] [CrossRef]
- Yuen, K.C.J.; E Masel, B.; Reifschneider, K.L.; Sheffield-Moore, M.; Urban, R.J.; Pyles, R.B. Alterations of the GH/IGF-I Axis and Gut Microbiome after Traumatic Brain Injury: A New Clinical Syndrome? J. Clin. Endocrinol. Metab. 2020, 105, e3054–e3064. [Google Scholar] [CrossRef]
- Dick, M.; Catford, S.R.; Kumareswaran, K.; Hamblin, P.; Topliss, D.J. Persistent syndrome of inappropriate antidiuretic hormone secretion following traumatic brain injury. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 150070. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, C.E.; Condon, A.M.; Ridgway, S.W.; Krishna, G.; Garcia-Filion, P.C.; Adelson, P.D.; Rowe, R.K.; Thomas, T.C. Sex-Dependent Pathology in the HPA Axis at a Sub-acute Period After Experimental Traumatic Brain Injury. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.H.; Wu, H.Y.; He, R.H.; Zheng, B.E.; Fan, J.Z. Sex Differences in Sex Hormone Profiles and Prediction of Consciousness Recovery After Severe Traumatic Brain Injury. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Dirlikov, B.; Lavoie, S.; Shem, K. Correlation between thyroid function, testosterone levels, and depressive symptoms in females with spinal cord injury. Spinal Cord Ser. Cases 2019, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kgosidialwa, O.; Agha, A. Hypopituitarism post traumatic brain injury (TBI): Review. Ir. J. Med. Sci. 2019, 188, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Garbe, A.; Graef, F.; Appelt, J.; Schmidt-Bleek, K.; Jahn, D.; Lünnemann, T.; Tsitsilonis, S.; Seemann, R. Leptin Mediated Pathways Stabilize Posttraumatic Insulin and Osteocalcin Patterns after Long Bone Fracture and Concomitant Traumatic Brain Injury and Thus Influence Fracture Healing in a Combined Murine Trauma Model. Int. J. Mol. Sci. 2020, 21, 9144. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-J.; Zhou, J.; Guo, M.; Yang, C.-S.; Xu, Q.-C.; Lv, Q.-W.; Yang, S.-B.; Huang, H.-B. Serum lipocalin-2 concentrations and mortality of severe traumatic brain injury. Clin. Chim. Acta 2017, 474, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.H.; Black, R.T.; Lee, N.N.; Doperalski, A.E.; Reeves, T.M.; Phillips, L.L. Time-dependent hemeoxygenase-1, lipocalin-2 and ferritin induction after non-contusion traumatic brain injury. Brain Res. 2019, 1725, 146466. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.I.; Barrett, J.P.; Stoica, B.A.; Henry, R.J. Bidirectional Brain-Systemic Interactions and Outcomes After TBI. Trends Neurosci. 2021, 44, 406–418. [Google Scholar] [CrossRef]
- Li, M.; Sirko, S. Traumatic Brain Injury: At the Crossroads of Neuropathology and Common Metabolic Endocrinopathies. J. Clin. Med. 2018, 7, 59. [Google Scholar] [CrossRef]
- McMillan, D.W.; Nash, M.S.; Gater, J.D.R.; Valderrábano, R.J. Neurogenic Obesity and Skeletal Pathology in Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2021, 27, 57–67. [Google Scholar] [CrossRef]
- Anderson, R.; Moses, R.; Lenherr, S.; Hotaling, J.M.; Myers, J. Spinal cord injury and male infertility—a review of current literature, knowledge gaps, and future research. Transl. Androl. Urol. 2018, 7, S373–S382. [Google Scholar] [CrossRef]
- Cruse, J.M.; Keith, J.C.; Bryant, M.L.; Lewis, R.E. Immune system-neuroendocrine dysregulation in spinal cord injury. Immunol. Res. 1996, 15, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; Sanders, V.M.; Popovich, P.G. Stress hormones collaborate to induce lymphocyte apoptosis after high level spinal cord injury. J. Neurochem. 2009, 110, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, D.I.; Bartlett, J.A.; Chatterton, R.; Keller, S.E. ADRENAL AND PITUITARY HORMONE PATTERNS AFTER SPINAL CORD INJURY. Am. J. Phys. Med. Rehabil. 1999, 78, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, J.; Mailhot, G. Vitamin D and spinal cord injury: Should we care? Spinal Cord 2016, 54, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Bigford, G.E.; Bracchi-Ricard, V.C.; Nash, M.S.; Bethea, J.R. Alterations in Mouse Hypothalamic Adipokine Gene Expression and Leptin Signaling following Chronic Spinal Cord Injury and with Advanced Age. PLoS ONE 2012, 7, e41073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaculik, J.; Wenchich, L.; Bobelyak, M.; Pavelka, K.; Stepan, J.J. Decrease in serum calcitriol (but not free 25-hydroxyvitamin D) concentration in hip fracture healing. J. Endocrinol. Investig. 2021, 1–9. [Google Scholar] [CrossRef]
- Ettehad, H.; Mirbolook, A.; Mohammadi, F.; Mousavi, M.; Ebrahimi, H.; Shirangi, A. Changes in the Serum Level of Vitamin D During Healing of Tibial and Femoral Shaft Fractures. Trauma Mon. 2014, 19, e10946. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, A.; Eckardt, K.-U.; Osswald, P.M.; Kruse, C. Perioperative plasma erythropoietin levels in hip arthroplasty. Ann. Hematol. 1994, 68, 117–124. [Google Scholar] [CrossRef]
- Tan, C.C.; Eckardt, K.U.; Firth, J.D.; Ratcliffe, P.J. Feedback modulation of renal and hepatic erythropoietin mRNA in response to graded anemia and hypoxia. Am. J. Physiol. Physiol. 1992, 263, F474–F481. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, W.-X.; Huang, J.-F.; Zheng, X.-Q.; Tian, H.-J.; Wang, B.; Fu, W.-L.; Wu, A.-M. Endocrine Therapy for the Functional Recovery of Spinal Cord Injury. Front. Neurosci. 2020, 14, 1324. [Google Scholar] [CrossRef]
- Bighinati, A.; Focarete, M.L.; Gualandi, C.; Pannella, M.; Giuliani, A.; Beggiato, S.; Ferraro, L.; Lorenzini, L.; Giardino, L.; Calzà, L. Improved Functional Recovery in Rat Spinal Cord Injury Induced by a Drug Combination Administered with an Implantable Polymeric Delivery System. J. Neurotrauma 2020, 37, 1708–1719. [Google Scholar] [CrossRef]
