From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma
Abstract
:1. Introduction
| Treatment(s) | Class | Setting | Indication | Key Data | Clinical Trial |
|---|---|---|---|---|---|
| Monotherapy | |||||
| Nivolumab | ICI | Second line | Advanced RCC after prior anti-angiogenic therapy | Nivolumab vs. Everolimus: • mOS: 25.0 mo (95% CI, 21.8–NE) vs. 19.6 mo (95% CI, 17.6–23.1) [HR 0.73; 98.5% CI, 0.57–0.93; p = 0.002] • mPFS: 4.6 mo (95% CI, 3.7–5.4) vs. 4.4 mo (95% CI, 3.7–5.5) [HR 0.88; 95% CI, 0.75–1.03; p = 0.11] • ORR: 25% vs. 5% [OR 5.98; 95% CI, 3.68–9.72; p < 0.001] | CheckMate 025 [18] |
| High-Dose IL-2 | Cytokine | First line | Metastatic RCC | Proleukin: • ORR: 14% (90% CI, 10–19) • CR: 12 (5%) CR • PR: 24 (9%, median response duration 19.0 mo) | [8] |
| Combination Therapy | |||||
| Ipilimumab + Nivolumab | ICI + ICI | First line | Intermediate/poor-risk advanced RCC | Ipilimumab + Nivolumab vs. Sunitinib: • mOS: NR (95% CI, 28.2–NE) vs. 26 mo (95% CI, 22.1–NE) [HR 0.63; 99.8%, CI 0.44–0.89; p < 0.001] • mPFS: 11.6 mo (95% CI, 8.7–15.5) vs. 8.4 mo (95% CI, 7.0–10.8) [HR 0.82; 99% CI, 0.64–1.05; p = 0.03] • ORR: 42% (95% CI, 37–47) vs. 27% (95% CI, 22–31) [p < 0.001] | CheckMate 214 [24] |
| Nivolumab + Cabozantinib | ICI + TKI | First line | Advanced RCC | Nivolumab + Cabozantinib vs. Sunitinib: • Probability of OS at 12 mo: 85.7% (95% CI, 81.3–89.1) vs. 75.6% (95% CI, 70.5–80.0) [HR 0.60; 98.89% CI, 0.40–0.89; p = 0.001] • mPFS: 16.6 mo (95% CI, 12.5–24.9) vs. 8.3 mo (95% CI, 7.0–9.7) [HR 0.51; 95% CI, 0.41–0.64; p < 0.001] • ORR: 55.7% (95% CI, 50.1–61.2) vs. 27.1% (95% CI, 22.4–32.3) [p < 0.001] | CheckMate 9ER [31] |
| Pembrolizumab + Lenvatinib | ICI + TKI | First line | Advanced RCC | Pembrolizumab + Lenvatinib vs. Sunitinib: • mOS: NR (33.6–NE) vs. NR (NE–NE) [HR 0.66; 95% CI, 0.49–0.88; p = 0.0049] • mPFS: 23.9 mo (95% CI, 20.8–27.7) vs. 9.2 mo (95% CI, 6.0–11.0) [HR 0.39; 95% CI, 0.32–0.49; p < 0.0001] • ORR: 71% (95% CI, 66–76) vs. 36% (95% CI, 31–41) [p < 0.0001] | KEYNOTE-581/CLEAR [30] |
| Pembrolizumab + Axitinib | ICI + TKI | First line | Advanced RCC | Pembrolizumab + Axitinib vs. Sunitinib: • mOS: NR vs. NR [HR 0.53; 95% CI, 0.38–0.74; p < 0.0001] • mPFS: 15.1 mo (95% CI, 12.6–17.7) vs. 11.1 mo (95% CI, 8.7–12.5) [HR 0.69; 95% CI, 0.57–0.84; p < 0.001) • ORR: 59.3% (95%, CI 54.5–63.9) vs. 35.7% (95% CI, 31.1–40.4) [p < 0.001] | KEYNOTE-426 [27] |
| Avelumab + Axitinib | ICI + TKI | First line | Advanced RCC | Avelumab + Axitinib vs. Sunitinib: • 12 mo OS (PD-L1+): 86% vs. 83% (HR 0.78; 95% CI, 0.55–1.08; p = 0.14) • mPFS (PD-L1+): 13.8 mo vs. 7.2 mo [HR 0.61; 95% CI, 0.47–0.79; p < 0.001] • mPFS (overall population): 13.8 mo (95% CI, 11.1–NE) vs. 8.4 mo (95% CI, 6.9–11.1) [HR 0.69; 95% CI, 0.56–0.84; p < 0.001] • ORR (PD-L1+): 55.2% (95% CI, 49.0–61.2) vs. 25.5% (95% CI, 20.6–30.9) | JAVELIN Renal 101 [28] |
| IFNα-2a + Bevacizumab | Cytokine + VEGF inhibitor | First line | Metastatic RCC | IFNα-2a + Bevacizumab vs. IFNα-2a + Placebo: • mOS: 23.3 mo vs. 21.3 mo [HR 0.91; 95% CI, 0.76 to 1.10; p = 0.3360] • mPFS: 10.2 mo vs. 5.4 mo [HR 0.63; 95% CI, 0.52 to 0.75; p = 0.0001] • ORR: 31% vs. 12% [p < 0.001] | AVOREN [9] |
2. Targeting the Tumor Microenvironment
2.1. Cellular Targets
2.1.1. Tumor-Infiltrating Lymphocytes: CD8 T Cells, CD4 T Cells, Regulatory T Cells, and B Cells
2.1.2. Dendritic Cells
2.1.3. Tumor-Associated Macrophages
2.1.4. NK Cells
2.1.5. Myeloid-Derived Suppressor Cells
2.2. Extracellular Targets
2.2.1. Tumor Mutational Burden
2.2.2. Neo-Antigens
2.2.3. DNA Repair
2.2.4. Tumor and Immune Metabolism
2.3. Immune Checkpoints
3. Current State of Biomarkers for Immunotherapy
3.1. Single Immune Checkpoints
3.2. Immune Cells and Immune Gene Signatures
3.3. TMB, Neo-Antigens, and DNA Repair
3.4. Genomic Profiles
3.5. Microbiome
3.6. Clinical Phenotypes
4. Future Directions: How Can We Develop Better Treatments by Creating a More Favorable Anti-Tumor Immune Microenvironment?
4.1. ICIs
4.2. TKIs and HIF-2α Inhibitors
4.3. Cellular Therapies
4.4. Vaccines
4.5. Cytokine Stimulation: IL-2
4.6. Microbiome
4.7. Other Immunomodulators
| Treatment(s) | Phase | Setting, Patient Population | Key Data * | Clinical Trial |
|---|---|---|---|---|
| ICIs | ||||
| Nivo ± Ipi | III | First-line, intermediate/poor-risk advanced ccRCC | NCT03873402 | |
| Pembro | III | Adjuvant, ccRCC with high-risk, intermediate–high-risk, or M1 NED | Pembro vs. Placebo: • 24 mo DFS: 77.3% vs. 68.1% [HR 0.68; 95% CI, 0.53–0.87; p = 0.002] | KEYNOTE-564, NCT03142334 [17] |
| Nivo ± Ipi | III | Adjuvant, high-risk ccRCC | CheckMate 914, NCT03138512 [161] | |
| Durva ± Treme | III | Adjuvant, RCC with high/intermediate risk of relapse | RAMPART, NCT03288532 [162] | |
| Pembro | II | First-line, advanced ccRCC or non-ccRCC | • mOS: NR • mPFS: 7.1 mo (95% CI, 5.6–11.0) • ORR: 36.4% | KEYNOTE-427, NCT02853344 [19,20] |
| Nivo followed by Nivo + Ipi | II | First-line and salvage, advanced ccRCC | • mPFS: 7.4 mo (5.5–10.9) • ORR: 35% | HCRN GU16-260, NCT03117309 [164] |
| Nivo ± followed by Ipi | II | First- or second-line and salvage, advanced ccRCC and non-ccRCC | Nivo with salvage Ipi (69% of patients in arm B): • Conversion from SD/PD to PR: 4% (90% CI, 1–11) | OMNIVORE, NCT03203473 [166] |
| Nivo ± followed by Nivo + Ipi | II | First- or second-line and salvage, metastatic or unresectable non-ccRCC | • mPFS: 4.0 mo (95% CI, 3.6–7.4) • ORR: 17% | UNISON/ANZUP 1602, NCT03177239 [167] |
| Nivo followed by Nivo + Ipi “boost” | II | First- and second-line, intermediate/high-risk advanced RCC | Nivo first-line vs. second-line: • mOS: 27.2 mo (95 % CI, 19.9–NE) vs. 20.2 mo (95 % CI, 15.6–NE) • ORR: 28 % vs. 17 % | TITAN-RCC, NCT02917772 [165] |
| Durva + Treme | II | ICI/CD137/cMet-naïve and VEGF treatment-refractory (advanced ccRCC) or VEGF treatment naïve or -refractory (advanced pRCC) | • mPFS: 4.9 months (95% CI, 2.5–10.0) | CALYPSO, NCT02819596 |
| Durva + Treme | Ib | Neoadjuvant and adjuvant, locally advanced RCC | NCT02762006 [163] | |
| MEDI0680 (PD-1) + Durva vs. Nivo | I/II | ICI-naïve, advanced ccRCC | MEDI0680 + Durva vs. Nivo: • mPFS 3.6 mo vs. 3.6 mo • ORR: 14.3% vs. 19.0% | NCT02118337 [171] |
| Nivo + Ipi, Relatlimab (LAG-3), BMS-986205 (IDO1), or BMS-813160 (CCR2/CCR5) | II | First- or second-line, advanced RCC | Nivo + Ipi: • ORR: 15.2% | FRACTION-RCC, NCT02996110 [168] |
| Relatlimab ± Nivo | I/II | ICI-naïve, RCC | NCT01968109 | |
| LAG525 (LAG-3) ± Spartalizumab (PD-1) | I/II | Second- or later-line, advanced RCC | NCT02460224 [169] | |
| Sabatolimab (TIM-3) ± Spartalizumab | I-Ib/II | ICI-naïve or pre-treated, advanced RCC | NCT02608268 [170] | |
| INCAGN02390 (TIM-3) | I | Later-line, advanced RCC | NCT03652077 | |
