Abstract
Immunotherapy targeting the PD-1–PD-L1 axis yielded good results in treating different immunologically ‘‘hot’’ tumors. A phase II study revealed good therapeutic activity of pembrolizumab in selected prostatic carcinoma (PC)-patients. We performed a systematic literature review (PRISMA guidelines), which analyzes the immunohistochemical expression of PD-L1 in human PC samples and highlights the pre-analytical and interpretation variables. Interestingly, 29% acinar PCs, 7% ductal PCs, and 46% neuroendocrine carcinomas/tumors were PD-L1+ on immunohistochemistry. Different scoring methods or cut-off criteria were applied on variable specimen-types, evaluating tumors showing different clinic-pathologic features. The positivity rate of different PD-L1 antibody clones in tumor cells ranged from 3% (SP142) to 50% (ABM4E54), excluding the single case tested for RM-320. The most tested clone was E1L3N, followed by 22C3 (most used for pembrolizumab eligibility), SP263, SP142, and 28-8, which gave the positivity rates of 35%, 11–41% (depending on different scoring systems), 6%, 3%, and 15%, respectively. Other clones were tested in <200 cases. The PD-L1 positivity rate was usually higher in tumors than benign tissues. It was higher in non-tissue microarray specimens (41–50% vs. 15%), as PC cells frequently showed heterogenous or focal PD-L1-staining. PD-L1 was expressed by immune or stromal cells in 12% and 69% cases, respectively. Tumor heterogeneity, inter-institutional preanalytics, and inter-observer interpretation variability may account for result biases.
1. Introduction
Prostatic carcinoma (PC) is one of the most common cancers in men, with a 5-year survival rate of 30% at advanced stage [1,2,3,4]. As for other tumors, the implementation of a molecular-based classification and the development of targeted-therapies are increasingly relevant topics of discussion and research, also concerning the possibility to make easy-to-use and cost-effective surrogate biomarkers available worldwide for diagnostic and therapeutic purposes [5].
Programmed death-1 (PD-1) and its ligands (PD-L1 and PD-L2) are type I transmembrane glycoproteins of the CD28/CTLA-4 and B7 families, respectively [6,7]. Microenvironmental conditions may influence the expression of PD-1 by hematopoietic cells (activated T, B, NK cells, and monocytes) and of the two ligands by hematopoietic and non-hematopoietic cells, by modulating their interaction and the subsequent activation of intracellular signaling cascades [6,7].
Immune checkpoint therapy targeting the PD-1–PD-L1 axis has yielded good results in the standard treatment of different immunologically ‘‘hot’’ tumors (such as pulmonary non-small cell carcinoma, renal or bladder cancer, melanoma, etc.). Multiple clinical trials demonstrated sustained survival benefit, sometimes with complete regression of the metastatic disease [6,7,8]. Conversely, immunologically “cold” neoplasms seem relatively resistant to immune checkpoint therapy in some series, as they may show a relatively low somatic mutation frequency and few tumor-infiltrating T cells [6,7,8]. Some studies have been performed on the usually “cold” metastatic castration-resistant prostate cancer (mCRPC), with limited results; treatment doses and schedules could be implemented, especially in selected subgroups of patients, and larger series of patients should be recruited [4,8]. Recently, a phase II study (KEYNOTE-199) seemed to reveal a good therapeutic activity of pembrolizumab (an anti-PD-1 antibody) in monotherapy, and the 2021 United States National Comprehensive Cancer Network (NCCN) guidelines have allowed the use of this drug in selected PC-patients [4,9].
To better clarify the role of PD-L1 expression in PC, we performed a systematic literature review, analyzing the results of human tissue-based studies (immunohistochemical, molecular, etc.), experimental researches (cell lines, mouse models), and clinical trials. As there were many data to discuss, we have split the presentation of our results into different articles, focusing on different sub-topics. In this part, we present the literature data concerning the PD-L1 immunohistochemical expression in human PC samples, highlighting the pre-analytical and interpretation variables.
2. Materials and Methods
We conducted our systematic literature review according to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines (http://www.prisma-statement.org/, accessed on 8 May 2021) (Figure 1).
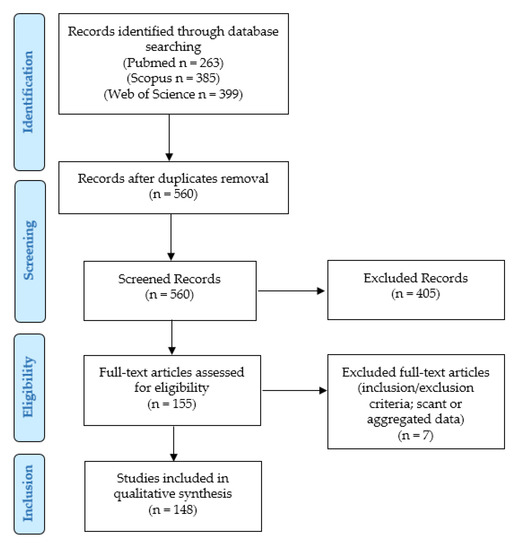
Figure 1.
Review of the literature: PRISMA flow-chart.
Our aim was to update and summarize the literature concerning the role of PD-L1 in PC and to report any clinic-pathologic information of the published cases. We tried to answer the following “Population, Intervention, Comparison, Outcomes” (PICO) questions:
- Population: Patients, tumor cell lines, or mouse models included in studies concerning the role of PD-L1 in PC.
- Intervention: Any type of treatment.
- Comparison: No comparisons were expected.
- Outcomes: Patient’s status at the last follow-up (no evidence of disease, alive with disease, dead of disease), response to therapy, biochemical recurrence-free survival, metastasis-free survival, cancer-specific survival, disease-free survival, clinical failure-free survival, overall-survival, and progression-free survival. For experiments on PC cell lines and mouse models: any reported effects on cancer and immune cell migration, proliferation, viability, growth, resistance or response to therapy, cytotoxic or anti-tumor activity, PD-L1 expression, and mice/cell lines survival.
Our research was designed as a retrospective observational study:
- Eligibility/inclusion criteria: experimental studies (tumor cell lines, mouse models) or clinic-pathologic studies on human patients (case series, case reports, clinical trials) concerning the role of PD-L1 in PC.
- Exclusion criteria: non-prostatic tumors; non-carcinomatous histotypes; studies not examining PD-L1; cases with uncertain diagnosis; review articles without new cases.
We searched for (PD-L1 AND (prostate OR prostatic) AND (adenocarcinoma OR adenocarcinomas OR cancer)) in Pubmed (all fields; 263 results; https://pubmed.ncbi.nlm.nih.gov, accessed on 8 May 2021), Web of Science (Topic/Title; 399 results; https://login.webofknowledge.com, accessed on 8 May 2021), and Scopus (Title/Abstract/Keywords; 385 results; https://www.scopus.com/home.uri, accessed on 8 May 2021) databases. No limitations or additional filters were set. The bibliographic research ended on 8 May 2021. After duplicates were excluded, 560 records underwent first-step screening by two independent reviewers, who checked the titles and abstracts of the articles to verify the satisfaction of the eligibility/inclusion criteria. They excluded clearly irrelevant studies, allowing articles of doubtful relevance to proceed to the following step, in order to avoid the potential missing of pertinent papers. The full texts of the 155 eligible articles were retrieved, and read by two other authors: (1) to look for additional relevant references; and (2) to confirm the satisfaction of the inclusion criteria. After their evaluation, seven papers were excluded, as they were unfit according to the inclusion criteria, or because they presented scant or aggregated data. Two other authors checked the extracted data, and 148 articles were finally included in our study [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155].
Data collection was study-related (authors and year of study publication) and case-related (PD-L1 expression; tumor stage at presentation; Grade Group/Gleason score; type of specimen; treatment; test methods; and results of PD-L1 expression, follow-up, outcomes, and experiment type).
Statistical analysis: the collected data were reported as continuous or categorical variables. Categorical variables were analyzed by frequencies and percentages; continuous variables were summarized by ranges, mean, and median values. Time-to-recurrence was the time from primary treatment to disease recurrence. The survival status was the time from primary treatment to the last follow-up.
3. Results
3.1. PD-L1 Immunohistochemical Expression in Tumor Tissue: Benign Glands vs. Adenocarcinoma
Haffner et al. [66] found that benign atrophic glands (especially those associated with inflammation) were frequently PD-L1-positive. Conversely, PD-L1 expression was rarely reported in benign prostatic tissue or hyperplasia in the remaining studies: Sharma et al., 1/201 (0.5%) cases [27,35]; Richter et al., 1/24 (4%) cases (focal expression) [55]; and Sun et al., 0/30 (0%) cases [13].
In some studies, PD-L1 expression was significantly higher in PCs of all stages compared to benign prostatic hyperplasia or normal prostatic tissue (Wagle et al., p < 0.0001 [28]; Sharma et al., p < 0.001 [35]). Richter et al. also found focal PD-L1 staining in <1% cells of a prostatic intraepithelial neoplasia [55].
Scimeca et al. described a significant increase in the number of PD-L1+ (p = 0.0047) and PTX3+ (p < 0.0001) cells in PCs (vs. benign lesions) [48]. Hahn et al. [57] reported that the immune infiltrates were higher in PCs than benign prostatic tissues (lymphocytes p < 0.001, macrophages p = 0.01).
3.2. PD-L1 Immunohistochemical Expression in Prostate Cancer
Table 1 summarizes the results of the studies concerning the immunohistochemical expression of PD-L1 in tumor cells of acinar PCs. Further details of the cases are provided in Supplementary Materials Table S1.

Table 1.
PD-L1 immunohistochemical expression in tumor cells of prostatic acinar adenocarcinomas.
1369/4708 (29%) PCs were overall classified as positive but different antibody clones, scoring systems, and/or cut-offs for positivity were used, with a range as wide as 0–100% in single studies. 687/2676 (26%) cases were considered PD-L1-positive if at least ≥1% of tumor cells were stained [8,13,27,29,35,36,37,38,39,41,42,43,54,62,66,67,74,75,77,78,79,84], while 93/1062 (9%) PCs were counted as positive for ≥5% of stained tumor cells [17,39,43,57,66,94,98,99,100,120]. 9/523 (2%) cases expressed PD-L1 on >50% of tumor cells [66,92], while Wang et al. used a cut-off of ≥25% (0/21, 0%) [61]. The remaining studies applied different scoring criteria, also including tumor proportion score (TPS) (9/51 PD-L1+ PCs, 18%) [59] or combined positive score (CPS ≥ 1: 362/464 cases, 78%) [9,154].
In other series, it was unclear how many PCs were tested or resulted PD-L1+. In human tumors, mutations in the PD-L1 C-tail are mutually exclusive with mutations in the substrate-interacting MATH domain of the “Speckle-type BTB/POZ protein” (SPOP) [73]. In the series of Zhang et al. (n = 97), 80% of 15 SPOPmutant tumors exhibited strong PD-L1 staining (clone 29E.12B1), compared to only 10% of 82 SPOPwild-type PCs. SPOP-deficient PCs correlated with increased PD-L1 expression and a decreased number of tumor infiltrating lymphocytes (TILs) [73]. In another large series [47], PD-L1 was expressed at lower levels than PD-L2 across all 9393 radical prostatectomy samples (tissue microarrays, TMA) (p < 0.001). The authors obtained data from six previously published retrospective cohorts (n = 1567) and one prospective cohort (n = 47826), but the method of PD-L1 testing and scoring was not fully disclosed. Using a median value cut-off, other researchers [89,93] found that PD-L1 was highly expressed in 486/820 (59%) PCs of their series, positively correlating with elevated (>median value) Ki-67 index and AR expression (p < 0.001).
Calagua et al. [75] reported 18/130 (14%) PD-L1+ hormone-naïve PCs associated with increased CD3+, CD8+, and PD-1+ TILs. Tumor cells showed three patterns of PD-L1 positivity:
- “scattered” pattern (10 cases, 56%) (small clusters);
- “nodular” pattern (7 cases, 39%) (distinct nodules): the PD-L1high and PD-L1low components showed consistently concordant ERG status but variable PTEN staining patterns;
- “interface” pattern (1 case, 5%): positive tumor cells were enriched at the tumor–stroma interface bordering the leading edge of a tumor nodule.
In their series, all the three examined PCs treated with neoadjuvant abiraterone acetate, prednisone and leuprolide revealed the “scattered” pattern of PD-L1 staining on tumor cells [75].
The positivity rate of different PD-L1 antibody clones in PC cells ranged from 3% (SP142) to 50% (ABM4E54), excluding the single case tested for RM-320. The most tested clone was E1L3N, followed by 22C3, SP263, SP142, and 28-8. The positivity rates were 35%, 11–41% (depending on different scoring systems), 6%, 3%, and 15%, respectively. The other clones were tested in less than 200 cases (Table 2).

Table 2.
PD-L1 antibody clones: positivity rate in tumor cells.
TILs were variably present in histologic PC specimens; few studies reported a quantification and characterization of the inflammatory infiltrate. Some authors described 156/396 (39%) cases showing sparse intratumoral PD-1+ lymphocytes (few intraepithelial) [42,79]. Iacovelli et al. [39] found macrophages and CD3+ T-lymphocytes in 10% of PD-L1− cases and in 21% of PD-L1+ PCs, while neutrophils were rarely observed (two cases) and only 1/5 PCs showed scattered PD-L1+ dendritic cells. Petitprez et al. [77] reported a significant association of PD-L1 expression with CD8+ T cell density (p = 0.040), but not with CD20+ B-cell density. In another study [50], FOXP3+, CD3+, and CD8+ T cells were observed in 74.6% (47/63), 98.4% (62/63), and 100% (61/61) of the cases, respectively. Finally, Massari et al. found TILs in 11/16 (69%) cases [92].
