A Step-by-Step Refined Strategy for Highly Efficient Generation of Neural Progenitors and Motor Neurons from Human Pluripotent Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. General Supplies
- Cell culture incubator (ThermoFisher, Waltham, MA, USA, HERAcell 150i).
- 6-well cell culture plates (Jet Biofil, Guangzhou, China, #TCP010006).
- 24-well cell culture plates (Jet Biofil, #TCP010024).
- Cryotube vials (ThermoFisher, #375418PK).
- 15 mL conical tubes (Jet Biofil, #CFT-011150).
- Coverslips for cell culture (Citotest, Haimen, China, #0346-0910).
- Microscope slides (Citotest, #188105W).
- DPBS (Beyotime, Shanghai, China, #C0221D).
- 96-well Q-PCR plates (Axygen, Union City, CA, USA, #PCR-96M2-HS-C).
- ABI QuantStudio Dx (Applied Biosystems, Waltham, MA, USA).
- Fluorescence microscope (Leica, Wetzlar, Germany, DMi8).
- MultiClamp 700B amplifier (Molecular Devices, San Jose, CA, USA).
- Clampex10.3 software (Molecular Devices).
2.2. Cell Lines Used
- iPSC-WT (Nuwacell, Hefei, China, #RC01001-A; #RC01001-B).
- iPSC-ALS (Coriell, Camden, NJ, USA, #ND35660; #ND35664).
- iPSC-DYT1 (CSUi002-A) [32].
- iPSC-HD (Coriell, #GM23225).
2.3. Culture and Maintenance of hPSCs
- hPSC medium: ncEpic hPSC Medium (Nuwacell, #RP01001).
- hPSC Dissociation Buffer (Nuwacell, #RP01007).
- Accutase (Millipore sigma, Burlington, MA, USA, #SCR005).
- Matrigel (Corning, Kakegawa, Japan, #354277); aliquot and store at −80 °C.
- Y-27632 (Selleck, Houston, TX, USA, #S1049); aliquot and store at −80 °C.
2.4. Differentiation of hPSCs to NPCs
- SB431542 (Sigma, St. Louis, MO, USA, #S4317); aliquot and store at −80 °C; avoid exposure to light.
- LDN193189 (Miltenyi, Bergisch Gladbach, Germany, #130-103-925); aliquot and store at −80 °C; avoid exposure to light.
- MGCD0103 (Selleck, #S1122); aliquot and store at −80 °C.
- RA (Sigma, #R2625); aliquot and store at −80 °C; avoid exposure to light.
- Knockout serum replacement (KOSR, ThermoFisher, #A3181502); aliquot and store at −80 °C.
- DMEM/F12 (Procell, Wuhan, China, #PM150312).
- Neurobasal medium (ThermoFisher, #21103049).
- GlutaMax (Gibco, Amarillo, TX, USA, #35050061).
- NEAA (Gibco, #11140050).
- β-ME (Procell, #PB180633).
- Penicillin/Streptomycin (P/S, Solarbio, Beijing, China, #P1400).
- DMSO (Solarbio, #D8371).
2.5. Differentiation of NPCs to Neurons
- DMEM (Procell, #PM150210).
- DMEM/F12 (Procell, #PM150312).
- Neurobasal medium (ThermoFisher, #21103049).
- Fetal bovine serum (FBS, TransGen Biotech, Beijing, China, #PS201-02); aliquot and store at −80 °C.
- Poly-L-ornithine (PLO, Sigma, #P4957).
- Mouse laminin (Corning, #354232); aliquot and store at −80 °C.
- Fibronectin (R&D Systems, Tokyo, Japan, #1918-FN); aliquot and store at −80 °C.
- AraC (Sigma, #C1768); aliquot and store at −80 °C.
- Purmorphamine (Calbiochem, San Diego, CA, USA, #540220); aliquot and store at −80 °C.
- B27 supplement (Gibco, #17504044).
- N2 supplement (Gibco, #17502048).
- L-ascorbic acid (L-AA, Sigma, #A4403); aliquot and store at −80 °C.
- Forskolin (Sigma, #F6886); aliquot and store at −80 °C.
- Puromycin (Solarbio, #P8230); aliquot and store at −80 °C.
- BDNF (Novoprotein, Shanghai, China, #C076); aliquot and store at −80 °C.
- GDNF (Novoprotein, #C226); aliquot and store at −80 °C.
2.6. Immunostaining
- 4% Paraformaldehyde (PFA, Servicebio, Wuhan, China, #G1101).
- Triton X-100 (Solarbio, #T8200).
- Tween-20 (Macklin, Rochelle, IL, USA, #C10232628).
- Bovine serum albumin (BSA, BioFroxx, Guangzhou, China, #4240).
- Hoechst 33342 (Beyotime, Shanghai, China, #C1025).
- Poly(vinyl alcohol) (PVA, Macklin, P816862).
- Primary and secondary antibodies (Table A1).
2.7. Q-PCR
- TransZol (TransGen Biotech, #ET101-01).
