Mechanosensing and the Hippo Pathway in Microglia: A Potential Link to Alzheimer’s Disease Pathogenesis?
Abstract
1. Introduction
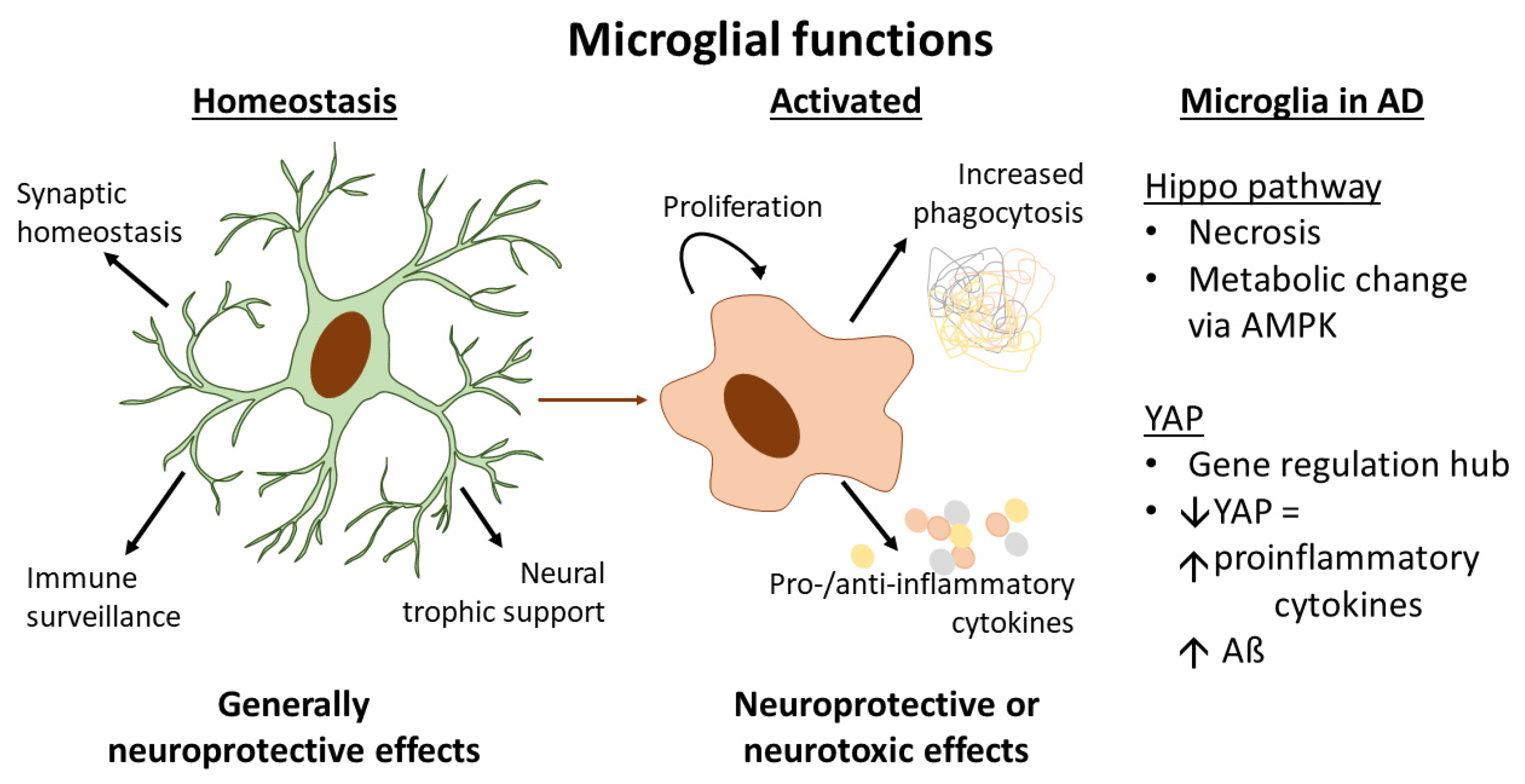
2. Microglia and Their Role in the Pathogenesis of Alzheimer’s Disease
3. Mechanosensation in Microglia
3.1. Mechanobiology of the Microglial Microenvironment in the Brain during Development and in Alzheimer’s Disease
3.2. Molecular Mechanisms of Mechanotransduction in Microglia
4. The Hippo Signalling Pathway
4.1. Activation of the Hippo Pathway by Mechanotransduction
4.2. Evidence for Hippo Signalling Pathway Function in Microglia, from Cerebral Ischaemia and Nerve Injury Studies
4.3. The Hippo Pathway in Microglia and Links to AD Pathogenesis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Chi, H.; Kinashi, T. Editorial: Hippo signaling in the immune system. Front. Immunol. 2020, 11, 587514. [Google Scholar] [CrossRef]
- Qi, Y.; Sun, D.; Yang, W.; Xu, B.; Lv, D.; Han, Y.; Sun, M.; Jiang, S.; Hu, W.; Yang, Y. Mammalian sterile 20-like kinase (MST) 1/2: Crucial players in nervous and immune system and neurological disorders. J. Mol. Biol. 2020, 432, 3177–3190. [Google Scholar] [CrossRef]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef] [PubMed]
- Boban, M.; Babić Leko, M.; Miškić, T.; Hof, P.R.; Šimić, G. Human neuroblastoma SH-SY5Y cells treated with okadaic acid express phosphorylated high molecular weight tau-immunoreactive protein species. J. Neurosci. Methods 2019, 319, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Dudiki, T.; Mahajan, G.; Liu, H.; Zhevlakova, I.; Bertagnolli, C.; Nascimento, D.W.; Kothapalli, C.R.; Byzova, T.V. Kindlin3 regulates biophysical properties and mechanics of membrane to cortex attachment. Cell. Mol. Life Sci. 2021, 78, 4003–4018. [Google Scholar] [CrossRef]
- Bennett, J.L.; Ajami, B.; Krieger, C.; Rossi, F.M.V.; Tetzlaff, W. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K. Microglial cell origin and phenotypes in health and disease. Nature reviews. Immunology 2011, 11, 775–787. [Google Scholar] [CrossRef]
- Alekseeva, O.S.; Kirik, O.V.; Gilerovich, E.G.; Korzhevskii, D.E. Microglia of the brain: Origin, structure, functions. J. Evol. Biochem. Phys. 2019, 55, 257–268. [Google Scholar] [CrossRef]
- Rio-Hortega, P.D. Microglia in Cytology and Cellularpathology of the Nervous System; Penfield, W., Ed.; Hoeber: New York, NY, USA, 1932; pp. 480–534. [Google Scholar]
- Ling, E.A.; Ling, E.A.; Penney, D.; Penney, D.; Leblond, C.P.; Leblond, C.P. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the ‘ameboid cells’ present in the corpus callosum of postnatal rats. J. Comp. Neurol. 1980, 193, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Kierdorf, K.; Prinz, M. Microglia in steady state. J. Clin. Investig. 2017, 127, 3201–3209. [Google Scholar] [CrossRef]
- Wright-Jin, E.; Gutmann, D.H. Microglia as dynamic cellular mediators of brain function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kang, J.Y.; Yun, J.I.; Chung, J.Y.; Hwang, I.K.; Won, M.H.; Choi, J.H. Age-related change of iba-1 immunoreactivity in the adult and aged gerbil spinal cord. Anat. Cell Biol. 2017, 50, 135–142. [Google Scholar] [CrossRef]
