Dynamic 3D On-Chip BBB Model Design, Development, and Applications in Neurological Diseases
Abstract
:1. Introduction
2. Current Design of BBB Models
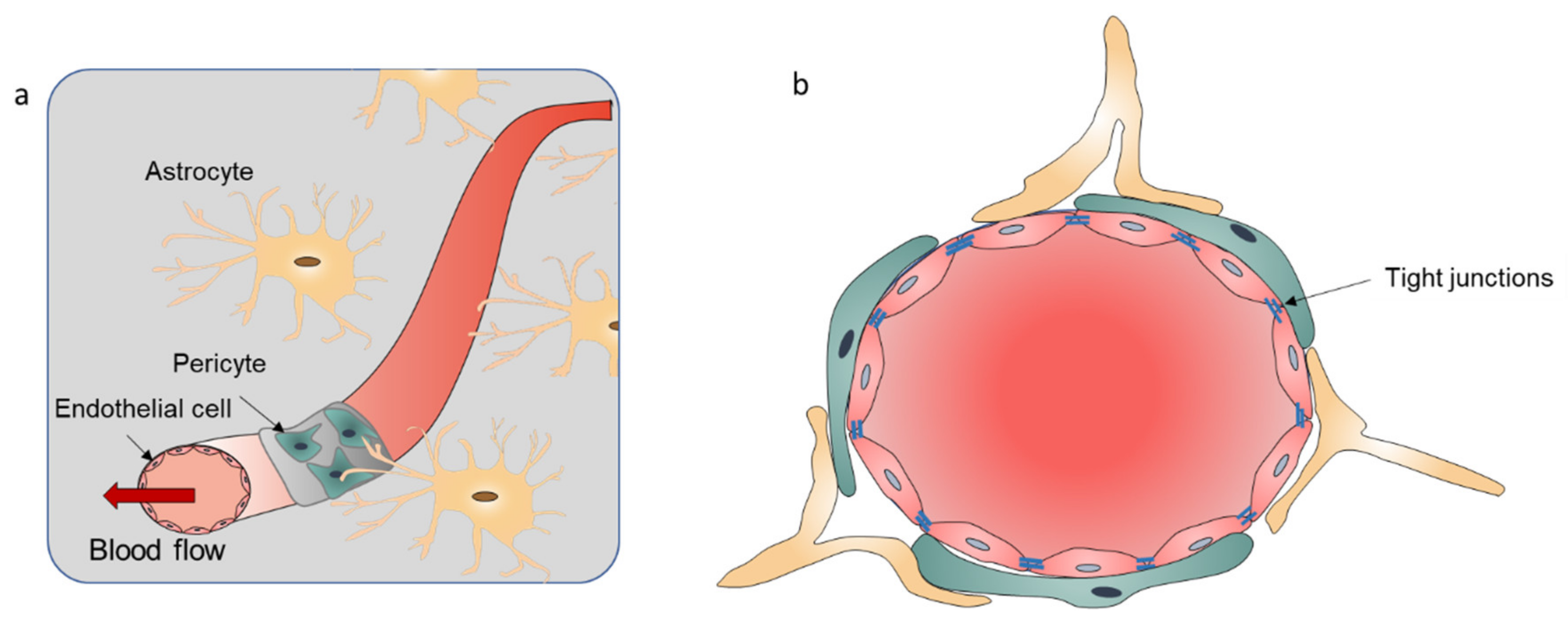
2.1. Introduction of the BBB
2.2. Computation Models
2.3. In Vivo Models
2.4. In Vitro Models
2.4.1. Static In Vitro BBB Models
2.4.2. Dynamic In Vitro BBB Models
3. Principles of Microfluidic Device Design
3.1. Chip Materials
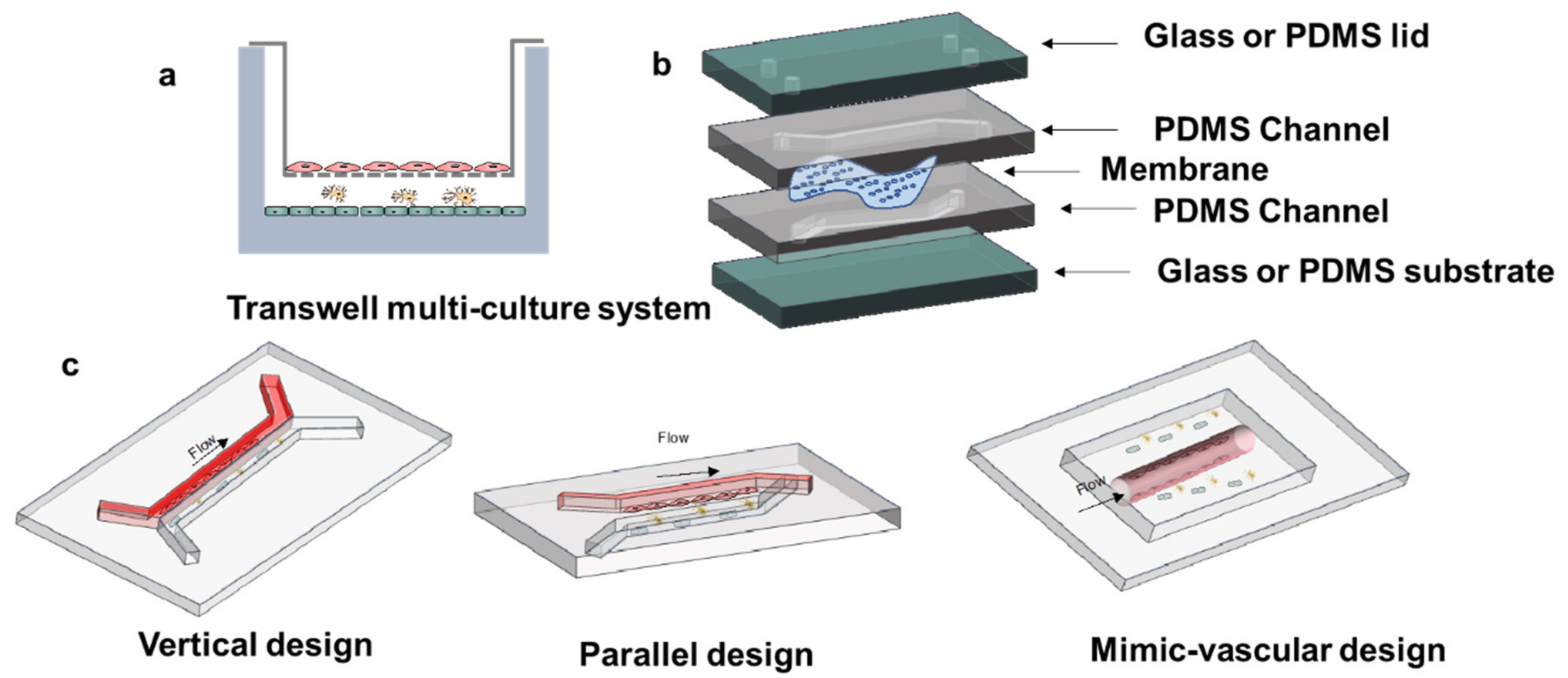
3.2. Microfluidic Device Structure Design
3.3. Porous Membrane
3.4. Cell Source for In Vitro BBB Models
3.5. Incorporation of Shear Stress
4. Characterizations to Examine the Model Integrity
4.1. Transepithelial/Endothelial Electrical Resistance Measurement
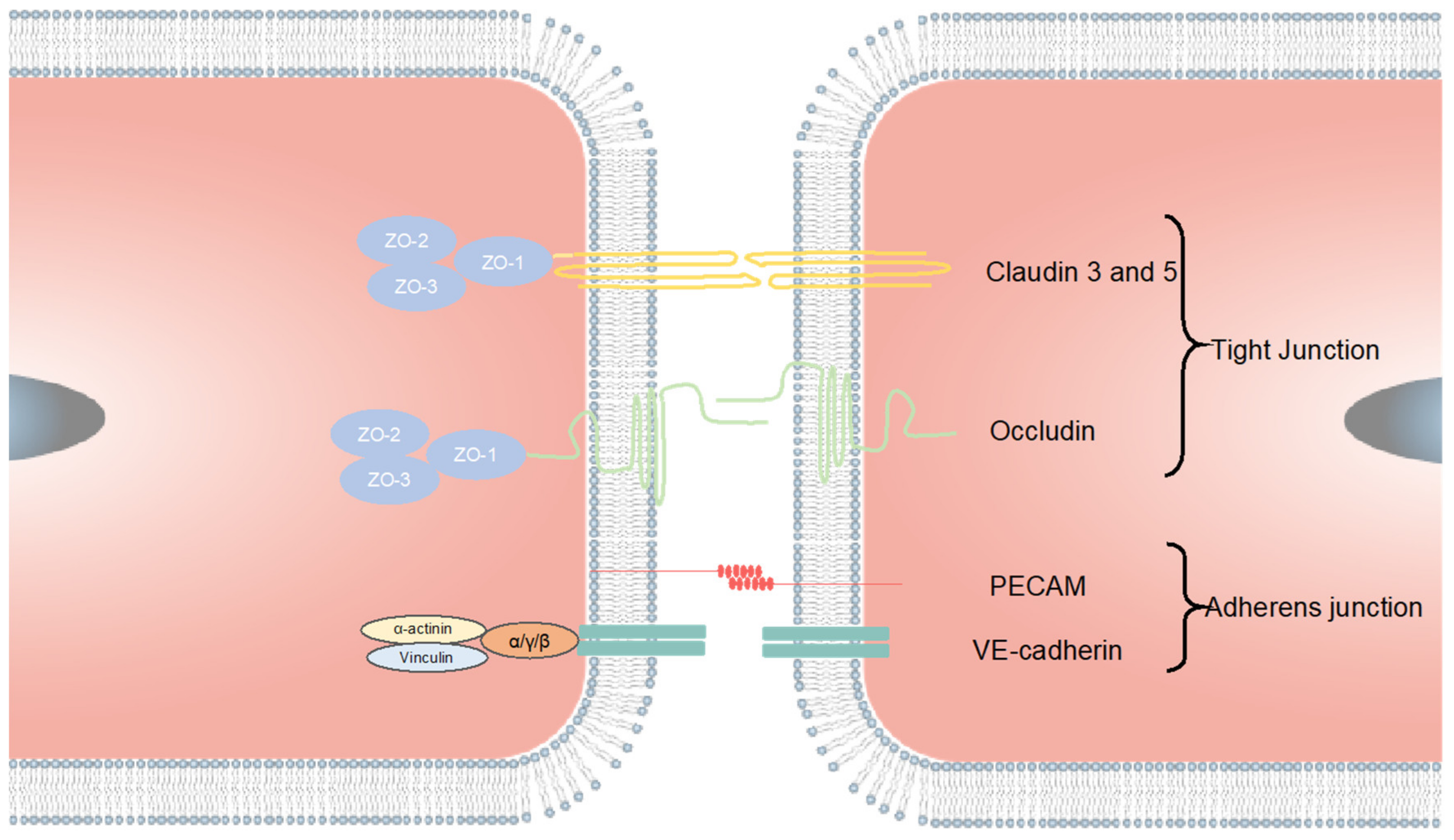
4.2. Tight Junction Markers
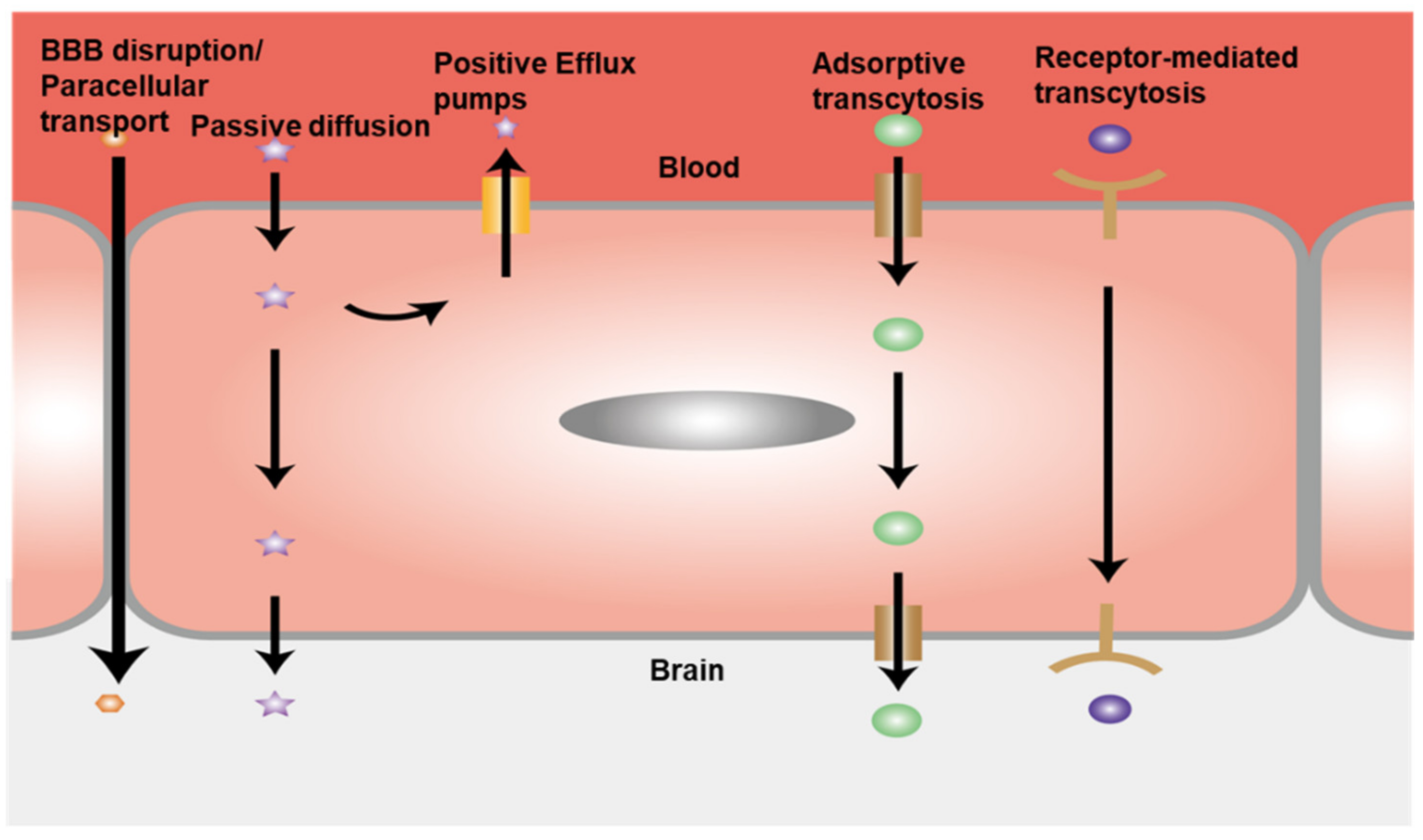
4.3. Permeability
5. Applications of In Vitro BBB Models in Neurological Diseases
5.1. Brain Tumor Research
5.2. Drug-Screening and Efficacy Evaluation
5.3. Stem Cell-Based BBB Models in Personalized Medicine
5.4. Neurological Disorder Disease Modeling
5.5. Neurobiology Research
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zehendner, C.M.; White, R.; Hedrich, J.; Luhmann, H.J. A Neurovascular Blood-Brain Barrier In Vitro Model. In Cerebral Angiogenesis: Methods and Protocols; Milner, R., Ed.; Humana Press: New York, NY, USA, 2014; Volume 1135, pp. 403–413. [Google Scholar]
- Patel, M.M.; Goyal, B.R.; Bhadada, S.V.; Bhatt, J.S.; Amin, A.F. Getting into the brain. CNS Drugs 2009, 23, 35–58. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Zhao, P.; Nandakumar, K.; Wang, L.; Song, Y. Microfluidics-Based Systems in Diagnosis of Alzheimer’s Disease and Biomimetic Modeling. Micromachines 2020, 11, 787. [Google Scholar] [CrossRef]
- Pangalos, M.N.; Schechter, L.E.; Hurko, O. Drug development for CNS disorders: Strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discov. 2007, 6, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Mortsdorf, T.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef]
- Huber, J.D.; Egleton, R.D.; Davis, T.P. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci. 2001, 24, 719–725. [Google Scholar] [CrossRef]
- Jiang, L.; Li, S.; Zheng, J.; Li, Y.; Huang, H. Recent Progress in Microfluidic Models of the Blood-Brain Barrier. Micromachines 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musafargani, S.; Mishra, S.; Gulyas, M.; Mahalakshmi, P.; Archunan, G.; Padmanabhan, P.; Gulyas, B. Blood brain barrier: A tissue engineered microfluidic chip. J. Neurosci. Methods 2020, 331, 108525. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Lecuyer, M.-A.; Kebir, H.; Prat, A. Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.I.; Amaral, M.H.; Costa, P.C.; Lopes, C.M.; Lamprou, D.A. Recent developments in microfluidic technologies for central nervous system targeted studies. Pharmaceutics 2020, 12, 542. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Ai, X.; Sun, J.; Xu, B.; Meng, X.; Zhang, Y.; Zhang, S. Potential applications of microfluidics based blood brain barrier (BBB)-on-chips for in vitro drug development. Biomed. Pharmacother. 2020, 132, 110822. [Google Scholar] [CrossRef]
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in microfluidic blood–brain barrier (BBB) models. Trends Biotechnol. 2019, 37, 1295–1314. [Google Scholar] [CrossRef] [PubMed]
- Nag, S. Blood brain barrier, exchange of metabolites and gases. In Pathology and Genetics: Cerebrovascular Diseases; ISN Neuropath Press: Basel, Switzerland, 2005; pp. 22–29. [Google Scholar]
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-conjugated poly (ethylene glycol)-co-poly (ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32, 4293–4305. [Google Scholar] [CrossRef]
- Sei, Y.; Justus, K.; LeDuc, P.; Kim, Y. Engineering living systems on chips: From cells to human on chips. Microfluid. Nanofluidics 2014, 16, 907–920. [Google Scholar] [CrossRef]
- Hajal, C.; Campisi, M.; Mattu, C.; Chiono, V.; Kamm, R.D. In vitro models of molecular and nano-particle transport across the blood-brain barrier. Biomicrofluidics 2018, 12, 042213. [Google Scholar] [CrossRef]