- Shultz, R.B.; Wang, Z.; Nong, J.; Zhang, Z.; Zhong, Y. Local delivery of thyroid hormone enhances oligodendrogenesis and myelination after spinal cord injury. J. Neural Eng. 2017, 14, 036014. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rojas, T.; Sastre-Oliva, T.; Esclarín-Ruz, A.; Gil-Dones, F.; Mourino-Alvarez, L.; Corbacho-Alonso, N.; Moreno-Luna, R.; Hernandez-Fernandez, G.; Lopez, J.A.; Oliviero, A.; et al. Effects of Growth Hormone Treatment and Rehabilitation in Incomplete Chronic Traumatic Spinal Cord Injury: Insight from Proteome Analysis. J. Pers. Med. 2020, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Mureşanu, D.F.; Sharma, A.; Lafuente, J.-V.; Patnaik, R.; Tian, Z.R.; Nyberg, F.; Sharma, H.S. Nanowired Delivery of Growth Hormone Attenuates Pathophysiology of Spinal Cord Injury and Enhances Insulin-Like Growth Factor-1 Concentration in the Plasma and the Spinal Cord. Mol. Neurobiol. 2015, 52, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Gorio, A.; Gökmen, N.; Erbayraktar, S.; Yilmaz, O.; Madaschi, L.; Cichetti, C.; Di Giulio, A.M.; Vardar, E.; Cerami, A.; Brines, M. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc. Natl. Acad. Sci. USA 2002, 99, 9450–9455. [Google Scholar] [CrossRef]
- Calderón-Vallejo, D.; Quintanar-Stephano, A.; Hernández-Jasso, I.; Jiménez-Hernández, V.; Ruiz-Ornelas, J.; Jiménez, I.; Quintanar, J.L. Functional and Structural Recovery of the Injured Spinal Cord in Rats Treated with Gonadotropin-Releasing Hormone. Neurochem. Res. 2015, 40, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. Critical illness and flat batteries. Crit. Care 2017, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Rogobete, A.F.; Grintescu, I.M.; Bratu, T.; Bedreag, O.H.; Papurica, M.; Crainiceanu, Z.P.; Popovici, S.E.; Sandesc, D. Assessment of Metabolic and Nutritional Imbalance in Mechanically Ventilated Multiple Trauma Patients: From Molecular to Clinical Outcomes. Diagnostics 2019, 9, 171. [Google Scholar] [CrossRef]
- Simsek, T.; Simsek, H.U.; Cantürk, N.Z. Response to trauma and metabolic changes: Posttraumatic metabolism. Turk. J. Surg. 2014, 30, 153–159. [Google Scholar] [CrossRef]
- Bosarge, P.L.; Kerby, J.D. Stress-induced Hyperglycemia. Adv. Surg. 2013, 47, 287–297. [Google Scholar] [CrossRef]
- Krakau, K.; Omne-Pontén, M.; Karlsson, T.; Borg, J. Metabolism and nutrition in patients with moderate and severe traumatic brain injury: A systematic review. Brain Inj. 2006, 20, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.L.; Matsumoto, J.H. The collective therapeutic potential of cerebral ketone metabolism in traumatic brain injury. J. Lipid Res. 2014, 55, 2450–2457. [Google Scholar] [CrossRef]
- Giza, C.C.; Hovda, D.A. The New Neurometabolic Cascade of Concussion. Neurosurgery 2014, 75, S24–S33. [Google Scholar] [CrossRef]
- Xu, X.-J.; Yang, M.-S.; Zhang, B.; Niu, F.; Dong, J.-Q.; Liu, B.-Y. Glucose metabolism: A link between traumatic brain injury and Alzheimer’s disease. Chin. J. Traumatol. 2021, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Gajavelli, S.; Sinha, V.K.; Mazzeo, A.T.; Spurlock, M.S.; Lee, S.W.; Ahmed, A.I.; Yokobori, S.; Bullock, R.M. Evidence to support mitochondrial neuroprotection, in severe traumatic brain injury. J. Bioenerg. Biomembr. 2014, 47, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The Neurometabolic Cascade of Concussion. J. Athl. Train. 2001, 36, 228–235. [Google Scholar] [CrossRef]
- Gajavelli, S.; Kentaro, S.; Diaz, J.; Yokobori, S.; Spurlock, M.; Diaz, D.; Jackson, C.; Wick, A.; Zhao, W.; Leung, L.Y.; et al. Glucose and Oxygen Metabolism after Penetrating Ballistic-Like Brain Injury. Br. J. Pharmacol. 2015, 35, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Spungen, A.M. Metabolic Changes in Persons After Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2000, 11, 109–140. [Google Scholar] [CrossRef]
- A Bauman, W.; Wecht, J.M.; Biering-Sørensen, F. International spinal cord injury endocrine and metabolic extended data set. Spinal Cord 2017, 55, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Spungen, A.M. Invited Review Carbohydrate and Lipid Metabolism In Chronic Spinal Cord Injury. J. Spinal Cord Med. 2001, 24, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Dolbow, D.R.; Dolbow, J.D.; Khalil, R.K.; Castillo, C.; Gater, D.R. Effects of spinal cord injury on body composition and metabolic profile – Part I. J. Spinal Cord Med. 2014, 37, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.W.; Dayton, A.; Walsh, J.; Rutkowski, S.B.; Leong, G.; Duong, S. Life expectancy after spinal cord injury: A 50-year study. Spinal Cord 2012, 50, 803–811. [Google Scholar] [CrossRef]
- Amorini, A.M.; Lazzarino, G.; Di Pietro, V.; Signoretti, S.; Lazzarino, G.; Belli, A.; Tavazzi, B. Metabolic, enzymatic and gene involvement in cerebral glucose dysmetabolism after traumatic brain injury. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.E.; Scafidi, J.; Scafidi, S. Metabolic perturbations after pediatric TBI: It’s not just about glucose. Exp. Neurol. 2019, 316, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Nessel, I.; Michael-Titus, A.T. Lipid profiling of brain tissue and blood after traumatic brain injury. Semin. Cell Dev. Biol. 2021, 112, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Mannino, C.; Glenn, T.; Hovda, D.A.; Vespa, P.M.; McArthur, D.L.; Van Horn, J.D.; Wright, M.J. Acute glucose and lactate metabolism are associated with cognitive recovery following traumatic brain injury. J. Neurosci. Res. 2018, 96, 696–701. [Google Scholar] [CrossRef]
- Kobayakawa, K.; Kumamaru, H.; Saiwai, H.; Kubota, K.; Ohkawa, Y.; Kishimoto, J.; Yokota, K.; Ideta, R.; Shiba, K.; Tozaki-Saitoh, H.; et al. Acute hyperglycemia impairs functional improvement after spinal cord injury in mice and humans. Sci. Transl. Med. 2014, 6, 256ra137. [Google Scholar] [CrossRef]
- Park, K.-S.; Kim, J.B.; Keung, M.; Seo, Y.J.; Seo, S.Y.; Mun, S.A.; Lee, Y.-S.; Cho, D.-C.; Hwang, J.-H.; Han, I.; et al. Chronic Hyperglycemia before Spinal Cord Injury Increases Inflammatory Reaction and Astrogliosis after Injury: Human and Rat Studies. J. Neurotrauma 2020, 37, 1165–1181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, H.; Lu, Z.; Sun, K.; Jin, Q. Hyperglycemia aggravates spinal cord injury through endoplasmic reticulum stress mediated neuronal apoptosis, gliosis and activation. Biomed. Pharmacother. 2019, 112, 108672. [Google Scholar] [CrossRef]