PD-L1+ immune cells (mainly TILs) were identified in 76/634 (12%) cases [21,27,32,35,41,51,57,67,74,90]. Sater et al. [34] reported that neoadjuvant prostate specific-antigen (PSA)-targeted vaccination with PROSTVAC prior to radical prostatectomy enhanced T cell infiltration into the tumor microenvironment of PCs. PD-L1 expression (by immunofluorescence) was focal in the immune (macrophage-like) cells, and the PD-L1+ status correlated to higher post/pre-ratio of immune-cell infiltrates. CD4+ T cells were significantly increased in post-vaccination radical prostatectomy specimens compared to baseline biopsies by non-compartmentalized analysis. By compartmentalized analysis, an increase in CD4+ T cells at the tumor infiltrative margins, as well as of CD8+ T cells at the tumor core, was noted in post-vaccination specimens rather than in baseline biopsies. In addition, 13/25 (52%) patients developed peripheral T cell responses to any of the three tested tumor-associated antigens (PSA, brachyury, MUC-1).
PD-L1 was expressed by stromal cells in 349/505 (69%) cases [42,50,62,79]. Mo et al. [50] found that 69/73 (94.5%) regionally localized PCs (RLPCs) and 2/7 (29%) CRPCs were positive for PD-L1 in tumor-associated stroma (nerve branches), supported by colocalization with axonal marker PGP9.5. The density of PD-L1+ tumor-associated nerves inversely correlated to that of CD8+ TILs. In another study [42,79], the expression of PD-L1+ intratumoral stromal cells (267/402, 66%) was significantly associated with PD-L1+ tumor cells (p < 0.001), showing a weak correlation with intratumoral PD-1+ lymphocytes (p < 0.001). Li et al. [44] also reported that stroma and TILs partially expressed PD-L1: PD-1 was mainly positive at the membrane of the stromal infiltrating lymphocytes (31/127 cases, 24.4%).
3.3. PD-L1 Immunohistochemical Expression in Different Specimen-Types of Prostatic Adenocarcinoma
PD-L1 expression was investigated in different types of specimens, including biopsies, radical prostatectomies (RPs), transurethral prostatic resections (TURPs), metastases, and/or material retrieved from autopsies. Finally, TMAs were used by some authors [27,29,35,39,41,53,66,67,74,78,83,92,112,120]. This method allowed the evaluation of larger series, but reduced the number of tumor cells evaluable for PD-L1, which usually showed heterogeneous or focal expression. In many series, the tested specimen-type was not specified. Other studies analyzed different samples, globally reporting the positivity rate of PD-L1 without highlighting the data for each specimen type. Finally, some articles just described the results of the statistical comparisons among various subgroups, without providing detailed information for each of them (Table S1). Haffner et al. [66] reported no variation of the PD-L1 positivity rate in their TMA series (n = 508) despite including different samples (core biopsies, surgical resections, post-mortem rapid autopsies), while Shaw et al. found a significantly higher PD-L1 expression in resection specimens compared to biopsies (p = 0.045; n = 63) [36].
When data were available and analyzable, the PD-L1 positivity rate of non-TMA specimens was 50% (240/483) for biopsies [9,11,12,21,32,62,90] and 41% (587/1427) for RPs [18,38,42,43,44,57,61,75,79,94]. In TMA series (variably including RPs, biopsies, TURPs, metastases, autopsies), the expression rate was lower (258/1728, 15%) [27,29,35,39,41,53,66,67,74,78,83,92,112,120]. Considering only RPs, PD-L1 was more frequently positive in non-TMA specimens (587/1427, 41%) than in TMA-RPs (130/762, 17%) (total: 717/2189, 33%) [18,27,29,35,38,42,43,44,57,61,67,75,79,92,94,112,120].
3.4. PD-L1 Immunohistochemical Expression in Rare PC-Histotypes
Previous data referred to the acinar histotype of PC, which is by far the most common [1]. Only few studies focused on PD-L1 expression in rare PC-histotypes:
- Ductal carcinoma (8/119 PD-L1+ cases, 7%) [36,41,66,78].
In all the studies, positivity was assessed for ≥1% of tumor cells stained for PD-L1. The positivity rate ranged from 3% to 17% among various series. It was higher for clone SP263 (5/58, 9%: Lindh et al., 1/34, 3% [41]; Haffner et al., 4/24, 17% [66]), while clone E1L3N resulted positive only in 2/34 (6%) cases [78], and an unspecified clone was used by Shaw et al. (1/27 PD-L1+ cases, 4%) [36]. In addition, Hashimoto et al. reported 2/110 (2%) PD-L1+ cases: the series included 105 acinar, 4 ductal, and 1 signet ring PC, but it was unclear which histotypes resulted positive [85]. Lindh et al. [41] found 10/34 (29%) ductal adenocarcinomas showing PD-L1+ tumor-infiltrating cells (positivity in 1–20% of cells), including the case with PD-L1+ tumor cells, while PD-L2 was negative in both tumor cells and tumor-infiltrating immune cells in all cases. Ductal adenocarcinomas showed a significantly increased number of CD8+ lymphocytes compared to acinar adenocarcinomas (p = 0.04).
- Neuroendocrine carcinomas (25/48 PD-L1+ cases, 46%).
In the abstract of Sun et al. [111], PD-L1 was rarely expressed in primary acinar PCs, while increased rates of PD-L1 positivity were observed in prostatic small cell neuroendocrine carcinomas (SCNCs); however, the number of tested samples was unclear. In a subsequent paper [13], PD-L1 positivity was observed only in the SCNC group (n = 16) (which were N-cadherin-positive), while other samples seemed not to express PD-L1 (30 benign prostates, 15 “low level” adenocarcinomas, 15 “high level” adenocarcinomas, 18 CRPCs). “Low”- and “high”-level adenocarcinomas were not defined, while the PD-L1 clone and scoring systems were unclear.
Von Hardenberg et al. [52] tested a series including 7 adenocarcinomas with neuroendocrine differentiation (ND), 20 SCNCs, 2 well-differentiated neuroendocrine tumors (NET), and 10 adenocarcinomas. PD-L1 positivity (>1% of PD-L1+ tumor cells; clone E1L3N) was identified in 5/24 (20.8%) cases, but it was unclear which histotypes resulted positive. High PD-L1 expression was observed in 4/11 (36.4%) specimens. TILs (≥10%) were found in 9/24 (37.5%) cases. All the PCs were negative for clone 22C3. On gene expression analysis, positive PD-L1 status was observed in hot spots of 11 (61.1%) cases (4/5 adenocarcinomas with ND, 6/12 SCNC, 1/1 NET, and 4/4 adenocarcinoma). In addition, the PD-L1 mRNA expression in hot spots or TILs had no prognostic significance.
The neuroendocrine PC of Massari et al. revealed strong expression of CD3, PD-1, and PD-L1 (anti PD-L1 clone, Sino Biological, Eschborn, Germany; weak/strong positivity in >50% tumor cells) [92].
Finally, Haffner et al. found 3/7 (42.9%) PD-L1+ SCNCs (clone SP263) [66].
- Carcinosarcoma (1/1, 100%; clone SP142).
In the abstract of Sun et al. [111], PD-L1 was rarely expressed in primary acinar PCs, PCs, Salvi et al. [60] reported a prostatic carcinosarcoma in which the epithelial component was PD-L1-positive (“high” expression; Gleason Score 9, 4+5, Grade Group 5), while the sarcomatous areas were negative.
3.5. Evaluation of PD-L1 Expression Density in Tumor Tissue
Few studies [15,25,26,28,31,34,40,48] (Table S2) evaluated the PD-L1 expression density by counting the number of PD-L1+ tumor and/or immune cells in variably analyzed areas. Different methods of analysis and scoring were used. Moreover, the difference in PD-L1 expression (percentage of positive cells, intensity of staining) between tumor and immune cells groups was frequently unclear. In fact, the different cell types were cumulatively counted in some articles, while the analyzed cell type was unclear in other papers; thus, it was sometimes difficult to understand which cells resulted positive for PD-L1.
Ryan et al. [26] found that PD-L1 staining primarily occurred in tertiary lymphoid structures of high-risk PCs, while a non-significant decrease in the mean PD-L1 density was observed in rituximab-treated tissues (n = 8) vs. control samples (n = 11) (p = 0.36).
Scimeca et al. reported a significant increase in the number of PD-L1+ (p = 0.0047) and PTX3+ (p < 0.0001) cells in PCs (n = 50) (vs. benign lesions). PD-L1+ PCs showed a decrease in PD-1+ lymphocytes and tumor-infiltrating macrophages (mainly M2 subpopulation), while PTX3 expression inversely correlated to the number of PD-L1+ PC cells [48].
Kazantseva et al. [40] generated three different groups of PCs (n = 122) by clustering analysis. Compared to the other two groups, group A (43 cases, 35%) had significantly more PD-L1+ cells (p = 0.01), higher Δ133TP53 and TP53β, a significantly higher number of infiltrating T cells (CD3+, CD4+, and/or CD8+) and CD20+ B cells, as well as an increased number of PD-1+ T cells. Group A also showed significantly higher number of infiltrating CD163+ macrophages (compared to Group C), and higher Ki67 index (compared to Group B). Group C had less perineural invasion than Group B and lower Gleason Score than Groups A/B.
In another study [28], high tumor fusion burden (number of fusions/10,000 genes) correlated to high immune infiltration, PD-L1 expression on immune cells (negative on tumor cells), and immune signatures of T cells and M1 macrophages activation; conversely, it inversely correlated to immune suppressive signatures. Only late metastatic samples (n = 3) showed ≥10% of PD-L1 + inflammatory cells.
Ihle et al. reported higher PD-L1 expression in blastic (n = 5) than in lytic bone PC metastases (n = 10) [31].
Brady et al. [15] analyzed 27 mCRPC with digital spatial gene expression profiling. PD-L1 protein levels were not detectable above the background in any tumor or tumor regions of interest (ROI), and CTLA4/PD-1 expression were below measurable levels in >90% of ROI.
By using fluorescent immunohistochemistry on TMAs, Vicier et al. [25] analyzed the total density of PD-L1 positivity in 109 PCs. CD8 low and/or PD-L1high expression was associated with shorter time to biochemical recurrence and metastasis-free survival (median 10.8 vs. 18.4 years) compared to CD8high/PD-L1low cases. However, this association was not significant on multivariate analysis.
4. Discussion
The implementation of a molecular-based classification and the development of targeted therapies are increasingly relevant topics of discussion and research in numerous tumors, especially regarding the search for cost-effective, surrogate diagnostic, prognostic, and treatment-predictive biomarkers [1,2,3,4,5,156,157,158,159,160,161,162,163,164,165]. Promising pre-clinical models, early clinical results, and some case reports provided proof of concept for considering selected mCRPC patients as suitable candidates for treatment with immune checkpoint blockade [4,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155]. Moreover, PC is one of the first adult solid tumors demonstrating to benefit from a cancer vaccine, further supporting the potential usefulness for immunotherapy. The 2021 NCCN guidelines have allowed immunotherapy in men with asymptomatic or minimally symptomatic mCRPCs [4]. Sipuleucel-T is a dentritic cell-based autologous cancer vaccine approved by the Federal Drug Administration (FDA) and the NCCN guidelines for treatment of mCRPCs. However, the survival benefit and efficacy are modest, and further progress is required in understanding which patients may benefit from treatment [4].
As better delineated in other parts of our review (see the end of the Discussion section), experimental studies revealed that the intracellular ERK/MEK, Akt-mTOR, NF-kB, WNT, and JAK/STAT pathways are involved in PD-L1 upregulation in PC cells. PD-L1 interacts with its receptor PD-1, which can be expressed by inflammatory cells (T, B, NK, myeloid, etc.), favoring the process of immune evasion by tumor cells. The PD-1–PD-L1 interaction inhibits the T cell receptor signaling, suppressing the activity and proliferation of cytotoxic TILs, and promoting the immunosuppressive effects of regulatory T cells. Moreover, the PD-1–PD-L1 axis influences all the components of the tumor microenvironment, regulating the inhibition of NK cells (which can kill tumor cells directly), the secretion of cytokines, and the immunosuppressive effects of tumor-associated macrophages, dendritic cells, and myeloid-derived suppressive cells.
Blocking the PD-1–PD-L1 signaling can prevent tumor immune escape, and increase the anti-tumor activity of immune cells, despite some clinical trials reported limited or controversial benefits (especially if including unselected patients) [4,108,123,126]. Pembrolizumab can be administered to men with docetaxel- or hormone-resistant mCRPCs showing high microsatellite instability or mismatch repair system deficiency (MSI-H/MMR-d), or tumor mutation burden >10 mutations/Mb [4]. However, MSI-H/MMR-d PCs occur with a low frequency, and the immunotherapy administration is still limited in the routine management of PC-patients [4]. Moreover, the MMR-d/MSI-H status does not necessarily correlate to the response to immunotherapy [46,59].
The NCCN guidelines do not report the need of a histochemical evaluation of PD-L1 in PCs and there are no clear indications concerning the PD-L1 clone to use for evaluating PC samples [4]. So, PD-L1 expression may not be useful alone for patient selection in PCs. The focal PD-L1 positivity may not predict the responsiveness of the tumor, and clinical studies reported PD-L1+ PCs resistant to immunotherapy, as well as PD-L1 cases responding to treatment. Moreover, in some clinical trials, not all the samples were tested for PD-L1 or it was unclear how many cases were analyzed by immunohistochemistry [9,21,24,32,62,90,116]. Other immune checkpoints or targets that may be clinically relevant in PC (VISTA, PARP, etc.) could help for the selection of patients. Indeed, reliable biomarkers should guide the selection of patients for clinical trials, monitor treatment efficacy, and allow the development of more effective combinatorial strategies. Multiple targeting may improve the immunogenicity of the “cold” PC tumor cells. In other parts of our review, we will discuss the results of clinical trials testing anti-PD-1–PD-L1 drugs, as of studies investigating both the PD-L1 and MMR-d/MSI-H status.
Our systematic review on the expression of PD-L1 in PC highlights a high heterogeneity across studies [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155], causing limitations to our analysis.
However, these results provide ground to discuss various issues related to the assessment methodologies and processes of PD-L1 expression by PC in clinical practice.
