NSC828779 Alleviates Renal Tubulointerstitial Lesions Involving Interleukin-36 Signaling in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of NSC828779
2.2. UUO Animal Model and Treatment Experiments
2.3. Histopathology and Immunohistochemistry
2.4. Cultured Cells
2.5. Western Blot Analysis
2.6. ELISA
2.7. Real-Time PCR Assay
2.8. Mechanically Induced Constant Pressure Model
2.9. IL-36a-Mediated Activation of NLRP3 Inflammasome in Renal TECs and Macrophages
2.10. Reporter Assay for NF-κB Activation
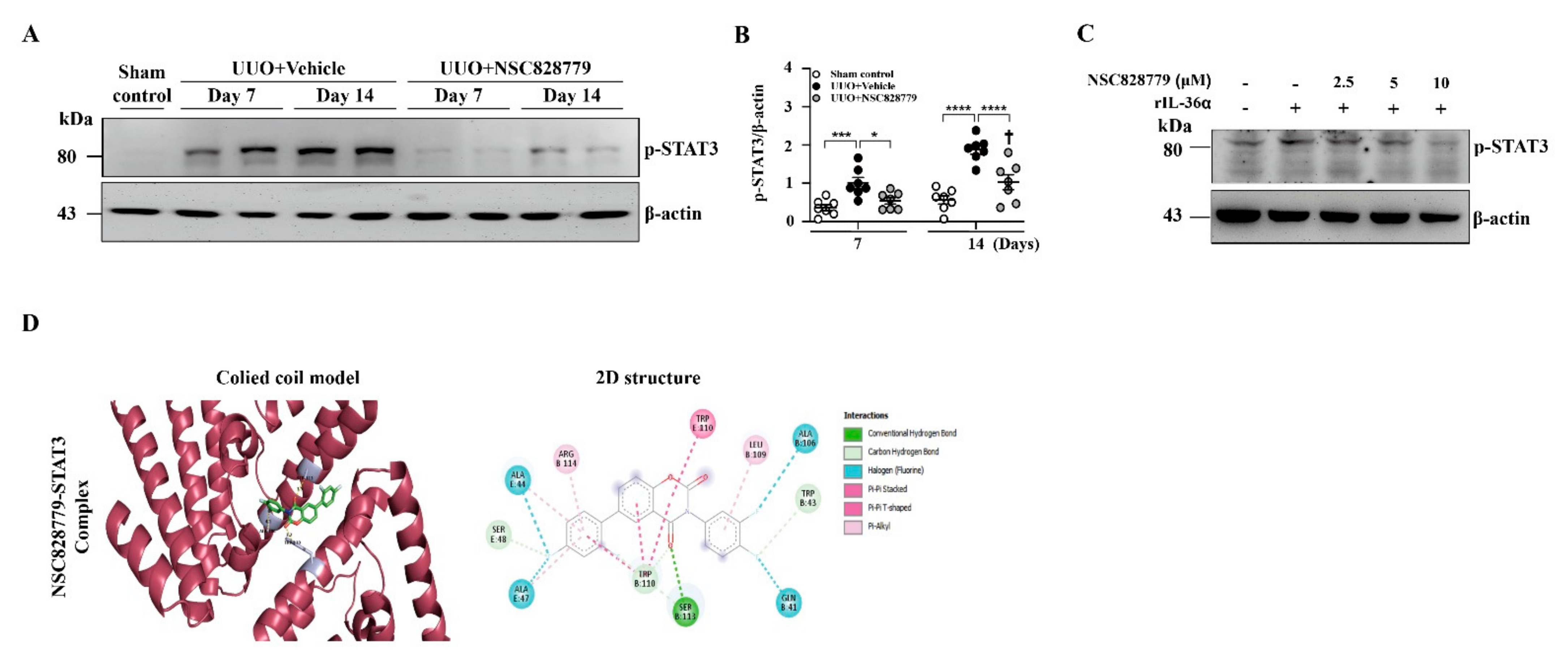
2.11. In-Silico Molecular Docking Analyses
2.12. Statistical Analysis
3. Results
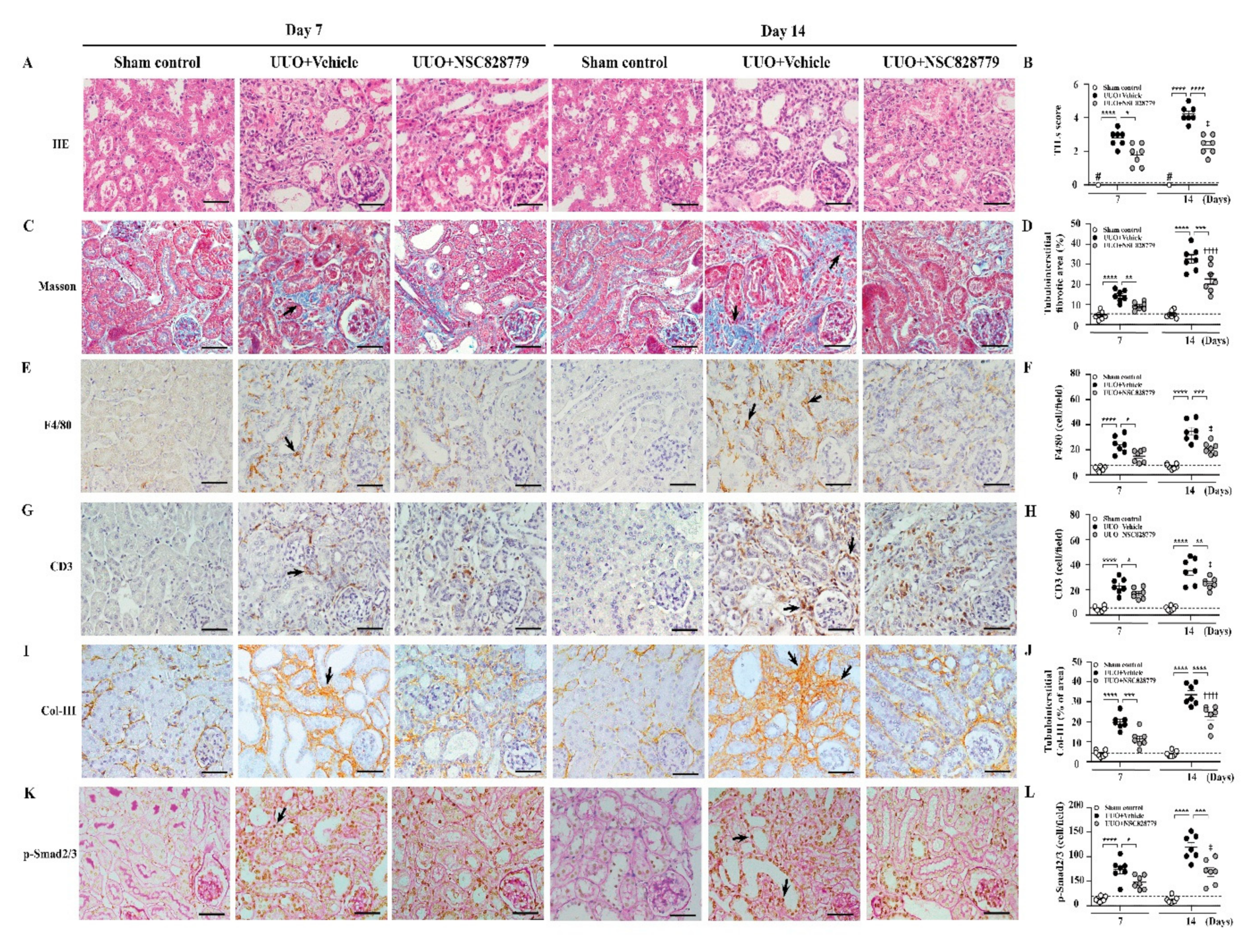
3.1. NSC828779 Improves Renal TILs in UUO Mice
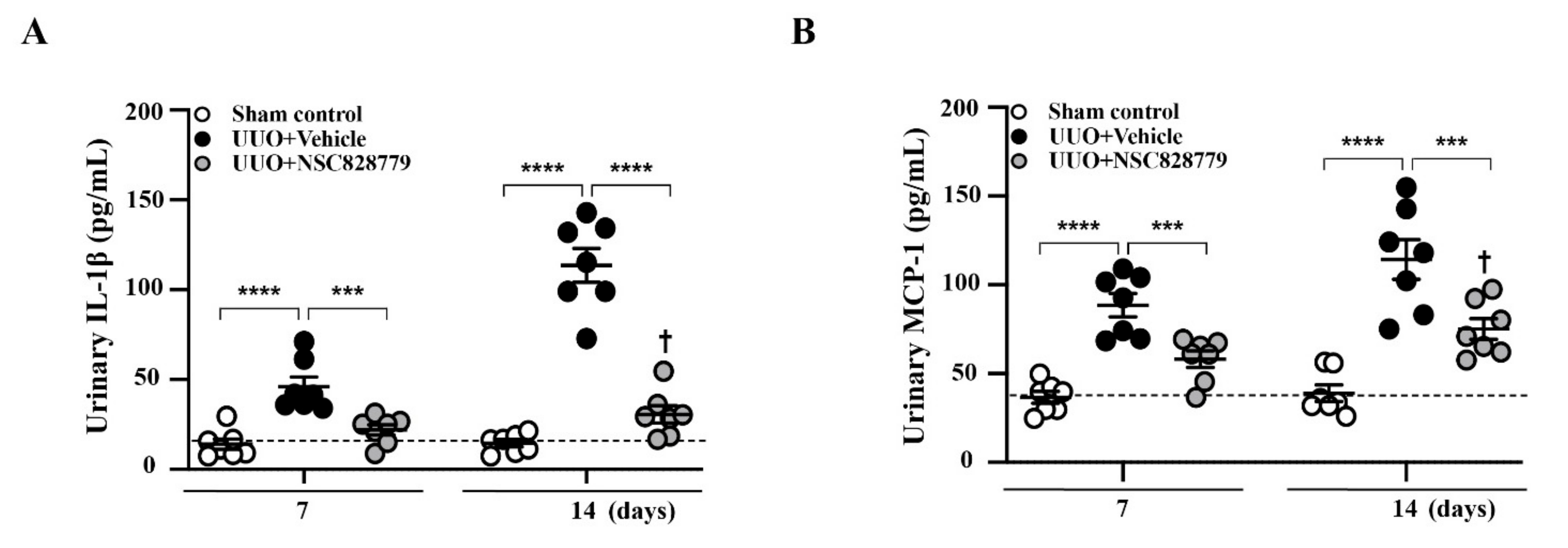
3.2. Decreased Urine Cytokine Levels
3.3. Alleviated Pathological Changes
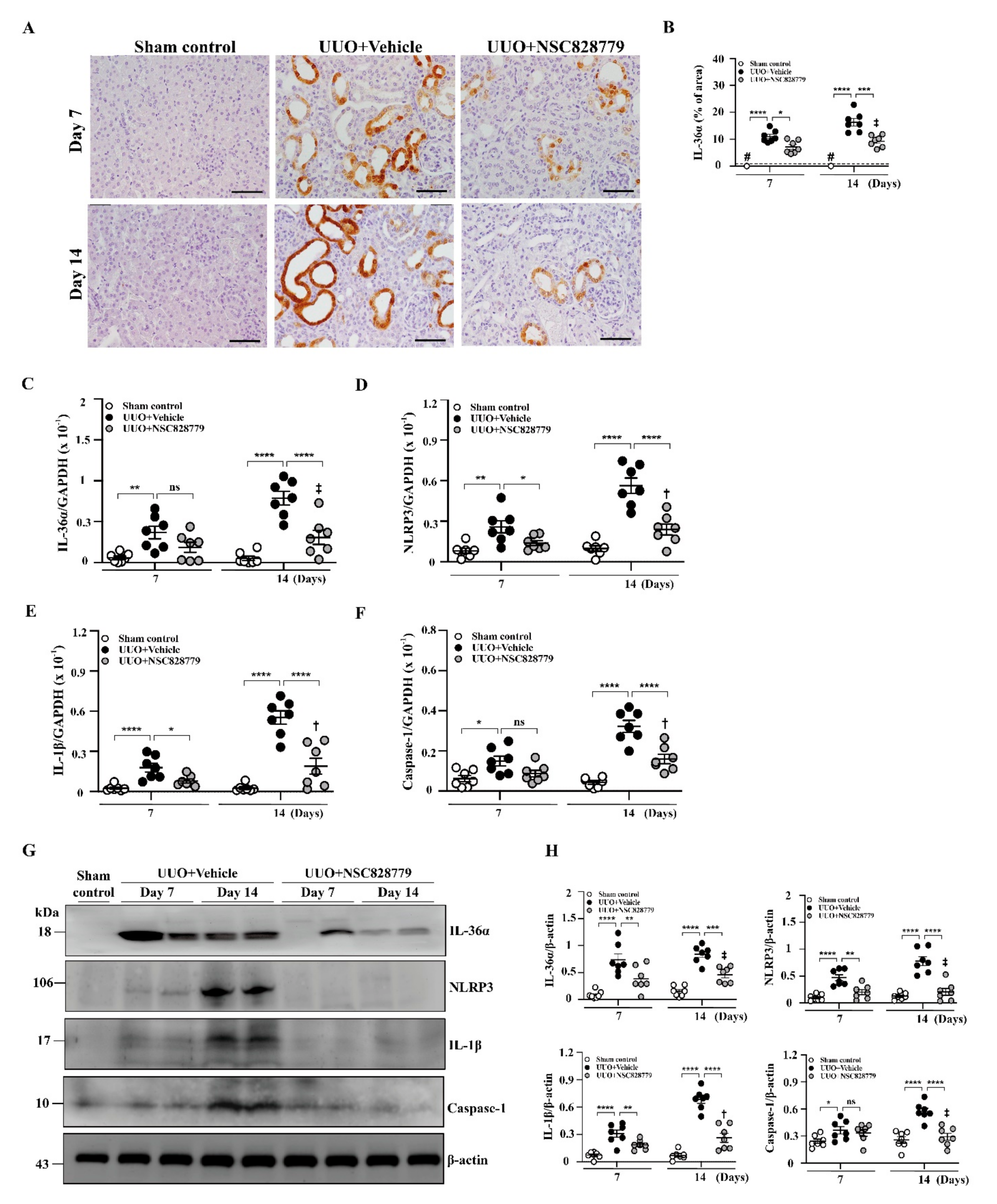
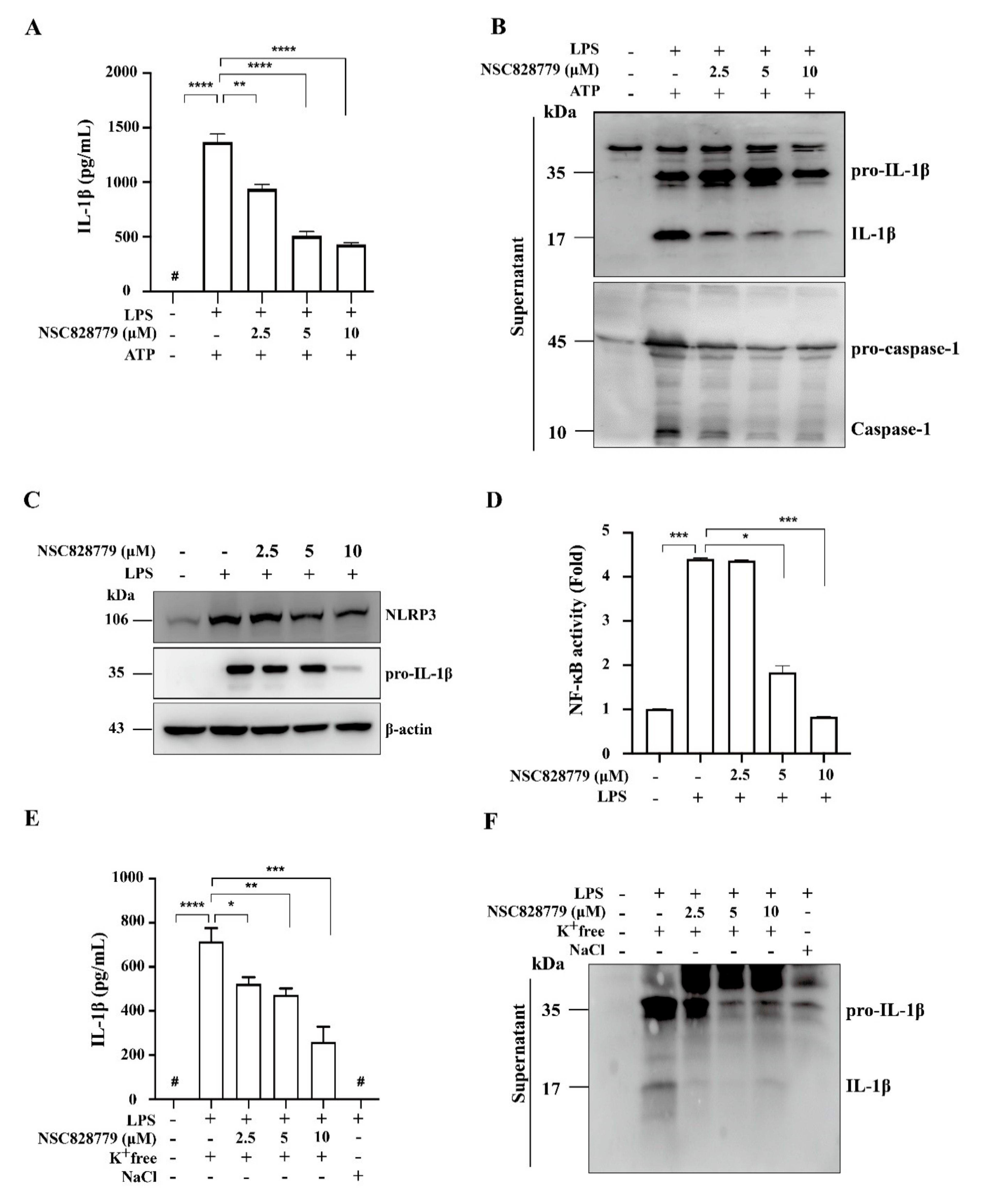
3.4. NSC828779 Inhibits the IL-36α/NLRP3 Inflammasome Pathway
3.4.1. Renal IL-36α Expression and NLRP3 Inflammasome Activation in UUO Mice
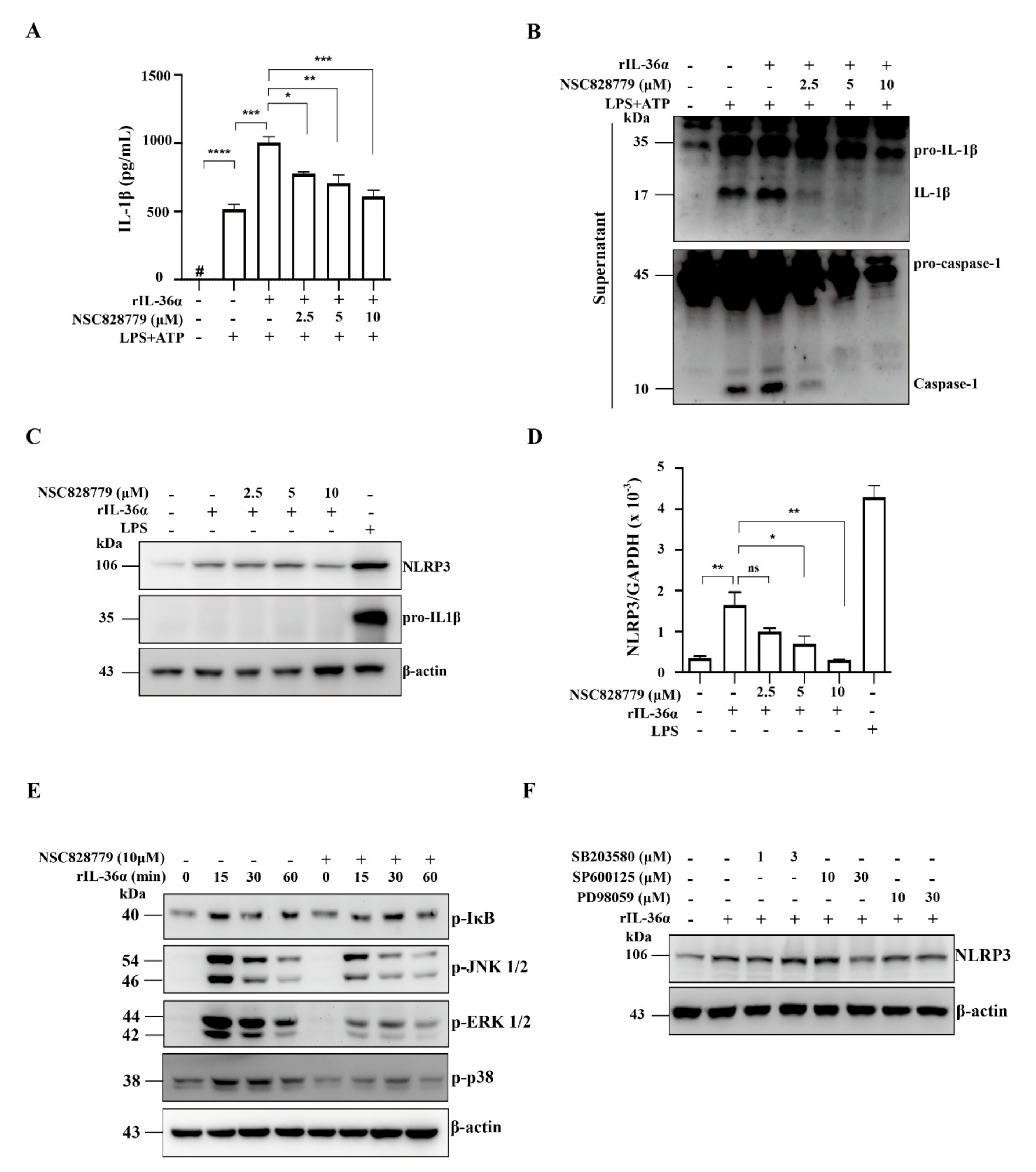
3.4.2. IL-36α Expression Mediated by a Cell-Based, MICP Model or H2O2 in Renal TECs
3.4.3. NSC828779 Inhibits NLRP3 Inflammasome Activation in IL-36α-Primed Macrophages
3.5. NSC828779 Inhibits STAT3 Signaling
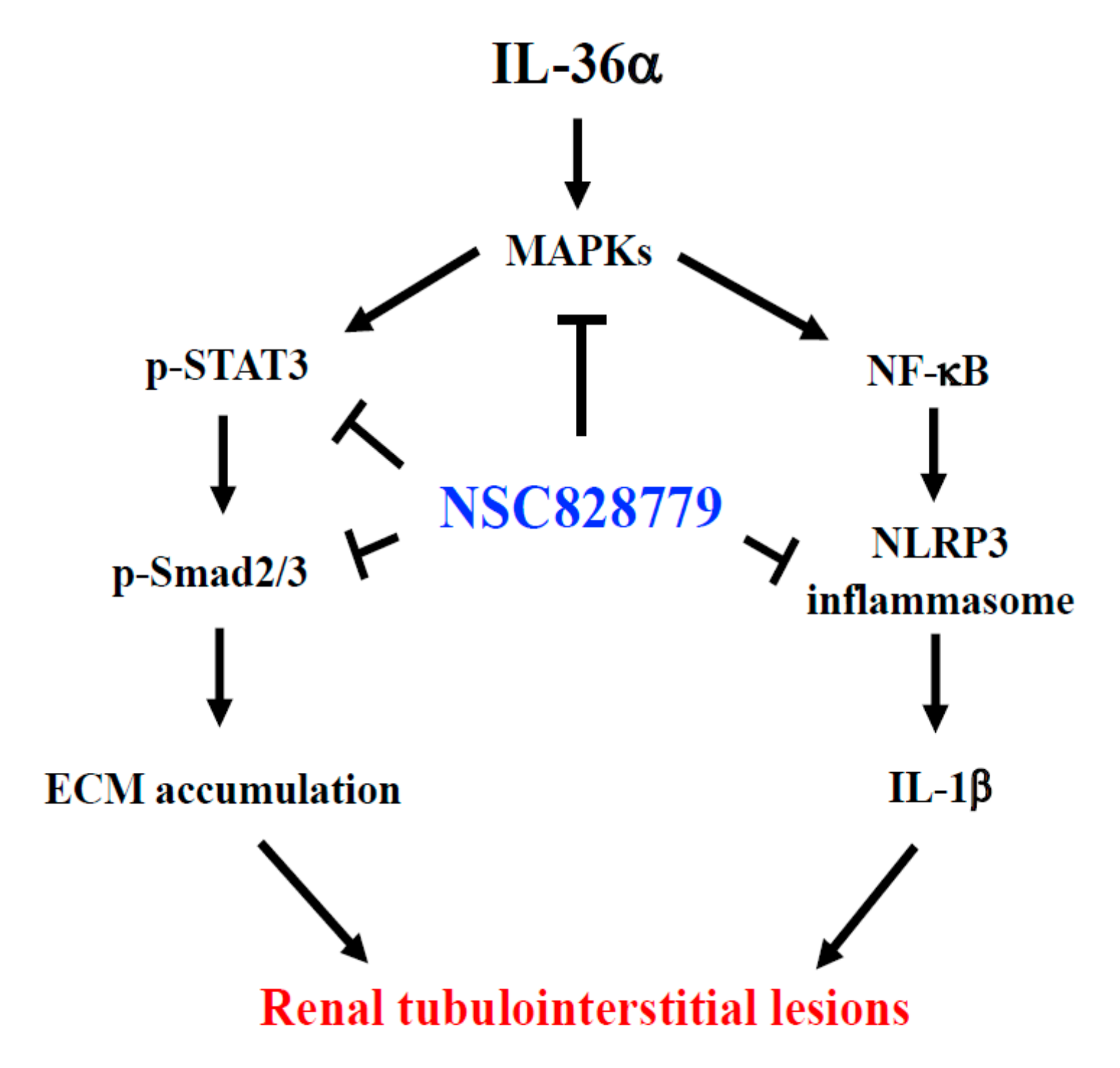
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Col | collagen |
| CKD | chronic kidney disease |
| TILs | tubulointerstitial lesions |
| TEC | tubular epithelial cell |
| UUO | unilateral ureteral obstruction |
| rIL-36α | recombinant IL-36α |
| PEG 400 | polyethylene glycol 400 |
| MICP | mechanically induced constant pressure |
References