- Mowery, N.T.; Gunter, O.L.; Guillamondegui, O.; Dossett, L.A.; Dortch, M.J.; Morris, J.A.; May, A.K. Stress Insulin Resistance is a Marker for Mortality in Traumatic Brain Injury. J. Trauma Inj. Infect. Crit. Care 2009, 66, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Moro, N.; Ghavim, S.; Harris, N.G.; Hovda, D.A.; Sutton, R.L. Glucose administration after traumatic brain injury improves cerebral metabolism and reduces secondary neuronal injury. Brain Res. 2013, 1535, 124–136. [Google Scholar] [CrossRef]
- Kurtz, P.; Rocha, E.E.M. Nutrition Therapy, Glucose Control, and Brain Metabolism in Traumatic Brain Injury: A Multimodal Monitoring Approach. Front. Neurosci. 2020, 14, 190. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Song, C.K.; Demas, G.E. SCN Efferents to Peripheral Tissues: Implications for Biological Rhythms. J. Biol. Rhythm. 2001, 16, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Palm, I.F.; La Fleur, S.E.; Scheer, F.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R.M. SCN Outputs and the Hypothalamic Balance of Life. J. Biol. Rhythm. 2006, 21, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Castriotta, R.; Wilde, M.C.; Lai, J.M.; Atanasov, S.; Masel, B.E.; Kuna, S.T. Prevalence and Consequences of Sleep Disorders in Traumatic Brain Injury. J. Clin. Sleep Med. 2007, 3, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Barshikar, S.; Bell, K. Sleep Disturbance After TBI. Curr. Neurol. Neurosci. Rep. 2017, 17, 87. [Google Scholar] [CrossRef]
- Zhanfeng, N.; Hechun, X.; Zhijun, Z.; Hongyu, X.; Zhou, F. Regulation of Circadian Clock Genes on Sleep Disorders in Traumatic Brain Injury Patients. World Neurosurg. 2019, 130, e475–e486. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Ayas, N.T.; Shea, S.A.; Brown, R.; Czeisler, C.A. Absence of Detectable Melatonin and Preservation of Cortisol and Thyrotropin Rhythms in Tetraplegia. J. Clin. Endocrinol. Metab. 2000, 85, 2189–2196. [Google Scholar] [CrossRef]
- Hultén, V.D.T.; Biering-Sørensen, F.; Jørgensen, N.R.; Jennum, P.J. Melatonin and cortisol in individuals with spinal cord injury. Sleep Med. 2018, 51, 92–98. [Google Scholar] [CrossRef]
- Baschieri, F.; Guaraldi, P.; Provini, F.; Chiogna, M.; Barletta, G.; Cecere, A.; De Scisciolo, G.; Cortelli, P.; Calandra-Buonaura, G. Circadian and state-dependent core body temperature in people with spinal cord injury. Spinal Cord 2021, 59, 538–546. [Google Scholar] [CrossRef]
- Kostovski, E.; Frigato, E.; Savikj, M.; Dahm, A.; Sandset, P.M.; Mowinckel, M.-C.; Skretting, G.; Østerud, B.; Bertolucci, C.; Iversen, P.O. Normalization of disrupted clock gene expression in males with tetraplegia: A crossover randomized placebo-controlled trial of melatonin supplementation. Spinal Cord 2018, 56, 1076–1083. [Google Scholar] [CrossRef]
- Yamakawa, G.; Brady, R.; Sun, M.; McDonald, S.; Shultz, S.; Mychasiuk, R. The interaction of the circadian and immune system: Desynchrony as a pathological outcome to traumatic brain injury. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100058. [Google Scholar] [CrossRef]
- Slomnicki, L.P.; Myers, S.A.; Ohri, S.S.; Parsh, M.V.; Andres, K.R.; Chariker, J.H.; Rouchka, E.C.; Whittemore, S.R.; Hetman, M. Improved locomotor recovery after contusive spinal cord injury in Bmal1−/− mice is associated with protection of the blood spinal cord barrier. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Killgore, W.D.; Vanuk, J.R.; Shane, B.R.; Weber, M.; Bajaj, S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol. Dis. 2020, 134, 104679. [Google Scholar] [CrossRef] [PubMed]
- Salva, M.-A.Q.; Azabou, E.; Hartley, S.; Sauvagnac, R.; Leotard, A.; Vaugier, I.; Diehl, P.P.; Vallat-Azouvi, C.; Barbot, F.; Azouvi, P. Blue-Enriched White Light Therapy Reduces Fatigue in Survivors of Severe Traumatic Brain Injury: A Randomized Controlled Trial. J. Head Trauma Rehabil. 2020, 35, E78–E85. [Google Scholar] [CrossRef]
- Grima, N.A.; Ponsford, J.L.; Hilaire, M.A.S.; Mansfield, D.; Rajaratnam, S.M. Circadian Melatonin Rhythm Following Traumatic Brain Injury. Neurorehabilit. Neural Repair 2016, 30, 972–977. [Google Scholar] [CrossRef]
- Shekleton, J.A.; Parcell, D.L.; Redman, J.R.; Phipps-Nelson, J.; Ponsford, J.L.; Rajaratnam, S.M.W. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology 2010, 74, 1732–1738. [Google Scholar] [CrossRef]
- Sankari, A.; Badr, M.S.; Martin, J.L.; Ayas, N.T.; Berlowitz, D.J. Impact of Spinal Cord Injury on Sleep: Current Perspectives. Nat. Sci. Sleep 2019, 11, 219–229. [Google Scholar] [CrossRef]
- Barlow, K.; Esser, M.M.J.; Veidt, M.; Boyd, R. Melatonin as a Treatment after Traumatic Brain Injury: A Systematic Review and Meta-Analysis of the Pre-Clinical and Clinical Literature. J. Neurotrauma 2019, 36, 523–537. [Google Scholar] [CrossRef]
- Grima, N.A.; Rajaratnam, S.M.W.; Mansfield, D.; Sletten, T.L.; Spitz, G.; Ponsford, J.L. Efficacy of melatonin for sleep disturbance following traumatic brain injury: A randomised controlled trial. BMC Med. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Wislet-Gendebien, S.; Laudet, E.; Neirinckx, V.; Rogister, B. Adult Bone Marrow: Which Stem Cells for Cellular Therapy Protocols in Neurodegenerative Disorders? J. Biomed. Biotechnol. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Bejargafshe, M.J.; Hedayati, M.; Zahabiasli, S.; Tahmasbpour, E.; Rahmanzadeh, S.; Nejad-Moghaddam, A. Safety and efficacy of stem cell therapy for treatment of neural damage in patients with multiple sclerosis. Stem Cell Investig. 2019, 6, 44. [Google Scholar] [CrossRef]
- Staff, N.P.; Jones, D.T.; Singer, W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin. Proc. 2019, 94, 892–905. [Google Scholar] [CrossRef]
- Genc, B.; Bozan, H.R.; Genc, S.; Genc, K. Stem Cell Therapy for Multiple Sclerosis. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1084, pp. 145–174. [Google Scholar]
- Kwak, K.-A.; Lee, S.-P.; Yang, J.-Y.; Park, Y.-S. Current Perspectives regarding Stem Cell-Based Therapy for Alzheimer’s Disease. Stem Cells Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Dabrowski, A.; Robinson, T.J.; Felling, R.J. Promoting Brain Repair and Regeneration After Stroke: A Plea for Cell-Based Therapies. Curr. Neurol. Neurosci. Rep. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Leak, R.; Chen, F.; Cao, G. Stem cell therapies in age-related neurodegenerative diseases and stroke. Ageing Res. Rev. 2017, 34, 39–50. [Google Scholar] [CrossRef]