| CA-170 (PD-L1, PD-L2, VISTA) | I | ICI-ineligible, advanced RCC | NCT02812875 [172] | |
| TKIs + ICIs | ||||
| Bev + Atezo | III | First-line, PD-L1+ metastatic RCC | Bev + Atezo vs. Sunitinib: • mPFS (PD-L1+): 11.2 mo vs. 7.7 mo [HR 0.74; 95% CI, 0.57–0.96; p = 0.0217] • mOS (ITT): [HR 0.93; 95% CI, 0.76–1.14] | IMmotion151, NCT02420821 [29] |
| Cabo + Atezo | III | Second- or third-line, ICI-refractory advanced RCC | CONTACT-03, NCT04338269 [180] | |
| Nivo + Ipi ± Cabo | III | First-line, intermediate/poor-risk advanced ccRCC | COSMIC-313, NCT03937219 [178] | |
| Nivo + Ipi followed by maintenance Nivo (CR), Cabo (PD), or Nivo ± Cabo (non-CR/PD) | III | First-line, intermediate/poor-risk advanced ccRCC | A031704/ PDIGREE, NCT03793166 [179] | |
| Nivo ± Ipi ± followed by Nivo or Sunitinib/Pazopanib | II | First-line, metastatic ccRCC stratified into one of four molecular subtypes | BIONIKK, NCT02960906 [143] | |
| Savolitinib + Durva | II | ICI/CD137/cMet naïve and VEGF treatment refractory (advanced ccRCC) or VEGF treatment naïve or refractory (advanced pRCC) | • mPFS (pRCC): 4.9 mo (95% CI, 2.5–10.0) • mPFS (pRCC, MET-driven disease): 10.5 mo (95% CI, 2.9–15.7) • mOS (pRCC, MET-driven disease: 27.4 mo (95% CI, 7.3–NR) • ORR (pRCC): 29% • ORR (pRCC, MET-driven disease): 57% | CALYPSO, NCT02819596 [181] |
| Sitravatinib + Nivo | II | Neoadjuvant, ccRCC | • ORR 11.8% (33.3% for 120 mg Sitravatinib) | NCT03680521 [174] |
| Cabo + Atezo | I/II | First-line (ccRCC) or prior VEGF TKI treatment (non-ccRCC) | 40 mg Cabo + Atezo vs. 60 mg Cabo + Atezo: • mPFS (ccRCC): 19.5 mo (95% CI, 11.0 –NE) vs. 15.1 mo (95% CI, 8.2–22.3) • ORR (ccRCC): 53% (80% CI, 41–65) vs. 58% (80% CI, 46–70) 40 mg Cabo: • ORR (non-ccRCC): 31% (80% CI, 20–44) • mPFS (non-ccRCC): 9.5 mo | COSMIC-021, NCT03170960 [175] |
| Cabo + Pembro | I/II | First- or later-line Pembro/Cabo-naïve, advanced RCC | • mPFS: 10.4 mo (95% CI, 6.3–NR) • mOS: NR • 17.8 mo ORR: 60% (95% CI, 0.458–1.00) | NCT03149822 [177] |
| Lenvatinib + Pembro | Ib/II | First- or later-line, metastatic ccRCC | ICI-pre-treated population: • mOS: NR (95% CI, NR–NR) • mPFS: 12.2 mo (95% CI, 9.5–17.7) • 24 wk ORR: 55.8% (95% CI, 45.7–65.5) | KEYNOTE-146, NCT02501096 [182] |
| Ibrutinib + Nivo | Ib/II | Second- or later-line, advanced RCC | NCT02899078 [173] | |
| Cabo + Nivo ± Ipi | I | Later-line, advanced ccRCC | NCT02496208 [176] | |
| Cellular Therapies | ||||
| VEGFR2 CAR T cells | I/II | Second- or later-line, metastatic RCC | NCT01218867 | |
| Anti-c-Met CAR T cells | I/II | PR/NR/recurrency if prior ICI, c-Met+ RCC | NCT03638206 | |
| ROR2, AXL CAR T cells | I/II | ROR2+ or AXL+ relapsed and refractory stage IV metastatic RCC | NCT03393936 | |
| CD70 CAR T cells | I/II | Second- or later-line, CD70+ ccRCC | NCT02830724 † | |
| D-CIK + Axitinib | II | First-line or after progression on anti-angiogenesis or cytokine therapy, advanced RCC | NCT03736330 | |
| HIF-2α + ICI | ||||
| PT2385 + Nivo or Cabo | I | Second- or later-line, advanced ccRCC | PT2385 + Nivo: • mPFS: 7.3 mo • ORR: 22% | NCT02293980 [184] |
| Vaccines | ||||
| IMA901 + Sunitinib | III | First-line, advanced ccRCC | IMA901 + Sunitinib vs. Sunitinib: • mOS: 33.17 mo (95% CI, 27.8–41.4) vs. NR (33.7–NR) [HR 1.34; 95% CI, 0.96–1.86; p = 0.087] | IMPRINT, NCT01265901 [187] |
| Rocapuldencel-T + Sunitinib | III | First-line, advanced RCC | Rocapuldencel-T + Sunitinib vs. SOC: • mOS: 27.7 mo (95% CI, 23.0–35.9) vs. 32.4 mo (95% CI, 22.5-NE) [HR 1.10; 95% CI, 0.83–1.40] • mPFS: 6.0 mo vs. 7.83 mo [HR 1.15; 95% CI, 0.92–1.44] • ORR: 42.7% (95% CI, 37.1–48.4) vs. 39.4% (95% CI, 31.6–47.5) | ADAPT, NCT01582672 † [189] |
| AGS-003 + Sunitinib | II | First-line, intermediate/poor-risk metastatic ccRCC | • mOS: 30.2 mo (95% CI, 9.4–57.1) • mPFS: 11.2 mo (95% CI, 6.0–19.4) | NCT00678119 [188] |
| INTUVAX/Ilixadencel (intra-tumoral) + Sunitinib | II | Neoadjuvant + adjuvant first-line, synchronous metastatic RCC | INTUVAX + Sunitinib vs. Sunitinib: • mPFS 11.8 mo vs. 11.0 mo • ORR 42.4% vs. 24.0% • mDOR 7.1 mo vs. 2.9 mo | MERECA, NCT02432846 [191] |
| GEN-009 Adjuvanted Vaccine + Nivo or Pembro | I/II | First-line (intermediate/poor-risk beginning Nivo + Ipi) or after anti-angiogenic therapy (beginning nivolumab), advanced RCC | NCT03633110 [192] | |
| VB10.NEO + Bempegaldesleukin | I/IIa | PR/SD/PD on ICI, advanced ccRCC | DIRECT-01, NCT03548467 [193] | |
| Pexastimogene Devacirepvec + Cemiplimab | I/II | First- or later-line ICI-naïve, advanced ccRCC | • ORR: 37.5% | NCT03294083 [194] |
| DC Tumor Fusion + GM-CSF | I/II | Chemotherapy- and biological therapy-naïve, stage IV RCC | NCT00458536 | |
| Autologous or Allogeneic tumor cells | I/II | Chemotherapy-refractory, metastatic RCC | NCT00722228 | |
| Treme + Durva + PolyICLC | I/II | Dual ICI-naïve, biopsy-accessible advanced RCC | NCT02643303 [195] | |
| COMBIG-DC/INTUVAX | I | Intermediate/poor-risk metastatic RCC | • mOS: NR | NCT01525017 [190] |
| Neovax ± Ipi | I | First- or later-line ICI-naïve, stage III/IV resectable ccRCC | NCT02950766 | |
| Cevumeran ± Atezo | Ia/Ib | First- or later-line ICI-naïve, advanced RCC | NCT03289962 | |
| PSMA plasmid DNA vaccine | I | Favorable-risk RCC | NCT00096629 | |
| IL-2 + ICI | ||||
| Bempegaldesleukin + Nivo | III | First-line, advanced ccRCC | PIVOT-09, NCT03729245 [199] | |
| High Dose IL-2 + Pembro | II | First- or later-line ICI-naïve, metastatic RCC | • Projected ORR: 69% | NCT02964078 [196] |
| High Dose IL-2 + Nivo | Ib/II | First-, second-, or third-line, IL-2-, IFN-, PD-1/PD-L1-ICI-naïve, metastatic ccRCC | NCT02989714 [197] | |
| Bempegaldesleukin + Nivo ± Ipi | I/II | First-, second-, or third-line IL-2-naïve, advanced RCC | PIVOT-02, NCT02983045 [198] | |
| Microbiome | ||||
| ± Deferred cytoreductive nephrectomy following Nivo + Ipi (microbiome analysis) | III | First-line, intermediate/poor-risk synchronous metastatic RCC | NORDIC-SUN, NCT03977571 | |
| Nivo or Pembro ± Metformin or Rosiglitazone (microbiome analysis) | II | PD-1/L1 ICI-naïve, advanced RCC | NCT04114136 | |
| Nivo + Ipi ± SBRT (microbiome analysis) | II | First-line, intermediate/poor-risk metastatic RCC | CYTOSHRINK, NCT04090710 | |
| FMT from ICI-responding donors + ICI | I/II | Receiving or eligible for ICI, advanced RCC | TACITO, NCT04758507 | |
| FMT + Nivo + Ipi (irAEs analysis) | I | First-line, intermediate/poor-risk advanced RCC | PERFORM, NCT04163289 | |
| MRx0518 (Enterococcus) | I | No therapy in past 2 years, RCC | MICROBIOME, NCT03934827 | |
| ICI/s ± other systemic therapy (microbiome analysis) | N/A | Eligible for ICI, stage I–IV RCC | PARADIGM, NCT05037825 | |
| Nivo ± Ipi, Durva ± Treme (microbiome analysis) | N/A | ICI-naïve, advanced RCC | NCT04107168 | |
| Other Immunomodulators | ||||