- Daneman, R. The blood–brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef]
- Ji, K.; Akgul, G.; Wollmuth, L.P.; Tsirka, S.E. Microglia actively regulate the number of functional synapses. PLoS ONE 2013, 8, e56293. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke HW, G.M.; Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Yun, H.J.; Yoon, J.H.; Lee, J.K.; Noh, K.T.; Yoon, K.W.; Oh, S.P.; Oh, H.J.; Chae, J.S.; Hwang, S.G.; Kim, E.H.; et al. Daxx mediates activation-induced cell death in microglia by triggering MST1 signalling. EMBO J. 2011, 30, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ni, H.; Rui, Q.; Gao, R.; Chen, G. Mst1: Function and mechanism in brain and myocardial ischemia reperfusion injury. Curr. Neuropharmacol. 2018, 16, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.; Lowery, R.L.; Majewska, A.K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010, 8, e1000527. [Google Scholar] [CrossRef] [PubMed]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Zhang, Y.; Seegobin, S.P.; Pruvost, M.; Wang, Q.; Purtell, K.; Zhang, B.; Yue, Z. Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat. Commun. 2020, 11, 1386. [Google Scholar] [CrossRef]
- Shen, X.-N.; Niu, L.-D.; Wang, Y.-J.; Cao, X.-P.; Liu, Q.; Tan, L.; Zhang, C.; Yu, J.-T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 590–598. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Hamelin, L.; Lagarde, J.; Dorothée, G.; Leroy, C.; Labit, M.; Comley, R.A.; de Souza, L.C.; Corne, H.; Dauphinot, L.; Bertoux, M.; et al. Early and protective microglial activation in alzheimer’s disease: A prospective study using 18F-DPA-714 PET imaging. Brain 2016, 139, 1252–1264. [Google Scholar] [CrossRef]
- Onuska, K.M. The dual role of microglia in the progression of alzheimer’s disease. J. Neurosci. 2020, 40, 1608–1610. [Google Scholar] [CrossRef]
- McQuade, A.; Blurton-Jones, M. Microglia in alzheimer’s disease: Exploring how genetics and phenotype influence risk. J. Mol. Biol. 2019, 431, 1805–1817. [Google Scholar] [CrossRef]
- Ayata, P.; Schaefer, A. Innate sensing of mechanical properties of brain tissue by microglia. Curr. Opin. Immunol. 2020, 62, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.M.; Moeendarbary, E.; Sheridan, G.K. Mechanobiology of the brain in ageing and alzheimer’s disease. Eur. J. Neurosci. 2020, 53, 3851–3878. [Google Scholar] [CrossRef]
- Bartlett, R.D.; Eleftheriadou, D.; Evans, R.; Choi, D.; Phillips, J.B. Mechanical properties of the spinal cord and brain: Comparison with clinical-grade biomaterials for tissue engineering and regenerative medicine. Biomaterials 2020, 258, 120303. [Google Scholar] [CrossRef]
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; Costa, L.D.; Guck, J.; et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Pillai, E.K.; Dimov, I.B.; Foster, S.K.; Holt, C.E.; Franze, K. Rapid changes in tissue mechanics regulate cell behaviour in the developing embryonic brain. eLife 2019, 8, e39356. [Google Scholar] [CrossRef]