- Zibara, K.; Ballout, N.; Mondello, S.; Karnib, N.; Ramadan, N.; Omais, S.; Nabbouh, A.; Caliz, D.; Clavijo, A.; Hu, Z.; et al. Combination of drug and stem cells neurotherapy: Potential interventions in neurotrauma and traumatic brain injury. Neuropharmacology 2019, 145, 177–198. [Google Scholar] [CrossRef]
- Cox, S.C., Jr.; Juranek, J.; Bedi, S. Clinical trials in traumatic brain injury: Cellular therapy and outcome measures. Transfusion 2019, 59, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, C.; Zurita, M. Cell-Based Therapies for Traumatic Brain Injury: Therapeutic Treatments and Clinical Trials. Biomedicine 2021, 9, 669. [Google Scholar] [CrossRef]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021, 30, 096368972198926. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal Stem Cells for Neurological Disorders. Adv. Sci. 2021, 8, 2002944. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Guan, L.-X.; Zhang, K.; Zhang, Q.; Dai, L.-J. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy 2008, 10, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Gajavelli, S.; Spurlock, M.S.; O Chieng, L.; Bullock, M.R. Stem cells for therapy in TBI. J. R. Army Med. Corps 2015, 162, 98–102. [Google Scholar] [CrossRef]
- Ramalho, B.D.S.; Pestana, F.M.; Prins, C.A.; Cardoso, F.S.D.S.; Cavalcante, D.R.; De Souza, S.A.L.; Gutfilen, B.; De Almeida, F.M.; Martinez, A.M.B. Effects of Different Doses of Mesenchymal Stem Cells on Functional Recovery After Compressive Spinal-Cord Injury in Mice. Neuroscience 2018, 400, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? STEM CELLS Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef]
- Pool, M.; Leuvenink, H.; Moers, C. Reparative and Regenerative Effects of Mesenchymal Stromal Cells-Promising Potential for Kidney Transplantation? Int. J. Mol. Sci. 2019, 20, 4614. [Google Scholar] [CrossRef]
- Klingemann, H.; Matzilevich, D.; Marchand, J. Mesenchymal Stem Cells – Sources and Clinical Applications. Transfus. Med. Hemotherapy 2008, 35, 2. [Google Scholar] [CrossRef]
- Sadan, O.; Melamed, E.; Offen, D. Bone-marrow-derived mesenchymal stem cell therapy for neurodegenerative diseases. Expert Opin. Biol. Ther. 2009, 9, 1487–1497. [Google Scholar] [CrossRef]
- Volkman, R.; Offen, D. Concise Review: Mesenchymal Stem Cells in Neurodegenerative Diseases. Stem Cells 2017, 35, 1867–1880. [Google Scholar] [CrossRef]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Vasic, V.; Barth, K.; Schmidt, M.H. Neurodegeneration and Neuro-Regeneration—Alzheimer’s Disease and Stem Cell Therapy. Int. J. Mol. Sci. 2019, 20, 4272. [Google Scholar] [CrossRef]
- Massoto, T.B.; Santos, A.C.R.; Ramalho, B.S.; Almeida, F.M.; Martinez, A.M.B.; Marques, S.A. Mesenchymal stem cells and treadmill training enhance function and promote tissue preservation after spinal cord injury. Brain Res. 2020, 1726, 146494. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, F.M.; Marques, S.A.; Ramalho, B.D.S.; Massoto, T.B.; Martinez, A.M.B. Chronic spinal cord lesions respond positively to tranplants of mesenchymal stem cells. Restor. Neurol. Neurosci. 2015, 33, 43–55. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.; Ortiz-Gonzalez, X.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nat. Cell Biol. 2002, 418, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Na Kim, H.; Park, H.-J.; Shin, J.Y.; Lee, P.H. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer’s Disease Model. Cell Transplant. 2015, 24, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Wang, X.; Wang, X.; Wang, L.; Wang, X.; Wu, S.; Wan, Z. Autologous Bone Marrow Mesenchymal Stem Cell Therapy in the Subacute Stage of Traumatic Brain Injury by Lumbar Puncture. Exp. Clin. Transplant. 2013, 11, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Gennai, S.; Monsel, A.; Hao, Q.; Liu, J.; Gudapati, V.; Barbier, E.; Lee, J. Cell-Based therapy for traumatic brain injury. Br. J. Anaesth. 2015, 115, 203–212. [Google Scholar] [CrossRef]
- Cox, C.S. Cellular therapy for traumatic neurological injury. Pediatr. Res. 2017, 83, 325–332. [Google Scholar] [CrossRef]
- Cox, S.C.; Hetz, R.A.; Liao, G.P.; Aertker, B.M.; Ewing-Cobbs, L.; Juranek, J.; Savitz, S.I.; Jackson, M.L.; Romanowska-Pawliczek, A.M.; Triolo, F.; et al. Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells. Stem Cells 2017, 35, 1065–1079. [Google Scholar] [CrossRef]
- Nezhad, R.H.B.; Asadi, F.; Froushani, S.M.A.; Hassanshahi, G.; Kaeidi, A.; Falahati-Pour, S.K.; Hashemi, Z.; Mirzaei, M.R. The effects of transplanted mesenchymal stem cells treated with 17-b estradiol on experimental autoimmune encephalomyelitis. Mol. Biol. Rep. 2019, 46, 6135–6146. [Google Scholar] [CrossRef]
- Martinez, A.M.B.; Ramalho, B.D.S.; De Almeida, F.M.; Sales, C.M.; De Lima, S. Injection of bone marrow mesenchymal stem cells by intravenous or intraperitoneal routes is a viable alternative to spinal cord injury treatment in mice. Neural Regen. Res. 2018, 13, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Yousefifard, M.; Maleki, S.N.; Askarian-Amiri, S.; Vaccaro, A.R.; Chapman, J.R.; Fehlings, M.G.; Hosseini, M.; Rahimi-Movaghar, V. A combination of mesenchymal stem cells and scaffolds promotes motor functional recovery in spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Spine 2020, 32, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Khodabandeh, Z.; Mehrabani, D.; Dehghani, F.; Gashmardi, N.; Erfanizadeh, M.; Zare, S.; Bozorg-Ghalati, F. Spinal cord injury repair using mesenchymal stem cells derived from bone marrow in mice: A stereological study. Acta Histochem. 2021, 123, 151720. [Google Scholar] [CrossRef]
- Abrams, M.B.; Dominguez, C.; Pernold, K.; Reger, R.; Wiesenfeld-Hallin, Z.; Olson, L.; Prockop, D. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor. Neurol. Neurosci. 2009, 27, 307–321. [Google Scholar] [CrossRef]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 2016, 78, 436–447. [Google Scholar] [CrossRef]