- Cho, H.; Seo, J.H.; Wong, K.H.; Terasaki, Y.; Park, J.; Bong, K.; Arai, K.; Lo, E.H.; Irimia, D. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. 2015, 5, 15222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.-E.; Sleeboom, J.J.; Ingber, D.E. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Lee, S.-R.; Ko, J.; Son, K.; Tahk, D.; Ahn, J.; Im, C.; Jeon, N.L. A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci. Rep. 2017, 7, 8083. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Shawahna, R.; Uchida, Y.; Decleves, X.; Ohtsuki, S.; Yousif, S.; Dauchy, S.; Jacob, A.; Chassoux, F.; Daumas-Duport, C.; Couraud, P.-O. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol. Pharm. 2011, 8, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Wang, Z.; Liu, Y.; Wang, P.; Xue, Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood–brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. 2011, 44, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood–brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K. Pericytes regulate the blood–brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Chen, Z.-L.; Norris, E.H.; Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014, 5, 3413. [Google Scholar] [CrossRef] [Green Version]
- Nagelhus, E.A.; Ottersen, O.P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 2013, 93, 1543–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolburg, H.; Noell, S.; Wolburg-Buchholz, K.; Mack, A.; Fallier-Becker, P. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neurosci. 2009, 15, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Almad, A.; Maragakis, N.J. A stocked toolbox for understanding the role of astrocytes in disease. Nat. Rev. Neurol. 2018, 14, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.F.; Teixeira, A.L.; Pinheiro, L.; Falcao, A.O. A Bayesian Approach to in Silico Blood-Brain Barrier Penetration Modeling. J. Chem. Inf. Modeling 2012, 52, 1686–1697. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Ibrahim, A.; Fish, P.V.; Cole, S.; Lewis, M.L.; de Groot, M.J.; Reynolds, D.P. Predicting penetration across the blood-brain barrier from simple descriptors and fragmentation schemes. J. Chem. Inf. Modeling 2007, 47, 170–175. [Google Scholar] [CrossRef]
- Ecker, G.F.; Noe, C.R. In silico prediction models for blood-brain barrier permeation. Curr. Med. Chem. 2004, 11, 1617–1628. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Xiao, J.F.; Zhou, N.N.; Zheng, M.Y.; Luo, X.M.; Jiang, H.L.; Chen, K.X. A Genetic Algorithm Based Support Vector Machine Model for Blood-Brain Barrier Penetration Prediction. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adenot, M.; Lahana, R. Blood-brain barrier permeation models: Discriminating between potential CNS and non-CNS drugs including P-glycoprotein substrates. J. Chem. Inf. Comput. Sci. 2004, 44, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Liu, T.; Fan, X.H.; Ai, N. In silico modeling on ADME properties of natural products: Classification models for blood-brain barrier permeability, its application to traditional Chinese medicine and in vitro experimental validation. J. Mol. Graph. Model. 2017, 75, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Terrell-Hall, T.B.; Ammer, A.G.; Griffith, J.I.G.; Lockman, P.R. Permeability across a novel microfluidic blood-tumor barrier model. Fluids Barriers CNS 2017, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.I.G.; Lockman, P.R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017, 8, 83734–83744. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Seo, J.-H.; Garud, K.S.; Park, S.W.; Lee, M.-Y. Numerical approach-based simulation to predict cerebrovascular shear stress in a blood-brain barrier organ-on-a-chip. Biosens. Bioelectron. 2021, 183, 113197. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood-brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Kuhnline Sloan, C.D.; Nandi, P.; Linz, T.H.; Aldrich, J.V.; Audus, K.L.; Lunte, S.M. Analytical and biological methods for probing the blood-brain barrier. Annu. Rev. Anal. Chem. 2012, 5, 505–531. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, T.; Miao, X.; Yi, X.; Wang, X.; Zhao, H.; Lee, S.M.-Y.; Zheng, Y. Zebrafish: A promising in vivo model for assessing the delivery of natural products, fluorescence dyes and drugs across the blood-brain barrier. Pharmacol. Res. 2017, 125, 246–257. [Google Scholar] [CrossRef]
- Liu, D.; Lin, B.; Shao, W.; Zhu, Z.; Ji, T.; Yang, C. In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood–brain barrier. ACS Appl. Mater. Interfaces 2014, 6, 2131–2136. [Google Scholar] [CrossRef]