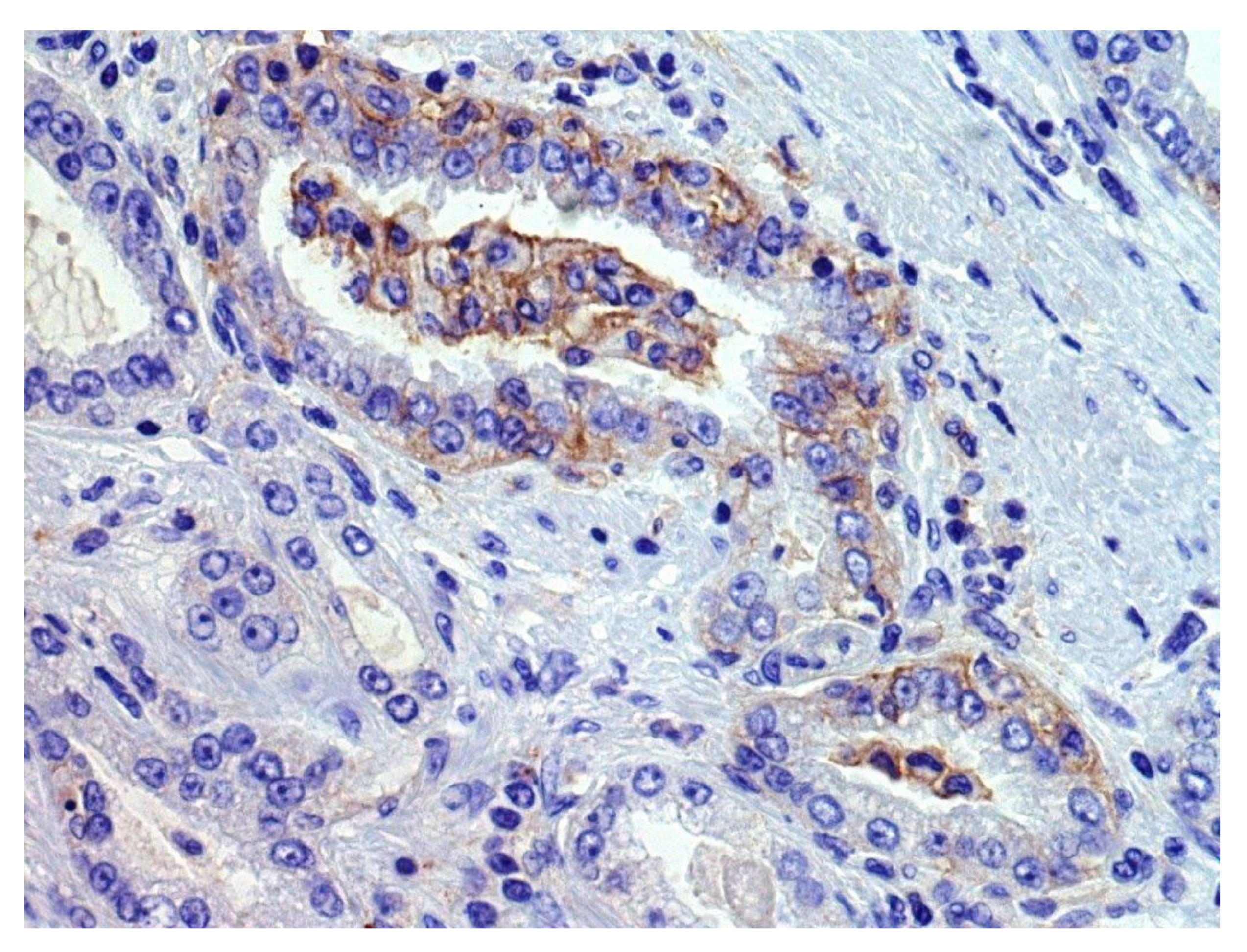
We found that 29% of acinar PCs, 7% of ductal PCs, and 46% of neuroendocrine carcinomas or tumors were classified as PD-L1-positive by immunohistochemical analysis on tumor tissue, despite these results may be influenced by some selection biases [8,9,11,12,13,17,21,27,29,32,35,36,37,38,39,41,42,43,44,50,51,52,53,54,55,57,59,60,61,62,66,67,74,75,77,78,79,83,84,85,89,90,92,93,94,98,99,100,111,112,120,154]. In fact, when reported, different scoring systems (CPS, TPS, semiquantitive analysis, percentage of positive cells × staining intensity, etc.) and cut-offs for positivity (<1%, ≥1%, ≥5%, ≥25%, ≥50%, ≥ median value, etc.) were applied. Various types of specimens were tested (radical prostatectomies, transurethral resections of the prostate, biopsies, autopsy samples; primary vs. metastatic tumors), revealing variable positivity rates also among the different sampled sites of a single patient. Moreover, PCs showing different clinic-pathologic features (Grade Group, stage, treatment type, etc.) were tested, but the potential association of PD-L1 expression with clinic-pathologic features was not always clearly investigated. In addition, various PD-L1 antibody clones were rarely simultaneously tested on the same series. Considering the heterogenous or focal expression of PD-L1 by PC cells (Figure 2), the use of TMA reduced the number of evaluable cells, probably causing false-negative results in some cases; conversely, some trials included only PD-L1+ cases, causing a selection bias for the abovementioned positivity rates [154].
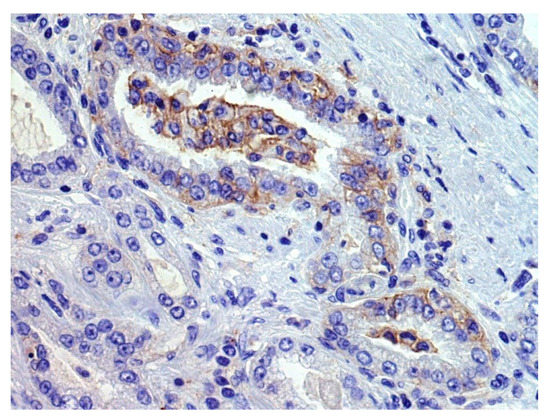
Figure 2.
An example of focal membranous PD-L1 immunohistochemical staining (previously unpublished, original photo; magnification 400×, Clone 22C3, Dako).
Inter-observer variability in the interpretation of PD-L1 immunohistochemical staining may frequently occur, but it could be decreased by the support of inter-institutional teams of experts, training, and quality controls [166]. An issue in assessing PD-L1 expression in PC samples may be represented by the concomitant staining of both immune (lymphocytes, plasma cells, granulocytes, mast cells) and stromal cells, which may be a confounding factor, especially when both the tumor and immune cell compartments need to be evaluated in a composite score. Our results showed that PD-L1+ immune cells (mainly TILs) were found in 12% cases, and that PD-L1 was expressed by stromal cells in as many as 69% cases. Moreover, T-helper lymphocytes were significantly increased in post-vaccination radical prostatectomy specimens compared to baseline biopsies in one study [34].
Plasticity of the immune response and the effects of the different tissue microenvironment components were not simultaneously evaluated in most of the PD-L1 testing series. The studies were usually retrospective, rarely providing multicenter validation cohorts.
Several clones of the PD-L1 antibody are currently feasible for immunohistochemical testing and are used in clinic-pathologic studies and clinical trials to identify patients that can benefit from immunotherapy. Few clones have been approved as companion or complementary diagnostics in various tumors [166]. However, there are different PD-L1 assays, each specific to a therapeutic drug for disparate tumors, without a common standard [166]. Moreover, various scoring systems are applied to different tumor types and indications. Finally, some issues are difficult to solve, including tumor heterogeneity of PD-L1 expression, inter-institutional preanalytics, and inter-observer variability in interpretation of the results [156,162,166].
Clone 22C3 is typically tested in non-small cell lung cancer (NSCLC) or gastro-esophageal adenocarcinoma for pembrolizumab treatment, clone 28-8 for nivolumab therapy in melanoma, clone SP263 for durvalumab administration in NSCLC, and clone SP142 for atezolizumab use in triple negative breast cancer [166]. Discrepancies in indications are also present among different countries. In the United States, the FDA restricted the application of pembrolizumab for advanced NSCLCs tested using the DAKO 22C3 pharmDx assay, while the DAKO platform is not as widely used in Europe, and the European Medicines Agency (EMA) did not demand a mandatory specific PD-L1 immunohistochemical platform or clone [166]. As regards to genito-urinary tumors, clones SP263, SP142, 28-8, and 22C3 have been approved as assays to identify urothelial carcinoma patients that are most likely to benefit from various immunomodulants. To select eligible patients, each clone should be evaluated on the basis of specific score system and/or cut-off criteria (SSCC), according to current guidelines based on the results from large clinical trials: Atezolizumab (SP142; SSCC: ≥5% of positive immune cells); avelumab, durvalumab, and tislelizumab (SP263; SSCC: ≥25% of positive tumor or immune cells); nivolumab (28-8; SSCC: ≥1% of positive tumor cells); and pembrolizumab (22C3; CPS score) [167,168]. Such discrepancies in the recommended algorithms of evaluation reveal defective harmonization of PD-L1 immunohistochemical testing.
As we previously said, the NCCN guidelines do not report the need of a histochemical evaluation of PD-L1 in PCs and there are no clear indications concerning the PD-L1 clone to use for evaluating PC samples [4]. In the studies analyzed in our review, the most frequently tested clone was E1L3N, followed by 22C3, SP263, SP142, and 28-8. Other clones were tested in less than 200 cases (Table 2). Of them, E1L3N, SP263, and SP142 target epitopes in the cytoplasmic domain, which is identical for SP263 and SP142. Conversely, 22C3, 28-8, and 5H1 target distinct epitopes within the extracellular domain [169]. Recently, Lawson et al. [169] performed linear and discontinuous epitope mapping of the binding sites of PD-L1 antibodies by peptide array. Their analysis revealed that immunohistochemical results are not significantly altered by differences in epitope binding.
Based on the results of our review, the positivity rate of different PD-L1 antibody clones in tumor cells ranged from 3% (SP142) to 50% (ABM4E54), excluding the single case tested for RM-320. The positivity rate of clone 22C3 (the most used to assess eligibility for administration of pembrolizumab) was 11% after excluding cases with a CPS score of ≥1 (as it was unclear if tumor cells resulted positive), 41% after including those cases, and 78% only considering the cases scored as CPS ≥1 [9,12,13,21,27,32,35,51,77,80,83,90,154].
In recent years, some evidence from the literature has suggested that substantial concordance in both staining results [170,171] and clinical outcomes [170,172] was yielded by the three FDA-approved clones (22C3, 28-8, and SP263) when tested on NSCLC specimens. Limited similar data are also available for urothelial carcinoma [173].
However, the performance of different diagnostic anti PD-L1 antibody clones in other tumors (such as head and neck squamous cell carcinoma) is less robust and interchangeable [166]. In our analysis, the staining rates of these three clones were 11–41%, 15%, and 6%, respectively; these results (with the abovementioned caveats of clone 22C3) may seem lower than for other clones, which, in turn, yielded ≥35% rates (E1L3N: 35%, PA5-18337: 39%; ABM4E54: 50%; 5H1: 46%; anti-PD-L1: 47%) with the exception of SP142 (3%). Calagua et al. [75] found that E1L3N and SP142 clones demonstrated excellent concordance (correlation coefficient r = 0.90; n = 174), while antibody 405.9A11 showed the lowest sensitivity (suboptimal).
Some studies have reported details on the staining pattern and/or intensity of each antibody, although substantial differences have been found in both cases, potentially affecting the final PD-L1 scoring [167]. Calagua et al. [75] showed three patterns of PD-L1 expression in PCs but further analysis was not performed in subsequent papers. In their study, clone E1L3N consistently stained nerve fibers, unlike SP142 or SP263 [75]. In contrast, SP142 showed non-specific staining in luminal secretions and in the cytoplasm of benign and malignant glands (absent with E1L3N). In another paper [74], clone SP263 showed a stronger membranous staining in tumor cells than clone E1L3N, but only five PD-L1 positive cases were found.
Features intrinsic to the tissue sample should be taken into account in assessing PD-L1 expression, including biological intratumor heterogeneity (as abovementioned) and pre-analytical variables, which may affect the final results [166]. While the former is largely unpredictable, the latter should be standardized by following strict laboratory protocols [167]. However, differences in staining concordance and interpretation may occur among various institutes [166,174]. Recently, Angels et al. [175] reported a significant rate of suboptimal PD-L1 immunostaining (clones SP142 and SP263) in tonsillectomy specimens exposed to more than 72 h in formaldehyde. As for other tumor tissues, overfixation may influence the staining results. Standardization of the type of platform and testing method by using proper positive and negative controls are of pivotal importance at this regard. It is advisable to provide all the technical information, along with the results and the used scoring system, in the final pathological report [167].
Finally, systematic literature reviews (SLRs) and meta-analyses have become increasingly important in health care as clinicians read SLRs to keep themselves up-to-date. Moreover, SLRs are often a starting point for developing clinical practice guidelines or further studies/trials, while granting agencies may require data of SLRs to justify research financial supports. For these reasons, medical journals frequently ask contributing authors to conduct their SLRs according to the PRISMA (http://www.prisma-statement.org/; accessed on 8 May 2021) guidelines (or similar), which include an evidence-based minimum set of items for reporting and are useful for the critical evaluation of the submitted manuscripts [176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206]. So, we have conducted our SLR according to these guidelines, as previously described in various topics/contexts in which they are applicable and could provide relevant benefits in dissemination of knowledge [176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206]. To better examine the multiple interesting facets of the role of PD-L1 in PCs, we have divided the discussion of our results into different articles. In the other papers, the readers will find further information on sub-topics not discussed in the present article, including correlations of PD-L1 expression with clinic-pathologic features in PC patients; genetic and epigenetic regulation of PD-L1; PD-L1 intracellular signaling pathways in PC and influence of the tumor microenvironment; data of pre-clinical studies (cell lines, mouse models) about the effects of experimental treatments on PD-L1 expression in PC cells; investigated correlations of PD-L1 expression with the status of mismatch repair system, BRCA, PTEN, and other main genes in PC; PD-L1 expression in liquid biopsy samples; and information of clinical trials [207,208,209,210].