- Johnson, L.; Pickard, M.; Johnson, W. The Comparative Effects of Mesenchymal Stem Cell Transplantation Therapy for Spinal Cord Injury in Humans and Animal Models: A Systematic Review and Meta-Analysis. Biology 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Liu, W.-F.; Bai, Y.-Y.; Zhou, Y.-Y.; Zhang, Y.; Wang, C.-M.; Lin, S.; He, H.-F. Transplantation of mesenchymal stem cells for spinal cord injury: A systematic review and network meta-analysis. J. Transl. Med. 2021, 19, 1–14. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Ahuja, C.S.; Fehlings, M.G. Time is spine: A review of translational advances in spinal cord injury. J. Neurosurg. Spine 2018, 30, 1–18. [Google Scholar] [CrossRef]
- Willison, A.G.; Smith, S.; Davies, B.M.; Kotter, M.R.N.; Barnett, S.C. A scoping review of trials for cell-based therapies in human spinal cord injury. Spinal Cord 2020, 58, 844–856. [Google Scholar] [CrossRef]
- Watzlawick, R.; Antonic, A.; Sena, E.S.; Kopp, M.A.; Rind, J.; Dirnagl, U.; MacLeod, M.; Howells, D.W.; Schwab, J.M. Outcome heterogeneity and bias in acute experimental spinal cord injury. Neurology 2019, 93, e40–e51. [Google Scholar] [CrossRef]
- Shao, A.; Tu, S.; Lu, J.; Zhang, J. Crosstalk between stem cell and spinal cord injury: Pathophysiology and treatment strategies. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Brennan, M.Á.; Layrolle, P.; Mooney, D.J. Biomaterials Functionalized with MSC Secreted Extracellular Vesicles and Soluble Factors for Tissue Regeneration. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef] [PubMed]
- Himes, B.T.; Neuhuber, B.; Coleman, C.; Kushner, R.; Swanger, S.; Kopen, G.C.; Wagner, J.; Shumsky, J.S.; Fischer, I. Recovery of Function Following Grafting of Human Bone Marrow-Derived Stromal Cells into the Injured Spinal Cord. Neurorehabilit. Neural Repair 2006, 20, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, C.; Ceresa, D.; Lesage, R.; Villa, F.; Reverberi, D.; Balbi, C.; Santamaria, S.; Cortese, K.; Malatesta, P.; Geris, L.; et al. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials 2021, 269, 120633. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-J.; Zhou, Y.; Wen, L.-L.; Li, Y.-F.; Wu, K.-M.; Duan, R.-R.; Yao, Y.-B.; Jing, L.-J.; Gong, Z.; Teng, J.-F. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen. Res. 2022, 17, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Falnikar, A.; Stratton, J.; Lin, R.; Andrews, C.E.; Tyburski, A.; Trovillion, V.A.; Gottschalk, C.; Ghosh, B.; Iacovitti, L.; Elliott, M.B.; et al. Differential Response in Novel Stem Cell Niches of the Brain after Cervical Spinal Cord Injury and Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2195–2207. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhang, P.; Liu, T.; Xu, J.; Fan, Z.; Shen, Y.; Li, W.; Zhang, H. Calcitonin gene-related peptide is a key factor in the homing of transplanted human MSCs to sites of spinal cord injury. Sci. Rep. 2016, 6, 27724. [Google Scholar] [CrossRef]
- Fischer, J.; Knoch, N.; Sims, T.; Rosshirt, N.; Richter, W. Time-dependent contribution of BMP, FGF, IGF, and HH signaling to the proliferation of mesenchymal stroma cells during chondrogenesis. J. Cell. Physiol. 2018, 233, 8962–8970. [Google Scholar] [CrossRef]
- Xia, X.; Tao, Q.; Ma, Q.; Chen, H.; Wang, J.; Yu, H. Growth Hormone-Releasing Hormone and Its Analogues: Significance for MSCs-Mediated Angiogenesis. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Palagano, E.; Villa, A.; Menale, C. Soluble Factors on Stage to Direct Mesenchymal Stem Cells Fate. Front. Bioeng. Biotechnol. 2017, 5, 32. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, F.; Xue, E.; Huang, L.; Yan, P.; Pan, X.; Zhou, Y. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1α in injured neuronal cells derived exosomes culture system. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Islas, J.F.; Abbasgholizadeh, R.; Dacso, C.; Potaman, V.N.; Navran, S.; Bond, R.A.; Iyer, D.; Birla, R.; Schwartz, R.J. β-Adrenergic stimuli and rotating suspension culture enhance conversion of human adipogenic mesenchymal stem cells into highly conductive cardiac progenitors. J. Tissue Eng. Regen. Med. 2019, 14, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Z.; Qian, M.; Wang, L.; Bai, C.; Wang, X. Adrenaline stimulates the proliferation and migration of mesenchymal stem cells towards the LPS -induced lung injury. J. Cell. Mol. Med. 2014, 18, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Hedderich, J.; El Bagdadi, K.; Angele, P.; Grässel, S.; Meurer, A.; Straub, R.H.; Zaucke, F.; Jenei-Lanzl, Z. Norepinephrine Inhibits the Proliferation of Human Bone Marrow-Derived Mesenchymal Stem Cells via β2-Adrenoceptor-Mediated ERK1/2 and PKA Phosphorylation. Int. J. Mol. Sci. 2020, 21, 3924. [Google Scholar] [CrossRef] [PubMed]
- Takahata, Y.; Takarada, T.; Iemata, M.; Yamamoto, T.; Nakamura, Y.; Kodama, A.; Yoneda, Y. Functional expression of β2adrenergic receptors responsible for protection against oxidative stress through promotion of glutathione synthesis after Nrf2 upregulation in undifferentiated mesenchymal C3H10T1/2 stem cells. J. Cell. Physiol. 2009, 218, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. Melatonin plays critical role in mesenchymal stem cell-based regenerative medicine in vitro and in vivo. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, Q.; Sun, J.; Xu, X.; Yang, Y.; Liu, N.; Shi, J. Protective effect of epigenetic silencing of CyclinD1 against spinal cord injury using bone marrow-derived mesenchymal stem cells in rats. J. Cell. Physiol. 2018, 233, 5361–5369. [Google Scholar] [CrossRef]
- Zvonic, S.; Ptitsyn, A.A.; Kilroy, G.; Wu, X.; Conrad, S.A.; Scott, L.K.; Guilak, F.; Pelled, G.; Gazit, D.; Gimble, J.M. Circadian Oscillation of Gene Expression in Murine Calvarial Bone. J. Bone Miner. Res. 2006, 22, 357–365. [Google Scholar] [CrossRef]
- Zvonic, S.; Ptitsyn, A.; A Conrad, S.; Scott, L.K.; Floyd, E.; Kilroy, G.; Wu, X.; Goh, B.; Mynatt, R.L.; Gimble, J.M. Characterization of Peripheral Circadian Clocks in Adipose Tissues. Diabetes 2006, 55, 962–970. [Google Scholar] [CrossRef]