- RevertAid First Strand cDNA Synthesis Kit (ThermoFisher, #K1622).
- PerfectStart Green qPCR SuperMix (TransGen Biotech, #AQ601-01).
- Primers (Table A2).
2.8. Medium and Solution Setup
- KOSR Medium: DMEM/F12 medium with 20% KOSR, 1% GlutaMax, 1% NEAA, 50 µM β-ME and 1% P/S.
- NSP Medium: DMEM/F12 medium containing 1% N2, 1% GlutaMax, 1% NEAA, 50 µM β-ME, 1% P/S, 8 µg/mL Heparin, 20 ng/mL bFGF and 20 ng/mL EGF.
- NPC Medium: DMEM/F12 and Neurobasal medium (1:1) containing 0.5% N2, 1% B27, 1% GlutaMax, 1% NEAA, 50 µM β-ME, 1% P/S, 20 ng/mL EGF and 20 ng/mL bFGF.
- Glia Medium: DMEM, 10% FBS.
- NPC Freeze medium: NPC Medium, 30% KOSR, 10% DMSO, 5 µM Y-27632.
- hPSC Freeze medium: 90% KOSR, 10% DMSO, 10 µM Y-27632.
- NDM Medium: Neurobasal medium containing 1% N2, 2% B27, 1% GlutaMax, 1% NEAA, 50 µM β-ME, 10 ng/mL BDNF and 10 ng/mL GDNF.
- C2 medium: DMEM/F12 and Neurobasal medium (2:1) containing 0.8% N2, 0.4% B27, 0.4 µg/mL L-AA, 5 µM forskolin, 10 ng/mL BDNF and 10 ng/mL GDNF.
- Blocking buffer: 3% BSA, 2% Triton X-100 in DPBS.
- PBST: DPBS with 0.1 % Tween-20.
- Tyrode solution: 150 mM NaCl, 4 mM KCl, 2 mM MgCl2, 3 mM CaCl2, 10 mM glucose and 10 mM HEPES at pH 7.4 (adjusted with KOH) and 300 mOsm.
- Intracellular solution: 0.2 mM EGTA, 130 mM K-Gluconate, 6 mM KCl, 3 mM NaCl, 10 mM HEPES, 4 mM ATP-Mg, 0.4 mM GTP-Na, 14 mM phosphocreatine-di(Tris) at pH 7.2 and 285 mOsm.
2.9. Differentiation Procedures
2.9.1. NPC Generation from hPSCs
2.9.2. Neural Differentiation from NPCs
2.10. Immunostaining Analysis
- Fix NPCs or neurons on coverslips with 4% PFA for 15 min at room temperature.
- Wash with DPBS 3 times (5 min each time).
- Incubate with blocking buffer for 30 min at room temperature.
- Incubate with primary antibodies (diluted with blocking buffer) overnight at 4 °C.
- Wash with PBST 3 times (5 min each time).
- Incubate with second antibodies for 1 h at room temperature.
- Wash with PBST 3 times (5 min each time).
- Incubate nucleus with Hoechst 33342 for 5–10 min at room temperature.
- Seal coverslips inverted onto microscope slides with 10% PVA.
- Observe under the fluorescence microscope.
2.11. Q-PCR Analysis
- Total mRNAs were extracted from NPCs using TransZol reagents according to the manufacturer’s instructions.
- Total mRNAs were reverse-transcripted into cDNAs using RevertAid First Strand cDNA Synthesis Kit.
- PCR was performed using PerfectStart Green qPCR SuperMix and carried out on a 96-well ABI QuantStudio Dx.
- The PCR program consisted of 30 s at 94 °C, followed by 42 cycles of 5 s at 94 °C, 15 s at 62 °C, and 15 s at 72 °C.
- All primers were verified by melting curve analysis containing a single melt curve peak.
- Relative gene expression levels were analyzed using the 2−∆∆Ct algorithm normalized to the housekeeping HPRT and relative to control samples.