| Epacadostat (IDO1) + Pembro | III | First-line, advanced ccRCC | KEYNOTE-679/ECHO-302, NCT03260894 | |
| Entinostat (HDAC) + Nivo + Ipi | II | Nivo + Ipi-refractory, metastatic RCC | NCT03552380 | |
| High Dose IL-2 ± Entinostat | II | Second- or third-line (including ICI), advanced ccRCC | NCT03501381 | |
| Atezo + Bev + Entinostat | I/II | ICI-naïve (II Cohort A) or ICI-refractory (II Cohort B), metastatic RCC | • mPFS: 7.6 mo (95% CI, 1.6–16.3) • ORR: 47.1% (95% CI, 23.0–72.2) | NCT03024437 [203] |
| Aldesleukin + Entinostat | I/II | First-, second-, or third-line, metastatic ccRCC | • ORR: 37% (90% CI, 24–51) [p = 0.010] • mPFS 13.8 mo (95% CI, 6.0–18.8) • mOS 65.3 mo (95% CI, 52.6–65.3) • Decreased Tregs associated with response | NCT01038778 [204] |
| HBI-8000 (HDAC) + Nivo | I/II | Advanced RCC | NCT02718066 | |
| NIR178 (A2AR) + Spartalizumab | II | Later-line, TKI-refractory, advanced RCC | NCT03207867 | |
| Dalantercept (ALK1/TGF-β) + Axitinib | II | Second- or third-line (including VEGF inhibitor) ICI-naïve, advanced ccRCC | Dalantercept + Axitinib vs. Axitinib + Placebo: • mOS: NR vs. NR [HR 1.39; 95% CI, 0.70–2.77; p = 0.349] • mPFS: 6.8 mo vs. 5.6 mo [HR 1.11; 95% CI, 0.71–1.73; p = 0.670] • ORR: 19.0% (95% CI, 9.9–31.4) vs. 24.6% (95% CI, 14.5–37.3) | DART, NCT01727336 [200,201] |
| Oleclumab (CD73) + Durva | II | No prior CD73/CD39/innate immune agonist, advanced RCC | DOMINATION, NCT04262375 | |
| Axitinib ± Ivuxolimab (OX40) | II | Second- (one prior TKI + ICI) or third-line (one prior non-axitinib TKI, one ICI), metastatic RCC | NCT03092856 | |
| INBRX-106 (OX40) ± Pembro | I | Later-line OX40 agonist-naïve, advanced RCC | NCT04198766 | |
| Feladilimab (ICOS) + Treme | I/II | CTLA-4/ICOS-treatment-naïve, advanced ccRCC | NCT03693612 [209] | |
| Varlilumab (CD27) + Nivo | I/II | Anti-angiogenic therapy-experienced, ICI-naïve, no CTLA-4/CD27 therapy in past 3 mo, advanced ccRCC | NCT02335918 | |
| INCAGN01876 (GITR) + Nivo or Ipi or Nivo + Ipi | I/II | Later-line, advanced RCC | NCT03126110 | |
| Axitinib ± Carotuximab (Endoglin) | I/II | Later-line, one prior TKI (other than axitinib) ± one prior ICI, advanced ccRCC | NCT01806064 [202] | |
| Telaglenastat (glutaminase) + Nivo | I/II | Later-line ± ICI-naïve, advanced ccRCC | NCT02771626 | |
| Utomilumab (4-1BB) + Pembro | I | Advanced RCC | KEYNOTE-0036, NCT02179918 [208] | |
| Mogamulizumab (CCR4) | I | RCC | NCT02946671 | |
| Dostarlimab (PD-1) + Niraparib (PARP) Cobolimab (TIM-3), Bev, or Platinum-based doublet chemotherapy | I | Advanced RCC | IOLite, NCT03307785 [205] | |
| Valemetostat (EZH1/2) + Ipi | I | Later-line ICI- and anti-angiogenic therapy-refractory, advanced ccRCC | NCT04388852 | |
| Ciforadenant (A2AR) | I | Second- or third-line ICI-refractory, ccRCC | NCT02655822 [206,207] | |
| Siremadlin (MDM2) + Spartalizumab | I | Later-line, advanced RCC | NCT02890069 | |
| Nivo + Stereotactic Body Radiotherapy | II | Second- or third-line PD-1/L1/L2-naïve, advanced RCC | • mOS: 22.1 mo (95% CI, 18.1-NR) • mPFS: 4 mo (95% CI, 2.8–7.1) • ORR: 19% | NIVES, NCT03469713 [211] |
| Guadecitabine + Durva | I/II | ICI-naïve (Cohort 1) or PD-1/L2-regractory (Cohort 2), advanced ccRCC | • mOS: NR • mPF: 17 mo | NCT03308396 [210] |
| Treme ± Cryoablation | N/A | CTLA-4 ICI-naïve, advanced ccRCC or non-ccRCC | NCT02626130 | |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal Cell Carcinoma. Nat. Rev. Dis. Primer 2017, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Brannon, A.R.; Reddy, A.; Seiler, M.; Arreola, A.; Moore, D.T.; Pruthi, R.S.; Wallen, E.M.; Nielsen, M.E.; Liu, H.; Nathanson, K.L.; et al. Molecular Stratification of Clear Cell Renal Cell Carcinoma by Consensus Clustering Reveals Distinct Subtypes and Survival Patterns. Genes Cancer 2010, 1, 152–163. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Şenbabaoğlu, Y.; Ciriello, G.; Yang, L.; Reznik, E.; Shuch, B.; Micevic, G.; De Velasco, G.; Shinbrot, E.; et al. Multilevel Genomics-Based Taxonomy of Renal Cell Carcinoma. Cell Rep. 2016, 14, 2476–2489. [Google Scholar] [CrossRef] [Green Version]
- Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [CrossRef] [Green Version]
- Dutcher, J.P.; Flippot, R.; Fallah, J.; Escudier, B. On the Shoulders of Giants: The Evolution of Renal Cell Carcinoma Treatment—Cytokines, Targeted Therapy, and Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2020, 418–435. [Google Scholar] [CrossRef]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of Treatment of 255 Patients with Metastatic Renal Cell Carcinoma Who Received High-Dose Recombinant Interleukin-2 Therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Pluzanska, A.; Koralewski, P.; Ravaud, A.; Bracarda, S.; Szczylik, C.; Chevreau, C.; Filipek, M.; Melichar, B.; Bajetta, E.; et al. Bevacizumab plus Interferon Alfa-2a for Treatment of Metastatic Renal Cell Carcinoma: A Randomised, Double-Blind Phase III Trial. Lancet Lond. Engl. 2007, 370, 2103–2111. [Google Scholar] [CrossRef]
- Minasian, L.M.; Motzer, R.J.; Gluck, L.; Mazumdar, M.; Vlamis, V.; Krown, S.E. Interferon Alfa-2a in Advanced Renal Cell Carcinoma: Treatment Results and Survival in 159 Patients with Long-Term Follow-Up. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 1368–1375. [Google Scholar] [CrossRef]
- Sharma, R.; Kadife, E.; Myers, M.; Kannourakis, G.; Prithviraj, P.; Ahmed, N. Determinants of Resistance to VEGF-TKI and Immune Checkpoint Inhibitors in Metastatic Renal Cell Carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 186. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Schwartz, J.C.; Zhang, X.; Fedorov, A.A.; Nathenson, S.G.; Almo, S.C. Structural Basis for Co-Stimulation by the Human CTLA-4/B7-2 Complex. Nature 2001, 410, 604–608. [Google Scholar] [CrossRef]
- Stamper, C.C.; Zhang, Y.; Tobin, J.F.; Erbe, D.V.; Ikemizu, S.; Davis, S.J.; Stahl, M.L.; Seehra, J.; Somers, W.S.; Mosyak, L. Crystal Structure of the B7-1/CTLA-4 Complex That Inhibits Human Immune Responses. Nature 2001, 410, 608–611. [Google Scholar] [CrossRef]
- Ravaud, A.; Motzer, R.J.; Pandha, H.S.; George, D.J.; Pantuck, A.J.; Patel, A.; Chang, Y.-H.; Escudier, B.; Donskov, F.; Magheli, A.; et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N. Engl. J. Med. 2016, 375, 2246–2254. [Google Scholar] [CrossRef]
- Haas, N.B.; Manola, J.; Uzzo, R.G.; Flaherty, K.T.; Wood, C.G.; Kane, C.; Jewett, M.; Dutcher, J.P.; Atkins, M.B.; Pins, M.; et al. Adjuvant Sunitinib or Sorafenib for High-Risk, Non-Metastatic Renal-Cell Carcinoma (ECOG-ACRIN E2805): A Double-Blind, Placebo-Controlled, Randomised, Phase 3 Trial. Lancet 2016, 387, 2008–2016. [Google Scholar] [CrossRef] [Green Version]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.-H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1510665 (accessed on 27 May 2021).