- Sato, F.; Sato, H.; Jin, D.; Bhawal, U.K.; Wu, Y.; Noshiro, M.; Kawamoto, T.; Fujimoto, K.; Seino, H.; Morohashi, S.; et al. Smad3 and Snail show circadian expression in human gingival fibroblasts, human mesenchymal stem cell, and in mouse liver. Biochem. Biophys. Res. Commun. 2012, 419, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.; Vanneaux, V.; Domet, T.; Parouchev, A.; Larghero, J. Circadian Clock Genes Modulate Human Bone Marrow Mesenchymal Stem Cell Differentiation, Migration and Cell Cycle. PLoS ONE 2016, 11, e0146674. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, B.; Yuan, F.; He, X.; Lin, X.; Wang, J.; Wang, Y.; Yang, G.-Y. Melatonin Pretreatment Improves the Survival and Function of Transplanted Mesenchymal Stem Cells after Focal Cerebral Ischemia. Cell Transplant. 2014, 23, 1279–1291. [Google Scholar] [CrossRef]
- Lee, S.-J.; Jung, Y.H.; Oh, S.Y.; Yun, S.P.; Han, H.J. Melatonin enhances the human mesenchymal stem cells motility via melatonin receptor 2 coupling with Gαq in skin wound healing. J. Pineal Res. 2014, 57, 393–407. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, C.; Zhang, P.; Jiang, H.; Chen, J. Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J. Cell. Mol. Med. 2020, 24, 25–33. [Google Scholar] [CrossRef]
- Wolfe, L.F.; Sahni, A.S.; Attarian, H. Sleep disorders in traumatic brain injury. NeuroreHabilitation 2018, 43, 257–266. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Ayala, M.T.; Bateman, E.M.; Schleicher, W.E.; Smith, E.J.; D’Angelo, H.M.; Maier, S.F.; Watkins, L.R. Spinal Cord Injury in Rats Disrupts the Circadian System. eneuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Yang, J.; Wang, H.; Sun, S.; Wu, H.; Li, L.; Li, M. Mechanism of mesenchymal stem cells in spinal cord injury repair through macrophage polarization. Cell Biosci. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Ma, Z.-J.; Li, J.-R.; Kang, X.-W. Mesenchymal stem cell-derived exosomes: Therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res. Ther. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Probst, C.; Mirzayan, M.J.; Mommsen, P.; Zeckey, C.; Tegeder, T.; Geerken, L.; Maegele, M.; Samii, A.; Van Griensven, M. Systemic Inflammatory Effects of Traumatic Brain Injury, Femur Fracture, and Shock: An Experimental Murine Polytrauma Model. Mediat. Inflamm. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liang, Y.; Wei, S. M2 macrophages are closely associated with accelerated clavicle fracture healing in patients with traumatic brain injury: A retrospective cohort study. J. Orthop. Surg. Res. 2018, 13, 213. [Google Scholar] [CrossRef]
- Furusawa, K.; Emoto, K. Scrap and Build for Functional Neural Circuits: Spatiotemporal Regulation of Dendrite Degeneration and Regeneration in Neural Development and Disease. Front. Cell. Neurosci. 2021, 14. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Sabirzhanov, B.; Stoica, B.A.; Kumar, A.; Luo, T.; Skovira, J.; Faden, A.I. Spinal Cord Injury Causes Brain Inflammation Associated with Cognitive and Affective Changes: Role of Cell Cycle Pathways. J. Neurosci. 2014, 34, 10989–11006. [Google Scholar] [CrossRef] [PubMed]
- Sandalic, D.; Craig, A.; Arora, M.; Pozzato, I.; Simpson, G.; Gopinath, B.; Kaur, J.; Shetty, S.; Weber, G.; Cameron, I.; et al. A prospective cohort study investigating contributors to mild cognitive impairment in adults with spinal cord injury: Study protocol. BMC Neurol. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Gao, F.; Chan, C.C.H.; Krassioukov, A.V. Cognitive function after spinal cord injury. Neurol. 2018, 91, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, T.; Zheng, M.M.Z.; Sachdeva, R.; Phillips, A.A.; Krassioukov, A.V. Diverse cognitive impairment after spinal cord injury is associated with orthostatic hypotension symptom burden. Physiol. Behav. 2020, 213, 112742. [Google Scholar] [CrossRef] [PubMed]
- Claus-Walker, J.; Halstead, L.S. Metabolic and endocrine changes in spinal cord injury: II (Section 1). Consequences of partial decentralization of the autonomic nervous system. Arch. Phys. Med. Rehabil. 1982, 63, 569. Available online: https://pubmed.ncbi.nlm.nih.gov/6753794/ (accessed on 8 August 2021). [PubMed]
- Claus-Walker, J.; Halstead, L.S. Metabolic and endocrine changes in spinal cord injury: II (Section 2). Partial decentralization of the autonomic nervous system. Arch. Phys. Med. Rehabil. 1982, 63, 576. Available online: https://pubmed.ncbi.nlm.nih.gov/6753795/ (accessed on 8 August 2021).
- Guha, A.; Tator, C.H.; Rochon, J. Spinal cord blood flow and systemic blood pressure after experimental spinal cord injury in rats. Stroke 1989, 20, 372–377. [Google Scholar] [CrossRef]
- Crawford, R.A.; Griffiths, I.R.; McCulloch, J. The effect of norepinephrine on the spinal cord circulation and its possible implications in the pathogenesis of acute spinal trauma. J. Neurosurg. 1977, 47, 567–576. [Google Scholar] [CrossRef]
- Parkinson, J. The Spinal Cord-injured Patient. Autonomic Hyperreflexia. J. Neurosci. Nurs. 1977, 9, 1–4. [Google Scholar] [CrossRef]
- Wallin, B.G.; Stjernberg, L. Sympathetic activity in man after spinal cord injury: Outflow to skin below the lesion. Brain 1984, 107, 183–198. [Google Scholar] [CrossRef]
- Lujan, H.L.; Dicarlo, S.E. Direct comparison of cervical and high thoracic spinal cord injury reveals distinct autonomic and cardiovascular consequences. J. Appl. Physiol. 2020, 128, 554–564. [Google Scholar] [CrossRef]
- Ueno, M. Restoring neuro-immune circuitry after brain and spinal cord injuries. Int. Immunol. 2021, 33, 311–325. [Google Scholar] [CrossRef]
- Squair, J.W.; Gautier, M.; Mahe, L.; Soriano, J.E.; Rowald, A.; Bichat, A.; Cho, N.; Anderson, M.A.; James, N.D.; Gandar, J.; et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nat. Cell Biol. 2021, 590, 308–314. [Google Scholar] [CrossRef]
- Mallah, K.; Zibara, K.; Kerbaj, C.; Eid, A.; Khoshman, N.; Ousseily, Z.; Kobeissy, A.; Cardon, T.; Cizkova, D.; Kobeissy, F.; et al. Neurotrauma investigation through spatial omics guided by mass spectrometry imaging: Target identification and clinical applications. Mass Spectrom. Rev. 2021. [Google Scholar] [CrossRef]