2.12. Electrophysiology
3. Results and Discussion
3.1. Rapid and Highly Efficient Generation of NPCs from hPSCs
3.2. Identity Comparisons of Generated NPCs
3.3. Spontaneous Neural Differentiation of Generated NPCs
3.4. Rapid and Highly Efficient Generation of MNs from NPCs
3.5. Modeling MNDs by Differentiated MNs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primary Antibody | Species | Dilution | Source |
|---|---|---|---|
| NESTIN | Ms | 1:400 | Millipore, #MAB5326 |
| PAX6 | Rb | 1:500 | Sigma, #HPA030775 |
| SOX2 | Gt | 1:50 | Santa Cruz, #sc-17320 |
| Ki67 | Rb | 1:1000 | Leica, #NCL-Ki67p |
| PSA-NCAM | Ms | 1:250 | DSHB, #5A5-a |
| TUBB3 | Rb | 1:500 | ABclonal, #A17074 |
| TUBB3 | Ms | 1:750 | Biolegend, #801201 |
| GFAP | Rb | 1:200 | ABclonal, #A14673 |
| SYN1 | Rb | 1:200 | Cell Signaling, #5297 |
| vGLUT1 | Ms | 1:500 | Synaptic System, #135303 |
| GABA | Rb | 1:1000 | Sigma, #A2052 |
| TH | Ck | 1:1000 | Aves, #TYH |
| HB9 | Ms | 1:100 | DSHB, 81.5C10-c |
| ChAT | Gt | 1:200 | Millipore, #AB144P |
| GFP | Ck | 1:1000 | Aves, #GFP-1020 |
| Secondary Antibody | |||
| Donkey anti-Mouse IgG (H + L), Alexa Fluor 488 | 1:500 | Invitrogen, #A-21202 | |
| Donkey anti-Rabbit IgG (H + L), Alexa Fluor 488 | 1:500 | Invitrogen, #A-21206 | |
| Goat anti-Chicken IgY (H + L), Alexa Fluor 488 | 1:500 | Invitrogen, #A-11039 | |
| Donkey anti-Mouse IgG (H + L), Alexa Fluor 555 | 1:500 | Invitrogen, #A-31570 | |
| Donkey anti-Rabbit IgG (H + L), Alexa Fluor 555 | 1:500 | Invitrogen, #A-31572 | |
| Donkey anti-Goat IgG (H + L), Alexa Fluor 555 | 1:500 | Invitrogen, #A-21432 | |
| Goat anti-Mouse IgM (H), Alexa Fluor 555 | 1:500 | Invitrogen, #A-21426 | |
| Donkey anti-Mouse IgG(H + L), Alexa Flour 647 | 1:500 | Invitrogen, #A-31571 | |
| Donkey anti-Rabbit IgG(H + L), Alexa Flour 647 | 1:500 | Invitrogen, #A-31573 | |
| Donkey anti-Goat IgG (H + L), Alexa Fluor 647 | 1:500 | Invitrogen, #A-21447 | |
Appendix B
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | bp |
|---|---|---|---|
| HPRT | GCTTTCCTTGGTCAGGCAGTA | GTCTGGCTTATATCCAACACTTCGT | 94 |
| ASCL1 | GTCCTGTCGCCCACCATCTC | CCCTCCCAACGCCACTGAC | 251 |
| DLX1 | TGCCAGAAAGTCTCAACAGCC | CGAGTGTAAACAGTGCATGGA | 125 |
| EN1 | CGTGGCTTACTCCCCATTTA | TCTCGCTGTCTCTCCCTCTC | 117 |
| FOXP1 | CTACCGCTTCCATGGGAAATC | CTGTTGTCACTAAGGACAGGG | 207 |
| GSX2 | ATGTCGCGCTCCTTCTATGTC | CAAGCGGGATGAAGAAATCCG | 106 |
| ISL1 | GCGGAGTGTAATCAGTATTTGGA | GCATTTGATCCCGTACAACCT | 102 |
| NKX2.1 | AGCACACGACTCCGTTCTC | GCCCACTTTCTTGTAGCTTTCC | 68 |
| OLIG2 | AGCTCCTCAAATCGCATCC | AAAAGGTCATCGGGCTCTG | 146 |