- McDermott, D.F.; Lee, J.-L.; Bjarnason, G.A.; Larkin, J.M.G.; Gafanov, R.A.; Kochenderfer, M.D.; Jensen, N.V.; Donskov, F.; Malik, J.; Poprach, A.; et al. Open-Label, Single-Arm Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2021, 39, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Lee, J.-L.; Ziobro, M.; Suarez, C.; Langiewicz, P.; Matveev, V.B.; Wiechno, P.; Gafanov, R.A.; Tomczak, P.; Pouliot, F.; et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A. Combination of CTLA-4 and PD-1 Blockers for Treatment of Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Verma, R.; Sznol, M.; Boddupalli, C.S.; Gettinger, S.N.; Kluger, H.; Callahan, M.; Wolchok, J.D.; Halaban, R.; Dhodapkar, M.V.; et al. Combination Therapy with Anti–CTLA-4 and Anti–PD-1 Leads to Distinct Immunologic Changes In Vivo. J. Immunol. 2015, 194, 950–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.C.; Anang, N.-A.A.S.; Sharma, R.; Andrews, M.C.; Reuben, A.; Levine, J.H.; Cogdill, A.P.; Mancuso, J.J.; Wargo, J.A.; Pe’er, D.; et al. Combination Anti–CTLA-4 plus Anti–PD-1 Checkpoint Blockade Utilizes Cellular Mechanisms Partially Distinct from Monotherapies. Proc. Natl. Acad. Sci. USA 2019, 116, 22699–22709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Regan, M.M.; Jegede, O.A.; Mantia, C.M.; Powles, T.; Werner, L.; Motzer, R.J.; Tannir, N.M.; Lee, C.-H.; Tomita, Y.; Voss, M.H.; et al. Treatment-Free Survival after Immune Checkpoint Inhibitor Therapy versus Targeted Therapy for Advanced Renal Cell Carcinoma: 42-Month Results of the CheckMate 214 Trial. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Voss, M.H.; Kuo, F.; Sanchez, A.; Liu, M.; Nixon, B.G.; Vuong, L.; Ostrovnaya, I.; Chen, Y.-B.; Reuter, V.; et al. Transcriptomic Profiling of the Tumor Microenvironment Reveals Distinct Subgroups of Clear Cell Renal Cell Cancer: Data from a Randomized Phase III Trial. Cancer Discov. 2019, 9, 510–525. [Google Scholar] [CrossRef] [Green Version]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus Bevacizumab versus Sunitinib in Patients with Previously Untreated Metastatic Renal Cell Carcinoma (IMmotion151): A Multicentre, Open-Label, Phase 3, Randomised Controlled Trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Şenbabaoğlu, Y.; Gejman, R.S.; Winer, A.G.; Liu, M.; Van Allen, E.M.; de Velasco, G.; Miao, D.; Ostrovnaya, I.; Drill, E.; Luna, A.; et al. Tumor Immune Microenvironment Characterization in Clear Cell Renal Cell Carcinoma Identifies Prognostic and Immunotherapeutically Relevant Messenger RNA Signatures. Genome Biol. 2016, 17, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, D.A.; Hou, Y.; Bakouny, Z.; Ficial, M.; Sant’ Angelo, M.; Forman, J.; Ross-Macdonald, P.; Berger, A.C.; Jegede, O.A.; Elagina, L.; et al. Interplay of Somatic Alterations and Immune Infiltration Modulates Response to PD-1 Blockade in Advanced Clear Cell Renal Cell Carcinoma. Nat. Med. 2020, 26, 909–918. [Google Scholar] [CrossRef]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018, 23, 313–326.e5. [Google Scholar] [CrossRef] [Green Version]
- Chevrier, S.; Levine, J.H.; Zanotelli, V.R.T.; Silina, K.; Schulz, D.; Bacac, M.; Ries, C.H.; Ailles, L.; Jewett, M.A.S.; Moch, H.; et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017, 169, 736–749.e18. [Google Scholar] [CrossRef] [Green Version]
- Luke, J.J.; Bao, R.; Sweis, R.; Spranger, S.; Gajewski, T.F. WNT/β-Catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3074–3083. [Google Scholar] [CrossRef] [Green Version]
- Geissler, K.; Fornara, P.; Lautenschläger, C.; Holzhausen, H.-J.; Seliger, B.; Riemann, D. Immune Signature of Tumor Infiltrating Immune Cells in Renal Cancer. Oncoimmunology 2015, 4, e985082. [Google Scholar] [CrossRef]
- Ock, C.-Y.; Keam, B.; Kim, S.; Lee, J.-S.; Kim, M.; Kim, T.M.; Jeon, Y.K.; Kim, D.-W.; Chung, D.H.; Heo, D.S. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. 2016, 22, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, C.; Zhao, Z.; Xu, B.; Zheng, M.; Zhang, C.; Min, Z.; Guo, J.; Rong, R. Skewed T-Helper (Th)1/2- and Th17/T Regulatory-cell Balances in Patients with Renal Cell Carcinoma. Mol. Med. Rep. 2015, 11, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and Genetic Properties of Tumors Associated with Local Immune Cytolytic Activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Braun, D.A.; Street, K.; Burke, K.P.; Cookmeyer, D.L.; Denize, T.; Pedersen, C.B.; Gohil, S.H.; Schindler, N.; Pomerance, L.; Hirsch, L.; et al. Progressive Immune Dysfunction with Advancing Disease Stage in Renal Cell Carcinoma. Cancer Cell 2021, 39, 632–648.e8. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression—Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Griffiths, R.W.; Elkord, E.; Gilham, D.E.; Ramani, V.; Clarke, N.; Stern, P.L.; Hawkins, R.E. Frequency of Regulatory T Cells in Renal Cell Carcinoma Patients and Investigation of Correlation with Survival. Cancer Immunol. Immunother. CII 2007, 56, 1743–1753. [Google Scholar] [CrossRef]
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B Cells, Plasma Cells and Antibody Repertoires in the Tumour Microenvironment. Nat. Rev. Immunol. 2020, 20, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, E.; Frödin, M.; Lövrot, J.; Mezheyeuski, A.; Johansson, M.; Harmenberg, U.; Egevad, L.; Sandström, P.; Östman, A. A Minority-Group of Renal Cell Cancer Patients with High Infiltration of CD20+B-Cells Is Associated with Poor Prognosis. Br. J. Cancer 2018, 119, 840–846. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, C.; Hu, G.; Ma, J.; Chen, Y.; Zhang, J.; Huang, Y.; Zheng, J.; Xue, W.; Xu, Y.; et al. Tumor-Educated B Cells Promote Renal Cancer Metastasis via Inducing the IL-1β/HIF-2α/Notch1 Signals. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Iglesia, M.D.; Parker, J.S.; Hoadley, K.A.; Serody, J.S.; Perou, C.M.; Vincent, B.G. Genomic Analysis of Immune Cell Infiltrates Across 11 Tumor Types. JNCI J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Gigante, M.; Blasi, A.; Loverre, A.; Mancini, V.; Battaglia, M.; Selvaggi, F.P.; Maiorano, E.; Napoli, A.; Castellano, G.; Storkus, W.J.; et al. Dysfunctional DC Subsets in RCC Patients: Ex Vivo Correction to Yield an Effective Anti-Cancer Vaccine. Mol. Immunol. 2009, 46, 893–901. [Google Scholar] [CrossRef] [Green Version]
- Figel, A.-M.; Brech, D.; Prinz, P.U.; Lettenmeyer, U.K.; Eckl, J.; Turqueti-Neves, A.; Mysliwietz, J.; Anz, D.; Rieth, N.; Muenchmeier, N.; et al. Human Renal Cell Carcinoma Induces a Dendritic Cell Subset That Uses T-Cell Crosstalk for Tumor-Permissive Milieu Alterations. Am. J. Pathol. 2011, 179, 436–451. [Google Scholar] [CrossRef]
- Kobayashi, M.; Suzuki, K.; Yashi, M.; Yuzawa, M.; Takayashiki, N.; Morita, T. Tumor Infiltrating Dendritic Cells Predict Treatment Response to Immmunotherapy in Patients with Metastatic Renal Cell Carcinoma. Anticancer Res. 2007, 27, 1137–1141. [Google Scholar]
- Voss, M.H.; Buros Novik, J.; Hellmann, M.D.; Ball, M.; Hakimi, A.A.; Miao, D.; Margolis, C.; Horak, C.; Wind-Rotolo, M.; De Velasco, G.; et al. Correlation of Degree of Tumor Immune Infiltration and Insertion-and-Deletion (Indel) Burden with Outcome on Programmed Death 1 (PD1) Therapy in Advanced Renal Cell Cancer (RCC). J. Clin. Oncol. 2018, 36, 4518. [Google Scholar] [CrossRef]