- Pandey, P.K.; Sharma, A.K.; Gupta, U. Blood brain barrier: An overview on strategies in drug delivery, realistic in vitro modeling and in vivo live tracking. Tissue Barriers 2016, 4, e1129476. [Google Scholar] [CrossRef] [Green Version]
- Appelt-Menzel, A.; Cubukova, A.; Günther, K.; Edenhofer, F.; Piontek, J.; Krause, G.; Stüber, T.; Walles, H.; Neuhaus, W.; Metzger, M. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri-and multipotent stem cells. Stem Cell Rep. 2017, 8, 894–906. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.-F.; Wolfe, J.M.; Fadzen, C.M.; Calligaris, D.; Hornburg, K.; Chiocca, E.A.; Agar, N.Y.; Pentelute, B.L.; Lawler, S.E. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017, 8, 15623. [Google Scholar] [CrossRef]
- Qi, D.; Wu, S.; Lin, H.; Kuss, M.A.; Lei, Y.; Krasnoslobodtsev, A.; Ahmed, S.; Zhang, C.; Kim, H.J.; Jiang, P. Establishment of a human iPSC-and nanofiber-based microphysiological blood–brain barrier system. ACS Appl. Mater. Interfaces 2018, 10, 21825–21835. [Google Scholar] [CrossRef]
- De Jong, E.; Williams, D.S.; Abdelmohsen, L.K.; Van Hest, J.C.; Zuhorn, I.S. A filter-free blood-brain barrier model to quantitatively study transendothelial delivery of nanoparticles by fluorescence spectroscopy. J. Control. Release 2018, 289, 14–22. [Google Scholar] [CrossRef]
- Chou, C.-H.; Sinden, J.D.; Couraud, P.-O.; Modo, M. In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells. PLoS ONE 2014, 9, e106346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Brookes, O.; Battaglia, G. Pericytes from Mesenchymal Stem Cells as a model for the blood-brain barrier. Sci. Rep. 2017, 7, 39676. [Google Scholar] [CrossRef]
- Stebbins, M.J.; Gastfriend, B.D.; Canfield, S.G.; Lee, M.-S.; Richards, D.; Faubion, M.G.; Li, W.-J.; Daneman, R.; Palecek, S.P.; Shusta, E.V. Human pluripotent stem cell–derived brain pericyte–like cells induce blood-brain barrier properties. Sci. Adv. 2019, 5, eaau7375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eilenberger, C.; Rothbauer, M.; Selinger, F.; Gerhartl, A.; Jordan, C.; Harasek, M.; Schädl, B.; Grillari, J.; Weghuber, J.; Neuhaus, W. A microfluidic multisize spheroid array for multiparametric screening of anticancer drugs and blood–brain barrier transport properties. Adv. Sci. 2021, 8, 2004856. [Google Scholar] [CrossRef]
- Sugihara, K.; Yamaguchi, Y.; Usui, S.; Nashimoto, Y.; Hanada, S.; Kiyokawa, E.; Uemura, A.; Yokokawa, R.; Nishiyama, K.; Miura, T. A new perfusion culture method with a self-organized capillary network. PLoS ONE 2020, 15, e0240552. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.L.; Triplett, M.; Simon, M.; Alvarado, J.; Booth, R.; Osburn, J.; Soscia, D.; Qian, F.; Fischer, N.O.; Kulp, K. A reconfigurable in vitro model for studying the blood–brain barrier. Ann. Biomed. Eng. 2020, 48, 780–793. [Google Scholar] [CrossRef]
- Partyka, P.P.; Godsey, G.A.; Galie, J.R.; Kosciuk, M.C.; Acharya, N.K.; Nagele, R.G.; Galie, P.A. Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier. Biomaterials 2017, 115, 30–39. [Google Scholar] [CrossRef]
- Santa-Maria, A.R.; Walter, F.R.; Figueiredo, R.; Kincses, A.; Vigh, J.P.; Heymans, M.; Culot, M.; Winter, P.; Gosselet, F.; Dér, A. Flow induces barrier and glycocalyx-related genes and negative surface charge in a lab-on-a-chip human blood-brain barrier model. J. Cereb. Blood Flow Metab. 2021, 41, 0271678X21992638. [Google Scholar] [CrossRef]
- Yu, F.; Kumar, N.D.O.S.; Foo, L.C.; Ng, S.H.; Hunziker, W.; Choudhury, D. A pump-free tricellular blood–brain barrier on-a-chip model to understand barrier property and evaluate drug response. Biotechnol. Bioeng. 2020, 117, 1127–1136. [Google Scholar] [CrossRef]
- D’Aversa, T.G.; Eugenin, E.A.; Lopez, L.; Berman, J.W. Myelin basic protein induces inflammatory mediators from primary human endothelial cells and blood–brain barrier disruption: Implications for the pathogenesis of multiple sclerosis. Neuropathol. Appl. Neurobiol. 2013, 39, 270–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, T.; Maguire, S.E.; Canfield, S.G.; Bao, X.; Olson, W.R.; Shusta, E.V.; Palecek, S.P. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci. Adv. 2017, 3, e1701679. [Google Scholar] [CrossRef] [Green Version]
- Linville, R.M.; DeStefano, J.G.; Sklar, M.B.; Xu, Z.; Farrell, A.M.; Bogorad, M.I.; Chu, C.; Walczak, P.; Cheng, L.; Mahairaki, V.; et al. Human iPSC-derived blood-brain barrier microvessels: Validation of barrier function and endothelial cell behavior. Biomaterials 2019, 190–191, 24–37. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e1006. [Google Scholar] [CrossRef]