| OTX2 | CAAAGTGAGACCTGCCAAAAAGA | TGGACAAGGGATCTGACAGTG | 179 |
| PAX6 | GCCCTCACAAACACCTACAG | TCATAACTCCGCCCATTCAC | 149 |
| SOX1 | GCGGAGCTCGTCGCATT | GCGGTAACAACTACAAAAAACTTGTAA | 62 |
| TBR1 | GCAGCAGCTACCCACATTCA | AGGTTGTCAGTGGTCGAGATA | 76 |
References
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [PubMed]
- YYang, J.; Tang, Y.; Liu, H.; Guo, F.; Ni, J.; Le, W. Suppression of histone deacetylation promotes the differentiation of human pluripotent stem cells towards neural progenitor cells. BMC Biol. 2014, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Nakashima, K.; Kuwabara, T.; Mejia, E.; Gage, F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16659–16664. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, R.; Yang, X.; Tang, K.; Jing, N. Dual roles of histone H3 lysine 9 acetylation in human embryonic stem cell pluripotency and neural differentiation. J. Biol. Chem. 2015, 290, 9949. [Google Scholar] [CrossRef]
- Mandai, M.; Kurimoto, Y.; Takahashi, M. Autologous Induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 377, 792–793. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-derived dopamine progenitor cells for parkinson’s disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Ichida, J.K.; Kiskinis, E. Probing disorders of the nervous system using reprogramming approaches. EMBO J. 2015, 34, 1456–1477. [Google Scholar] [CrossRef]
- Lorenz, C.; Lesimple, P.; Bukowiecki, R.; Zink, A.; Inak, G.; Mlody, B.; Singh, M.; Semtner, M.; Mah, N.; Aure, K.; et al. Human iPSC-derived neural progenitors are an effective drug discovery model for neurological mtdna disorders. Cell Stem Cell 2017, 20, 659–674.e9. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, M.L.; Zang, T.; Zhang, C.L. Direct reprogramming rather than iPSC-based reprogramming maintains aging hallmarks in human motor neurons. Front. Mol. Neurosci. 2017, 10, 359. [Google Scholar] [CrossRef]
- Ding, B.; Tang, Y.; Ma, S.; Akter, M.; Liu, M.L.; Zang, T.; Zhang, C.L. Disease modeling with human neurons reveals LMNB1 dysregulation underlying DYT1 dystonia. J. Neurosci. 2021, 41, 2024–2038. [Google Scholar] [CrossRef]
- Yang, Y.M.; Gupta, S.K.; Kim, K.J.; Powers, B.E.; Cerqueira, A.; Wainger, B.J.; Ngo, H.D.; Rosowski, K.A.; Schein, P.A.; Ackeifi, C.A.; et al. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell 2013, 12, 713–726. [Google Scholar] [CrossRef]