- Gewin, L.; Zent, R.; Pozzi, A. Progression of chronic kidney disease: Too much cellular talk causes damage. Kidney Int. 2017, 91, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Bienaimé, F.; Muorah, M.; Yammine, L.; Burtin, M.; Nguyen, C.; Baron, W.; Garbay, S.; Viau, A.; Broueilh, M.; Blanc, T.; et al. Stat3 controls tubulointerstitial communication during CKD. J. Am. Soc. Nephrol. 2016, 27, 3690–3705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripodi, D.; Conti, F.; Rosati, M.; Maccauro, G.; Saggini, A.; Cianchetti, E.; Angelucci, D.; Fulcheri, M.; Tetè, S.; Salini, V.; et al. IL-36 a new member of the IL-1 family cytokines. J. Biol. Regul. Homeost. Agents 2012, 26, 7–14. [Google Scholar]
- Müller, A.; Hennig, A.; Lorscheid, S.; Grondona, P.; Schulze-Osthoff, K.; Hailfinger, S.; Kramer, D. IκBζ is a key transcriptional regulator of IL-36–driven psoriasis-related gene expression in keratinocytes. Proc. Natl. Acad. Sci. USA 2018, 115, 10088–10093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, H.-H.; Hua, K.-F.; Lin, Y.-C.; Chu, C.-L.; Hsieh, C.-Y.; Hsu, Y.-J.; Ka, S.-M.; Tsai, Y.-L.; Liu, F.-C.; Chen, A. IL-36 signaling facilitates activation of the NLRP3 inflammasome and IL-23/IL-17 axis in renal inflammation and fibrosis. J. Am. Soc. Nephrol. 2017, 28, 2022–2037. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-M.; Kim, Y.G.; Kim, D.-J.; Park, S.H.; Jeong, K.-H.; Lee, Y.H.; Lim, S.J.; Lee, S.-H.; Moon, J.-Y. Inflammasome-independent role of NLRP3 mediates mitochondrial regulation in renal injury. Front. Immunol. 2018, 9, 2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Bi, X.; Zhou, P.; Zhu, S.; Ding, W. NLRP3 deficiency attenuates renal fibrosis and ameliorates mitochondrial dysfunction in a mouse unilateral ureteral obstruction model of chronic kidney disease. Mediat. Inflamm. 2017, 2017, 8316560. [Google Scholar] [CrossRef] [Green Version]