5. Conclusions
In this part of our review, we present some results that could be helpful for the design of further studies or trials. We have summarized the data retrieved from our SLR, which was performed according to the PRISMA guidelines, by searching in multiple databases. Immunohistochemical PD-L1 positivity was usually focal in the tumor cells of about 29% acinar PCs, 7% ductal PCs, and 46% neuroendocrine carcinomas/tumors, despite some limitations. Most of the available data were related to the acinar type of PC. Additional studies are required, especially for rare PC histotypes, such as ductal and neuroendocrine carcinomas, with the latter showing a higher PD-L1 positivity rate.
The assessment of PD-L1 immunohistochemical expression and the resulting positivity rate are influenced by various factors, including different antibody clones, scoring systems and cut-off criteria for positivity, tumor heterogeneity, sample type (primary vs. metastatic tumors, RPs, biopsies, autopsies, TMAs), inter-observer variability in interpretation, and other pre-analytical variables (fixation, etc.). This information was sometimes lacking or unclear, also in some clinical trials. Further studies are required to clarify the role (and the need) of PD-L1 immunohistochemical testing in PCs, and the eventual difference in clinical outcome among cases with limited vs. diffuse expression, also paying attention to differences in scoring systems. Indeed, in the analyzed studies, only tumor cells were usually evaluated, while the CPS score (also including immune cells in the count) was rarely applied.
The 2021 NCCN guidelines have allowed pembrolizumab administration in selected MSI-H/MMR-d mCRPC patients, progressing through prior docetaxel and/or novel hormone therapy [4]. However, these guidelines do not currently report the need of an immunohistochemical evaluation of PD-L1 in PC, and there are no clear indications concerning the PD-L1 clone to use for evaluating PC samples. Reasons for this lack of indication could include the following: (1) PC is a “cold” tumor sometimes showing limited intratumoral inflammation and response to immunotherapy; (2) some immunohistochemically PD-L1+ tumors may not respond to immunotherapy, while PD-L1 cases can respond; (3) the frequently focal PD-L1 immunohistochemical expression (positivity was usually assessed for ≥1% of stained cells) could not be a reliable predictor of the tumor responsiveness to immunotherapy; (4) it is unclear which is the best sample to test, as the positivity rate varies among different types of specimen; (5) data about the immunohistochemically tested cases (number of analyzed PCs; correlation with some clinic-pathologic features; and the abovementioned pre-clinical and interpretation variables) were occasionally missing or limited in some clinical studies/trials (sometimes lacking multicenter validation cohorts).
Future researches should clearly provide details of these pre-analytical variables, which can also be taken into consideration in making comparisons between the results of the different analyzed groups. However, PD-L1 immunohistochemical expression could be confirmed not to be useful (at least alone) to select PC patients for the inclusion in clinical trials. Other immune checkpoints or targets that may be clinically relevant in PCs should be investigated, supporting the trial selection and/or allowing the development of more effective combinatorial strategies for treatment. The intracellular ERK/MEK, Akt-mTOR, NF-kB, WNT, and JAK/STAT pathways are involved in PD-L1 upregulation in PC cells. An estimate of expression of PD-L1 and of markers involved in these (and other) signaling pathways may be combined in future clinical studies. Multiple targeting may improve the immunogenicity of PC.
Supplementary Materials
The following Tables are available online at https://www.mdpi.com/article/10.3390/cells10113166/s1. Table S1: Details of the cases tested by immunohistochemistry. Table S2: Evaluation of PD-L1 expression density in tumor tissue.
Author Contributions
Conceptualization, A.P., M.B., S.C., F.S., C.M.-G., S.C.-P., A.C. (Alcides Chaux), and M.Z. (Magda Zanelli); methodology, A.P., M.B., S.C., M.R., C.M.-G., S.C.-P., A.C. (Alcides Chaux), and M.P.B.; validation, A.P., A.C. (Alberto Cavazza), D.A., C.M.-G. and S.C.-P.; formal analysis, A.T., C.M.-G., A.P., D.A., D.M.B. and F.S.; investigation, A.C. (Alcides Chaux), A.P., S.C. and M.B.; resources, A.P., M.Z. (Maurizio Zizzo), and A.S.; data curation, A.T., A.C. (Alcides Chaux), A.P., S.A. and C.C.R.; writing—original draft preparation, A.P., M.B., S.C., F.S. and M.Z. (Magda Zanelli); writing—review and editing, C.M.-G., G.S., B.M., A.P., M.B., S.C., D.A., D.M.B., A.B. and E.Z.; visualization, A.T., D.D.B. and B.M.; supervision, C.M.-G., A.P., D.M.B., D.A., A.D.L., A.C. (Alberto Cavazza), and M.L.; project administration, A.P., G.G., J.G., L.C. and G.C.; funding acquisition, A.P. and M.Z. (Magda Zanelli). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Andrea Palicelli thanks his family for personal support. Daniel M. Berney is supported by Orchid and a PCUK grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moch, H.; Reuter, V.E. WHO Classification of Tumours of the Urinary System and Male Genital Organs; International Agency for Research on Cancer: Lyon, France, 2016.
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual; Springer International Publishing: Berlin, Germany, 2018. [Google Scholar]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Programs. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 29 May 2021).
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Prostate Cancer. Version 2.2021—17 February 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 29 May 2021).
- Santandrea, G.; Piana, S.; Valli, R.; Zanelli, M.; Gasparini, E.; De Leo, A.; Mandato, V.D.; Palicelli, A. Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature. Diagnostics 2021, 11, 199. [Google Scholar] [CrossRef]
- Dai, S.; Jia, R.; Zhang, X.; Fang, Q.; Huang, L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell. Immunol. 2014, 290, 72–79. [Google Scholar] [CrossRef]
- Patsoukis, N.; Wang, Q.; Strauss, L.; Boussiotis, V.A. Revisiting the PD-1 pathway. Sci. Adv. 2020, 6, eabd2712. [Google Scholar] [CrossRef]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, X.; He, H.; Peng, S.; Zhang, Y.; Zhang, J.; Cheng, L.; Liu, S.; Huang, M.; Xie, R.; et al. WD repeat domain 5 promotes chemoresistance and Programmed Death-Ligand 1 expression in prostate cancer. Theranostics 2021, 11, 4809–4824. [Google Scholar] [CrossRef]
- Vardaki, I.; Corn, P.; Gentile, E.; Song, J.H.; Madan, N.; Hoang, A.; Parikh, N.; Guerra, L.; Lee, Y.C.; Lin, S.C.; et al. Radium-223 Treatment Increases Immune Checkpoint Expression in Extracellular Vesicles from the Metastatic Prostate Cancer Bone Microenvironment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 3253–3264. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.H.; Kwon, J.E.; Park, S.G.; Choo, S.H.; Kim, S.J.; Kim, S.I. Cell membrane and nuclear expression of programmed death ligand-1 in prostate needle biopsy tissue in prostate cancer patients undergoing primary radiation therapy. Urol. Oncol. 2021, 39, 298.e13–298.e20. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jing, J.; Xu, H.; Xu, L.; Hu, H.; Tang, C.; Liu, S.; Wei, Q.; Duan, R.; Guo, J.; et al. N-cadherin inhibitor creates a microenvironment that protect TILs from immune checkpoints and Treg cells. J. Immunother. Cancer 2021, 9, e002138. [Google Scholar] [CrossRef]
- Zavridou, M.; Strati, A.; Bournakis, E.; Smilkou, S.; Tserpeli, V.; Lianidou, E. Prognostic Significance of Gene Expression and DNA Methylation Markers in Circulating Tumor Cells and Paired Plasma Derived Exosomes in Metastatic Castration Resistant Prostate Cancer. Cancers 2021, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- Brady, L.; Kriner, M.; Coleman, I.; Morrissey, C.; Roudier, M.; True, L.D.; Gulati, R.; Plymate, S.R.; Zhou, Z.; Birditt, B.; et al. Inter- and intra-tumor heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling. Nat. Commun. 2021, 12, 1426. [Google Scholar] [CrossRef]
- Zhang, T.; Agarwal, A.; Almquist, R.G.; Runyambo, D.; Park, S.; Bronson, E.; Boominathan, R.; Rao, C.; Anand, M.; Oyekunle, T.; et al. Expression of immune checkpoints on circulating tumor cells in men with metastatic prostate cancer. Biomark. Res. 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Loriot, Y.; Shaffer, D.R.; Braiteh, F.; Powderly, J.; Harshman, L.C.; Conkling, P.; Delord, J.P.; Gordon, M.; Kim, J.W.; et al. Safety and Clinical Activity of Atezolizumab in Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase I Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 3360–3369. [Google Scholar] [CrossRef]
- Imamura, R.; Kitagawa, S.; Kubo, T.; Irie, A.; Kariu, T.; Yoneda, M.; Kamba, T.; Imamura, T. Prostate cancer C5a receptor expression and augmentation of cancer cell proliferation, invasion, and PD-L1 expression by C5a. Prostate 2021, 81, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhou, Y.; Lu, X.; Bian, Z.; Chen, Y.; Zhou, J.; Zhang, L.; Hao, Z.; Zhang, M.; Liang, C. Immune response drives outcomes in prostate cancer: Implications for immunotherapy. Mol. Oncol. 2021, 15, 1358–1375. [Google Scholar] [CrossRef]
- Wong, J.K.; MacFarlane, A.; Devarajan, K.; Shulman, R.M.; Alpaugh, R.K.; Burbure, N.; Hallman, M.A.; Geynisman, D.M.; Horwitz, E.M.; Campbell, K.; et al. Hypofractionated Short Course Radiation Treatment Results in Systemic Immune Activation and Upregulation of the PD-1/PD-L1 Exhaustion Axis: A Prospective Pilot Study in Early Stage Prostate Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S120. [Google Scholar] [CrossRef]
- Graff, J.N.; Beer, T.M.; Alumkal, J.J.; Slottke, R.E.; Redmond, W.L.; Thomas, G.V.; Thompson, R.F.; Wood, M.A.; Koguchi, Y.; Chen, Y.; et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J. Immunother. Cancer 2020, 8, e000642. [Google Scholar] [CrossRef]
- Chen, Q.H.; Li, B.; Liu, D.G.; Zhang, B.; Yang, X.; Tu, Y.L. LncRNA KCNQ1OT1 sponges miR-15a to promote immune evasion and malignant progression of prostate cancer via up-regulating PD-L1. Cancer Cell Int. 2020, 20, 394. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, Y.; Yu, H.; Lin, S.H.; Tu, H.; Liang, D.; Chang, D.W.; Huang, M.; Wu, X. Immune checkpoint-related serum proteins and genetic variants predict outcomes of localized prostate cancer, a cohort study. Cancer Immunol. Immunother. CII 2021, 70, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Li, Y.R.; Roach, M., 3rd; Aggarwal, R. Dramatic response to combination pembrolizumab and radiation in metastatic castration resistant prostate cancer. Ther. Adv. Med Oncol. 2020, 12, 1758835920936084. [Google Scholar] [CrossRef]
- Vicier, C.; Ravi, P.; Kwak, L.; Werner, L.; Huang, Y.; Evan, C.; Loda, M.; Hamid, A.A.; Sweeney, C.J. Association between CD8 and PD-L1 expression and outcomes after radical prostatectomy for localized prostate cancer. Prostate 2021, 81, 50–57. [Google Scholar] [CrossRef]
- Ryan, S.T.; Zhang, J.; Burner, D.N.; Liss, M.; Pittman, E.; Muldong, M.; Shabaik, A.; Woo, J.; Basler, N.; Cunha, J.; et al. Neoadjuvant rituximab modulates the tumor immune environment in patients with high risk prostate cancer. J. Transl. Med. 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Yang, Z.; Miyamoto, H. Loss of DNA mismatch repair proteins in prostate cancer. Medicine 2020, 99, e20124. [Google Scholar] [CrossRef]