- Zhou, T.; Tan, L.; Cederquist, G.Y.; Fan, Y.; Hartley, B.J.; Mukherjee, S.; Tomishima, M.; Brennand, K.J.; Zhang, Q.; Schwartz, R.E.; et al. High-Content screening in hPSC-neural progenitors identifies drug candidates that inhibit zika virus infection in fetal-like organoids and adult brain. Cell Stem Cell 2017, 21, 274–283.e5. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Itsykson, P.; Turetsky, T.; Pera, M.F.; Reinhartz, E.; Itzik, A.; Ben-Hur, T. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1134–1140. [Google Scholar] [CrossRef]
- Carpenter, M.K.; Inokuma, M.S.; Denham, J.; Mujtaba, T.; Chiu, C.P.; Rao, M.S. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp. Neurol. 2001, 172, 383–397. [Google Scholar] [CrossRef]
- Li, X.J.; Du, Z.W.; Zarnowska, E.D.; Pankratz, M.; Hansen, L.O.; Pearce, R.A.; Zhang, S.C. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 2005, 23, 215–221. [Google Scholar] [CrossRef]
- Kozhich, O.A.; Hamilton, R.S.; Mallon, B.S. Standardized generation and differentiation of neural precursor cells from human pluripotent stem cells. Stem Cell Rev. Rep. 2013, 9, 531–536. [Google Scholar] [CrossRef]
- Vazin, T.; Chen, J.; Lee, C.T.; Amable, R.; Freed, W.J. Assessment of stromal-derived inducing activity in the generation of dopaminergic neurons from human embryonic stem cells. Stem Cells 2008, 26, 1517–1525. [Google Scholar] [CrossRef][Green Version]
- Perrier, A.L.; Tabar, V.; Barberi, T.; Rubio, M.E.; Bruses, J.; Topf, N.; Harrison, N.L.; Studer, L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 12543–12548. [Google Scholar] [CrossRef]
- Lee, H.; Shamy, G.A.; Elkabetz, Y.; Schofield, C.M.; Harrsion, N.L.; Panagiotakos, G.; Socci, N.D.; Tabar, V.; Studer, L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells 2007, 25, 1931–1939. [Google Scholar] [CrossRef]
- Kim, H.; Lee, G.; Ganat, Y.; Papapetrou, E.P.; Lipchina, I.; Socci, N.D.; Sadelain, M.; Studer, L. miR-371-3 expression predicts neural differentiation propensity in human pluripotent stem cells. Cell Stem Cell 2011, 8, 695–706. [Google Scholar] [CrossRef]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef]
- Shi, Y.; Kirwan, P.; Smith, J.; Robinson, H.P.; Livesey, F.J. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat. Neurosci. 2012, 15, 477–486. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Surmacz, B.; Fox, H.; Gutteridge, A.; Fish, P.; Lubitz, S.; Whiting, P. Directing differentiation of human embryonic stem cells toward anterior neural ectoderm using small molecules. Stem Cells 2012, 30, 1875–1884. [Google Scholar] [CrossRef]