- Pulskens, W.P.; Butter, L.M.; Teske, G.J.; Claessen, N.; Dessing, M.C.; Flavell, R.A.; Sutterwala, F.S.; Florquin, S.; Leemans, J.C. Nlrp3 prevents early renal interstitial edema and vascular permeability in unilateral ureteral obstruction. PLoS ONE 2014, 9, e85775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulay, S.R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 2019, 96, 58–66. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, F.Y.; Tesch, G.H.; Ozols, E.; Xie, M.; Schneider, M.D.; Nikolic-Paterson, D.J. TGF-β1-activated kinase-1 regulates inflammation and fibrosis in the obstructed kidney. Am. J. Physiol. Ren. Physiol. 2011, 300, F1410–F1421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, C.; Yang, H.; Deng, J.; Fan, D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol. Res. 2020, 155, 104746. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Fan, H.-W.; Li, K.; Fan, X.-D. Suppression of Elp2 prevents renal fibrosis and inflammation induced by unilateral ureter obstruction (UUO) via inactivating Stat3-regulated TGF-β1 and NF-κB pathways. Biochem. Biophys. Res. Commun. 2018, 501, 400–407. [Google Scholar] [CrossRef]
- Hassan, N.M.; Said, E.; Shehatou, G.S. Nifuroxazide suppresses UUO-induced renal fibrosis in rats via inhibiting STAT-3/NF-κB signaling, oxidative stress and inflammation. Life Sci. 2021, 272, 119241. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Hou, X.; Li, J.; Li, T.; Qiu, A.; Liu, N.; Zhuang, S. Histone deacetylase 6 inhibition mitigates renal fibrosis by suppressing TGF-β and EGFR signaling pathways in obstructive nephropathy. Am. J. Physiol. Ren. Physiol. 2020, 319, F1003–F1014. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Hua, K.-F.; Tuan, L.-H.; Tsai, Y.-L.; Chu, L.J.; Lee, Y.-C.; Wong, W.-T.; Lee, S.-L.; Lai, J.-H.; Chu, C.-L.; et al. Compound K inhibits priming and mitochondria-associated activating signals of NLRP3 inflammasome in renal tubulointerstitial lesions. Nephrol. Dial. Transplant. 2020, 35, 74–85. [Google Scholar] [CrossRef]