- Sack, I.; Beierbach, B.; Wuerfel, J.; Klatt, D.; Hamhaber, U.; Papazoglou, S.; Martus, P.; Braun, J. The impact of aging and gender on brain viscoelasticity. NeuroImage 2009, 46, 652–657. [Google Scholar] [CrossRef]
- Smith, J.F.; Tuomas, P.J.K.; Dobson, C.M.; MacPhee, C.E.; Welland, M.E. Characterization of the nanoscale properties of individual amyloid fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 15806–15811. [Google Scholar] [CrossRef]
- Hagestedt, T.; Lichtenberg, B.; Wille, H.; Mandelkow, E.-M.; Mandelkow, E. Tau Protein Becomes Long and Stiff upon Phosphorylation: Correlation between Paracrystalline Structure and Degree of Phosphorylation. J. Cell Biol. 1989, 109, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, A.-A.; Benilova, I.; Krylychkina, O.; Braeken, D.; De Strooper, B.; Van Haesendonck, C.; Dotti, C.G.; Bartic, C. Amyloid beta oligomers induce neuronal elasticity changes in age-dependent manner: A force spectroscopy study on living hippocampal neurons. Sci. Rep. 2016, 6, 25841. [Google Scholar] [CrossRef]
- Hiscox, L.V.; Schwarb, H.; McGarry, M.D.; Johnson, C.L. Aging brain mechanics: Progress and promise of magnetic resonance elastography. NeuroImage 2021, 232, 117889. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, L.; Koser, D.E.; Shahapure, R.; Gautier, H.O.B.; Holzapfel, G.A.; Scarcelli, G.; Gather, M.C.; Ulbricht, E.; Franze, K. Microglia mechanics: Immune activation alters traction forces and durotaxis. Front. Cell. Neurosci. 2015, 9, 363. [Google Scholar] [CrossRef]
- Lo, C.; Wang, H.; Dembo, M.; Wang, Y. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Dudiki, T.; Meller, J.; Mahajan, G.; Liu, H.; Zhevlakova, I.; Stefl, S.; Witherow, C.; Podrez, E.; Kothapalli, C.R.; Byzova, T.V. Microglia control vascular architecture via a TGFβ1 dependent paracrine mechanism linked to tissue mechanics. Nat. Commun. 2020, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, S.J.; Demir, S.; König, A.; Abraham, J.; Vay, S.U.; Rabenstein, M.; Olschewski, D.N.; Hoffmann, C.; Hoffmann, M.; Hersch, N.; et al. Substrate elasticity exerts functional effects on primary microglia. Front. Cell. Neurosci. 2020, 14, 590500. [Google Scholar] [CrossRef]
- Moshayedi, P.; Ng, G.; Kwok, J.C.F.; Yeo, G.S.H.; Bryant, C.E.; Fawcett, J.W.; Franze, K.; Guck, J. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials 2014, 35, 3919–3925. [Google Scholar] [CrossRef]
- Velasco-Estevez, M.; Mampay, M.; Boutin, H.; Chaney, A.; Warn, P.; Sharp, A.; Burgess, E.; Moeendarbary, E.; Dev, K.K.; Sheridan, G.K. Infection augments expression of mechanosensing Piezo1 channels in amyloid plaque-reactive astrocytes. Front. Aging Neurosci. 2018, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, S.; Rodriguez, M.J.; Torres, Y.P. Transient receptor potential channels in microglia: Roles in physiology and disease. Neurotox. Res. 2016, 30, 467–478. [Google Scholar] [CrossRef]