- Wagle, M.C.; Castillo, J.; Srinivasan, S.; Holcomb, T.; Yuen, K.C.; Kadel, E.E.; Mariathasan, S.; Halligan, D.L.; Carr, A.R.; Bylesjo, M.; et al. Tumor Fusion Burden as a Hallmark of Immune Infiltration in Prostate Cancer. Cancer Immunol. Res. 2020, 8, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, A.Z.; Dallos, M.C.; Zahurak, M.L.; Partin, A.W.; Schaeffer, E.M.; Ross, A.E.; Allaf, M.E.; Nirschl, T.R.; Liu, D.; Chapman, C.G.; et al. T-Cell Infiltration and Adaptive Treg Resistance in Response to Androgen Deprivation with or Without Vaccination in Localized Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Walle, T.; Cornish, A.E.; Basu, S.; Anandhan, S.; Fernandez, I.; Vence, L.; Blando, J.; Zhao, H.; Yadav, S.S.; et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat. Med. 2020, 26, 39–46. [Google Scholar] [CrossRef]
- Ihle, C.L.; Provera, M.D.; Straign, D.M.; Smith, E.E.; Edgerton, S.M.; Van Bokhoven, A.; Lucia, M.S.; Owens, P. Distinct tumor microenvironments of lytic and blastic bone metastases in prostate cancer patients. J. Immunother. Cancer 2019, 7, 293. [Google Scholar] [CrossRef]
- Ross, A.E.; Hurley, P.J.; Tran, P.T.; Rowe, S.P.; Benzon, B.; Neal, T.O.; Chapman, C.; Harb, R.; Milman, Y.; Trock, B.J.; et al. A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 184–193. [Google Scholar] [CrossRef]
- Bryce, A.H.; Dronca, R.S.; Costello, B.A.; Infante, J.R.; Ames, T.D.; Jimeno, J.; Karp, D.D. PT-112 in advanced metastatic castrate-resistant prostate cancer (mCRPC), as monotherapy or in combination with PD-L1 inhibitor avelumab: Findings from two phase I studies. J. Clin. Oncol. 2020, 38, 83. [Google Scholar] [CrossRef]
- Abdul Sater, H.; Marté, J.L.; Donahue, R.N.; Walter-Rodriguez, B.; Heery, C.R.; Steinberg, S.M.; Cordes, L.M.; Chun, G.; Karzai, F.; Bilusic, M.; et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. J. Immunother. Cancer 2020, 8, e000655. [Google Scholar] [CrossRef]
- Sharma, M.; Yang, Z.; Miyamoto, H. Immunohistochemistry of immune checkpoint markers PD-1 and PD-L1 in prostate cancer. Medicine 2019, 98, e17257. [Google Scholar] [CrossRef]
- Shaw, K.C.; Calagua, C.; Russo, J.; Einstein, D.; Balk, S.; Ye, H. Tumor PD-L1 Expression is Detected in a Significant Subset of High-Risk Localized and Metastatic Prostate Cancer but is Rare in Ductal Subtype. Abstracts from USCAP 2019: Genitourinary Pathology (including renal tumors) (776–992). Mod. Pathol. 2019, 32, 143–144. [Google Scholar]
- Matveev, V.B.; Kirichek, A.A.; Safronova, V.M.; Khafizov, K.O.; Filippova, M.G.; Lyubchenko, L.N. Impact of PD-L1 status on the long-term outcomes of radical treatment of patients with prostate cancer. Urologiia 2019, 4, 51–57. [Google Scholar] [CrossRef]
- Matveev, V.; Kirichek, A.; Safronova, V.; Kokosadze, N.; Khalmurzaev, O.; Kamolov, B.; Liubchenko, L. The prognostic value of tumor PD-L1 status in patients with metastatic prostate cancer. Cancer Urol. 2019, 15, 57–65. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Brunelli, M.; Bogina, G.; Munari, E.; Bimbatti, D.; Mosillo, C.; Fantinel, E.; Bria, E.; Martignoni, G.; et al. PD-L1 Expression in De Novo Metastatic Castration-sensitive Prostate Cancer. J. Immunother. 2019, 42, 269–273. [Google Scholar] [CrossRef]
- Kazantseva, M.; Mehta, S.; Eiholzer, R.A.; Gimenez, G.; Bowie, S.; Campbell, H.; Reily-Bell, A.L.; Roth, I.; Ray, S.; Drummond, C.J.; et al. The Δ133p53β isoform promotes an immunosuppressive environment leading to aggressive prostate cancer. Cell Death Dis. 2019, 10, 631. [Google Scholar] [CrossRef]
- Lindh, C.; Kis, L.; Delahunt, B.; Samaratunga, H.; Yaxley, J.; Wiklund, N.P.; Clements, M.; Egevad, L. PD-L1 expression and deficient mismatch repair in ductal adenocarcinoma of the prostate. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2019, 127, 554–560. [Google Scholar] [CrossRef]
- Richardsen, E.; Andersen, S.; Al-Saad, S.; Rakaee, M.; Nordby, Y.; Pedersen, M.I.; Ness, N.; Ingebriktsen, L.M.; Fassina, A.; Taskén, K.A.; et al. Low Expression of miR-424–3p is Highly Correlated with Clinical Failure in Prostate Cancer. Sci. Rep. 2019, 9, 10662. [Google Scholar] [CrossRef] [PubMed]
- Xian, P.; Ge, D.; Wu, V.J.; Patel, A.; Tang, W.W.; Wu, X.; Zhang, K.; Li, L.; You, Z. PD-L1 instead of PD-1 status is associated with the clinical features in human primary prostate tumors. Am. J. Clin. Exp. Urol. 2019, 7, 159–169. [Google Scholar] [PubMed]
- Li, H.; Wang, Z.; Zhang, Y.; Sun, G.; Ding, B.; Yan, L.; Liu, H.; Guan, W.; Hu, Z.; Wang, S.; et al. The Immune Checkpoint Regulator PDL1 is an Independent Prognostic Biomarker for Biochemical Recurrence in Prostate Cancer Patients Following Adjuvant Hormonal Therapy. J. Cancer 2019, 10, 3102–3111. [Google Scholar] [CrossRef]
- Pal, S.K.; Moreira, D.; Won, H.; White, S.W.; Duttagupta, P.; Lucia, M.; Jones, J.; Hsu, J.; Kortylewski, M. Reduced T-cell Numbers and Elevated Levels of Immunomodulatory Cytokines in Metastatic Prostate Cancer Patients De Novo Resistant to Abiraterone and/or Enzalutamide Therapy. Int. J. Mol. Sci. 2019, 20, 1831. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Zhao, S.G.; Lehrer, J.; Chang, S.L.; Das, R.; Erho, N.; Liu, Y.; Sjöström, M.; Den, R.B.; Freedland, S.J.; Klein, E.A.; et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. J. Natl. Cancer Inst. 2019, 111, 301–310. [Google Scholar] [CrossRef]
- Scimeca, M.; Bonfiglio, R.; Urbano, N.; Cerroni, C.; Anemona, L.; Montanaro, M.; Fazi, S.; Schillaci, O.; Mauriello, A.; Bonanno, E. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. 2019, 37, 297.e19–297.e31. [Google Scholar] [CrossRef]
- Jung, K.H.; LoRusso, P.; Burris, H.; Gordon, M.; Bang, Y.J.; Hellmann, M.D.; Cervantes, A.; Ochoa de Olza, M.; Marabelle, A.; Hodi, F.S.; et al. Phase I Study of the Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC-0919) Administered with PD-L1 Inhibitor (Atezolizumab) in Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3220–3228. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.J.; Han, Z.D.; Liang, Y.K.; Ye, J.H.; Wu, S.L.; Lin, S.X.; Zhang, Y.Q.; Song, S.D.; Jiang, F.N.; Zhong, W.D.; et al. Expression of PD-L1 in tumor-associated nerves correlates with reduced CD8(+) tumor-associated lymphocytes and poor prognosis in prostate cancer. Int. J. Cancer 2019, 144, 3099–3110. [Google Scholar] [CrossRef] [PubMed]
- Papanicolau-Sengos, A.; Yang, Y.; Pabla, S.; Lenzo, F.L.; Kato, S.; Kurzrock, R.; DePietro, P.; Nesline, M.; Conroy, J.; Glenn, S.; et al. Identification of targets for prostate cancer immunotherapy. Prostate 2019, 79, 498–505. [Google Scholar] [CrossRef] [PubMed]
- von Hardenberg, J.; Hartmann, S.; Nitschke, K.; Worst, T.S.; Ting, S.; Reis, H.; Nuhn, P.; Weis, C.A.; Erben, P. Programmed Death Ligand 1 (PD-L1) Status and Tumor-Infiltrating Lymphocytes in Hot Spots of Primary and Liver Metastases in Prostate Cancer with Neuroendocrine Differentiation. Clin. Genitourin. Cancer 2019, 17, 145–153.e5. [Google Scholar] [CrossRef]
- Jin, X.; Ding, D.; Yan, Y.; Li, H.; Wang, B.; Ma, L.; Ye, Z.; Ma, T.; Wu, Q.; Rodrigues, D.N.; et al. Phosphorylated RB Promotes Cancer Immunity by Inhibiting NF-κB Activation and PD-L1 Expression. Mol. Cell 2019, 73, 22–35.e6. [Google Scholar] [CrossRef] [PubMed]
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L.; et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6, 141. [Google Scholar] [CrossRef]
- Richter, I.; Jirasek, T.; Havlickova, I.; Curcikova, R.; Samal, V.; Dvorak, J.; Bartos, J. The expression of PD-L1 in patients with castrate prostate cancer treated with enzalutamide. J. BUON 2018, 23, 1796–1802. [Google Scholar] [PubMed]
- Xiong, W.; Deng, H.; Huang, C.; Zen, C.; Jian, C.; Ye, K.; Zhong, Z.; Zhao, X.; Zhu, L. MLL3 enhances the transcription of PD-L1 and regulates anti-tumor immunity. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 454–463. [Google Scholar] [CrossRef]
- Hahn, E.; Liu, S.K.; Vesprini, D.; Xu, B.; Downes, M.R. Immune infiltrates and PD-L1 expression in treatment-naïve acinar prostatic adenocarcinoma: An exploratory analysis. J. Clin. Pathol. 2018, 71, 1023–1027. [Google Scholar] [CrossRef]
- Redman, J.M.; Steinberg, S.M.; Gulley, J.L. Quick efficacy seeking trial (QuEST1): A novel combination immunotherapy study designed for rapid clinical signal assessment metastatic castration-resistant prostate cancer. J. Immunother. Cancer 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Nava Rodrigues, D.; Rescigno, P.; Liu, D.; Yuan, W.; Carreira, S.; Lambros, M.B.; Seed, G.; Mateo, J.; Riisnaes, R.; Mullane, S.; et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J. Clin. Investig. 2018, 128, 4441–4453. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Casadio, V.; Martignano, F.; Gurioli, G.; Tumedei, M.M.; Calistri, D.; Gunelli, R.; Costantini, M. Carcinosarcoma of the prostate: Case report with molecular and histological characterization. Int. J. Biol. Markers 2018, 33, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hahn, E.; Slodkowska, E.; Eskander, A.; Enepekides, D.; Higgins, K.; Vesprini, D.; Liu, S.K.; Downes, M.R.; Xu, B. Reproducibility of PD-L1 immunohistochemistry interpretation across various types of genitourinary and head/neck carcinomas, antibody clones, and tissue types. Hum. Pathol. 2018, 82, 131–139. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.C.; Wargowski, E.; Zahm, C.; Staab, M.J.; Straus, J.; Liu, G. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2018, 9, 25586–25596. [Google Scholar] [CrossRef]
- Ishiba, T.; Hoffmann, A.C.; Usher, J.; Elshimali, Y.; Sturdevant, T.; Dang, M.; Jaimes, Y.; Tyagi, R.; Gonzales, R.; Grino, M.; et al. Frequencies and expression levels of programmed death ligand 1 (PD-L1) in circulating tumor RNA (ctRNA) in various cancer types. Biochem. Biophys. Res. Commun. 2018, 500, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Ma, Q.; Zhu, J.; Li, J.; Xue, B.X.; Gao, J.; Sun, C.Y.; Zang, Y.C.; Zhou, Y.B.; Yang, D.R.; et al. Combined inhibition of JAK1,2/Stat3-PD-L1 signaling pathway suppresses the immune escape of castration-resistant prostate cancer to NK cells in hypoxia. Mol. Med. Rep. 2018, 17, 8111–8120. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Guner, G.; Taheri, D.; Netto, G.J.; Palsgrove, D.N.; Zheng, Q.; Guedes, L.B.; Kim, K.; Tsai, H.; Esopi, D.M.; et al. Comprehensive Evaluation of Programmed Death-Ligand 1 Expression in Primary and Metastatic Prostate Cancer. Am. J. Pathol. 2018, 188, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Nagaputra, J.; Thike, A.A.; Koh, V. Loss of Androgen Receptor Accompained by Paucity of PD-L1 in Prostate Cancer is Associated with Clinical Relapse. USCAP 2018 Abstracts: Genitourinary Pathology (894–1126). Meeting Abstract: 1033. Mod. Pathol. 2018, 31, 323–403. [Google Scholar]
- Tu, Y.N.; Tong, W.L.; Yavorski, J.M.; Blanck, G. Immunogenomics: A Negative Prostate Cancer Outcome Associated with TcR-γ/δ Recombinations. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2018, 11, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Xu, S.; Ruan, H.; Wang, T.; Song, W.; Qian, L.; Chen, K. MiR-195/-16 Family Enhances Radiotherapy via T Cell Activation in the Tumor Microenvironment by Blocking the PD-L1 Immune Checkpoint. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 48, 801–814. [Google Scholar] [CrossRef]
- Budczies, J.; Denkert, C.; Győrffy, B.; Schirmacher, P.; Stenzinger, A. Chromosome 9p copy number gains involving PD-L1 are associated with a specific proliferation and immune-modulating gene expression program active across major cancer types. BMC Med. Genom. 2017, 10, 74. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Schüffler, P.J.; Gillessen, S.; Omlin, A.; Rupp, N.J.; Rueschoff, J.H.; Hermanns, T.; Poyet, C.; Sulser, T.; Moch, H.; et al. Comprehensive immunohistochemical analysis of PD-L1 shows scarce expression in castration-resistant prostate cancer. Oncotarget 2018, 9, 10284–10293. [Google Scholar] [CrossRef]
- Truillet, C.; Oh, H.L.J.; Yeo, S.P.; Lee, C.Y.; Huynh, L.T.; Wei, J.; Parker, M.F.L.; Blakely, C.; Sevillano, N.; Wang, Y.H.; et al. Imaging PD-L1 Expression with ImmunoPET. Bioconjugate Chem. 2018, 29, 96–103. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, X.; Wang, H.; Zhu, Y.; Geng, Y.; Nihira, N.T.; Tan, Y.; Ci, Y.; Wu, F.; Dai, X.; et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 2018, 553, 91–95. [Google Scholar] [CrossRef]