- Liu, F.-C.; Huang, H.-S.; Huang, C.-Y.; Yang, R.; Chang, D.-M.; Lai, J.-H.; Ho, L.-J. A benzamide-linked small molecule HS-Cf inhibits TNF-α-induced interferon regulatory factor-1 in porcine chondrocytes: A potential disease-modifying drug for osteoarthritis therapeutics. J. Clin. Immunol. 2011, 31, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-T.; Huang, H.-S.; Chiang, M.-L.; Lin, C.-S.; Yang, S.-P.; Ho, L.-J.; Lai, J.-H. A salicylate-based small molecule HS-Cm exhibits immunomodulatory effects and inhibits dipeptidyl peptidase-IV activity in human T cells. Eur. J. Pharmacol. 2014, 726, 124–132. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lo, Y.; Ho, L.-J.; Lai, J.-H.; Lien, S.-B.; Lin, L.-C.; Chen, C.-L.; Chen, T.-C.; Liu, F.-C.; Huang, H.-S. A new application of parallel synthesis strategy for discovery of amide-linked small molecules as potent chondroprotective agents in TNF-α-stimulated chondrocytes. PLoS ONE 2016, 11, e0149317. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-C.; Liu, F.-L.; Chen, C.-L.; Chen, T.-C.; Liu, F.-C.; Ali, A.A.A.; Chang, D.-M.; Huang, H.-S. Novel inhibitors of RANKL-induced osteoclastogenesis: Design, synthesis, and biological evaluation of 6-(2,4-difluorophenyl)-3-phenyl-2H-benzo [e][1, 3] oxazine-2, 4 (3H)-diones. Bioorganic Med. Chem. 2015, 23, 4522–4532. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Liu, F.-L.; Chen, C.-L.; Chen, T.-C.; Chang, D.-M.; Huang, H.-S. Discovery of 5-(2′,4′-difluorophenyl)-salicylanilides as new inhibitors of receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis. Eur. J. Med. Chem. 2015, 98, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-C.; Lu, J.-W.; Chien, C.-Y.; Huang, H.-S.; Lee, C.-C.; Lien, S.-B.; Lin, L.-C.; Chen, L.W.; Ho, Y.-J.; Shen, M.-C.; et al. Arthroprotective Effects of Cf-02 Sharing Structural Similarity with Quercetin. Int. J. Mol. Sci. 2018, 19, 1453. [Google Scholar] [CrossRef] [Green Version]