- Miyake, T.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Activation of mitochondrial transient receptor potential vanilloid 1 channel contributes to microglial migration. Glia 2015, 63, 1870–1882. [Google Scholar] [CrossRef]
- Sappington, R.M.; Calkins, D.J. Contribution of TRPV1 to microglia-derived IL-6 and NFκB translocation with elevated hydrostatic pressure. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3004–3017. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, R.; Derouiche, S.; Eto, K.; Deveci, A.; Kashio, M.; Kimori, Y.; Matsuoka, Y.; Morimatsu, H.; Nabekura, J.; Tominaga, M. Thermosensitive TRPV4 channels mediate temperature-dependent microglia movement. Proc. Natl. Acad. Sci. USA 2021, 118, e2012894118. [Google Scholar] [CrossRef]
- Redmon, S.N.; Yarishkin, O.; Lakk, M.; Jo, A.; Mustafić, E.; Tvrdik, P.; Križaj, D. TRPV4 channels mediate the mechanoresponse in retinal microglia. Glia 2021, 69, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, R.G.; Abraham, S.; Niese, K.A.; Southern, B.D.; Grove, L.M.; Hite, R.D.; McDonald, C.; Hamilton, T.A.; Olman, M.A. TRPV4 mechanosensitive ion channel regulates lipopolysaccharide-stimulated macrophage phagocytosis. J. Immunol. 2016, 196, 428–436. [Google Scholar] [CrossRef]
- Meotti, F.C.; Figueiredo, C.P.; Manjavachi, M.; Calixto, J.B. The transient receptor potential ankyrin-1 mediates mechanical hyperalgesia induced by the activation of B1 receptor in mice. Biochem. Pharm. 2017, 125, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bian, W.; Yang, D.; Yang, M.; Luo, H. Inhibiting the Piezo1 channel protects microglia from acute hyperglycaemia damage through the JNK1 and mTOR signalling pathways. Life Sci. 2021, 264, 118667. [Google Scholar] [CrossRef]
- Satoh, K.; Hata, M.; Takahara, S.; Tsuzaki, H.; Yokota, H.; Akatsu, H.; Yamamoto, T.; Kosaka, K.; Yamada, T. A novel membrane protein, encoded by the gene covering KIAA0233, is transcriptionally induced in senile plaque-associated astrocytes. Brain Res. 2006, 1108, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Dai Trang, T.L.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef]
- Sahu, M.R.; Mondal, A.C. The emerging role of hippo signaling in neurodegeneration. J. Neurosci. Res. 2020, 98, 796–814. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Dong, J.; Pan, D. Hippo encodes a ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003, 114, 445–456. [Google Scholar] [CrossRef]
- Shimizu, T.; Lai, Z.; Wei, X. Mob as tumor suppressor is activated by hippo kinase for growth inhibition in drosophila. EMBO J. 2007, 26, 1772–1781. [Google Scholar] [CrossRef]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- Di Cara, F.; Maile, T.M.; Parsons, B.D.; Magico, A.; Basu, S.; Tapon, N.; King-Jones, K. The Hippo pathway promotes cell survival in response to chemical stress. Cell Death Differ. 2015, 22, 1526–1539. [Google Scholar] [CrossRef]