- Pera, M.F.; Andrade, J.; Houssami, S.; Reubinoff, B.; Trounson, A.; Stanley, E.G.; Ward-van Oostwaard, D.; Mummery, C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J. Cell Sci. 2004, 117, 1269–1280. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, J.S.; Leem, J.W.; Huh, Y.J.; Kim, J.Y.; Kim, H.S.; Park, I.H.; Daley, G.Q.; Hwang, D.Y.; Kim, D.W. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. Rep. 2010, 6, 270–281. [Google Scholar] [CrossRef]
- Kiskinis, E.; Sandoe, J.; Williams, L.A.; Boulting, G.L.; Moccia, R.; Wainger, B.J.; Han, S.; Peng, T.; Thams, S.; Mikkilineni, S.; et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 2014, 14, 781–795. [Google Scholar] [CrossRef]
- Chen, H.; Qian, K.; Du, Z.; Cao, J.; Petersen, A.; Liu, H.; Blackbourn, L.W.T.; Huang, C.L.; Errigo, A.; Yin, Y.; et al. Modeling ALS with iPSCs reveals that mutant SOD1 misregulates neurofilament balance in motor neurons. Cell Stem Cell 2014, 14, 796–809. [Google Scholar] [CrossRef]
- Zhang, Y.; Pak, C.; Han, Y.; Ahlenius, H.; Zhang, Z.; Chanda, S.; Marro, S.; Patzke, C.; Acuna, C.; Covy, J.; et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 2013, 78, 785–798. [Google Scholar] [CrossRef]
- Yang, N.; Chanda, S.; Marro, S.; Ng, Y.H.; Janas, J.A.; Haag, D.; Ang, C.E.; Tang, Y.; Flores, Q.; Mall, M.; et al. Generation of pure GABAergic neurons by transcription factor programming. Nat. Methods 2017, 14, 621–628. [Google Scholar] [CrossRef]
- Hester, M.E.; Murtha, M.J.; Song, S.; Rao, M.; Miranda, C.J.; Meyer, K.; Tian, J.; Boulting, G.; Schaffer, D.V.; Zhu, M.X.; et al. Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ren, J.; Luo, H.; Zuo, X.; Tang, Y. Generation of patient-specific induced pluripotent stem cell line (CSUi002-A) from a patient with isolated dystonia carrying TOR1A mutation. Stem Cell Res. 2021, 53, 102277. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-Specific induced pluripotent stem cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Dhara, S.K.; Stice, S.L. Neural differentiation of human embryonic stem cells. J. Cell Biochem. 2008, 105, 633–640. [Google Scholar] [CrossRef]
- Bissonnette, C.J.; Lyass, L.; Bhattacharyya, B.J.; Belmadani, A.; Miller, R.J.; Kessler, J.A. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells 2011, 29, 802–811. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Lee, Y.J.; Shin, H.A.; Cho, H.Y.; Wang, K.C.; Kim, Y.S.; Lee, H.T.; Chung, K.S.; Kim, E.Y.; et al. Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci. Lett. 2004, 359, 99–103. [Google Scholar] [CrossRef]
- Erceg, S.; Lainez, S.; Ronaghi, M.; Stojkovic, P.; Perez-Arago, M.A.; Moreno-Manzano, V.; Moreno-Palanques, R.; Planells-Cases, R.; Stojkovic, M. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS ONE 2008, 3, e2122. [Google Scholar] [CrossRef]