- Chen, Y.P.; Zhang, Y.; Lv, J.W.; Li, Y.Q.; Wang, Y.Q.; He, Q.M.; Yang, X.J.; Sun, Y.; Mao, Y.P.; Yun, J.P.; et al. Genomic Analysis of Tumor Microenvironment Immune Types across 14 Solid Cancer Types: Immunotherapeutic Implications. Theranostics 2017, 7, 3585–3594. [Google Scholar] [CrossRef]
- Calagua, C.; Russo, J.; Sun, Y.; Schaefer, R.; Lis, R.; Zhang, Z.; Mahoney, K.; Bubley, G.J.; Loda, M.; Taplin, M.E.; et al. Expression of PD-L1 in Hormone-naïve and Treated Prostate Cancer Patients Receiving Neoadjuvant Abiraterone Acetate plus Prednisone and Leuprolide. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6812–6822. [Google Scholar] [CrossRef] [PubMed]
- Schott, D.S.; Pizon, M.; Pachmann, U.; Pachmann, K. Sensitive detection of PD-L1 expression on circulating epithelial tumor cells (CETCs) could be a potential biomarker to select patients for treatment with PD-1/PD-L1 inhibitors in early and metastatic solid tumors. Oncotarget 2017, 8, 72755–72772. [Google Scholar] [CrossRef]
- Petitprez, F.; Fossati, N.; Vano, Y.; Freschi, M.; Becht, E.; Lucianò, R.; Calderaro, J.; Guédet, T.; Lacroix, L.; Rancoita, P.M.V.; et al. PD-L1 Expression and CD8(+) T-cell Infiltrate are Associated with Clinical Progression in Patients with Node-positive Prostate Cancer. Eur. Urol. Focus 2019, 5, 192–196. [Google Scholar] [CrossRef]
- Li, G.; Ross, J.; Yang, X. Mismatch Repair (MMR) Deficiency and PD-L1 Expression in the Prostatic Ductal Adenocarcinoma. Abstracts from USCAP 2019: Genitourinary Pathology (including renal tumors) (776–992). Meeting Abstract: 893. Mod. Pathol. 2019, 32, 91. [Google Scholar]
- Ness, N.; Andersen, S.; Khanehkenari, M.R.; Nordbakken, C.V.; Valkov, A.; Paulsen, E.E.; Nordby, Y.; Bremnes, R.M.; Donnem, T.; Busund, L.T.; et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget 2017, 8, 26789–26801. [Google Scholar] [CrossRef]
- Baas, W.; Gershburg, S.; Dynda, D.; Delfino, K.; Robinson, K.; Nie, D.; Yearley, J.H.; Alanee, S. Immune Characterization of the Programmed Death Receptor Pathway in High Risk Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, 577–581. [Google Scholar] [CrossRef]
- Gao, J.; Ward, J.F.; Pettaway, C.A.; Shi, L.Z.; Subudhi, S.K.; Vence, L.M.; Zhao, H.; Chen, J.; Chen, H.; Efstathiou, E.; et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017, 23, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef]
- Tretiakova, M.; Fulton, R.; Kocherginsky, M. Comparison of 4 PD-L1 Antibodies in 560 Kidney, Bladder and Prostate Cancers. Abstracts from USCAP 2019: Genitourinary Pathology (including Renal tumors). Meeting Abstract: 1062. Mod. Pathol. 2017, 30, 210–271. [Google Scholar]
- Najjar, S.N.; Kallakury, B.V.S.; Sheehan, C.E. Infrequent PD-L1 Protetin Expression and Gene Amplification in Prostatic Adenocarcinomas (PACs). Abstracts from USCAP 2019: Genitourinary Pathology (including Renal tumors). Meeting Abstract: 992. Mod. Pathol. 2017, 30, 246A. [Google Scholar]
- Hashimoto, Y.; Imai, A.; Hatakeyama, S.; Yoneyama, T.; Koie, T.; Ohyama, C. PD-L1 over expression may predict disease aggressiveness in prostate cancer. Meeting Abstract: 291P. Ann. Oncol. 2016, 27, ix91–ix92. [Google Scholar] [CrossRef]
- Gevensleben, H.; Holmes, E.E.; Goltz, D.; Dietrich, J.; Sailer, V.; Ellinger, J.; Dietrich, D.; Kristiansen, G. PD-L1 promoter methylation is a prognostic biomarker for biochemical recurrence-free survival in prostate cancer patients following radical prostatectomy. Oncotarget 2016, 7, 79943–79955. [Google Scholar] [CrossRef]
- Zhou, Q.Z.; Liu, C.D.; Yang, J.K.; Guo, W.B.; Zhou, J.H.; Bian, J. Changed percentage of myeloid-derived suppressor cells in the peripheral blood of prostate cancer patients and its clinical implication. Natl. J. Androl. 2016, 22, 963–967. [Google Scholar]
- Sharma, V.; Dong, H.; Kwon, E.; Karnes, R.J. Positive Pelvic Lymph Nodes in Prostate Cancer Harbor Immune Suppressor Cells to Impair Tumor-reactive T Cells. Eur. Urol. Focus 2018, 4, 75–79. [Google Scholar] [CrossRef]
- Goltz, D.; Holmes, E.E.; Gevensleben, H.; Sailer, V.; Dietrich, J.; Jung, M.; Röhler, M.; Meller, S.; Ellinger, J.; Kristiansen, G.; et al. CXCL12 promoter methylation and PD-L1 expression as prognostic biomarkers in prostate cancer patients. Oncotarget 2016, 7, 53309–53320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Graff, J.N.; Alumkal, J.J.; Drake, C.G.; Thomas, G.V.; Redmond, W.L.; Farhad, M.; Cetnar, J.P.; Ey, F.S.; Bergan, R.C.; Slottke, R.; et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016, 7, 52810–52817. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Batth, I.S.; Brownlee, Z.; Rojas, C.; Meng, Q.H.; Kopetz, S.; Li, S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci. Rep. 2016, 6, 28910. [Google Scholar] [CrossRef]
- Massari, F.; Ciccarese, C.; Caliò, A.; Munari, E.; Cima, L.; Porcaro, A.B.; Novella, G.; Artibani, W.; Sava, T.; Eccher, A.; et al. Magnitude of PD-1, PD-L1 and T Lymphocyte Expression on Tissue from Castration-Resistant Prostate Adenocarcinoma: An Exploratory Analysis. Target. Oncol. 2016, 11, 345–351. [Google Scholar] [CrossRef]
- Gevensleben, H.; Dietrich, D.; Golletz, C.; Steiner, S.; Jung, M.; Thiesler, T.; Majores, M.; Stein, J.; Uhl, B.; Müller, S.; et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1969–1977. [Google Scholar] [CrossRef]
- Martin, A.M.; Nirschl, T.R.; Nirschl, C.J.; Francica, B.J.; Kochel, C.M.; van Bokhoven, A.; Meeker, A.K.; Lucia, M.S.; Anders, R.A.; DeMarzo, A.M.; et al. Paucity of PD-L1 expression in prostate cancer: Innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015, 18, 325–332. [Google Scholar] [CrossRef]
- Shalapour, S.; Font-Burgada, J.; Di Caro, G.; Zhong, Z.; Sanchez-Lopez, E.; Dhar, D.; Willimsky, G.; Ammirante, M.; Strasner, A.; Hansel, D.E.; et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 2015, 521, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Spary, L.K.; Salimu, J.; Webber, J.P.; Clayton, A.; Mason, M.D.; Tabi, Z. Tumor stroma-derived factors skew monocyte to dendritic cell differentiation toward a suppressive CD14(+) PD-L1(+) phenotype in prostate cancer. Oncoimmunology 2014, 3, e955331. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M. Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology 2014, 3, e963413. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Dulos, J.; Carven, G.J.; van Boxtel, S.J.; Evers, S.; Driessen-Engels, L.J.; Hobo, W.; Gorecka, M.A.; de Haan, A.F.; Mulders, P.; Punt, C.J.; et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J. Immunother. 2012, 35, 169–178. [Google Scholar] [CrossRef]
- Zhou, J.E.; Yu, J.; Wang, Y.; Wang, H.; Wang, J.; Wang, Y.; Yu, L.; Yan, Z. ShRNA-mediated silencing of PD-1 augments the efficacy of chimeric antigen receptor T cells on subcutaneous prostate and leukemia xenograft. Biomed. Pharmacother. 2021, 137, 111339. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, J.; Jin, X.; Lenchine, R.V.; Wang, X.; Fang, D.M.; Nassar, Z.D.; Butler, L.M.; Li, J.; Proud, C.G. eEF2K enhances expression of PD-L1 by promoting the translation of its mRNA. Biochem. J. 2020, 477, 4367–4381. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, X.; Chen, R.; Zhu, Y.; Peng, S.; Chang, Y.; Nian, X.; Xiao, G.; Fang, Z.; Li, Y.; et al. Novel Long Non-coding RNA lncAMPC Promotes Metastasis and Immunosuppression in Prostate Cancer by Stimulating LIF/LIFR Expression. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 2473–2487. [Google Scholar] [CrossRef]
- Rennier, K.; Shin, W.J.; Krug, E.; Virdi, G.; Pachynski, R.K. Chemerin Reactivates PTEN and Suppresses PD-L1 in Tumor Cells via Modulation of a Novel CMKLR1-mediated Signaling Cascade. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5019–5035. [Google Scholar] [CrossRef] [PubMed]
- Philippou, Y.; Sjoberg, H.T.; Murphy, E.; Alyacoubi, S.; Jones, K.I.; Gordon-Weeks, A.N.; Phyu, S.; Parkes, E.E.; Gillies McKenna, W.; Lamb, A.D.; et al. Impacts of combining anti-PD-L1 immunotherapy and radiotherapy on the tumour immune microenvironment in a murine prostate cancer model. Br. J. Cancer 2020, 123, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, L.; Yuan, Z.; Tao, Z. Wee1 kinase inhibitor AZD1775 potentiates CD8+ T cell-dependent antitumour activity via dendritic cell activation following a single high dose of irradiation. Med. Oncol. 2020, 37, 66. [Google Scholar] [CrossRef]
- Papaevangelou, E.; Smolarek, D.; Smith, R.A.; Dasgupta, P.; Galustian, C. Targeting Prostate Cancer Using Intratumoral Cytotopically Modified Interleukin-15 Immunotherapy in a Syngeneic Murine Model. ImmunoTargets Therapy 2020, 9, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, Y.H.; Lee, C.Y.; Truillet, C.; Oh, D.Y.; Xu, Y.; Ruggero, D.; Flavell, R.R.; VanBrocklin, H.F.; Seo, Y.; et al. An Analysis of Isoclonal Antibody Formats Suggests a Role for Measuring PD-L1 with Low Molecular Weight PET Radiotracers. Mol. Imaging Biol. 2020, 22, 1553–1561. [Google Scholar] [CrossRef]
- Ding, H.; Wang, Z.; Pascal Laura, E.; Chen, W.; Wang, Z.; Wang, Z. ELL2 Deficiency Upregulates PD-L1 Expression via JAK2 Signaling in Prostate Cancer Cells. Meeting Abstract: MP51–12. J. Urol. 2020, 203 (Suppl. S4), e768. [Google Scholar]
- Sun, Y.; Wei, Q.; Huang, J.; Yang, L. Methylation can Regulate the Expression of PD-L1 in Small Cell Prostate Cancer. Meeting Abstract: MP16–12. J. Urol. 2020, 203 (Suppl. S4), e219–e220. [Google Scholar]
- Liu, J.; He, D.; Cheng, L.; Huang, C.; Zhang, Y.; Rao, X.; Kong, Y.; Li, C.; Zhang, Z.; Liu, J.; et al. p300/CBP inhibition enhances the efficacy of programmed death-ligand 1 blockade treatment in prostate cancer. Oncogene 2020, 39, 3939–3951. [Google Scholar] [CrossRef]
- Yamazaki, T.; Buqué, A.; Ames, T.D.; Galluzzi, L. PT-112 induces immunogenic cell death and synergizes with immune checkpoint blockers in mouse tumor models. Oncoimmunology 2020, 9, 1721810. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Li, G.; Zhou, X.; Shi, Y.; Zou, F.; Chen, Y.; Gao, J.; Yang, S.; Wu, S.; et al. Increased Tim-3 expression on TILs during treatment with the Anchored GM-CSF vaccine and anti-PD-1 antibodies is inversely correlated with response in prostate cancer. J. Cancer 2020, 11, 648–656. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Y.; Zhang, J.; Jin, X.; Wu, H.; Huang, H. Fructose-1,6-bisphosphatase loss modulates STAT3-dependent expression of PD-L1 and cancer immunity. Theranostics 2020, 10, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Orme, J.J.; Jazieh, K.A.; Xie, T.; Harrington, S.; Liu, X.; Ball, M.; Madden, B.; Charlesworth, M.C.; Azam, T.U.; Lucien, F.; et al. ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance. Oncoimmunology 2020, 9, 1744980. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Ye, J.; Li, J.; Hu, C.; Wang, J.; Xu, D.; Pan, X.; Chu, C.; Chu, J.; Zhang, J.; et al. LRP11 activates β-catenin to induce PD-L1 expression in prostate cancer. J. Drug Target. 2020, 28, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Jung, H.; Yu, B.; Choi, H.; Lee, J.; Kim, D.H. Sequential MR Image-Guided Local Immune Checkpoint Blockade Cancer Immunotherapy Using Ferumoxytol Capped Ultralarge Pore Mesoporous Silica Carriers after Standard Chemotherapy. Small 2019, 15, e1904378. [Google Scholar] [CrossRef]
- Mao, W.; Ghasemzadeh, A.; Freeman, Z.T.; Obradovic, A.; Chaimowitz, M.G.; Nirschl, T.R.; McKiernan, E.; Yegnasubramanian, S.; Drake, C.G. Immunogenicity of prostate cancer is augmented by BET bromodomain inhibition. J. Immunother. Cancer 2019, 7, 277. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiong, W.; Zhou, X.; Gao, R.S.; Lin, Q.F.; Liu, H.Y.; Li, J.N.; Tian, X.F. CTHRC1 and PD-1/PD-L1 expression predicts tumor recurrence in prostate cancer. Mol. Med. Rep. 2019, 20, 4244–4252. [Google Scholar] [CrossRef]