- Motoshima, T.; Miura, Y.; Wakigami, N.; Kusada, N.; Takano, T.; Inoshita, N.; Okaneya, T.; Sugiyama, Y.; Kamba, T.; Takeya, M.; et al. Phenotypical Change of Tumor-Associated Macrophages in Metastatic Lesions of Clear Cell Renal Cell Carcinoma. Med. Mol. Morphol. 2018, 51, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Rutigliano, M.; Lucarelli, G.; Annese, T.; Ruggieri, S.; Cascardi, E.; Napoli, A.; Battaglia, M.; Ribatti, D. Microvascular Density, Macrophages, and Mast Cells in Human Clear Cell Renal Carcinoma with and without Bevacizumab Treatment. Urol. Oncol. 2019, 37, 355.e11–355.e19. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Xu, L.; Wang, Y.; Jiang, Q.; Liu, Z.; Zhang, J.; Zhou, Q.; Zeng, H.; Tong, S.; Wang, T.; et al. Tumor-Associated Macrophage-Derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction with Immune Evasion. Eur. Urol. 2019, 75, 752–763. [Google Scholar] [CrossRef]
- Schleypen, J.S.; Von Geldern, M.; Weiss, E.H.; Kotzias, N.; Rohrmann, K.; Schendel, D.J.; Falk, C.S.; Pohla, H. Renal Cell Carcinoma-Infiltrating Natural Killer Cells Express Differential Repertoires of Activating and Inhibitory Receptors and Are Inhibited by Specific HLA Class I Allotypes. Int. J. Cancer 2003, 106, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Remark, R.; Alifano, M.; Cremer, I.; Lupo, A.; Dieu-Nosjean, M.-C.; Riquet, M.; Crozet, L.; Ouakrim, H.; Goc, J.; Cazes, A.; et al. Characteristics and Clinical Impacts of the Immune Environments in Colorectal and Renal Cell Carcinoma Lung Metastases: Influence of Tumor Origin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 4079–4091. [Google Scholar] [CrossRef] [Green Version]
- Prinz, P.U.; Mendler, A.N.; Brech, D.; Masouris, I.; Oberneder, R.; Noessner, E. NK-Cell Dysfunction in Human Renal Carcinoma Reveals Diacylglycerol Kinase as Key Regulator and Target for Therapeutic Intervention. Int. J. Cancer 2014, 135, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, Q.; Zhen, Q.; Zhao, Y.; Liu, N.; Li, T.; Hao, Y.; Zhang, Y.; Luo, C.; Wu, X. Negative Regulation of Tumor-Infiltrating NK Cell in Clear Cell Renal Cell Carcinoma Patients through the Exosomal Pathway. Oncotarget 2017, 8, 37783–37795. [Google Scholar] [CrossRef] [Green Version]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Guan, X.; Liu, Z.; Zhang, J.; Jin, X. Myeloid-Derived Suppressor Cell Accumulation in Renal Cell Carcinoma Is Correlated with CCL2, IL-17 and IL-18 Expression in Blood and Tumors. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2018, 27, 947–953. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Ernstoff, M.S.; Hernandez, C.; Atkins, M.; Zabaleta, J.; Sierra, R.; Ochoa, A.C. Arginase I-Producing Myeloid-Derived Suppressor Cells in Renal Cell Carcinoma Are a Subpopulation of Activated Granulocytes. Cancer Res. 2009, 69, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.L.; Simmons, R.M.; Franke-Welch, S.; Nguyen, T.H.; Korman, A.J.; Dillon, S.R.; Gilbertson, D.G. Conditioned Media from the Renal Cell Carcinoma Cell Line 786.O Drives Human Blood Monocytes to a Monocytic Myeloid-Derived Suppressor Cell Phenotype. Cell. Immunol. 2018, 323, 49–58. [Google Scholar] [CrossRef]
- Najjar, Y.G.; Rayman, P.; Jia, X.; Pavicic, P.G.; Rini, B.I.; Tannenbaum, C.; Ko, J.; Haywood, S.; Cohen, P.; Hamilton, T.; et al. Myeloid-Derived Suppressor Cell Subset Accumulation in Renal Cell Carcinoma Parenchyma Is Associated with Intratumoral Expression of IL1β, IL8, CXCL5, and Mip-1α. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2346–2355. [Google Scholar] [CrossRef] [Green Version]
- Orillion, A.; Hashimoto, A.; Damayanti, N.; Shen, L.; Adelaiye-Ogala, R.; Arisa, S.; Chintala, S.; Ordentlich, P.; Kao, C.; Elzey, B.; et al. Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumor Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5187–5201. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sun, J.; Rong, R.; Li, L.; Shang, W.; Song, D.; Feng, G.; Luo, F. HMGB1 Promotes Myeloid-Derived Suppressor Cells and Renal Cell Carcinoma Immune Escape. Oncotarget 2017, 8, 63290–63298. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.S.; Zea, A.H.; Rini, B.I.; Ireland, J.L.; Elson, P.; Cohen, P.; Golshayan, A.; Rayman, P.A.; Wood, L.; Garcia, J.; et al. Sunitinib Mediates Reversal of Myeloid-Derived Suppressor Cell Accumulation in Renal Cell Carcinoma Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 2148–2157. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.S.; Rayman, P.; Ireland, J.; Swaidani, S.; Li, G.; Bunting, K.D.; Rini, B.; Finke, J.H.; Cohen, P.A. Direct and Differential Suppression of Myeloid-Derived Suppressor Cell Subsets by Sunitinib Is Compartmentally Constrained. Cancer Res. 2010, 70, 3526–3536. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-Small Cell Lung Cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [Green Version]
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Foppen, M.H.G.; Goldinger, S.M.; et al. Genomic Correlates of Response to CTLA-4 Blockade in Metastatic Melanoma. Science 2015, 350, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational Heterogeneity in Cancer and the Search for New Cancer-Associated Genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Qi, F.; Hu, X.; Luo, J. Exploration of the Relationships between Tumor Mutation Burden with Immune Infiltrates in Clear Cell Renal Cell Carcinoma. Ann. Transl. Med. 2019, 7. [Google Scholar] [CrossRef]
- Wang, X.; Li, M. Correlate Tumor Mutation Burden with Immune Signatures in Human Cancers. BMC Immunol. 2019, 20, 4. [Google Scholar] [CrossRef] [Green Version]
- Turajlic, S.; Litchfield, K.; Xu, H.; Rosenthal, R.; McGranahan, N.; Reading, J.L.; Wong, Y.N.S.; Rowan, A.; Kanu, N.; Al Bakir, M.; et al. Insertion-and-Deletion-Derived Tumour-Specific Neoantigens and the Immunogenic Phenotype: A Pan-Cancer Analysis. Lancet Oncol. 2017, 18, 1009–1021. [Google Scholar] [CrossRef] [Green Version]
- Chae, Y.K.; Anker, J.F.; Carneiro, B.A.; Chandra, S.; Kaplan, J.; Kalyan, A.; Santa-Maria, C.A.; Platanias, L.C.; Giles, F.J. Genomic Landscape of DNA Repair Genes in Cancer. Oncotarget 2016, 7, 23312–23321. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch-Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Ged, Y.; Chaim, J.L.; DiNatale, R.G.; Knezevic, A.; Kotecha, R.R.; Carlo, M.I.; Lee, C.-H.; Foster, A.; Feldman, D.R.; Teo, M.Y.; et al. DNA Damage Repair Pathway Alterations in Metastatic Clear Cell Renal Cell Carcinoma and Implications on Systemic Therapy. J. Immunother. Cancer 2020, 8, e000230. [Google Scholar] [CrossRef]
- Na, J.C.; Nagaya, N.; Rha, K.H.; Han, W.K.; Kim, I.Y. DNA Damage Response Pathway Alteration in Locally Advanced Clear-Cell Renal-Cell Carcinoma Is Associated with a Poor Outcome. Clin. Genitourin. Cancer 2019, 17, 299–305.e1. [Google Scholar] [CrossRef]
- Hartman, T.R.; Demidova, E.V.; Lesh, R.W.; Hoang, L.; Richardson, M.; Forman, A.; Kessler, L.; Speare, V.; Golemis, E.A.; Hall, M.J.; et al. Prevalence of Pathogenic Variants in DNA Damage Response and Repair Genes in Patients Undergoing Cancer Risk Assessment and Reporting a Personal History of Early-Onset Renal Cancer. Sci. Rep. 2020, 10, 13518. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.; Wu, C.; Ming, J.; Zhang, W.; Zhang, L.; Hu, G. The Clinical Significance of DNA Damage Repair Signatures in Clear Cell Renal Cell Carcinoma. Front. Genet. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Powell, J.D. Metabolism of Immune Cells in Cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Lucarelli, G.; Loizzo, D.; Franzin, R.; Battaglia, S.; Ferro, M.; Cantiello, F.; Castellano, G.; Bettocchi, C.; Ditonno, P.; Battaglia, M. Metabolomic Insights into Pathophysiological Mechanisms and Biomarker Discovery in Clear Cell Renal Cell Carcinoma. Expert Rev. Mol. Diagn. 2019, 19, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ragone, R.; Sallustio, F.; Piccinonna, S.; Rutigliano, M.; Vanessa, G.; Palazzo, S.; Lucarelli, G.; Ditonno, P.; Battaglia, M.; Fanizzi, F.P.; et al. Renal Cell Carcinoma: A Study through NMR-Based Metabolomics Combined with Transcriptomics. Diseases 2016, 4, 7. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Sallustio, F.; Ribatti, D.; Giglio, A.; Lepore Signorile, M.; Grossi, V.; Sanese, P.; Napoli, A.; Maiorano, E.; et al. Integrated Multi-Omics Characterization Reveals a Distinctive Metabolic Signature and the Role of NDUFA4L2 in Promoting Angiogenesis, Chemoresistance, and Mitochondrial Dysfunction in Clear Cell Renal Cell Carcinoma. Aging 2018, 10, 3957–3985. [Google Scholar] [CrossRef]