- Baharvand, H.; Mehrjardi, N.Z.; Hatami, M.; Kiani, S.; Rao, M.; Haghighi, M.M. Neural differentiation from human embryonic stem cells in a defined adherent culture condition. Int. J. Dev. Biol. 2007, 51, 371–378. [Google Scholar] [CrossRef]
- Kim, T.W.; Koo, S.Y.; Studer, L. Pluripotent stem cell therapies for parkinson disease: Present challenges and future opportunities. Front. Cell Dev. Biol. 2020, 8, 729. [Google Scholar] [CrossRef]
- Ahfeldt, T.; Ordureau, A.; Bell, C.; Sarrafha, L.; Sun, C.; Piccinotti, S.; Grass, T.; Parfitt, G.M.; Paulo, J.A.; Yanagawa, F.; et al. Pathogenic pathways in early-onset autosomal recessive parkinson’s disease discovered using isogenic human dopaminergic neurons. Stem Cell Rep. 2020, 14, 75–90. [Google Scholar] [CrossRef]
- Du, Z.W.; Chen, H.; Liu, H.; Lu, J.; Qian, K.; Huang, C.L.; Zhong, X.; Fan, F.; Zhang, S.C. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015, 6, 6626. [Google Scholar] [CrossRef]
- Verrier, L.; Davidson, L.; Gierlinski, M.; Dady, A.; Storey, K.G. Neural differentiation, selection and transcriptomic profiling of human neuromesodermal progenitor-like cells in vitro. Development 2018, 145, dev166215. [Google Scholar] [CrossRef]
- Maroof, A.M.; Keros, S.; Tyson, J.A.; Ying, S.W.; Ganat, Y.M.; Merkle, F.T.; Liu, B.; Goulburn, A.; Stanley, E.G.; Elefanty, A.G.; et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 2013, 12, 559–572. [Google Scholar] [CrossRef]
- Li, W.; Sun, W.; Zhang, Y.; Wei, W.; Ambasudhan, R.; Xia, P.; Talantova, M.; Lin, T.; Kim, J.; Wang, X.; et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 8299–8304. [Google Scholar] [CrossRef]
- Chen, C.Y.; Plocik, A.; Anderson, N.C.; Moakley, D.; Boyi, T.; Dundes, C.; Lassiter, C.; Graveley, B.R.; Grabel, L. Transcriptome and in vitro differentiation profile of human embryonic stem cell derived NKX2.1-positive neural progenitors. Stem Cell Rev. Rep. 2016, 12, 744–756. [Google Scholar] [CrossRef]
- Bergsland, M.; Werme, M.; Malewicz, M.; Perlmann, T.; Muhr, J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 2006, 20, 3475–3486. [Google Scholar] [CrossRef]
- Thein, D.C.; Thalhammer, J.M.; Hartwig, A.C.; Crenshaw, E.B., 3rd; Lefebvre, V.; Wegner, M.; Sock, E. The closely related transcription factors Sox4 and Sox11 function as survival factors during spinal cord development. J. Neurochem. 2010, 115, 131–141. [Google Scholar] [CrossRef]