- Wainwright, L.; Hargreaves, I.P.; Georgian, A.R.; Turner, C.; Dalton, R.N.; Abbott, N.J.; Heales, S.J.; Preston, J.E. CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport. J. Clin. Med. 2020, 9, 3236. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Zahid, A.A.; Mraiche, F.; Alam, K.; Al Moustafa, A.-E.; Hasan, A. Gelatin-methacryloyl hydrogel based in vitro blood–brain barrier model for studying breast cancer-associated brain metastasis. Pharm. Dev. Technol. 2021, 26, 490–500. [Google Scholar] [CrossRef]
- Cucullo, L.; Hossain, M.; Rapp, E.; Manders, T.; Marchi, N.; Janigro, D. Development of a humanized in vitro blood–brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia 2007, 48, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Cucullo, L.; Marchi, N.; Hossain, M.; Janigro, D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J. Cereb. Blood Flow Metab. 2011, 31, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Li, Z.; Yu, Y.; Sizdahkhani, S.; Ho, W.S.; Yin, F.; Wang, L.; Zhu, G.; Zhang, M.; Jiang, L. A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci. Rep. 2016, 6, 36670. [Google Scholar] [CrossRef] [Green Version]
- Prabhakarpandian, B.; Shen, M.-C.; Nichols, J.B.; Mills, I.R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. SyM-BBB: A microfluidic blood brain barrier model. Lab Chip 2013, 13, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.A.; Kim, H.N.; Im, S.-K.; Chung, S.; Kang, J.Y.; Choi, N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015, 9, 024115. [Google Scholar] [CrossRef] [Green Version]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Pensabene, V.; Markov, D.A.; Allwardt, V.; Neely, M.D.; Shi, M.; Britt, C.M.; Hoilett, O.S.; Yang, Q.; Brewer, B.M. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015, 9, 054124. [Google Scholar] [CrossRef] [Green Version]
- Booth, R.; Kim, H. Permeability analysis of neuroactive drugs through a dynamic microfluidic in vitro blood–brain barrier model. Ann. Biomed. Eng. 2014, 42, 2379–2391. [Google Scholar] [CrossRef]
- Deosarkar, S.P.; Prabhakarpandian, B.; Wang, B.; Sheffield, J.B.; Krynska, B.; Kiani, M.F. A novel dynamic neonatal blood-brain barrier on a chip. PLoS ONE 2015, 10, e0142725. [Google Scholar]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef]
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; Van Vught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G. A perfused human blood–brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 2018, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Buchroithner, B.; Mayr, S.; Hauser, F.; Priglinger, E.; Stangl, H.; Santa-Maria, A.R.; Deli, M.A.; Der, A.; Klar, T.A.; Axmann, M. Dual Channel Microfluidics for Mimicking the Blood–Brain Barrier. ACS Nano 2021, 15, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab Chip 2017, 17, 448–459. [Google Scholar] [CrossRef]
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G. A 3D Real-Scale, Biomimetic, and Biohybrid Model of the Blood-Brain Barrier Fabricated through Two-Photon Lithography. Small 2018, 14, 1702959. [Google Scholar] [CrossRef]
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.-E.; Dauth, S.; Mannix, R. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Hawkins, B.T.; Yun, Y. Three-dimensional (3D) tetra-culture brain on chip platform for organophosphate toxicity screening. Sci. Rep. 2018, 8, 2841. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.; Buonocore, J.; Park, J.; Welsh, C.J.; Li, J.; Han, A. A three-dimensional arrayed microfluidic blood–brain barrier model with integrated electrical sensor array. IEEE Trans. Biomed. Eng. 2017, 65, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Walter, F.R.; Valkai, S.; Kincses, A.; Petneházi, A.; Czeller, T.; Veszelka, S.; Ormos, P.; Deli, M.A.; Dér, A. A versatile lab-on-a-chip tool for modeling biological barriers. Sens. Actuators B Chem. 2016, 222, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Achyuta, A.K.H.; Conway, A.J.; Crouse, R.B.; Bannister, E.C.; Lee, R.N.; Katnik, C.P.; Behensky, A.A.; Cuevas, J.; Sundaram, S.S. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip 2013, 13, 542–553. [Google Scholar] [CrossRef]