- Zheng, M.; Jacob, J.; Hung, S.-H.; Wang, J. The Hippo Pathway in Cardiac Regeneration and Homeostasis: New Perspectives for Cell-Free Therapy in the Injured Heart. Biomolecules 2020, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Wu, L.; Grivennikov, S.I.; De Jong, P.R.; Lian, I.; Yu, F.; Wang, K.; Ho, S.B.; Boland, B.S.; Chang, J.T.; et al. A gp130-src-YAP module links inflammation to epithelial regeneration. Nature 2015, 519, 57–62. [Google Scholar] [CrossRef]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.V.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Elbediwy, A.; Vanyai, H.; Diaz-de-la-Loza, M.D.; Frith, D.; Snijders, A.P.; Thompson, B.J. Enigma proteins regulate YAP mechanotransduction. J. Cell Sci. 2018, 131, jcs221788. [Google Scholar] [CrossRef] [PubMed]
- Ffrench-Constant, C.; Colognato, H. Integrins: Versatile integrators of extracellular signals. Trends Cell Biol. 2004, 14, 678–686. [Google Scholar] [CrossRef]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Spencer-Dene, B.; Stone, R.K.; Boeing, S.; Wculek, S.K.; Cordero, J.; Tan, E.H.; Ridgway, R.; Brunton, V.G.; et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 2016, 143, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Emoto, K. The growing role of the hippo-NDR kinase signalling in neuronal development and disease. J. Biochem. 2011, 150, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, E.; O’Driscoll, N.A.; Matallanas, D. The MST/hippo pathway and cell death: A non-canonical affair. Genes 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, Y.; Xu, S.; Reis, C.; Zhang, J. Mammalian Sterile20-like kinases: Signalings and roles in central nervous system. Aging Dis. 2018, 9, 537–552. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, S.; Dong, Y.; Yuan, Z. The role and regulatory mechanism of hippo signaling components in the neuronal system. Front. Immunol. 2020, 11, 281. [Google Scholar] [CrossRef]
- Montilla, A.; Zabala, A.; Matute, C.; Domercq, M. Functional and Metabolic Characterization of Microglia Culture in a Defined Medium. Front. Cell. Neurosci. 2020, 14, 22. [Google Scholar] [CrossRef]
- Soares, J.; Araujo, G.R.S.; Santana, C.; Matias, D.; Moura-Neto, V.; Farina, M.; Frases, S.; Viana, N.B.; Romão, L.; Nussenzveig, H.M.; et al. Membrane Elastic Properties During Neural Precursor Cell Differentiation. Cells 2020, 9, 1323. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Bhadriraju, K.; Yang, M.; Alom Ruiz, S.; Pirone, D.; Tan, J.; Chen, C.S. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp. Cell Res. 2007, 313, 3616–3623. [Google Scholar] [CrossRef]
- Roser, A.E.; Tönges, L.; Lingor, P. Modulation of Microglial Activity by Rho-Kinase (ROCK) Inhibition as Therapeutic Strategy in Parkinson’s Disease and Amyotrophic Lateral Sclerosis. Front. Aging Neurosci. 2017, 9, 94. [Google Scholar] [CrossRef]
- Saraceno, C.; Catania, M.; Paterlini, A.; Fostinelli, S.; Ciani, M.; Zanardini, R.; Binetti, G.; Di Fede, G.; Caroppo, P.; Benussi, L.; et al. Altered Expression of Circulating Cdc42 in Frontotemporal Lobar Degeneration. J. Alzheimers Dis. 2018, 61, 1477–1483. [Google Scholar] [CrossRef]
- Gu, Q.F.; Yu, J.Z.; Wu, H.; Li, Y.H.; Liu, C.Y.; Feng, L.; Zhang, G.X.; Xiao, B.G.; Ma, C.G. Therapeutic effect of Rho kinase inhibitor FSD-C10 in a mouse model of Alzheimer’s disease. Exp. Ther. Med. 2018, 16, 3929–3938. [Google Scholar] [CrossRef]