- Bombelli, S.; Torsello, B.; De Marco, S.; Lucarelli, G.; Cifola, I.; Grasselli, C.; Strada, G.; Bovo, G.; Perego, R.A.; Bianchi, C. 36-KDa Annexin A3 Isoform Negatively Modulates Lipid Storage in Clear Cell Renal Cell Carcinoma Cells. Am. J. Pathol. 2020, 190, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Netti, G.S.; Lucarelli, G.; Spadaccino, F.; Castellano, G.; Gigante, M.; Divella, C.; Rocchetti, M.T.; Rascio, F.; Mancini, V.; Stallone, G.; et al. PTX3 Modulates the Immunoflogosis in Tumor Microenvironment and Is a Prognostic Factor for Patients with Clear Cell Renal Cell Carcinoma. Aging 2020, 12, 7585–7602. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Meregalli, C.; Bombelli, S.; Di Stefano, V.; Salerno, F.; Torsello, B.; De Marco, S.; Bovo, G.; Cifola, I.; Mangano, E.; et al. The Glucose and Lipid Metabolism Reprogramming Is Grade-Dependent in Clear Cell Renal Cell Carcinoma Primary Cultures and Is Targetable to Modulate Cell Viability and Proliferation. Oncotarget 2017, 8, 113502–113515. [Google Scholar] [CrossRef] [Green Version]
- Lucarelli, G.; Galleggiante, V.; Rutigliano, M.; Sanguedolce, F.; Cagiano, S.; Bufo, P.; Lastilla, G.; Maiorano, E.; Ribatti, D.; Giglio, A.; et al. Metabolomic Profile of Glycolysis and the Pentose Phosphate Pathway Identifies the Central Role of Glucose-6-Phosphate Dehydrogenase in Clear Cell-Renal Cell Carcinoma. Oncotarget 2015, 6, 13371–13386. [Google Scholar] [CrossRef] [Green Version]
- Lucarelli, G.; Rutigliano, M.; Ferro, M.; Giglio, A.; Intini, A.; Triggiano, F.; Palazzo, S.; Gigante, M.; Castellano, G.; Ranieri, E.; et al. Activation of the Kynurenine Pathway Predicts Poor Outcome in Patients with Clear Cell Renal Cell Carcinoma. Urol. Oncol. 2017, 35, 461.e15–461.e27. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bullock, K.; Gurjao, C.; Braun, D.; Shukla, S.A.; Bossé, D.; Lalani, A.-K.A.; Gopal, S.; Jin, C.; Horak, C.; et al. Metabolomic Adaptations and Correlates of Survival to Immune Checkpoint Blockade. Nat. Commun. 2019, 10, 4346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, K.; He, M.X.; Bakouny, Z.; Kanodia, A.; Napolitano, S.; Wu, J.; Grimaldi, G.; Braun, D.A.; Cuoco, M.S.; Mayorga, A.; et al. Tumor and Immune Reprogramming during Immunotherapy in Advanced Renal Cell Carcinoma. Cancer Cell 2021, 39, 649–661.e5. [Google Scholar] [CrossRef] [PubMed]
- Beckermann, K.; Siska, P.; Mason, F.; Rathmell, K.; Rathmell, J.C. Metabolic Barriers to Immunotherapy in Renal Cell Carcinoma. J. Clin. Oncol. 2017, 35, 11560. [Google Scholar] [CrossRef]
- Carretero-González, A.; Lora, D.; Martín Sobrino, I.; Sáez Sanz, I.; Bourlon, M.T.; Anido Herranz, U.; Martínez Chanzá, N.; Castellano, D.; de Velasco, G. The Value of PD-L1 Expression as Predictive Biomarker in Metastatic Renal Cell Carcinoma Patients: A Meta-Analysis of Randomized Clinical Trials. Cancers 2020, 12, 1945. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Fishman, M.N.; Escudier, B.; McDermott, D.F.; Drake, C.G.; Kluger, H.; Stadler, W.M.; Perez-Gracia, J.L.; McNeel, D.G.; Curti, B.; et al. Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2016, 22, 5461–5471. [Google Scholar] [CrossRef] [Green Version]
- Kahlmeyer, A.; Stöhr, C.G.; Hartmann, A.; Goebell, P.J.; Wullich, B.; Wach, S.; Taubert, H.; Erlmeier, F. Expression of PD-1 and CTLA-4 Are Negative Prognostic Markers in Renal Cell Carcinoma. J. Clin. Med. 2019, 8, 743. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.H.; Dong, H.; Lohse, C.M.; Leibovich, B.C.; Blute, M.L.; Cheville, J.C.; Kwon, E.D. PD-1 Is Expressed by Tumor-Infiltrating Immune Cells and Is Associated with Poor Outcome for Patients with Renal Cell Carcinoma. Clin. Cancer Res. 2007, 13, 1757–1761. [Google Scholar] [CrossRef] [Green Version]
- Callea, M.; Albiges, L.; Gupta, M.; Cheng, S.-C.; Genega, E.M.; Fay, A.P.; Song, J.; Carvo, I.; Bhatt, R.S.; Atkins, M.B.; et al. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol. Res. 2015, 3, 1158–1164. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.H.; Kuntz, S.M.; Leibovich, B.C.; Dong, H.; Lohse, C.M.; Webster, W.S.; Sengupta, S.; Frank, I.; Parker, A.S.; Zincke, H.; et al. Tumor B7-H1 Is Associated with Poor Prognosis in Renal Cell Carcinoma Patients with Long-Term Follow-Up. Cancer Res. 2006, 66, 3381–3385. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.H.; Gillett, M.D.; Cheville, J.C.; Lohse, C.M.; Dong, H.; Webster, W.S.; Krejci, K.G.; Lobo, J.R.; Sengupta, S.; Chen, L.; et al. Costimulatory B7-H1 in Renal Cell Carcinoma Patients: Indicator of Tumor Aggressiveness and Potential Therapeutic Target. Proc. Natl. Acad. Sci. USA 2004, 101, 17174–17179. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Xu, L.; Wang, Q.; An, G.; Feng, G.; Liu, F. Clinicopathological and Prognostic Value of Programmed Death Ligand-1 (PD-L1) in Renal Cell Carcinoma: A Meta-Analysis. Int. J. Clin. Exp. Med. 2015, 8, 14595–14603. [Google Scholar]
- Liao, G.; Wang, P.; Wang, Y. Identification of the Prognosis Value and Potential Mechanism of Immune Checkpoints in Renal Clear Cell Carcinoma Microenvironment. Front. Oncol. 2021, 11, 720125. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, F.; Tan, W.; Zhang, L.; Dai, F.; Wang, Y.; Fan, Y.; Yuan, M.; Yang, D.; Zheng, Y.; et al. CTLA4 Has a Profound Impact on the Landscape of Tumor-Infiltrating Lymphocytes with a High Prognosis Value in Clear Cell Renal Cell Carcinoma (CcRCC). Cancer Cell Int. 2020, 20, 519. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Du, Q.; Jin, J.; Wei, Y.; Lu, Y.; Li, Q. LAG3 and Its Emerging Role in Cancer Immunotherapy. Clin. Transl. Med. 2021, 11, e365. [Google Scholar] [CrossRef]
- Klümper, N.; Ralser, D.J.; Bawden, E.G.; Landsberg, J.; Zarbl, R.; Kristiansen, G.; Toma, M.; Ritter, M.; Hölzel, M.; Ellinger, J.; et al. LAG3 (LAG-3, CD223) DNA Methylation Correlates with LAG3 Expression by Tumor and Immune Cells, Immune Cell Infiltration, and Overall Survival in Clear Cell Renal Cell Carcinoma. J. Immunother. Cancer 2020, 8, e000552. [Google Scholar] [CrossRef] [Green Version]
- Zelba, H.; Bedke, J.; Hennenlotter, J.; Mostböck, S.; Zettl, M.; Zichner, T.; Chandran, A.; Stenzl, A.; Rammensee, H.-G.; Gouttefangeas, C. PD-1 and LAG-3 Dominate Checkpoint Receptor–Mediated T-Cell Inhibition in Renal Cell Carcinoma. Cancer Immunol. Res. 2019, 7, 1891–1899. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X.; Wang, T.; Zhang, X. Correlation of T Cell Immunoglobulin and ITIM Domain (TIGIT) and Programmed Death 1 (PD-1) with Clinicopathological Characteristics of Renal Cell Carcinoma May Indicate Potential Targets for Treatment. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 6861–6872. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8 + T Cell Effector Function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Park, A.I.; Srivastava, M.; Mayes, E.; Jie, H.-B.; Yun, R.; Murriel, C.; Xie, M.; Lam, A.; Ji, M.; Axelrod, F.; et al. Abstract 2003: Antibody against TIGIT (T Cell Immunoreceptor with Ig and ITIM Domains) Induces Anti-Tumor Immune Response and Generates Long-Term Immune Memory. Cancer Res. 2017, 77, 2003. [Google Scholar] [CrossRef]