- Ophascharoensuk, V.; Giachelli, C.M.; Gordon, K.; Hughes, J.; Pichler, R.; Brown, P.; Liaw, L.; Schmidt, R.; Shankland, S.J.; Alpers, C.E.; et al. Obstructive uropathy in the mouse: Role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 1999, 56, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Saito, Y.; Yoshioka, T.; Ogawa, Y.; Imamaki, H.; Kusakabe, T.; et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009, 75, 285–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadbelt, N.V.; Stahl, P.J.; Chen, J.; Mizrahi, M.; Lal, A.; Bozkurt, A.; Poppas, D.P.; Felsen, D. Early upregulation of iNOS mRNA expression and increase in NO metabolites in pressurized renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2007, 293, F1877–F1888. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.-W.; Li, L.-H.; Hsieh, C.-Y.; Rao, Y.K.; Chen, F.-H.; Chen, A.; Ka, S.-M.; Hua, K.-F.J.S.R. Glucosamine inhibits IL-1β expression by preserving mitochondrial integrity and disrupting assembly of the NLRP3 inflammasome. Sci. Rep. 2019, 9, 5603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Visualizer, D.S. Biovia—Dassault Systèmes BIOVIA Workbatch Release; Dassault Systèmes: Waltham, MA, USA, 2020. [Google Scholar]
- Ichii, O.; Otsuka, S.; Sasaki, N.; Yabuki, A.; Ohta, H.; Takiguchi, M.; Hashimoto, Y.; Endoh, D.; Kon, Y. Local overexpression of interleukin-1 family, member 6 relates to the development of tubulointerstitial lesions. Lab. Investig. 2010, 90, 459–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutet, M.