- Gómez-Nicola, D.; Fransen, N.L.; Suzzi, S.; Hugh Perry, V. Regulation of microglial proliferation during chronic neurodegeneration. J. Neurosci. 2013, 33, 2481–2493. [Google Scholar] [CrossRef]
- Nehme, N.T.; Schmid, J.P.; Debeurme, F.; André-Schmutz, I.; Lim, A.; Nitschke, P.; Rieux-Laucat, F.; Lutz, P.; Picard, C.; Mahlaoui, N.; et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 2012, 119, 3458–3468. [Google Scholar] [CrossRef]
- Bagherzadeh Yazdchi, S.; Witalis, M.; Meli, A.P.; Leung, J.; Li, X.; Panneton, V.; Chang, J.; Li, J.; Nutt, S.L.; Johnson, R.L.; et al. Hippo Pathway Kinase Mst1 Is Required for Long-Lived Humoral Immunity. J. Immunol. 2019, 202, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ding, Q.; Hu, J.; He, S.; Shi, F.; Ma, L. GPER expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav. 2016, 6, e00449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yin, J.; Zhou, L.; Yan, F.; He, Q.; Huang, L.; Peng, S.; Jia, J.; Cheng, J.; Chen, H.; et al. Hippo/MST1 signaling mediates microglial activation following acute cerebral ischemia–reperfusion injury. Brain Behav. Immun. 2015, 55, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhang, Z.; Zou, C.; Tian, Q.; Chen, X.; Hong, M.; Liu, X.; Chen, Q.; Xu, Z.; Li, M.; et al. Hippo/YAP signaling pathway mitigates blood-brain barrier disruption after cerebral ischemia/reperfusion injury. Behav. Brain Res. 2019, 356, 8–17. [Google Scholar] [CrossRef]
- Elena GArias-Salgado Lizano, S.; Sarkar, S.; Brugge, J.S.; Ginsberg, M.H.; Shattil, S.J. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl. Acad. Sci. USA 2003, 100, 13298–13302. [Google Scholar] [CrossRef]
- Xu, Z.S.; Lee, R.J.; Chu, S.S.; Yao, A.; Paun, M.K.; Murphy, S.P.; Mourad, P.D. Evidence of Changes in Brain Tissue Stiffness After Ischemic Stroke Derived From Ultrasound-Based Elastography. J. Ultrasound Med. 2013, 32, 485–494. [Google Scholar] [CrossRef]
- Li, N.; Lim, G.; Chen, L.; McCabe, M.F.; Kim, H.; Zhang, S.; Mao, J. Spinal expression of hippo signaling components YAP and TAZ following peripheral nerve injury in rats. Brain Res. 2013, 1535, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, S.W.; Lin, K.C.; Moore, J.L.; Tan, F.E.; Ma, S.; Ye, Z.; Qiu, Y.; Ren, B.; Guan, K.L. The hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem. 2018, 293, 11230–11240. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Patassini, S.; Rustogi, N.; Riba-Garcia, I.; Hale, B.D.; Phillips, A.M.; Waldvogel, H.; Haines, R.; Bradbury, P.; Stevens, A.; et al. Regional protein expression in human alzheimer’s brain correlates with disease severity. Commun. Biol. 2019, 2, 43. [Google Scholar] [CrossRef]
- Friedman, B.A.; Srinivasan, K.; Ayalon, G.; Meilandt, W.J.; Lin, H.; Huntley, M.A.; Cao, Y.; Lee, S.H.; Haddick, P.; Ngu, H.; et al. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s Disease not evident in mouse models. Cell Rep. 2018, 22, 832–847. [Google Scholar] [CrossRef]