- Falanga, A.P.; Pitingolo, G.; Celentano, M.; Cosentino, A.; Melone, P.; Vecchione, R.; Guarnieri, D.; Netti, P.A. Shuttle-mediated nanoparticle transport across an in vitro brain endothelium under flow conditions. Biotechnol. Bioeng. 2017, 114, 1087–1095. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Jenkins, G. Rapid prototyping of PDMS devices using SU-8 lithography. In Microfluidic Diagnostics; Springer: Heidelberg, Germany, 2013; pp. 153–168. [Google Scholar]
- Menon, N.V.; Chuah, Y.J.; Cao, B.; Lim, M.; Kang, Y. A microfluidic co-culture system to monitor tumor-stromal interactions on a chip. Biomicrofluidics 2014, 8, 064118. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Fang, Q.; den Toonder, J.M. Microfluidics for cell-based high throughput screening platforms—A review. Anal. Chim. Acta 2016, 903, 36–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, A.M.; Devadas, D.; Young, E.W. Recycled polymethylmethacrylate (PMMA) microfluidic devices. Sens. Actuators B Chem. 2017, 253, 738–744. [Google Scholar] [CrossRef]
- Murphy, A.R.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 2017, 54, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.-T.; Ta, H.T. Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef]
- Vera, D.; García-Díaz, M.; Torras, N.; Álvarez, M.; Villa, R.; Martinez, E. Engineering Tissue Barrier Models on Hydrogel Microfluidic Platforms. ACS Appl. Mater. Interfaces 2021, 13, 13920–13933. [Google Scholar] [CrossRef] [PubMed]
- Aday, S.; Cecchelli, R.; Hallier-Vanuxeem, D.; Dehouck, M.; Ferreira, L. Stem cell-based human blood–brain barrier models for drug discovery and delivery. Trends Biotechnol. 2016, 34, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.; Robey, P.G. Human pluripotent stem cell culture: Considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Yan, Y.; Marzano, M.; Li, Y. Studying Heterotypic Cell–cell interactions in the human brain using pluripotent stem cell models for neurodegeneration. Cells 2019, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Jeske, R.; Albo, J.; Marzano, M.; Bejoy, J.; Li, Y. Engineering brain-specific pericytes from human pluripotent stem cells. Tissue Eng. Part B Rev. 2020, 26, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-Azpiazu, P.; Panagiotou, S.; Jose, G.; Saha, S. A novel dynamic multicellular co-culture system for studying individual blood-brain barrier cell types in brain diseases and cytotoxicity testing. Sci. Rep. 2018, 8, 8784. [Google Scholar] [CrossRef]
- Ghosh, C.; Gonzalez-Martinez, J.; Hossain, M.; Cucullo, L.; Fazio, V.; Janigro, D.; Marchi, N. Pattern of P450 expression at the human blood–brain barrier: Roles of epileptic condition and laminar flow. Epilepsia 2010, 51, 1408–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaisar, M.A.; Sajja, R.K.; Prasad, S.; Abhyankar, V.V.; Liles, T.; Cucullo, L. New experimental models of the blood-brain barrier for CNS drug discovery. Expert Opin. Drug Discov. 2017, 12, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Wang, P.; Liu, Z.-H.; Ye, P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. Int. J. Oral Sci. 2018, 10, e8. [Google Scholar] [CrossRef] [Green Version]
- Sawada, N. Tight junction-related human diseases. Pathol. Int. 2013, 63, 1–12. [Google Scholar] [CrossRef]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006, 126, 741–754. [Google Scholar] [CrossRef] [Green Version]
- Aryal, M.; Fischer, K.; Gentile, C.; Gitto, S.; Zhang, Y.-Z.; McDannold, N. Effects on P-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS ONE 2017, 12, e0166061. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.; Ye, M.; Levy, A.; Rothstein, J.; Bergles, D.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, H.; Olszewski, J. Spread of sodium fluorescein in normal brain tissue: A study of the mechanism of the blood-brain barrier. Neurology 1961, 11, 1081. [Google Scholar] [CrossRef]
- Yi, H.-G.; Jeong, Y.H.; Kim, Y.; Choi, Y.-J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Gao, D.; Chen, Y.; Jin, F.; Hu, G.; Jiang, Y.; Liu, H. Development of a blood-brain barrier model in a membrane-based microchip for characterization of drug permeability and cytotoxicity for drug screening. Anal. Chim. Acta 2016, 934, 186–193. [Google Scholar] [CrossRef]