- Xu, Q.; Long, Q.; Zhu, D.; Fu, D.; Zhang, B.; Han, L.; Qian, M.; Guo, J.; Xu, J.; Cao, L.; et al. Targeting amphiregulin (AREG) derived from senescent stromal cells diminishes cancer resistance and averts programmed cell death 1 ligand (PD-L1)-mediated immunosuppression. Aging Cell 2019, 18, e13027. [Google Scholar] [CrossRef]
- Dudzinski, S.O.; Cameron, B.D.; Wang, J.; Rathmell, J.C.; Giorgio, T.D.; Kirschner, A.N. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J. Immunother. Cancer 2019, 7, 218. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Huang, J.; Luo, H.; Zhu, S.; Yi, H.; Zheng, L.; Hu, B.; Yu, L.; Li, L.; et al. Specific zinc finger-induced methylation of PD-L1 promoter inhibits its expression. FEBS Open Bio 2019, 9, 1063–1070. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, Z.; Gao, H.; Yang, F.; Qian, Y.; Jin, H.; Guo, Y.; Liu, Y.; Li, H.; Zhang, C.; et al. JQ1, a BET-bromodomain inhibitor, inhibits human cancer growth and suppresses PD-L1 expression. Cell Biol. Int. 2019, 43, 642–650. [Google Scholar] [CrossRef]
- Xu, N.; Huang, L.; Li, X.; Watanabe, M.; Li, C.; Xu, A.; Liu, C.; Li, Q.; Araki, M.; Wada, K.; et al. The Novel Combination of Nitroxoline and PD-1 Blockade, Exerts a Potent Antitumor Effect in a Mouse Model of Prostate Cancer. Int. J. Biol. Sci. 2019, 15, 919–928. [Google Scholar] [CrossRef]
- Yoneda, T.; Kunimura, N.; Kitagawa, K.; Fukui, Y.; Saito, H.; Narikiyo, K.; Ishiko, M.; Otsuki, N.; Nibu, K.I.; Fujisawa, M.; et al. Overexpression of SOCS3 mediated by adenovirus vector in mouse and human castration-resistant prostate cancer cells increases the sensitivity to NK cells in vitro and in vivo. Cancer Gene Ther. 2019, 26, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Fenerty, K.E.; Padget, M.; Wolfson, B.; Gameiro, S.R.; Su, Z.; Lee, J.H.; Rabizadeh, S.; Soon-Shiong, P.; Hodge, J.W. Immunotherapy utilizing the combination of natural killer- and antibody dependent cellular cytotoxicity (ADCC)-mediating agents with poly (ADP-ribose) polymerase (PARP) inhibition. J. Immunother. Cancer 2018, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.E.; Thorek, D.L.J.; Meeker, A.K.; Isaacs, J.T.; Brennen, W.N. Tumor-infiltrating mesenchymal stem cells: Drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate 2019, 79, 320–330. [Google Scholar] [CrossRef]
- Medina Enríquez, M.M.; Félix, A.J.; Ciudad, C.J.; Noé, V. Cancer immunotherapy using PolyPurine Reverse Hoogsteen hairpins targeting the PD-1/PD-L1 pathway in human tumor cells. PLoS ONE 2018, 13, e0206818. [Google Scholar]
- Moreira, D.; Adamus, T.; Zhao, X.; Su, Y.L.; Zhang, Z.; White, S.V.; Swiderski, P.; Lu, X.; DePinho, R.A.; Pal, S.K.; et al. STAT3 Inhibition Combined with CpG Immunostimulation Activates Antitumor Immunity to Eradicate Genetically Distinct Castration-Resistant Prostate Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5948–5962. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.J.; Zhu, J.; Li, J.; Xue, B.X.; Gao, J.; Sun, C.Y.; Zang, Y.C.; Zhou, Y.B.; Yang, D.R.; et al. ATM-JAK-PD-L1 signaling pathway inhibition decreases EMT and metastasis of androgen-independent prostate cancer. Mol. Med. Rep. 2018, 17, 7045–7054. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wang, Y.; Ji, J.; Cai, B.; Chen, H.; Yang, Z.; Wang, K.; Luo, C.; Zhang, W.; Yuan, C.; et al. Molecular Profiling of Pooled Circulating Tumor Cells from Prostate Cancer Patients Using a Dual-Antibody-Functionalized Microfluidic Device. Anal. Chem. 2018, 90, 3744–3751. [Google Scholar] [CrossRef]
- Ahern, E.; Harjunpää, H.; O’Donnell, J.S.; Allen, S.; Dougall, W.C.; Teng, M.W.L.; Smyth, M.J. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. Oncoimmunology 2018, 7, e1431088. [Google Scholar] [CrossRef]
- Xu, L.; Shen, M.; Chen, X.; Yang, D.R.; Tsai, Y.; Keng, P.C.; Lee, S.O.; Chen, Y. In vitro-induced M2 type macrophages induces the resistance of prostate cancer cells to cytotoxic action of NK cells. Exp. Cell Res. 2018, 364, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, X.; Shen, M.; Yang, D.R.; Fang, L.; Weng, G.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O. Inhibition of IL-6-JAK/Stat3 signaling in castration-resistant prostate cancer cells enhances the NK cell-mediated cytotoxicity via alteration of PD-L1/NKG2D ligand levels. Mol. Oncol. 2018, 12, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, M.; Chen, X.; Zhu, R.; Yang, D.R.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O. Adipocytes affect castration-resistant prostate cancer cells to develop the resistance to cytotoxic action of NK cells with alterations of PD-L1/NKG2D ligand levels in tumor cells. Prostate 2018, 78, 353–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, S.; Qian, P.; Wang, X.; Xu, Z.; Sun, W.; Xu, Y. RelB upregulates PD-L1 in advanced prostate cancer: An insight into tumor immunoescape. Meeting Abstract 2791. Cancer Res. 2019, 79, 2791. [Google Scholar]
- Shimizu, N.; Velasco, M.A.D.; Kura, Y. PD-L1 immune checkpoint blockade in genetically engineered mouse models of prostate cancer. Cancer Sci. 2018, 109 (Suppl. S1), 292. [Google Scholar]
- Maher, C.M.; Thomas, J.D.; Haas, D.A.; Longen, C.G.; Oyer, H.M.; Tong, J.Y.; Kim, F.J. Small-Molecule Sigma1 Modulator Induces Autophagic Degradation of PD-L1. Mol. Cancer Res. MCR 2018, 16, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, X.; Li, J.; Zhao, H.; Mo, L.; Shi, X.; Hu, Z.; Gao, J.; Tan, W. PD-1/PD-L1 blockade enhances the efficacy of SA-GM-CSF surface-modified tumor vaccine in prostate cancer. Cancer Lett. 2017, 406, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cappuccini, F.; Pollock, E.; Stribbling, S.; Hill, A.V.S.; Redchenko, I. 5T4 oncofoetal glycoprotein: An old target for a novel prostate cancer immunotherapy. Oncotarget 2017, 8, 47474–47489. [Google Scholar] [CrossRef]
- De Velasco, M.A.; Kura, Y.; Ando, N.; Sato, N.; Sakai, K.; Davies, B.R.; Sugimoto, K.; Nozawa, M.; Yoshimura, K.; Yoshikawa, K.; et al. PD-L1 blockade in preclinical models of PTEN-deficient prostate cancer. Meeting Abstract: 4702. Cancer Res. 2017, 77, 4702. [Google Scholar]
- Liu, Z.; Zhao, Y.; Fang, J.; Cui, R.; Xiao, Y.; Xu, Q. SHP2 negatively regulates HLA-ABC and PD-L1 expression via STAT1 phosphorylation in prostate cancer cells. Oncotarget 2017, 8, 53518–53530. [Google Scholar] [CrossRef]
- Tanoue, K.; Rosewell Shaw, A.; Watanabe, N.; Porter, C.; Rana, B.; Gottschalk, S.; Brenner, M.; Suzuki, M. Armed Oncolytic Adenovirus-Expressing PD-L1 Mini-Body Enhances Antitumor Effects of Chimeric Antigen Receptor T Cells in Solid Tumors. Cancer Res. 2017, 77, 2040–2051. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Serganova, I.; Moroz, E.; Cohen, I.; Moroz, M.; Mane, M.; Zurita, J.; Shenker, L.; Ponomarev, V.; Blasberg, R. Enhancement of PSMA-Directed CAR Adoptive Immunotherapy by PD-1/PD-L1 Blockade. Mol. Ther. Oncolytics 2017, 4, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Rekoske, B.T.; Olson, B.M.; McNeel, D.G. Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology 2016, 5, e1165377. [Google Scholar] [CrossRef] [PubMed]
- Rekoske, B.T.; Smith, H.A.; Olson, B.M.; Maricque, B.B.; McNeel, D.G. PD-1 or PD-L1 Blockade Restores Antitumor Efficacy Following SSX2 Epitope-Modified DNA Vaccine Immunization. Cancer Immunol. Res. 2015, 3, 946–955. [Google Scholar] [CrossRef]
- Black, M.; Barsoum, I.B.; Truesdell, P.; Cotechini, T.; Macdonald-Goodfellow, S.K.; Petroff, M.; Siemens, D.R.; Koti, M.; Craig, A.W.; Graham, C.H. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget 2016, 7, 10557–10567. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Liu, S.; Wang, A.R.; You, Z. PD-1, PD-L1 and PD-L2 expression in mouse prostate cancer. Am. J. Clin. Exp. Urol. 2016, 4, 1–8. [Google Scholar]
- Carbotti, G.; Barisione, G.; Airoldi, I.; Mezzanzanica, D.; Bagnoli, M.; Ferrero, S.; Petretto, A.; Fabbi, M.; Ferrini, S. IL-27 induces the expression of IDO and PD-L1 in human cancer cells. Oncotarget 2015, 6, 43267–43280. [Google Scholar] [CrossRef]
- Bernstein, M.B.; Garnett, C.T.; Zhang, H.; Velcich, A.; Wattenberg, M.M.; Gameiro, S.R.; Kalnicki, S.; Hodge, J.W.; Guha, C. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother. Radiopharm. 2014, 29, 153–161. [Google Scholar] [CrossRef]
- Yu, P.; Steel, J.C.; Zhang, M.; Morris, J.C.; Waitz, R.; Fasso, M.; Allison, J.P.; Waldmann, T.A. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc. Natl. Acad. Sci. USA 2012, 109, 6187–6192. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Q.; Zeng, X.; Yu, W.; Xu, G. Pembrolizumab with or without enzalutamide in selected populations of men with previously untreated metastatic castration-resistant prostate cancer harbouring programmed cell death ligand-1 staining: A retrospective study. BMC Cancer 2021, 21, 399. [Google Scholar] [CrossRef]
- Morel, K.L.; Sheahan, A.V.; Burkhart, D.L.; Baca, S.C.; Boufaied, N.; Liu, Y.; Qiu, X.; Cañadas, I.; Roehle, K.; Heckler, M.; et al. EZH2 inhibition activates a dsRNA-STING-interferon stress axis that potentiates response to PD-1 checkpoint blockade in prostate cancer. Nat. Cancer 2021, 2, 444–456. [Google Scholar] [CrossRef]
- Fan, J.; Shen, X.; Wang, Y.; Zhou, H.L.; Liu, G.; Li, Y.L.; Xu, Z.X. Biomarkers for immune checkpoint therapy targeting programmed death 1 and programmed death ligand 1. Biomed. Pharmacother. 2020, 130, 110621. [Google Scholar] [CrossRef]
- Alkan, A.; Hofving, T.; Angenete, E.; Yrlid, U. Biomarkers and cell-based models to predict the outcome of neoadjuvant therapy for rectal cancer patients. Biomark. Res. 2021, 9, 60. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, S.; Yang, Z.; Song, Y.; Ding, J.; Hou, D.; Wang, L.; Zhang, Z.; Li, N.; Wang, H. Identification of docetaxel-related biomarkers for prostate cancer. Andrologia 2021, 53, e14079. [Google Scholar] [CrossRef]
- Huang, H.; Tang, Y.; Li, P.; Ye, X.; Chen, W.; Xie, H.; Zheng, Y. Significance of TP53 and immune-related genes to prostate cancer. Transl. Androl. Urol. 2021, 10, 1754–1768. [Google Scholar] [CrossRef] [PubMed]
- Wing-Cheuk Wong, R.; Palicelli, A.; Hoang, L.; Singh, N. Interpretation of p16, p53 and mismatch repair protein immunohistochemistry in gynaecological neoplasia. Diagn. Histopathol. 2020, 26, 257–277. [Google Scholar] [CrossRef]
- Bhasin, J.M.; Lee, B.H.; Matkin, L.; Taylor, M.G.; Hu, B.; Xu, Y.; Magi-Galluzzi, C.; Klein, E.A.; Ting, A.H. Methylome-wide Sequencing Detects DNA Hypermethylation Distinguishing Indolent from Aggressive Prostate Cancer. Cell Rep. 2015, 13, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, S.M.; Ferro, M.; Bollito, E.; Klein, E.A.; Carrieri, G.; Magi-Galluzzi, C. Novel biomarkers and genomic tests in prostate cancer: A critical analysis. Minerva Urol. Nefrol. 2015, 67, 211–231. [Google Scholar]
- Tomlins, S.A.; Alshalalfa, M.; Davicioni, E.; Erho, N.; Yousefi, K.; Zhao, S.; Haddad, Z.; Den, R.B.; Dicker, A.P.; Trock, B.J.; et al. Characterization of 1577 Primary Prostate Cancers Reveals Novel Biological and Clinicopathologic Insights into Molecular Subtypes. Eur. Urol. 2015, 68, 555–567. [Google Scholar]
- De Leo, A.; Santini, D.; Ceccarelli, C.; Santandrea, G.; Palicelli, A.; Acquaviva, G.; Chiarucci, F.; Rosini, F.; Ravegnini, G.; Pession, A.; et al. What Is New on Ovarian Carcinoma: Integrated Morphologic and Molecular Analysis Following the New 2020 World Health Organization Classification of Female Genital Tumors. Diagnostics 2021, 11, 697. [Google Scholar] [CrossRef]
- Foda, A.A.; Palicelli, A.; Shebl, A.; Boldorini, R.; Elnaghi, K.; ElHawary, A.K. Role of ERCC1 expression in colorectal adenoma-carcinoma sequence and relation to other mismatch repair proteins expression, clinicopathological features and prognosis in mucinous and non-mucinous colorectal carcinoma. Indian J. Pathol. Microbiol. 2019, 62, 405–412. [Google Scholar] [CrossRef]