- Qing, J.; Liu, X.; Wu, Q.; Zhou, M.; Zhang, Y.; Mazhar, M.; Huang, X.; Wang, L.; He, F. Hippo/YAP pathway plays a critical role in effect of GDNF against aβ-induced inflammation in microglial cells. DNA Cell Biol. 2020, 39, 1064–1071. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, D.; Luo, R.; Wu, Y.; Zhou, H.; Kong, L.; Bi, R.; Yao, Y.G. A systematic integrated analysis of brain expression profiles reveals YAP1 and other prioritized hub genes as important upstream regulators in alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 215–229. [Google Scholar] [CrossRef]
- Blalock, E.M.; Buechel, H.M.; Popovic, J.; Geddes, J.W.; Landfield, P.W. Microarray analyses of laser-captured hippocampus reveal distinct gray and white matter signatures associated with incipient alzheimer’s disease. J. Chem. Neuroanat. 2011, 42, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Homma, H.; Fujita, K.; Kondo, K.; Yamada, S.; Jin, X.; Waragai, M.; Ohtomo, G.; Iwata, A.; Tagawa, K.; et al. YAP-dependent necrosis occurs in early stages of alzheimer’s disease and regulates mouse model pathology. Nat. Commun. 2020, 11, 507. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, X.; Xu, M.; Fujita, K.; Motoki, K.; Sasabe, T.; Homma, H.; Murata, M.; Tagawa, K.; Tamura, T.; et al. Targeting TEAD/YAP-transcription-dependent necrosis, TRIAD, ameliorates Huntington’s disease pathology. Hum. Mol. Genet. 2016, 25, 4749–4770. [Google Scholar] [CrossRef]
- Yamanishi, E.; Hasegawa, K.; Fujita, K.; Ichinose, S.; Yagishita, S.; Murata, M.; Tagawa, K.; Akashi, T.; Eishi, Y.; Okazawa, H. A novel form of necrosis, TRIAD, occurs in human Huntington’s disease. Acta Neuropathol. Commun. 2017, 5, 19. [Google Scholar] [CrossRef]
- Kery, R.; Chen, A.P.F.; Kirschen, G.W. Genetic targeting of astrocytes to combat neurodegenerative disease. Neural Regen. Res. 2020, 15, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, Y.; Hu, G.; Zhou, J.; Mei, L.; Xiong, W. YAP is a critical inducer of SOCS3, preventing reactive astrogliosis. Cereb. Cortex 2016, 26, 2299–2310. [Google Scholar] [CrossRef]
- Makowski, L.; Chaib, M.; Rathmell, J.C. Immunometabolism: From basic mechanisms to translation. Immunol. Rev. 2020, 295, 5–14. [Google Scholar] [CrossRef]
- Cheng, S.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 346. [Google Scholar] [CrossRef]
- Kalsbeek, M.J.T.; Mulder, L.; Yi, C. Microglia energy metabolism in metabolic disorder. Mol. Cell. Endocrinol. 2016, 438, 27–35. [Google Scholar] [CrossRef]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.; Mook-Jung, I. A breakdown in metabolic reprogramming causes microglia dysfunction in alzheimer’s disease. Cell Metab. 2019, 30, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Lauro, C.; Chece, G.; Monaco, L.; Antonangeli, F.; Peruzzi, G.; Rinaldo, S.; Paone, A.; Cutruzzolà, F.; Limatola, C. Fractalkine modulates microglia metabolism in brain ischemia. Front. Cell. Neurosci. 2019, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, A.; Lupse, B.; Maedler, K. Hippo signaling: Key emerging pathway in cellular and whole-body metabolism. Trends Endocrinol. Metab. 2018, 29, 492–509. [Google Scholar] [CrossRef]