- Sances, S.; Ho, R.; Vatine, G.; West, D.; Laperle, A.; Meyer, A.; Godoy, M.; Kay, P.S.; Mandefro, B.; Hatata, S. Human iPSC-derived endothelial cells and microengineered organ-chip enhance neuronal development. Stem Cell Rep. 2018, 10, 1222–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chonan, Y.; Taki, S.; Sampetrean, O.; Saya, H.; Sudo, R. Endothelium-induced three-dimensional invasion of heterogeneous glioma initiating cells in a microfluidic coculture platform. Integr. Biol. 2017, 9, 762–773. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Monge, R.; Martínez-González, A.; Virumbrales-Muñoz, M.; Llamazares, G.A.; Berganzo, J.; Hernández-Laín, A.; Santolaria, J.; Doblaré, M.; Hubert, C. Glioblastoma on a microfluidic chip: Generating pseudopalisades and enhancing aggressiveness through blood vessel obstruction events. Neuro-Oncology 2017, 19, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Nguyen, D.T.; Akay, Y.; Xu, F.; Akay, M. Engineering a brain cancer chip for high-throughput drug screening. Sci. Rep. 2016, 6, 25062. [Google Scholar] [CrossRef] [Green Version]
- Stowe, A.M.; Adair-Kirk, T.L.; Gonzales, E.R.; Perez, R.S.; Shah, A.R.; Park, T.S.; Gidday, J.M. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol. Dis. 2009, 35, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, J.W.; Bula, M.; Davila-Velderrain, J.; Akay, L.A.; Zhu, L.; Frank, A.; Victor, M.B.; Bonner, J.M.; Mathys, H.; Lin, Y.T.; et al. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat. Med. 2020, 26, 952–963. [Google Scholar] [CrossRef]
- Park, J.; Wetzel, I.; Marriott, I.; Dréau, D.; D’Avanzo, C.; Kim, D.Y.; Tanzi, R.E.; Cho, H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 941–951. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Newbold, M.A.; Gao, Z.; Haynes, C.L. A versatile microfluidic platform for the study of cellular interactions between endothelial cells and neutrophils. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, M.; Wasson, E.M.; Lee, Y.W.; Davalos, R.V. Electroporation of brain endothelial cells on chip toward permeabilizing the blood-brain barrier. Biophys. J. 2016, 110, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishihara, H.; Gastfriend, B.D.; Soldati, S.; Perriot, S.; Mathias, A.; Sano, Y.; Shimizu, F.; Gosselet, F.; Kanda, T.; Palecek, S.P.; et al. Advancing human induced pluripotent stem cell-derived blood-brain barrier models for studying immune cell interactions. FASEB J. 2020, 34, 16693–16715. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Soroush, F.; Sun, S.; Liverani, E.; Langston, J.C.; Yang, Q.; Kilpatrick, L.E.; Kiani, M.F. Protein kinase C-delta inhibition protects blood-brain barrier from sepsis-induced vascular damage. J. Neuroinflammation 2018, 15, 309. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.T.; Chutna, O.; Chu, V.; Conde, J.P.; Outeiro, T.F. A novel microfluidic cell co-culture platform for the study of the molecular mechanisms of Parkinson’s disease and other synucleinopathies. Front. Neurosci. 2016, 10, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, H.J.; Kang, M.W.; Jeon, N.L. Advances in microfluidics-based experimental methods for neuroscience research. Lab Chip 2013, 13, 509–521. [Google Scholar] [CrossRef]
- Tong, Z.; Rajeev, G.; Guo, K.; Ivask, A.; McCormick, S.; Lombi, E.; Priest, C.; Voelcker, N.H. Microfluidic cell microarray platform for high throughput analysis of particle–cell interactions. Anal. Chem. 2018, 90, 4338–4347. [Google Scholar] [CrossRef]

| Types of BBB Model | Culture System Conditions | Architecture for Culture | Limitations | Application | Ref. |
|---|---|---|---|---|---|
| static 3D model | multi-culture in transwell | Establish a coculture model by iPSCs derived neurons, astrocytes, pericytes to mimic in vivo neurovascular units | no shear stress | Confirmation of the relevant role of claudin subtypes for cellular tightness. | [53] |
| static 3D model | self-assembling multicellular BBB spheroids model | The spheroid core is comprised mainly of astrocytes, while brain endothelial cells and pericytes encase the surface, acting as a barrier that regulates transport of molecules | no shear stress and difficult to control the test | Screening and identifying BBB-penetrant cell-penetrating peptides. | [54] |
| static 2D model | polymer transwell membrane model | PLGA nanofiber mesh replace the traditional transwell membrane culture with hiPSC-EC and Astrocytes | no shear stress | A new, powerful tool for research on human BBB physiology and pathology higher TEER value and good barrier functions. | [55] |