- Mallah, K.; Quanico, J.; Raffo-Romero, A.; Cardon, T.; Aboulouard, S.; Devos, D.; Kobeissy, F.; Zibara, K.; Salzet, M.; Fournier, I. Mapping Spatiotemporal Microproteomics Landscape in Experimental Model of Traumatic Brain Injury Unveils a link to Parkinson’s Disease. Mol. Cell. Proteom. 2019, 18, 1669–1682. [Google Scholar] [CrossRef]
- Ong, W.; Pinese, C.; Chew, S.Y. Scaffold-mediated sequential drug/gene delivery to promote nerve regeneration and remyelination following traumatic nerve injuries. Adv. Drug Deliv. Rev. 2019, 149–150, 19–48. [Google Scholar] [CrossRef]
- Bretherton, R.C.; DeForest, C.A. The Art of Engineering Biomimetic Cellular Microenvironments. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Badeau, B.A.; Comerford, M.P.; Arakawa, C.K.; Shadish, J.A.; Deforest, C.A. Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem. 2018, 10, 251–258. [Google Scholar] [CrossRef]
- Li, L.; Adnan, H.; Xu, B.; Wang, J.; Wang, C.; Li, F.; Tang, K. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: A systematic review and meta-analysis. Eur. Spine J. 2014, 24, 919–930. [Google Scholar] [CrossRef]
- Lee, T.E.; Kim, A.; Jang, M.; Jeon, B. Underregistration and Underreporting of Stem Cell Clinical Trials in Neurological Disorders. J. Clin. Neurol. 2018, 14, 215–224. [Google Scholar] [CrossRef]
- Kramer, A.S.; Harvey, A.R.; Plant, G.W.; Hodgetts, S.I. Systematic Review of Induced Pluripotent Stem Cell Technology as a Potential Clinical Therapy for Spinal Cord Injury. Cell Transplant. 2013, 22, 571–617. [Google Scholar] [CrossRef]
- Khalid, S.I.; Nunna, R.S.; Maasarani, S.; Kelly, B.R.; Sroussi, H.; Mehta, A.I.; Adogwa, O. Pharmacologic and cellular therapies in the treatment of traumatic spinal cord injuries: A systematic review. J. Clin. Neurosci. 2020, 79, 12–20. [Google Scholar] [CrossRef]
- Kang, X.-W.; Hu, X.-C.; Lu, Y.-B.; Yang, Y.-N.; Wang, Y.-G.; Ma, B.; Xing, S. Progress in clinical trials of cell transplantation for the treatment of spinal cord injury: How many questions remain unanswered? Neural Regen. Res. 2021, 16, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sakai, T. Current Concepts of Stem Cell Therapy for Chronic Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 7435. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.A.; Sackey, M. Elucidating the Pivotal Neuroimmunomodulation of Stem Cells in Spinal Cord Injury Repair. Stem Cells Int. 2021, 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kawabori, M.; Seki, T.; Houkin, K. Clinical Trials of Stem Cell Treatment for Spinal Cord Injury. Int. J. Mol. Sci. 2020, 21, 3994. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Martin, J.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950.e6. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; O Okonkwo, D.; Park, P.; Jenkins, A.L.; Kurpad, S.N.; Parr, A.M.; Ganju, A.; Aarabi, B.; Kim, D.; Casha, S.; et al. Emerging Safety of Intramedullary Transplantation of Human Neural Stem Cells in Chronic Cervical and Thoracic Spinal Cord Injury. Neurosurgery 2017, 82, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Curt, A.; Hsieh, J.; Schubert, M.; Hupp, M.; Friedl, S.; Freund, P.; Huber, E.; Pfyffer, D.; Sutter, R.; Jutzeler, C.; et al. The Damaged Spinal Cord Is a Suitable Target for Stem Cell Transplantation. Neurorehabilit. Neural Repair 2020, 34, 758–768. [Google Scholar] [CrossRef]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.N.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2019, 36, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Khan, S.; Kumar, S.V.; Rajak, R.; Sultana, A.; Pasha, S.A.; Gauba, D.; Ghosh, P.; Khurana, T.; Kulkarni, A.; et al. Efficacy and safety of neural stem cell therapy for spinal cord injury: A systematic literature review. Therapies 2021, 76, 201–210. [Google Scholar] [CrossRef]
- Maher, J.L.; Anderson, K.D.; Gant, K.L.; Cowan, R.E. Development and deployment of an at-home strength and conditioning program to support a phase I trial in persons with chronic spinal cord injury. Spinal Cord 2021, 59, 44–54. [Google Scholar] [CrossRef]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bunge, M.B.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, H.S.; Sarda, K.; Arora, M.; Sharawat, R.; Singh, V.; Nanda, A.; Sangodimath, G.M.; Tandon, V. Autologous bone marrow cell transplantation in acute spinal cord injury—an Indian pilot study. Spinal Cord 2015, 54, 57–64. [Google Scholar] [CrossRef]
- Saito, F.; Nakatani, T.; Iwase, M.; Maeda, Y.; Murao, Y.; Suzuki, Y.; Fukushima, M.; Ide, C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: A pilot study. Restor. Neurol. Neurosci. 2012, 30, 127–136. [Google Scholar] [CrossRef]
- Li, X.C.; Zhong, C.F.; Bin Deng, G.; Liang, R.W.; Huang, C.M. Efficacy and safety of bone marrow-derived cell transplantation for spinal cord injury: A systematic review and meta-analysis of clinical trials. Clin. Transplant. 2015, 29, 786–795. [Google Scholar] [CrossRef]
- Cox, C.S.; Baumgartner, J.E.; Harting, M.T.; Worth, L.L.; Walker, P.A.; Shah, S.K.; Ewing-Cobbs, L.; Hasan, K.M.; Day, M.-C.; Lee, D.; et al. Autologous Bone Marrow Mononuclear Cell Therapy for Severe Traumatic Brain Injury in Children. Neurosurgery 2011, 68, 588–600. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sane, H.M.; Kulkarni, P.P.; Gokulchandran, N.; Biju, H.; Badhe, P.B. Autologous bone marrow mononuclear cell transplantation in patients with chronic traumatic brain injury- a clinical study. Cell Regen. 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sane, H.; Kulkarni, P.; Yadav, J.; Gokulchandran, N.; Biju, H.; Badhe, P. Cell therapy attempted as a novel approach for chronic traumatic brain injury – a pilot study. SpringerPlus 2015, 4, 26. [Google Scholar] [CrossRef][Green Version]
- Aghayan, H.R.; Arjmand, B.; Yaghoubi, M.; Moradi-Lakeh, M.; Kashani, H.; Shokraneh, F. Clinical outcome of autologous mononuclear cells transplantation for spinal cord injury: A systematic review and meta-analysis. Med J. Islam. Repub. Iran 2014, 28, 112. [Google Scholar] [PubMed]