- Liu, M.L.; Zang, T.; Zou, Y.; Chang, J.C.; Gibson, J.R.; Huber, K.M.; Zhang, C.L. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat. Commun. 2013, 4, 2183. [Google Scholar] [CrossRef]
- Marchetto, M.C.; Muotri, A.R.; Mu, Y.; Smith, A.M.; Cezar, G.G.; Gage, F.H. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 2008, 3, 649–657. [Google Scholar] [CrossRef]
- De Santis, R.; Garone, M.G.; Pagani, F.; de Turris, V.; Di Angelantonio, S.; Rosa, A. Direct conversion of human pluripotent stem cells into cranial motor neurons using a piggyBac vector. Stem Cell Res. 2018, 29, 189–196. [Google Scholar] [CrossRef]
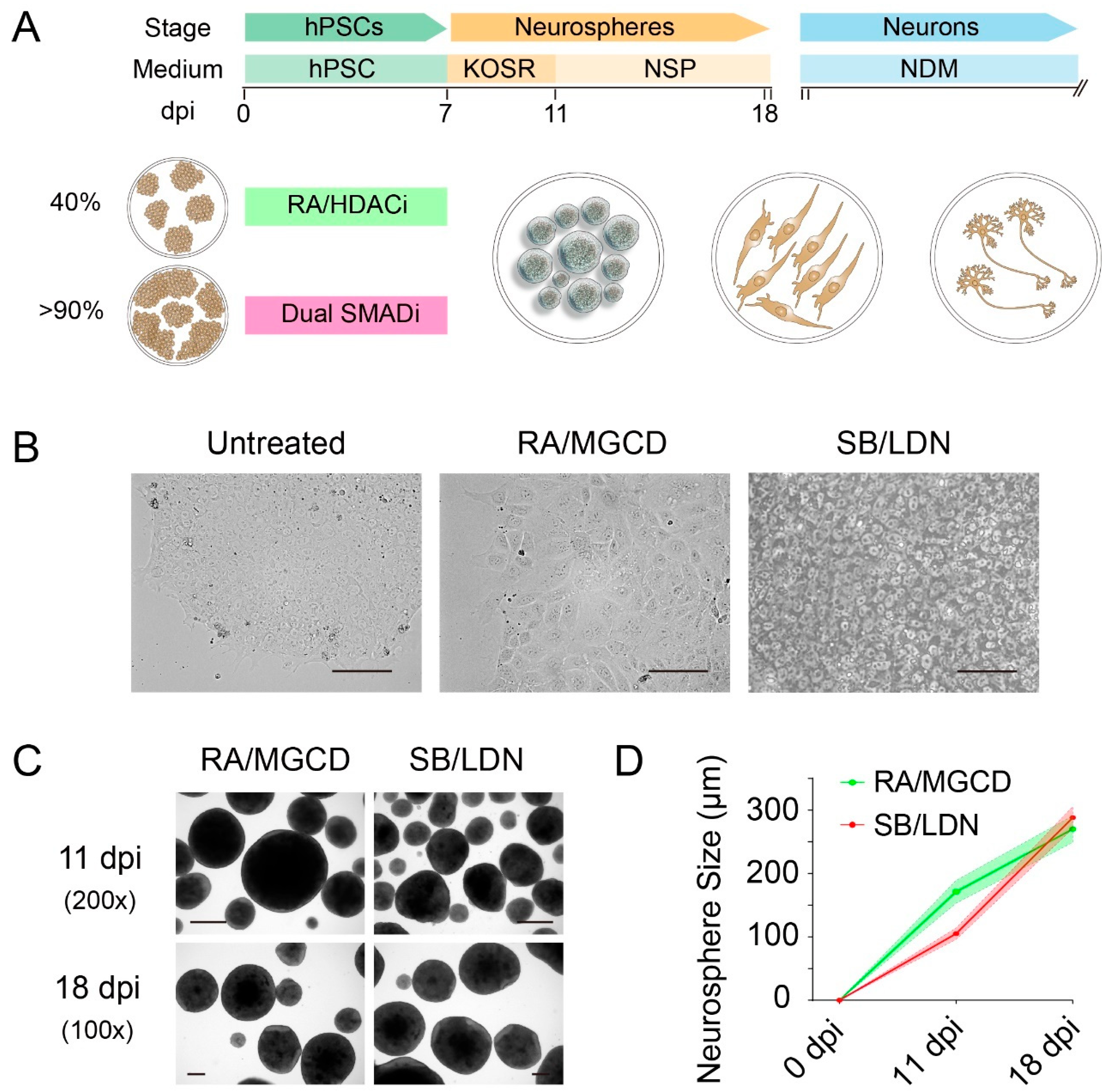

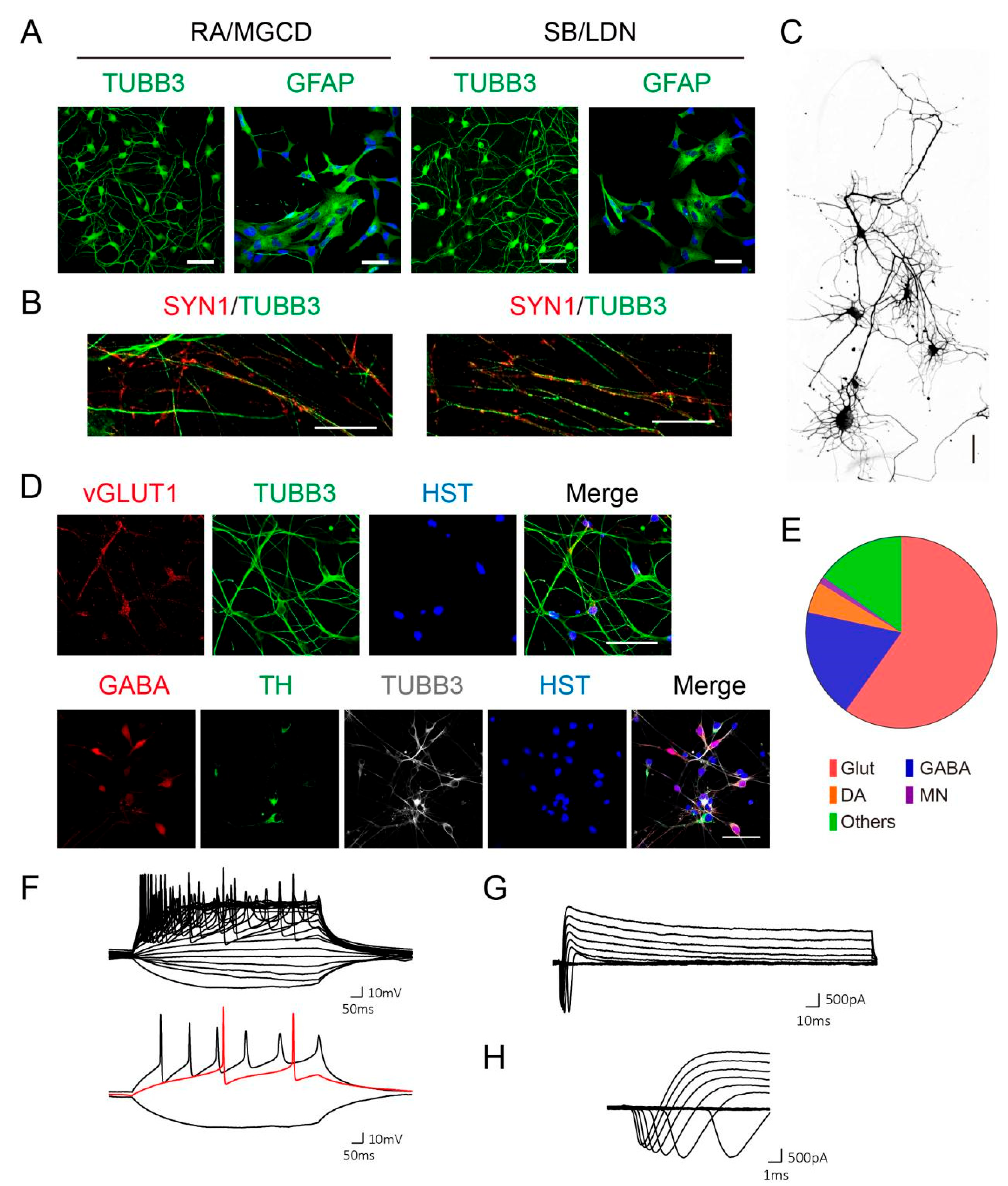

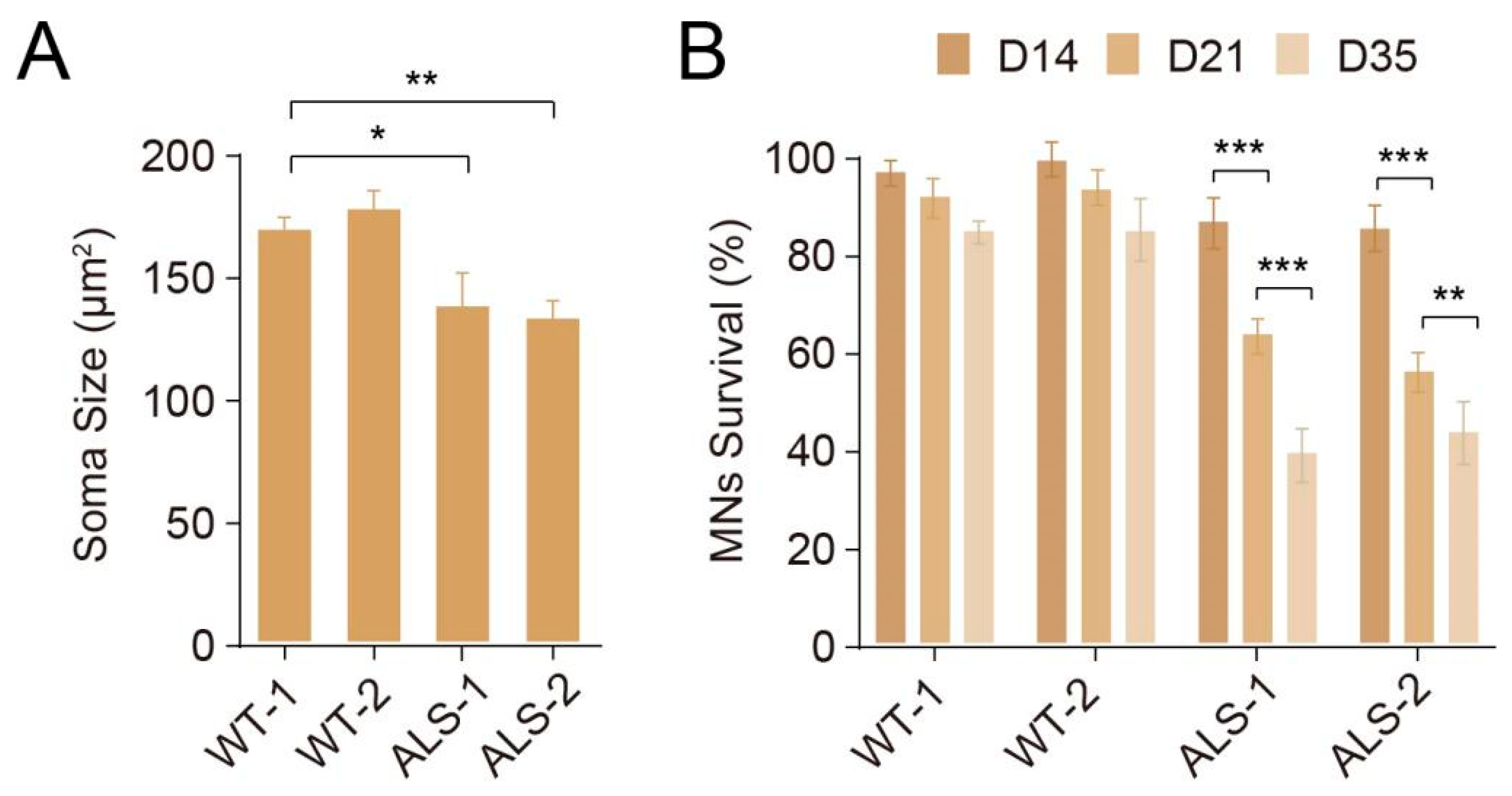
| Methods | Instructive Factors | Starting Density | Treatment Days | Purity (Pax6+/Sox2+/Nestin+) | Induction Capabilities | Differentiation Capabilities | Notes |
|---|---|---|---|---|---|---|---|
| Dual SMAD inhibition | SB431542 (10 μM) LDN193189 (0.1 μM) | >90% hPSCs | 7 days | >97% | Superior in general | Both neurons and astrocytes | Work with other patterning factors (such as purmorphamine, CHIR99021, FGF8, etc.) to generate region-specific NPCs |
| RA treatment | RA (10 μM) MGCD0103 (0.8 μM) | ~40% hPSCs | 7 days | >97% | Superior in inducing NKX2.1, but incapable of inducing SOX1 with 10 μM RA | Both neurons and astrocytes | MGCD0103 can be replaced as other HDACi such as sodium butyrate, trichostatin A and valproic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Li, C.; Zhang, M.; Wang, H.; Xie, Y.; Tang, Y. A Step-by-Step Refined Strategy for Highly Efficient Generation of Neural Progenitors and Motor Neurons from Human Pluripotent Stem Cells. Cells 2021, 10, 3087. https://doi.org/10.3390/cells10113087
Ren J, Li C, Zhang M, Wang H, Xie Y, Tang Y. A Step-by-Step Refined Strategy for Highly Efficient Generation of Neural Progenitors and Motor Neurons from Human Pluripotent Stem Cells. Cells. 2021; 10(11):3087. https://doi.org/10.3390/cells10113087
Chicago/Turabian StyleRen, Jie, Chaoyi Li, Mengfei Zhang, Huakun Wang, Yali Xie, and Yu Tang. 2021. "A Step-by-Step Refined Strategy for Highly Efficient Generation of Neural Progenitors and Motor Neurons from Human Pluripotent Stem Cells" Cells 10, no. 11: 3087. https://doi.org/10.3390/cells10113087
APA StyleRen, J., Li, C., Zhang, M., Wang, H., Xie, Y., & Tang, Y. (2021). A Step-by-Step Refined Strategy for Highly Efficient Generation of Neural Progenitors and Motor Neurons from Human Pluripotent Stem Cells. Cells, 10(11), 3087. https://doi.org/10.3390/cells10113087