| static 2D model | membrane free hydrogel BBB model | A collagen gel covered with a monolayer of brain microvascular endothelial cells | no shear stress and only ECs | Quantification of nanoparticle transcytosis and assessment of transendothelialdelivery of PEG-P(CL-g-TMC) polymersomes. | [56] |
| static 2D model | From mono- to transwell- to coculture BBB model | from the culture system with EC only, NSC only, EC and NSC transwell, to hECs/hNSC coculture | no shear stress with no pericytes and astrocytes | Assaying dynamic cellular interactions between hECs and NSCs and forming NVU. | [57] |
| static 2D model | Transwell model | Substituting pericytes with MSCs in fabricating BBB system | no shear stress and no astrocytes | Retaining the BBB phenotypes with TJ and permeability and up-regulating the pericytes mark. | [58] |
| static 2D model | Transwell model | iPSC-BMECs, astrocytes, pericyte, and neurons to form an isogenic human model | No fluidic flow and shear stress | Combining the BMECs, neurons, astrocytes, and brain pericyte-like cells from a single iPSC cell line to form an isogenic NVU model with optimal TEER. | [59] |
| Dynamic 3D spheroid model | microtiter plate | human primary astrocytes, human primary pericytes, hCMEC/D3 | Difficult for integration test of BBB organoids | Developing a method for generation 90-multi-sized organoids reliably and reproducibly. Fabricating multi-sized BBB organoids and characterizing the drug dose response. | [60] |
| Dynamic 3D spheroid model | Hydrogel with glass dish | HUVECs, LM-4 cells, HL-60 cells | Complex fabrication method for large numbers of experiments | Establishing a new culture system in the lumen of glass culture dish. Observation of endothelial cells formation with different cell lines. | [61] |
| DIV-model | 3D vasculogenic model | Human astrocyte and hECs | Too thick for the porous fiber | New platform for studying BBB. | [62] |
| DIV-model | QV-600 chamber multi-chamber perfusion system | PBMECs | Can only apply for the shear stress research | enhancing and maintaining TEER for longer. | [63] |
| microfluidics 2D model | sandwich design model | ECs and pericytes coculture with consistent fluid flow | low contact area between neuronal and vascular channels | Showing mechanical stimuli exerted by blood flow mediate both the permeability of the endothelial barrier and waste transport along the basement membrane. | [64] |
| microfluidics 3D model | 3D vasculogenic hydrogel model | ECs coculture system with pericyte and astrocytes in collagen I gel | difficult to apply different shear stress | Build a new simple, cost-effective, and scalable in vitro platform for targeting neuroinflammatory conditions. | [65] |
| Culture Structure | Materials Used | Cell Type | Membrane | EC Layer Integrity Marker | TEER Value | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Vertical 2D culture | PDMS | hBMECs, pericytes, astrocytes, hiNPCs | PC | ZO-1 | N/A | Provide a novel platform for modeling of BBB function and testing of drug toxicity and permeability regarding the CNS. | [78] |
| Tubular 3D culture | PDMS collagen gel | hMVECs, human astrocyte, human pericytes | N/A | ZO-1, VE-cadherin | 40–50 Ω·cm−2 | Astrocytes and pericytes coculture system enhances the integrity of BBB and provides better G-CSF and IL-6 secretion level than transwell. | [26] |
| Vertical chambers | PDMS | C6 astrocytes and bEnd.3 cells | PC | ZO-1 | 223–280 Ω·cm−2 | Permeability of seven neuroactive drugs and TEER and predicting of BBB clearance of pharmaceuticals. | [79] |
| Parallel 3D chambers | PDMS | RBE4 cells and astrocytes | pores generated by lithography between two chambers | ZO-1 | 250 Ω·cm−2 | Mimicking the in vivo microenvironment closely and showing better barrier properties. | [80] |
| Vertical 2D chambers | PDMS, 3D printed plastic, Ag/AgCl pellet electrode | iPSC-BMECs and astrocytes | 0.4 µm PC | ZO-1, Claudin-5 | 4,000 Ω·cm−2 | Evaluating the capacity of our microfluidic BBB model to be used for drug permeability studies using large molecules (FITC-dextrans) and model drugs. | [81] |
| Parallel 3D chambers | Organo Plate | hBMECs(TY10), human pericytes, human astrocytes | ECM gel | PECAM-1, Claudin-5, VE-Cadherin | N/A | Integrating a human BBB microfluidic model in a high-throughput plate-based format that can be used for drug-screening purposes. | [82] |