- Pal, R.; Venkataramana, N.K.; Bansal, A.; Balaraju, S.; Jan, M.; Chandra, R.; Dixit, A.; Rauthan, A.; Murgod, U.; Totey, S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009, 11, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Bonilla, C.; Marin, E.; Tapiador, N.; Sevilla, M.; Vazquez, D.; Carballido, J.; et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 2018, 20, 806–819. [Google Scholar] [CrossRef]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T.; Satti, T.M.; Shahbaz, N.; Khan, M.A.; Malik, S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.V.P.; Larocca, T.F.; Souza, B.S.D.F.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.P.; Martinez, A.C.D.O.M.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Larocca, T.F.; Macêdo, C.T.; Souza, B.S.D.F.; Andrade-Souza, Y.M.; Villarreal, C.F.; Matos, A.C.; Silva, D.; da Silva, K.N.; Souza, C.L.E.M.D.; Paixão, D.D.S.; et al. Image-guided percutaneous intralesional administration of mesenchymal stromal cells in subjects with chronic complete spinal cord injury: A pilot study. Cytotherapy 2017, 19, 1189–1196. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous Bone Marrow-Derived Cell Therapy Combined with Physical Therapy Induces Functional Improvement in Chronic Spinal Cord Injury Patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Fernández, C.; Tapiador, N.; Sevilla, M.; Morejón, C.; Montilla, J.; et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy 2017, 19, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Montilla, J.; Bustamante, S.; Carballido, J.; Marin, E.; Martinez, F.; et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy 2016, 18, 1025–1036. [Google Scholar] [CrossRef]
- Kawabori, M.; Weintraub, A.H.; Imai, H.; Zinkevych, I.; McAllister, P.; Steinberg, G.K.; Frishberg, B.M.; Yasuhara, T.; Chen, J.W.; Cramer, S.C.; et al. Cell Therapy for Chronic TBI. Neurol. 2021, 96, e1202–e1214. [Google Scholar] [CrossRef]
- Lindsay, S.; Barnett, S. Therapeutic Potential of Niche-Specific Mesenchymal Stromal Cells for Spinal Cord Injury Repair. Cells 2021, 10, 901. [Google Scholar] [CrossRef]
- Shahror, R.A.; Wu, C.-C.; Chiang, Y.-H.; Chen, K.-Y. Genetically Modified Mesenchymal Stem Cells: The Next Generation of Stem Cell-Based Therapy for TBI. Int. J. Mol. Sci. 2020, 21, 4051. [Google Scholar] [CrossRef] [PubMed]
- Willing, A.E.; Das, M.; Howell, M.; Mohapatra, S.S.; Mohapatra, S. Potential of mesenchymal stem cells alone, or in combination, to treat traumatic brain injury. CNS Neurosci. Ther. 2020, 26, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.A. Mesenchymal stromal cell secretome as a therapeutic strategy for traumatic brain injury. BioFactors 2019, 45, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Mayilsamy, K.; Mohapatra, S.S.; Mohapatra, S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: Progress and prospects. Rev. Neurosci. 2019, 30, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.W.; Cho, T.-H.; Park, D.-H.; Lee, J.-B.; Park, J.-Y.; Chung, Y.-G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2015, 39, 655–664. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Animals and Humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Bydon, M.; Dietz, A.B.; Goncalves, S.; Moinuddin, F.M.; Alvi, M.A.; Goyal, A.; Yolcu, Y.; Hunt, C.L.; Garlanger, K.L.; Del Fabro, A.S.; et al. CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Mayo Clin. Proc. 2020, 95, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pang, M.; Du, C.; Liu, Z.-Y.; Chen, Z.-H.; Wang, N.-X.; Zhang, L.-M.; Chen, Y.-Y.; Mo, J.; Dong, J.-W.; et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: A phase 1/2 pilot study. Cytotherapy 2021, 23, 57–64. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poon, W.; Liu, Y.; Leung, G.K.K.; Wong, Y.; Feng, Y.; Ng, S.C.P.; Tsang, K.S.; Sun, D.T.F.; Yeung, D.K.; et al. Phase I–II Clinical Trial Assessing Safety and Efficacy of Umbilical Cord Blood Mononuclear Cell Transplant Therapy of Chronic Complete Spinal Cord Injury. Cell Transplant. 2016, 25, 1925–1943. [Google Scholar] [CrossRef]
- Albu, S.; Kumru, H.; Coll, R.; Vives, J.; Vallés, M.; Benito-Penalva, J.; Rodríguez, L.; Codinach, M.; Hernández, J.; Navarro, X.; et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: A randomized controlled study. Cytotherapy 2021, 23, 146–156. [Google Scholar] [CrossRef]
- Lammertse, D.P.; Jones, L.A.T.; Charlifue, S.B.; Kirshblum, S.C.; Apple, D.F.; Ragnarsson, K.T.; Falci, S.; Heary, R.F.; Choudhri, T.F.; Jenkins, A.L.; et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: Results of the phase 2 randomized controlled multicenter trial. Spinal Cord 2012, 50, 661–671. [Google Scholar] [CrossRef] [PubMed]
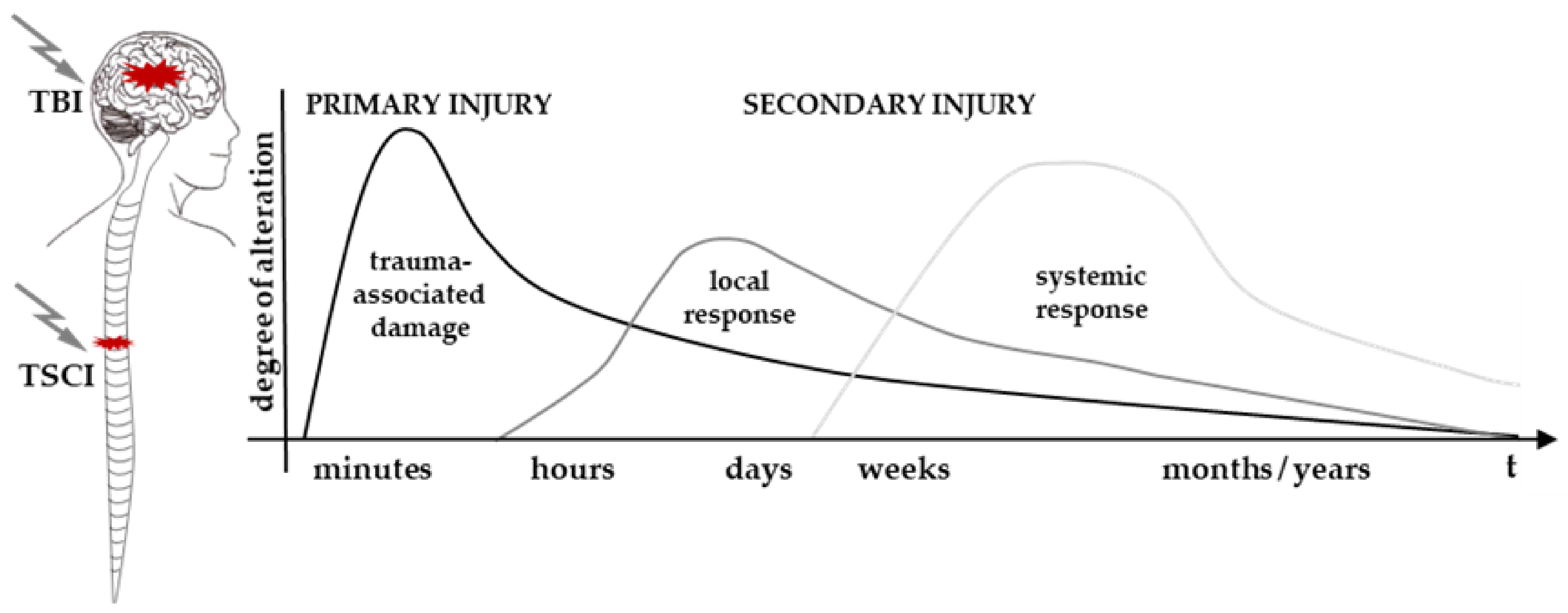
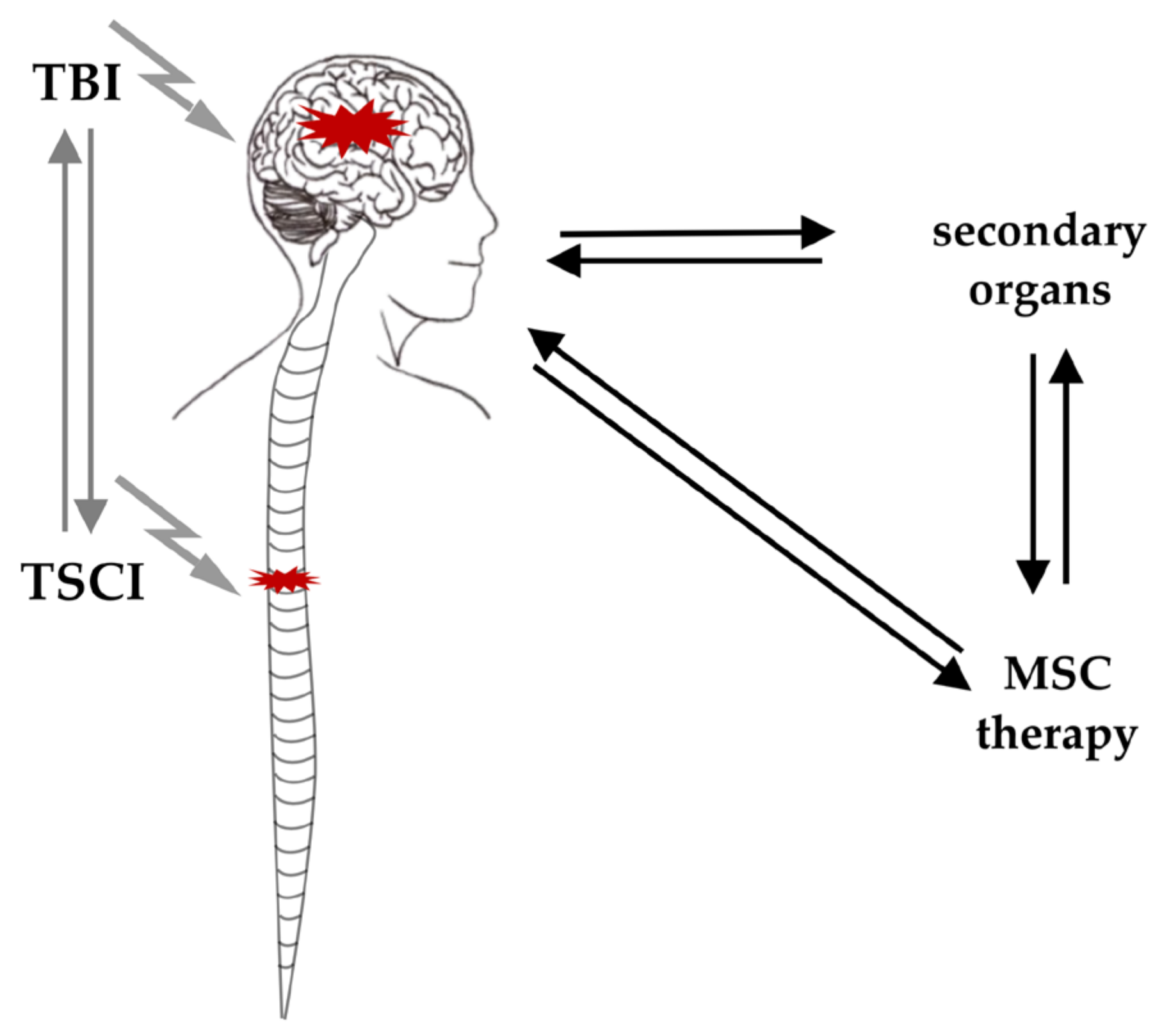
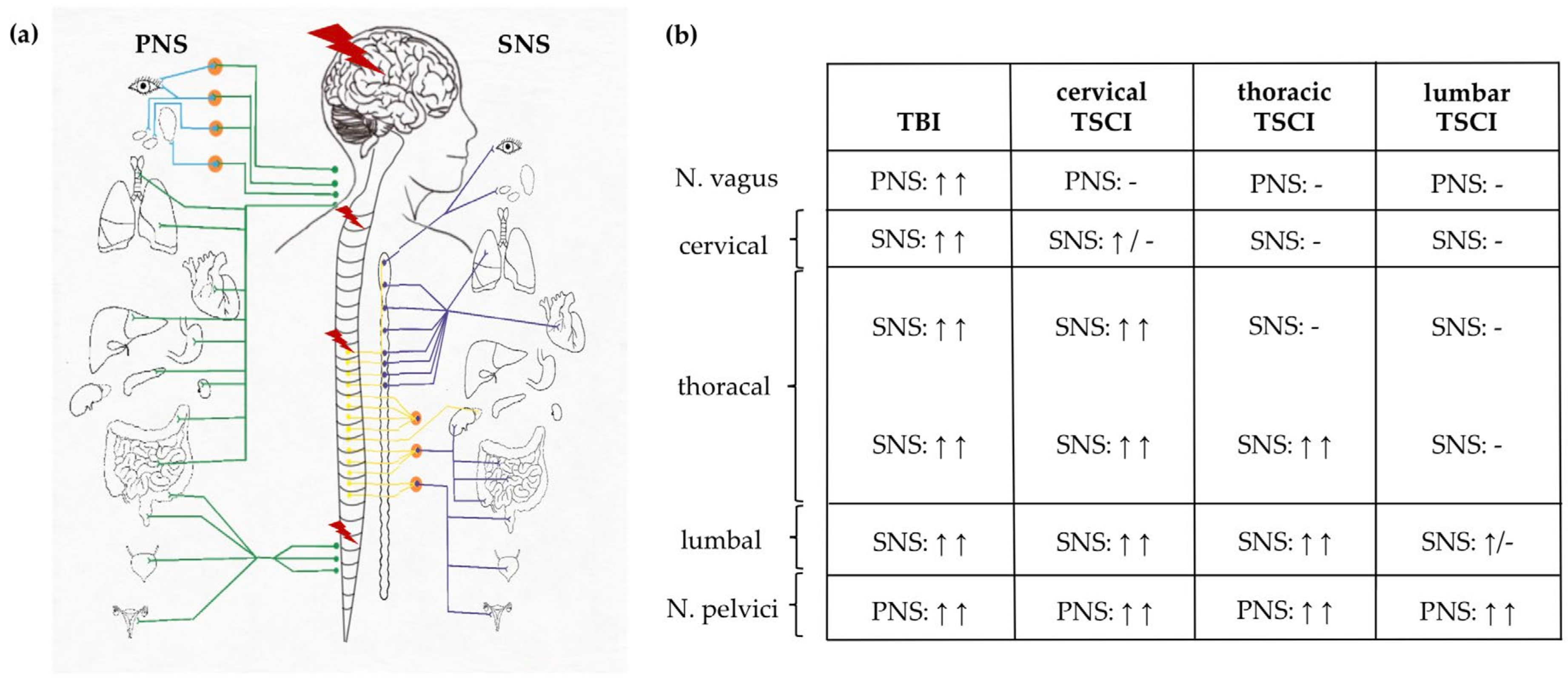
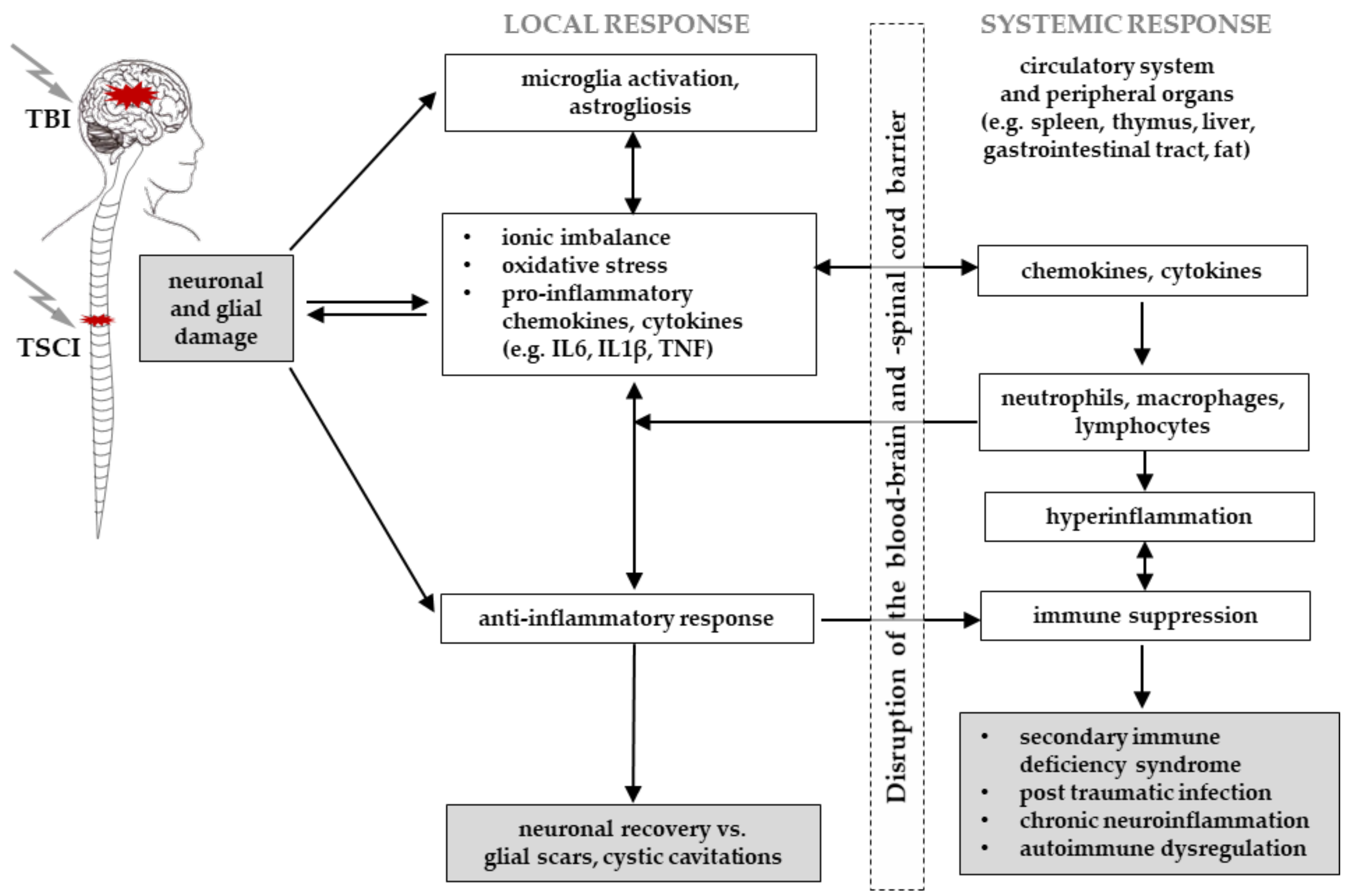
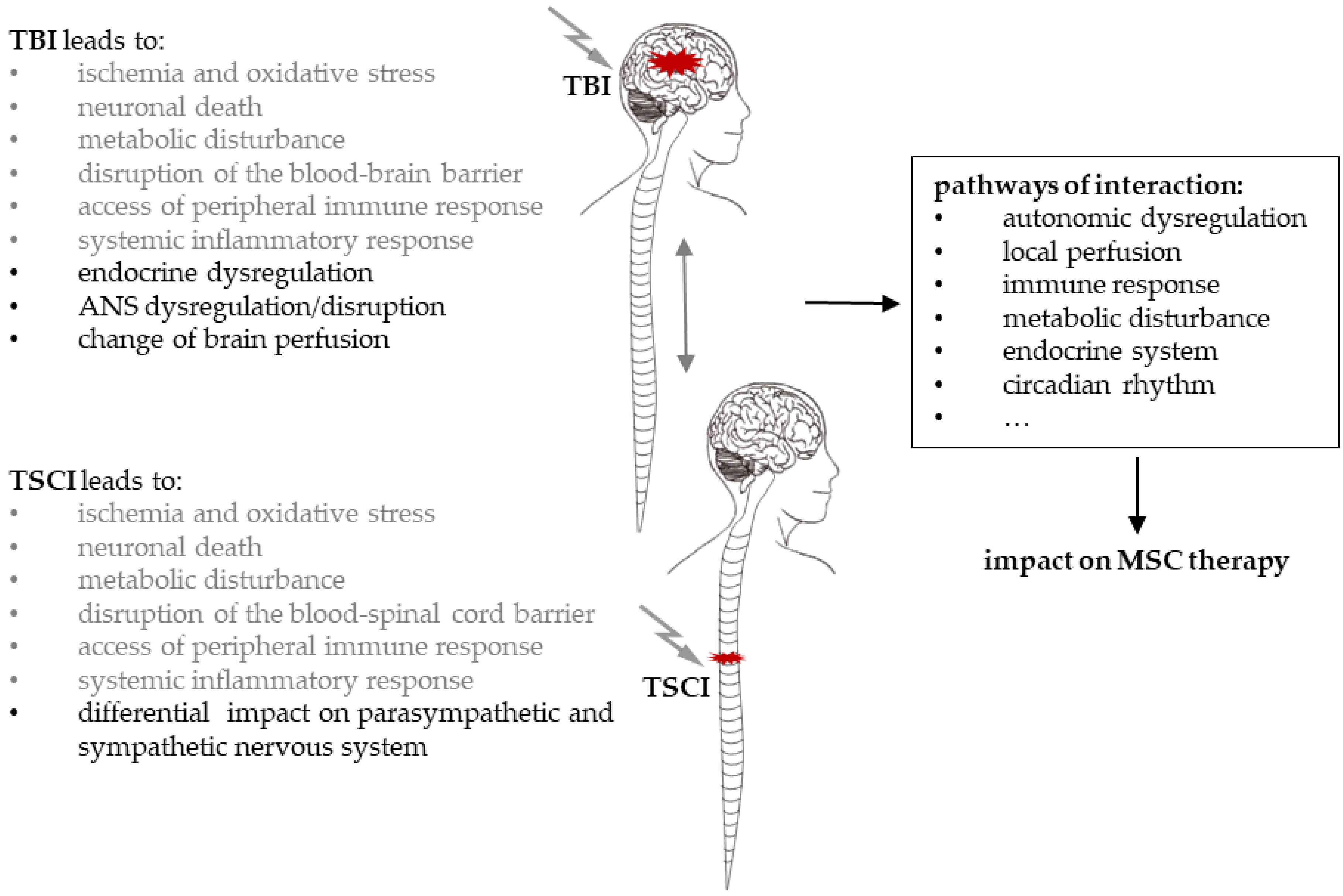
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhli, P.; Otto, E.; Jahn, D.; Reisener, M.-J.; Appelt, J.; Rahmani, A.; Taheri, N.; Keller, J.; Pumberger, M.; Tsitsilonis, S. Future Perspectives in Spinal Cord Repair: Brain as Saviour? TSCI with Concurrent TBI: Pathophysiological Interaction and Impact on MSC Treatment. Cells 2021, 10, 2955. https://doi.org/10.3390/cells10112955
Köhli P, Otto E, Jahn D, Reisener M-J, Appelt J, Rahmani A, Taheri N, Keller J, Pumberger M, Tsitsilonis S. Future Perspectives in Spinal Cord Repair: Brain as Saviour? TSCI with Concurrent TBI: Pathophysiological Interaction and Impact on MSC Treatment. Cells. 2021; 10(11):2955. https://doi.org/10.3390/cells10112955
Chicago/Turabian StyleKöhli, Paul, Ellen Otto, Denise Jahn, Marie-Jacqueline Reisener, Jessika Appelt, Adibeh Rahmani, Nima Taheri, Johannes Keller, Matthias Pumberger, and Serafeim Tsitsilonis. 2021. "Future Perspectives in Spinal Cord Repair: Brain as Saviour? TSCI with Concurrent TBI: Pathophysiological Interaction and Impact on MSC Treatment" Cells 10, no. 11: 2955. https://doi.org/10.3390/cells10112955
APA StyleKöhli, P., Otto, E., Jahn, D., Reisener, M.-J., Appelt, J., Rahmani, A., Taheri, N., Keller, J., Pumberger, M., & Tsitsilonis, S. (2021). Future Perspectives in Spinal Cord Repair: Brain as Saviour? TSCI with Concurrent TBI: Pathophysiological Interaction and Impact on MSC Treatment. Cells, 10(11), 2955. https://doi.org/10.3390/cells10112955