-A.; Nerviani, A.; Lliso-Ribera, G.; Lucchesi, D.; Prediletto, E.; Ghirardi, G.M.; Goldmann, K.; Lewis, M.; Pitzalis, C. Interleukin-36 family dysregulation drives joint inflammation and therapy response in psoriatic arthritis. Rheumatology 2020, 59, 828–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towne, J.E.; Garka, K.E.; Renshaw, B.R.; Virca, G.D.; Sims, J.E. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-κB and MAPKs. J. Biol. Chem. 2004, 279, 13677–13688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadas, R.A.; Ewart, S.L.; Iwakura, Y.; Medoff, B.D.; LeVine, A.M. IL-36α exerts pro-inflammatory effects in the lungs of mice. PLoS ONE 2012, 7, e45784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wu, C.; Boye, A.; Wu, J.; Wang, J.; Yang, X.; Yang, Y. MAPK inhibitors modulate Smad2/3/4 complex cyto-nuclear translocation in myofibroblasts via Imp7/8 mediation. Mol. Cell. Biochem. 2015, 406, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, I.; Nethery, D.; Kern, J.A. Role of Smad2/3 and p38 MAP kinase in TGF-β1-induced epithelial–mesenchymal transition of pulmonary epithelial cells. J. Cell. Physiol. 2011, 226, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Massagué, J.; Chen, Y.-G. Controlling TGF-β signaling. Genes Dev. 2000, 14, 627–644. [Google Scholar]
- Kretzschmar, M.; Massagué, J. SMADs: Mediators and regulators of TGF-β signaling. Curr. Opin. Genet. Dev. 1998, 8, 103–111. [Google Scholar] [CrossRef]
- Ka, S.; Yeh, Y.; Huang, X.; Chao, T.; Hung, Y.; Yu, C.; Lin, T.; Wu, C.; Lan, H.; Chen, A. Kidney-targeting Smad7 gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear factor κB (NF-κB) signalling pathways, and improves diabetic nephropathy in mice. Diabetologia 2012, 55, 509–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, H.Y.; Mu, W.; Tomita, N.; Huang, X.R.; Li, J.H.; Zhu, H.