| Vertical 3D Chambers | PDMS | hBMECs, human astrocytes, human pericytes | 8 µm PC | ZO-1, α-SMA | 150 Ω·cm−2 | Building an on-chip-BBB structure and function by cellular interactions, key gene expressions, low permeability, and 3D astrocytic network. Investigate the nanoparticles mechanism. | [8] |
| Layer-by-layer Sandwich coculture device | PMMA (Acrylic glass) | hBMECs, hUVEC, human pericytes | PET grids (laser cutting) | CD146, CD31 | N/A | Constructing a dual channels microfluidic BBB model for high-resolution 3D localization microscopy of the cytoskeleton and 3D single-molecule-sensitive tracing of lipoprotein particles. | [83] |
| Vertical 2D Chambers | PDMS | hBMECs, human astrocytes, human pericytes | 0.4 µm PET | ZO-1, Claudin-5, PECAM-1, GLUT-1, P-glycoprotein | 17,000–27,000 Ω (hypoxia)/400–23,000 Ω (normaxia) | The hypoxia condition enhances the integrity of BBB model and this model provides a more precise model for drug-screening. | [77] |
| Parallel 3D multi-channels culture | PDMS | hUVEC, rat astrocytes in gel, rat neurons in gel | N/A | ZO-1, VE-cadherin | N/A | Inventing a new platform for the development of a more sophisticated and complex 3D in vitro neurovascular model and has good observation of neurons. | [84] |
| 3D biomimetic vessel parallel microtubes | N/A | bEnd.3, U87 glioblastoma cells | porous microtube | ZO-1 | 71–75 Ω·cm−2 | Fabricating a 1:1 scale biomimetic BBB model with satisfied TEER and capability for drug-screening. | [85] |
| 2D vertical tandem multichambers | PDMS | hBMECs, human astrocytes, human pericytes | PC | VE-cadherin | N/A | The link system mimics the effects of intravascular administration of the psychoactive drug methamphetamine and determines the previously unknown metabolic coupling between the BBB and neurons. | [86] |
| 3D vertical culture | n/A | bEnd.3 (murine ECs), N2a (murine brain neuroblastoma), C8-D1A (murine astrocytes), BV-2 (murine microglia) | Gel-cell matrix | claudin-5 | N/A | Building a platform by measuring Organophosphate-based compounds (OPs) effects on barrier integrity, acetylcholinesterase (AChE) inhibition, viability and residual OP concentration with four model Ops. | [87] |
| 3D vertical culture | PDMS, PC, Titanium elecrode | mBMECs, mouse astrocytes, | PC | ZO-1 | 3.6–4.5 kΩ (coculture) | Coculture system with multielectrodes integrated system and the enhance the TJ under shear stress. | [88] |
| 3D 3 parrallel channels | PDMS, glass | hiPSC-ECs, human astrocytes, human pericytes | PDMS with 120 μm pores by fabrication | CD-31, F-actin | N/A | The microvascular model is fabricated by the vasculogenesis and provides transport of molecules. | [89] |
| 3D 3 parrallel channels | PDMS, microhydrogel | hUVEC, Astrocytes | PDMS porous structure | CD-31, ZO-1 | N/A | A NVU model was fabricated by perivascular network morphology and synaptic structures and test the permeability. | [74] |
| Vertical 2D channels | PDMS | hCMEC/D3 cell line or rEC, rat pericytes, rat astrocytes | 0.45 PET | ZO-1, β-catenin | 175 Ω·cm−2 | The 2 or 3 cells coculture make it easy to observe the cell growth and primary cells show better BBB integration. | [90] |
| Vertical 2D channels | PDMS | RBE4 cell, rat neurons, rat pericytes, rat astrocytes | 0.8 um PC | ZO-1 | N/A | Isolation culture with the different chambers and test the neuroinflammation. | [91] |
| Vertical 2D channels | PDMS | rBMEC, rat astrocytes | collagen I gel | ZO-1, VE-cadherin | 1300 Ω·cm−2 | Replicating of the key structural, functional and mechanical properties of the blood–brain barrier. The interaction of cancer cells and astrocytes decrease the migration of the tumor. | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, C.; Muok, L.; Zeng, C.; Li, Y. Dynamic 3D On-Chip BBB Model Design, Development, and Applications in Neurological Diseases. Cells 2021, 10, 3183. https://doi.org/10.3390/cells10113183
Chen X, Liu C, Muok L, Zeng C, Li Y. Dynamic 3D On-Chip BBB Model Design, Development, and Applications in Neurological Diseases. Cells. 2021; 10(11):3183. https://doi.org/10.3390/cells10113183
Chicago/Turabian StyleChen, Xingchi, Chang Liu, Laureana Muok, Changchun Zeng, and Yan Li. 2021. "Dynamic 3D On-Chip BBB Model Design, Development, and Applications in Neurological Diseases" Cells 10, no. 11: 3183. https://doi.org/10.3390/cells10113183
APA StyleChen, X., Liu, C., Muok, L., Zeng, C., & Li, Y. (2021). Dynamic 3D On-Chip BBB Model Design, Development, and Applications in Neurological Diseases. Cells, 10(11), 3183. https://doi.org/10.3390/cells10113183