- Ribbat-Idel, J.; Dressler, F.F.; Krupar, R.; Watermann, C.; Paulsen, F.O.; Kuppler, P.; Klapper, L.; Offermann, A.; Wollenberg, B.; Rades, D.; et al. Performance of Different Diagnostic PD-L1 Clones in Head and Neck Squamous Cell Carcinoma. Front. Med. 2021, 8, 640515. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; López-Rios, F.; Montironi, R.; Wildsmith, S.; Eckstein, M. Immune Checkpoint Inhibitors in Urothelial Carcinoma: Recommendations for Practical Approaches to PD-L1 and Other Potential Predictive Biomarker Testing. Cancers 2021, 13, 1424. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Calò, B.; Mancini, V.; Zanelli, M.; Palicelli, A.; Zizzo, M.; Ascani, S.; Carrieri, G.; Cormio, L. Non-Muscle Invasive Bladder Cancer with Variant Histology: Biological Features and Clinical Implications. Oncology 2021, 99, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.L.; Dix, C.I.; Scorer, P.W.; Stubbs, C.J.; Wong, E.; Hutchinson, L.; McCall, E.J.; Schimpl, M.; DeVries, E.; Walker, J.; et al. Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies. Mod. Pathol. 2020, 33, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef]
- Koomen, B.M.; Badrising, S.K.; van den Heuvel, M.M.; Willems, S.M. Comparability of PD-L1 immunohistochemistry assays for non-small-cell lung cancer: A systematic review. Histopathology 2020, 76, 793–802. [Google Scholar] [CrossRef]
- Fujimoto, D.; Sato, Y.; Uehara, K.; Ishida, K.; Fukuoka, J.; Morimoto, T.; Kawachi, H.; Mori, R.; Ito, M.; Teraoka, S.; et al. Predictive Performance of Four Programmed Cell Death Ligand 1 Assay Systems on Nivolumab Response in Previously Treated Patients with Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 377–386. [Google Scholar] [CrossRef]
- Lee, K.S.; Choe, G. Programmed cell death-ligand 1 assessment in urothelial carcinoma: Prospect and limitation. J. Pathol. Transl. Med. 2021, 55, 163–170. [Google Scholar] [CrossRef]
- Keppens, C.; Dequeker, E.M.; Pauwels, P.; Ryska, A.; ’t Hart, N.; von der Thüsen, J.H. PD-L1 immunohistochemistry in non-small-cell lung cancer: Unraveling differences in staining concordance and interpretation. Virchows Arch. 2021, 478, 827–839. [Google Scholar] [CrossRef]
- Barberà, A.; Marginet Flinch, R.; Martin, M.; Mate, J.L.; Oriol, A.; Martínez-Soler, F.; Santalucia, T.; Fernández, P.L. The Immunohistochemical Expression of Programmed Death Ligand 1 (PD-L1) Is Affected by Sample Overfixation. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Horton, R. Putting clinical trials into context. Lancet 2005, 366, 107–108. [Google Scholar] [CrossRef]
- Zanelli, M.; Sanguedolce, F.; Zizzo, M.; Palicelli, A.; Bassi, M.C.; Santandrea, G.; Martino, G.; Soriano, A.; Caprera, C.; Corsi, M.; et al. Primary effusion lymphoma occurring in the setting of transplanted patients: A systematic review of a rare, life-threatening post-transplantation occurrence. BMC Cancer 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Giaccherini, L.; Zanelli, M.; Bonasoni, M.P.; Gelli, M.C.; Bisagni, A.; Zanetti, E.; De Marco, L.; Torricelli, F.; Manzotti, G.; et al. How Can We Treat Vulvar Carcinoma in Pregnancy? A Systematic Review of the Literature. Cancers 2021, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Zanelli, M.; Zizzo, M.; Bisagni, A.; Soriano, A.; Cocco, G.; Palicelli, A.; Santandrea, G.; Caprera, C.; Corsi, M.; et al. Primary Pulmonary B-Cell Lymphoma: A Review and Update. Cancers 2021, 13, 415. [Google Scholar] [CrossRef]
- Bonasoni, M.P.; Palicelli, A.; Dalla Dea, G.; Comitini, G.; Pazzola, G.; Russello, G.; Bertoldi, G.; Bardaro, M.; Zuelli, C.; Carretto, E. Kingella kingae Intrauterine Infection: An Unusual Cause of Chorioamnionitis and Miscarriage in a Patient with Undifferentiated Connective Tissue Disease. Diagnostics 2021, 11, 243. [Google Scholar] [CrossRef]
- Bonasoni, M.P.; Palicelli, A.; Dalla Dea, G.; Comitini, G.; Nardini, P.; Vizzini, L.; Russello, G.; Bardaro, M.; Carretto, E. Klebsiella pneumoniae Chorioamnionitis: An Underrecognized Cause of Preterm Premature Rupture of Membranes in the Second Trimester. Microorganisms 2021, 9, 96. [Google Scholar] [CrossRef]
- Olivadese, R.; Ramponi, A.; Boldorini, R.; Dalla Dea, G.; Palicelli, A. Mitotically Active Cellular Fibroma of the Ovary Recurring After the Longest Interval of Time (16 yr): A Challenging Case with Systematic Literature Review. Int. J. Gynecol. Pathol. 2021, 40, 441–447. [Google Scholar] [CrossRef]
- Zanelli, M.; Ricci, S.; Zizzo, M.; Sanguedolce, F.; De Giorgi, F.; Palicelli, A.; Martino, G.; Ascani, S. Systemic Mastocytosis Associated with “Smoldering” Multiple Myeloma. Diagnostics 2021, 11, 88. [Google Scholar] [CrossRef]
- Palicelli, A. What do we know about the cytological features of pure intraductal carcinomas of the salivary glands? Cytopathology 2020, 31, 185–192. [Google Scholar] [CrossRef]
- Palicelli, A. Intraductal carcinomas of the salivary glands: Systematic review and classification of 93 published cases. APMIS 2020, 128, 191–200. [Google Scholar] [CrossRef]
- Ardighieri, L.; Palicelli, A.; Ferrari, F.; Bugatti, M.; Drera, E.; Sartori, E.; Odicino, F. Endometrial Carcinomas with Intestinal-Type Metaplasia/Differentiation: Does Mismatch Repair System Defects Matter? Case Report and Systematic Review of the Literature. J. Clin. Med. 2020, 9, 2552. [Google Scholar] [CrossRef]
- D’Agostino, C.; Surico, D.; Monga, G.; Palicelli, A. Pregnancy-related decidualization of subcutaneous endometriosis occurring in a post-caesarean section scar: Case study and review of the literature. Pathol. Res. Pract. 2019, 215, 828–831. [Google Scholar] [CrossRef]
- Palicelli, A.; Barbieri, P.; Mariani, N.; Re, P.; Galla, S.; Sorrentino, R.; Locatelli, F.; Salfi, N.; Valente, G. Unicystic high-grade intraductal carcinoma of the parotid gland: Cytological and histological description with clinic-pathologic review of the literature. APMIS 2018, 126, 771–776. [Google Scholar] [CrossRef]
- Palicelli, A.; Neri, P.; Marchioro, G.; De Angelis, P.; Bondonno, G.; Ramponi, A. Paratesticular seminoma: Echographic features and histological diagnosis with review of the literature. APMIS 2018, 126, 267–272. [Google Scholar] [CrossRef]
- Disanto, M.G.; Mercalli, F.; Palicelli, A.; Arnulfo, A.; Boldorini, R. A unique case of bilateral ovarian splenosis and review of the literature. APMIS 2017, 125, 844–848. [Google Scholar] [CrossRef]
- Palicelli, A.; Disanto, M.G.; Panzarasa, G.; Veggiani, C.; Galizia, G.; Dal Cin, S.; Gruppioni, E.; Boldorini, R. Orbital meningeal melanocytoma: Histological, immunohistochemical and molecular characterization of a case and review of the literature. Pathol. Res. Pract. 2016, 212, 946–953. [Google Scholar] [CrossRef]
- Zanelli, M.; Smith, M.; Zizzo, M.; Carloni, A.; Valli, R.; De Marco, L.; Foroni, M.; Palicelli, A.; Martino, G.; Ascani, S. A tricky and rare cause of pulmonary eosinophilia: Myeloid/lymphoid neoplasm with eosinophilia and rearrangement of PDGFRA. BMC Pulm. Med. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Boldorini, R.; Campisi, P.; Disanto, M.G.; Gatti, L.; Portigliotti, L.; Tosoni, A.; Rivasi, F. Tungiasis in Italy: An imported case of Tunga penetrans and review of the literature. Pathol. Res. Pract. 2016, 212, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, F.; Palicelli, A.; Bulfamante, G.; Rivasi, F. Langer mesomelic dysplasia in early fetuses: Two cases and a literature review. Fetal Pediatr. Pathol. 2014, 33, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Mastrofilippo, V.; Palicelli, A.; Silvotti, M.; Serra, S.; Giaccherini, L.; Aguzzoli, L. Solitary vulvar metastasis from early-stage endometrial cancer: Case report and literature review. Medicine 2021, 100, e25863. [Google Scholar] [CrossRef]
- Ardighieri, L.; Palicelli, A.; Ferrari, F.; Ragnoli, M.; Ghini, I.; Bugatti, M.; Bercich, L.; Sartori, E.; Odicino, F.E. Risk Assessment in Solitary Fibrous Tumor of the Uterine Corpus: Report of a Case and Systematic Review of the Literature. Int. J. Surg. Pathol. 2021, 28, 10668969211025759. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Pizzi, M.; Sanguedolce, F.; Zizzo, M.; Palicelli, A.; Soriano, A.; Bisagni, A.; Martino, G.; Caprera, C.; Moretti, M.; et al. Gastrointestinal Manifestations in Systemic Mastocytosis: The Need of a Multidisciplinary Approach. Cancers 2021, 13, 3316. [Google Scholar] [CrossRef] [PubMed]
- Donegani, E.; Ambassa, J.C.; Mvondo, C.; Giamberti, A.; Ramponi, A.; Palicelli, A.; Chelo, D. Linfoma di Burkitt cardiaco primitivo in un giovane ragazzo africano [Primary cardiac Burkitt lymphoma in an African child]. G. Ital. Cardiol. 2013, 14, 481–484. [Google Scholar]
- Zanelli, M.; Ragazzi, M.; Marchetti, G.; Bisagni, A.; Principi, M.; Fanni, D.; Froio, E.; Serra, S.; Zanetti, E.; De Marco, L.; et al. Primary histiocytic sarcoma presenting as diffuse leptomeningeal disease: Case description and review of the literature. Neuropathology 2017, 37, 517–525. [Google Scholar] [CrossRef]
- Minni, F.; Casadei, R.; Santini, D.; Verdirame, F.; Zanelli, M.; Vesce, G.; Marrano, D. Gastrointestinal autonomic nerve tumor of the jejunum. Case report and review of the literature. Ital. J. Gastroenterol. Hepatol. 1997, 6, 558–563. [Google Scholar]
- Bonasoni, M.P.; Comitini, G.; Barbieri, V.; Palicelli, A.; Salfi, N.; Pilu, G. Fetal Presentation of Mediastinal Immature Teratoma: Ultrasound, Autopsy and Cytogenetic Findings. Diagnostics 2021, 11, 1543. [Google Scholar] [CrossRef]
- Zanelli, M.; Sanguedolce, F.; Palicelli, A.; Zizzo, M.; Martino, G.; Caprera, C.; Fragliasso, V.; Soriano, A.; Valle, L.; Ricci, S.; et al. EBV-Driven Lymphoproliferative Disorders and Lymphomas of the Gastrointestinal Tract: A Spectrum of Entities with a Common Denominator (Part 1). Cancers 2021, 13, 4578. [Google Scholar] [CrossRef]
- Zanelli, M.; Sanguedolce, F.; Palicelli, A.; Zizzo, M.; Martino, G.; Caprera, C.; Fragliasso, V.; Soriano, A.; Valle, L.; Ricci, S.; et al. EBV-Driven Lymphoproliferative Disorders and Lymphomas of the Gastrointestinal Tract: A Spectrum of Entities with a Common Denominator (Part 2). Cancers 2021, 13, 4527. [Google Scholar] [CrossRef]
- Zanelli, M.; Zizzo, M.; Bisagni, A.; Froio, E.; De Marco, L.; Valli, R.; Filosa, A.; Luminari, S.; Martino, G.; Massaro, F.; et al. Germinotropic lymphoproliferative disorder: A systematic review. Ann. Hematol. 2020, 99, 2243–2253. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Zizzo, M.; Luminari, S.; Martino, G.; Soriano, A.; Ricci, L.; Caprera, C.; Ascani, S. Indolent T-cell lymphoproliferative disorders of the gastrointestinal tract (iTLPD-GI): A review. Cancers 2021, 13, 2790. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Mengoli, M.C.; Del Sordo, R.; Cagini, A.; De Marco, L.; Simonetti, E.; Martino, G.; Zizzo, M.; Ascani, S. Intravascular NK/T-cell lymphoma, Epstein-Barr virus positive with multiorgan involvement: A clinical dilemma. BMC Cancer 2018, 18, 1115. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Bonacini, M.; Croci, S.; Magi-Galluzzi, C.; Cañete-Portillo, S.; Chaux, A.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; et al. What do we have to know about PD-L1 expression in prostate cancer? A systematic literature review. Part 2: Clinic-pathologic correlations. Cells 2021, 10, 3156. [Google Scholar] [CrossRef]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What do we have to know about PD-L1 expression in prostate cancer? A systematic literature review. Part 3: PD-L1, intracellular signaling pathways and tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 12330. [Google Scholar] [CrossRef]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What do we have to know about PD-L1 expression in prostate cancer? A systematic literature review. Part 4: Experimental treatments in pre-clinical studies (cell lines and mouse models). Int. J. Mol. Sci. 2021, 22, 12297. [Google Scholar] [CrossRef]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What do we have to know about PD-L1 expression in prostate cancer? A systematic literature review. Part 5: Epigenetic regulation of PD-L1. Int. J. Mol. Sci. 2021, 22, 12314. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).