-J.; Morishita, R.; Johnson, R.J. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J. Am. Soc. Nephrol. 2003, 14, 1535–1548. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.-C.; Wang, W.; Huang, X.R.; Fu, P.; Chen, T.-H.; Sheikh-Hamad, D.; Lan, H.Y. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-β signaling and fibrosis in rat remnant kidney. Am. J. Pathol. 2005, 166, 761–771. [Google Scholar] [CrossRef]
- Ng, Y.-Y.; Hou, C.-C.; Wang, W.; Huang, X.R.; Lan, H.Y. Blockade of NFκB activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int. 2005, 67, S83–S91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Wei, J.; Lu, C.; Chen, H.; Zhong, X.; Lu, Y.; Li, L.; Huang, H.; Dai, Z.; Han, L. Genistein suppresses psoriasis-related inflammation through a STAT3–NF-κB-dependent mechanism in keratinocytes. Int. Immunopharmacol. 2019, 69, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Huang, L.; Luo, W.; Yu, W.; Hu, X.; Guan, X.; Cai, Y.; Zou, C.; Yin, H.; Xu, Z.; et al. Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice. Cell Death Dis. 2019, 10, 848. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Ren, J.; Gui, Y.; Wei, W.; Shu, B.; Lu, Q.; Xue, X.; Sun, X.; He, W.; Yang, J.; et al. Wnt/β-catenin–promoted macrophage alternative activation contributes to kidney fibrosis. J. Am. Soc. Nephrol. 2018, 29, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, K.; Huang, Z.-Q.; Raska, M.; Reily, C.; Anderson, J.C.; Suzuki, H.; Ueda, H.; Moldoveanu, Z.; Kiryluk, K.; Suzuki, Y.; et al. Inhibition of STAT3 signaling reduces IgA1 autoantigen production in IgA nephropathy. Kidney Int. Rep. 2017, 2, 1194–1207. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Finkelman, F.D.; Shao, W.H. Mechanism of Mer receptor tyrosine kinase inhibition of glomerular endothelial cell inflammation. J. Leukoc. Biol. 2018, 103, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, Y.; Liu, G.; Zhang, X.; Xiao, B.; Huang, S.; Liu, H.; He, L. Role of Stat3 signaling in control of EMT of tubular epithelial cells during renal fibrosis. Cell. Physiol. Biochem. 2017, 42, 2552–2558. [Google Scholar] [CrossRef]
- Gwon, M.-G.; An, H.-J.; Gu, H.; Kim, Y.-A.; Han, S.M.; Park, K.-K. Apamin inhibits renal fibrosis via suppressing TGF-β1 and STAT3 signaling in vivo and in vitro. J. Mol. Med. 2021, 99, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Carrasco, R.; Silva-Palacios, A.; Rojas-Morales, P.; Aparicio-Trejo, O.E.; Medina-Reyes, E.I.; Hernández-Cruz, E.Y.; Sánchez-Garibay, C.; Salinas-Lara, C.; Pavón, N.; Roldán, F.J.; et al. Unilateral Ureteral Obstruction for 28 Days in Rats Is Not Associated with Changes in Cardiac Function or Alterations in Mitochondrial Function. Biology 2021, 10, 671. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-R.; Hung, S.-C.; Chu, L.J.; Hua, K.-F.; Wei, C.-W.; Tsai, I.-L.; Kao, C.-C.; Sung, C.-C.; Chu, P.; Wu, C.-Y.; et al. NSC828779 Alleviates Renal Tubulointerstitial Lesions Involving Interleukin-36 Signaling in Mice. Cells 2021, 10, 3060. https://doi.org/10.3390/cells10113060
Yang S-R, Hung S-C, Chu LJ, Hua K-F, Wei C-W, Tsai I-L, Kao C-C, Sung C-C, Chu P, Wu C-Y, et al. NSC828779 Alleviates Renal Tubulointerstitial Lesions Involving Interleukin-36 Signaling in Mice. Cells. 2021; 10(11):3060. https://doi.org/10.3390/cells10113060
Chicago/Turabian StyleYang, Shin-Ruen, Szu-Chun Hung, Lichieh Julie Chu, Kuo-Feng Hua, Chyou-Wei Wei, I-Lin Tsai, Chih-Chin Kao, Chih-Chien Sung, Pauling Chu, Chung-Yao Wu, and et al. 2021. "NSC828779 Alleviates Renal Tubulointerstitial Lesions Involving Interleukin-36 Signaling in Mice" Cells 10, no. 11: 3060. https://doi.org/10.3390/cells10113060