- Takamatsu, K.; Tanaka, N.; Hakozaki, K.; Takahashi, R.; Teranishi, Y.; Murakami, T.; Kufukihara, R.; Niwa, N.; Mikami, S.; Shinojima, T.; et al. Profiling the Inhibitory Receptors LAG-3, TIM-3, and TIGIT in Renal Cell Carcinoma Reveals Malignancy. Nat. Commun. 2021, 12, 5547. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, X. A Genomic Instability-Derived Risk Index Predicts Clinical Outcome and Immunotherapy Response for Clear Cell Renal Cell Carcinoma. Bioengineered 2021, 12, 1642–1662. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, X.; Fan, Z.; Zhan, S.; Jiang, X.; Huang, J. Integrated Analysis on the N6-Methyladenosine-Related Long Noncoding RNAs Prognostic Signature, Immune Checkpoints, and Immune Cell Infiltration in Clear Cell Renal Cell Carcinoma. Immun. Inflamm. Dis. 2021, 9, 1596–1612. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Jiang, W.; Zeng, H.; Liu, Z.; Lin, Z.; Qu, Y.; Xiong, Y.; Wang, J.; Chang, Y.; et al. Tumor-Infiltrating TNFRSF9+ CD8+ T Cells Define Different Subsets of Clear Cell Renal Cell Carcinoma with Prognosis and Immunotherapeutic Response. Oncoimmunology 2020, 9, 1838141. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zeng, H.; Liu, Z.; Jin, K.; Jiang, W.; Wang, Z.; Lin, Z.; Xiong, Y.; Wang, J.; Chang, Y.; et al. Intratumoral CXCL13+CD8+T Cell Infiltration Determines Poor Clinical Outcomes and Immunoevasive Contexture in Patients with Clear Cell Renal Cell Carcinoma. J. Immunother. Cancer 2021, 9, e001823. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical Activity and Molecular Correlates of Response to Atezolizumab Alone or in Combination with Bevacizumab versus Sunitinib in Renal Cell Carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef]
- Motzer, R.J.; Robbins, P.B.; Powles, T.; Albiges, L.; Haanen, J.B.; Larkin, J.; Mu, X.J.; Ching, K.A.; Uemura, M.; Pal, S.K.; et al. Avelumab plus Axitinib versus Sunitinib in Advanced Renal Cell Carcinoma: Biomarker Analysis of the Phase 3 JAVELIN Renal 101 Trial. Nat. Med. 2020, 26, 1733–1741. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Xu, Y.; Zhu, M.; Xiao, Y.; Shen, Y.; Zhu, S.; Cao, C.; Xu, X. Upregulated Immune Checkpoint HHLA2 in Clear Cell Renal Cell Carcinoma: A Novel Prognostic Biomarker and Potential Therapeutic Target. J. Med. Genet. 2019, 56, 43–49. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Becht, E.; Pagès, F.; Skliris, G.; Verkarre, V.; Vano, Y.; Mejean, A.; Saint-Aubert, N.; Lacroix, L.; Natario, I.; et al. Orchestration and Prognostic Significance of Immune Checkpoints in the Microenvironment of Primary and Metastatic Renal Cell Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3031–3040. [Google Scholar] [CrossRef] [Green Version]
- de Velasco, G.; Miao, D.; Shukla, S.; Voss, M.H.; Wu, C.; Murray, B.; Meyerson, M.; Signoretti, S.; Motzer, R.J.; Van Allen, E.M.; et al. Integrated Genomic Correlates of Response to PD-1 Inhibitor Nivolumab in Metastatic Renal Cell Carcinoma (MRCC). J. Clin. Oncol. 2016, 34, 545. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Tu, H.; Liang, D.; Chang, D.W.; Ye, Y.; Wu, X. Soluble Immune Checkpoint-Related Proteins as Predictors of Tumor Recurrence, Survival, and T Cell Phenotypes in Clear Cell Renal Cell Carcinoma Patients. J. Immunother. Cancer 2019, 7. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Cai, X.; Xie, Z.; Zhou, L.; Cheng, B.; Zhong, R.; Xiong, S.; Li, J.; Chen, Z.; et al. The Association between CD8+ Tumor-Infiltrating Lymphocytes and the Clinical Outcome of Cancer Immunotherapy: A Systematic Review and Meta-Analysis. EClinicalMedicine 2021, 41. [Google Scholar] [CrossRef] [PubMed]
- Becht, E.; Giraldo, N.A.; Beuselinck, B.; Job, S.; Marisa, L.; Vano, Y.; Oudard, S.; Zucman-Rossi, J.; Laurent-Puig, P.; Sautès-Fridman, C.; et al. Prognostic and Theranostic Impact of Molecular Subtypes and Immune Classifications in Renal Cell Cancer (RCC) and Colorectal Cancer (CRC). Oncoimmunology 2015, 4, e1049804. [Google Scholar] [CrossRef]
- Bromwich, E.J.; McArdle, P.A.; Canna, K.; McMillan, D.C.; McNicol, A.-M.; Brown, M.; Aitchison, M. The Relationship between T-Lymphocyte Infiltration, Stage, Tumour Grade and Survival in Patients Undergoing Curative Surgery for Renal Cell Cancer. Br. J. Cancer 2003, 89, 1906–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo, N.A.; Becht, E.; Vano, Y.; Petitprez, F.; Lacroix, L.; Validire, P.; Sanchez-Salas, R.; Ingels, A.; Oudard, S.; Moatti, A.; et al. Tumor-Infiltrating and Peripheral Blood T-Cell Immunophenotypes Predict Early Relapse in Localized Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 4416–4428. [Google Scholar] [CrossRef] [Green Version]
- Nakano, O.; Sato, M.; Naito, Y.; Suzuki, K.; Orikasa, S.; Aizawa, M.; Suzuki, Y.; Shintaku, I.; Nagura, H.; Ohtani, H. Proliferative Activity of Intratumoral CD8(+) T-Lymphocytes as a Prognostic Factor in Human Renal Cell Carcinoma: Clinicopathologic Demonstration of Antitumor Immunity. Cancer Res. 2001, 61, 5132–5136. [Google Scholar] [PubMed]
- Ross-Macdonald, P.; Walsh, A.M.; Chasalow, S.D.; Ammar, R.; Papillon-Cavanagh, S.; Szabo, P.M.; Choueiri, T.K.; Sznol, M.; Wind-Rotolo, M. Molecular Correlates of Response to Nivolumab at Baseline and on Treatment in Patients with RCC. J. Immunother. Cancer 2021, 9, e001506. [Google Scholar] [CrossRef]
- Rini, B.I.; Huseni, M.; Atkins, M.B.; McDermott, D.F.; Powles, T.B.; Escudier, B.; Banchereau, R.; Liu, L.-F.; Leng, N.; Fan, J.; et al. Molecular Correlates Differentiate Response to Atezolizumab (Atezo) + Bevacizumab (Bev) vs Sunitinib (Sun): Results from a Phase III Study (IMmotion151) in Untreated Metastatic Renal Cell Carcinoma (MRCC). Ann. Oncol. 2018, 29, viii724–viii725. [Google Scholar] [CrossRef]
- Desnoyer, A.; Larive, A.; Drubay, D.; Lanoy, E.; Vano, Y.; Rioux-Leclercq, N.; Chouaib, S.; Beuselinck, B.; Tantot, F.; Escudier, B.; et al. Fresh Blood Immune Cell Monitoring in Patients Treated with Nivolumab in the GETUG-AFU26 NIVOREN Study: Association with Toxicity and Treatment Outcome. Ann. Oncol. 2019, 30, v394. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Y.; Chen, L.; An, H.; Zhang, W.; Wang, G.; Lin, Z.; Xu, J. Prognostic Value of Diametrically Polarized Tumor-Associated Macrophages in Renal Cell Carcinoma. Ann. Surg. Oncol. 2014, 21, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, R.; Kapur, P.; Jaiswal, B.S.; Hannan, R.; Zhang, Z.; Pedrosa, I.; Luke, J.J.; Zhang, H.; Goldstein, L.D.; et al. An Empirical Approach Leveraging Tumorgrafts to Dissect the Tumor Microenvironment in Renal Cell Carcinoma Identifies Missing Link to Prognostic Inflammatory Factors. Cancer Discov. 2018, 8, 1142–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High Tumor Mutation Burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Carril-Ajuria, L.; Santos, M.; Roldán-Romero, J.M.; Rodriguez-Antona, C.; de Velasco, G. Prognostic and Predictive Value of PBRM1 in Clear Cell Renal Cell Carcinoma. Cancers 2019, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, D.A.; Ishii, Y.; Walsh, A.M.; Van Allen, E.M.; Wu, C.J.; Shukla, S.A.; Choueiri, T.K. Clinical Validation of PBRM1 Alterations as a Marker of Immune Checkpoint Inhibitor Response in Renal Cell Carcinoma. JAMA Oncol. 2019, 5, 1631–1633. [Google Scholar] [CrossRef]