- Koo, J.H.; Guan, K. Interplay between YAP/TAZ and metabolism. Cell Metab. 2018, 28, 196–206. [Google Scholar] [CrossRef]
- Wang, X.; Zimmermann, H.R.; Ma, T. Therapeutic potential of AMP-activated protein kinase in Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 68, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiao, Z.; Li, X.; Aziz, K.E.; Gan, B.; Johnson, R.L.; Chen, J. AMPK modulates hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 2015, 17, 490–499. [Google Scholar] [CrossRef]
- Bays, J.L.; Campbell, H.K.; Heidema, C.; Sebbagh, M.; DeMali, K.A. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat. Cell Biol. 2017, 19, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Sun, J.; Lau, A.; Curtis, S.; Goldsmith, J.; Fox, V.L.; Wei, C.; Frazier, M.; Samson, O.; Wong, K.K.; et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat. Cell Biol. 2014, 16, 108–117. [Google Scholar] [CrossRef]
- Khalafalla, F.G.; Greene, S.; Khan, H.; Ilves, K.; Monsanto, M.M.; Alvarez, R., Jr.; Chavarria, M.; Nguyen, J.; Norman, B.; Dembitsky, W.P.; et al. P2Y2 Nucleotide Receptor Prompts Human Cardiac Progenitor Cell Activation by Modulating Hippo Signaling. Circ. Res. 2017, 121, 1224–1236. [Google Scholar] [CrossRef]
- de Diego García, L.; Sebastián-Serrano, Á.; Hernández, I.H.; Pintor, J.; Lucas, J.J.; Díaz-Hernández, M. The regulation of proteostasis in glial cells by nucleotide receptors is key in acute neuroinflammation. FASEB J. 2018, 32, 3020–3032. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L. Disease implication of hyper-hippo signalling. Open Biol. 2016, 6, 160119. [Google Scholar] [CrossRef] [PubMed]
- Gogia, N.; Chimata, A.; Deshpande, P.; Singh, A.; Singh, A. Hippo signaling: Bridging the gap between cancer and neurodegenerative disorders. Neural Regen. Res. 2021, 16, 643–652. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, L.; Karagil, S.; Mahmood, A.; Elbediwy, A.; Stolinski, M.; Mackenzie, F.E. Mechanosensing and the Hippo Pathway in Microglia: A Potential Link to Alzheimer’s Disease Pathogenesis? Cells 2021, 10, 3144. https://doi.org/10.3390/cells10113144
Bruno L, Karagil S, Mahmood A, Elbediwy A, Stolinski M, Mackenzie FE. Mechanosensing and the Hippo Pathway in Microglia: A Potential Link to Alzheimer’s Disease Pathogenesis? Cells. 2021; 10(11):3144. https://doi.org/10.3390/cells10113144
Chicago/Turabian StyleBruno, Lucrezia, Simge Karagil, Almas Mahmood, Ahmed Elbediwy, Michael Stolinski, and Francesca E. Mackenzie. 2021. "Mechanosensing and the Hippo Pathway in Microglia: A Potential Link to Alzheimer’s Disease Pathogenesis?" Cells 10, no. 11: 3144. https://doi.org/10.3390/cells10113144
APA StyleBruno, L., Karagil, S., Mahmood, A., Elbediwy, A., Stolinski, M., & Mackenzie, F. E. (2021). Mechanosensing and the Hippo Pathway in Microglia: A Potential Link to Alzheimer’s Disease Pathogenesis? Cells, 10(11), 3144. https://doi.org/10.3390/cells10113144