- Miao, D.; Margolis, C.A.; Gao, W.; Voss, M.H.; Li, W.; Martini, D.J.; Norton, C.; Bossé, D.; Wankowicz, S.M.; Cullen, D.; et al. Genomic Correlates of Response to Immune Checkpoint Therapies in Clear Cell Renal Cell Carcinoma. Science 2018, 359, 801–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yao, C.; Qiao, N.; Ge, Y.; Li, J.; Lin, Y.; Yao, S. Development and Validation of a PBRM1-Associated Immune Prognostic Model for Clear Cell Renal Cell Carcinoma. Cancer Med. 2021, 10, 6590–6609. [Google Scholar] [CrossRef]
- Liu, X.-D.; Kong, W.; Peterson, C.B.; McGrail, D.J.; Hoang, A.; Zhang, X.; Lam, T.; Pilie, P.G.; Zhu, H.; Beckermann, K.E.; et al. PBRM1 Loss Defines a Nonimmunogenic Tumor Phenotype Associated with Checkpoint Inhibitor Resistance in Renal Carcinoma. Nat. Commun. 2020, 11, 2135. [Google Scholar] [CrossRef] [PubMed]
- Dias Carneiro, A.P.C.; Marques Monteiro, F.S.; Soares, A. PBRM1 Mutations as a Predictive Biomarker for Immunotherapy in Metastatic Renal Cell Carcinoma: A Systematic Review. Kidney Cancer 2021, 5, 79–92. [Google Scholar] [CrossRef]
- Ascierto, M.L.; McMiller, T.L.; Berger, A.E.; Danilova, L.; Anders, R.A.; Netto, G.J.; Xu, H.; Pritchard, T.S.; Fan, J.; Cheadle, C.; et al. The Intratumoral Balance between Metabolic and Immunologic Gene Expression Is Associated with Anti–PD-1 Response in Patients with Renal Cell Carcinoma. Cancer Immunol. Res. 2016, 4, 726–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zizzari, I.G.; Napoletano, C.; Botticelli, A.; Caponnetto, S.; Calabrò, F.; Gelibter, A.; Rughetti, A.; Ruscito, I.; Rahimi, H.; Rossi, E.; et al. TK Inhibitor Pazopanib Primes DCs by Downregulation of the β-Catenin Pathway. Cancer Immunol. Res. 2018, 6, 711–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epaillard, N.; Simonaggio, A.; Elaidi, R.; Azzouz, F.; Braychenko, E.; Thibault, C.; Sun, C.-M.; Moreira, M.; Oudard, S.; Vano, Y.-A. BIONIKK: A Phase 2 Biomarker Driven Trial with Nivolumab and Ipilimumab or VEGFR Tyrosine Kinase Inhibitor (TKI) in Naïve Metastatic Kidney Cancer. Bull. Cancer 2020, 107, eS22–eS27. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative Association of Antibiotics on Clinical Activity of Immune Checkpoint Inhibitors in Patients with Advanced Renal Cell and Non-Small-Cell Lung Cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Routy, B.; Chatelier, E.L.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgia, N.J.; Bergerot, P.G.; Maia, M.C.; Dizman, N.; Hsu, J.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; et al. Stool Microbiome Profiling of Patients with Metastatic Renal Cell Carcinoma Receiving Anti–PD-1 Immune Checkpoint Inhibitors. Eur. Urol. 2020, 78, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Hsu, J.; Bergerot, P.G.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; Pal, S.K. Randomized Trial Assessing Impact of Probiotic Supplementation on Gut Microbiome and Clinical Outcome from Targeted Therapy in Metastatic Renal Cell Carcinoma. Cancer Med. 2020, 10, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lalani, A.-K.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Allen, E.M.V.; et al. Change in Neutrophil-to-Lymphocyte Ratio (NLR) in Response to Immune Checkpoint Blockade for Metastatic Renal Cell Carcinoma. J. Immunother. Cancer 2018, 6, 5. [Google Scholar] [CrossRef]
- Escudier, B.; Sharma, P.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. CheckMate 025 Randomized Phase 3 Study: Outcomes by Key Baseline Factors and Prior Therapy for Nivolumab Versus Everolimus in Advanced Renal Cell Carcinoma. Eur. Urol. 2017, 72, 962–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudier, B.; Motzer, R.J.; Tannir, N.M.; Porta, C.; Tomita, Y.; Maurer, M.A.; McHenry, M.B.; Rini, B.I. Efficacy of Nivolumab plus Ipilimumab According to Number of IMDC Risk Factors in CheckMate 214. Eur. Urol. 2020, 77, 449–453. [Google Scholar] [CrossRef] [PubMed]
- de Velasco, G.; Miao, D.; Voss, M.H.; Hakimi, A.A.; Hsieh, J.J.; Tannir, N.M.; Tamboli, P.; Appleman, L.J.; Rathmell, W.K.; Van Allen, E.M.; et al. Tumor Mutational Load and Immune Parameters across Metastatic Renal Cell Carcinoma Risk Groups. Cancer Immunol. Res. 2016, 4, 820–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgi, U.D.; Procopio, G.; Giannarelli, D.; Sabbatini, R.; Bearz, A.; Buti, S.; Basso, U.; Mitterer, M.; Ortega, C.; Bidoli, P.; et al. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clin. Cancer Res. 2019, 25, 3839–3846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, R.R.; Bossé, D.; Xie, W.; Wankowicz, S.A.M.; Flaifel, A.; Brandao, R.; Lalani, A.-K.A.; Martini, D.J.; Wei, X.X.; Braun, D.A.; et al. The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non-Clear Cell Renal Cell Carcinoma. Cancer Immunol. Res. 2018, 6, 758–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.A.; Gupta, S.; Tickoo, S.K.; Chan, T.A.; Russo, P.; Motzer, R.J.; Karam, J.A.; Hakimi, A.A. Sarcomatoid Renal Cell Carcinoma: Biology, Natural History and Management. Nat. Rev. Urol. 2020, 17, 659–678. [Google Scholar] [CrossRef]
- Kawakami, F.; Sircar, K.; Rodriguez-Canales, J.; Fellman, B.M.; Urbauer, D.L.; Tamboli, P.; Tannir, N.M.; Jonasch, E.; Wistuba, I.I.; Wood, C.G.; et al. Programmed Cell Death Ligand 1 and Tumor-Infiltrating Lymphocyte Status in Patients with Renal Cell Carcinoma and Sarcomatoid Dedifferentiation. Cancer 2017, 123, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Cheville, J.C.; Jungbluth, A.A.; Zhang, Y.; Zhang, L.; Chen, Y.-B.; Tickoo, S.K.; Fine, S.W.; Gopalan, A.; Al-Ahmadie, H.A.; et al. JAK2/PD-L1/PD-L2 (9p24.1) Amplifications in Renal Cell Carcinomas with Sarcomatoid Transformation: Implications for Clinical Management. Mod. Pathol. 2019, 32, 1344–1358. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Larkin, J.M.G.; Pal, S.K.; Motzer, R.J.; Venugopal, B.; Alekseev, B.Y.; Miyake, H.; Gravis, G.; Bilen, M.A.; Chudnovsky, A.; et al. Efficacy and Biomarker Analysis of Patients (Pts) with Advanced Renal Cell Carcinoma (ARCC) with Sarcomatoid Histology (SRCC): Subgroup Analysis from the Phase III JAVELIN Renal 101 Trial of First-Line Avelumab plus Axitinib (A + Ax) vs Sunitinib (S). Ann. Oncol. 2019, 30, v361. [Google Scholar] [CrossRef]
- McDermott, D.F.; Choueiri, T.K.; Motzer, R.J.; Aren, O.R.; George, S.; Powles, T.; Donskov, F.; Harrison, M.R.; Rodriguez Cid, J.R.R.; Ishii, Y.; et al. CheckMate 214 Post-Hoc Analyses of Nivolumab plus Ipilimumab or Sunitinib in IMDC Intermediate/Poor-Risk Patients with Previously Untreated Advanced Renal Cell Carcinoma with Sarcomatoid Features. J. Clin. Oncol. 2019, 37, 4513. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Soulieres, D.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab (Pembro) plus Axitinib (Axi) versus Sunitinib as First-Line Therapy for Metastatic Renal Cell Carcinoma (MRCC): Outcomes in the Combined IMDC Intermediate/Poor Risk and Sarcomatoid Subgroups of the Phase 3 KEYNOTE-426 Study. J. Clin. Oncol. 2019, 37, 4500. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Bria, E.; Bracarda, S.; Porta, C.; Procopio, G.; Tortora, G. Patients with Sarcomatoid Renal Cell Carcinoma–Re-Defining the First-Line of Treatment: A Meta-Analysis of Randomised Clinical Trials with Immune Checkpoint Inhibitors. Eur. J. Cancer 2020, 136, 195–203. [Google Scholar] [CrossRef]
- Bex, A.; Russo, P.; Tomita, Y.; Grünwald, V.; Ramirez, L.-M.; McHenry, B.M.; Motzer, R.J. A Phase III, Randomized, Placebo-Controlled Trial of Nivolumab or Nivolumab plus Ipilimumab in Patients with Localized Renal Cell Carcinoma at High-Risk of Relapse after Radical or Partial Nephrectomy (CheckMate 914). J. Clin. Oncol. 2020, 38, TPS5099. [Google Scholar] [CrossRef]
- Oza, B.; Frangou, E.; Smith, B.; Bryant, H.; Kaplan, R.; Choodari-Oskooei, B.; Powles, T.; Stewart, G.D.; Albiges, L.; Bex, A.; et al. RAMPART: A Phase III Multi-Arm Multi-Stage Trial of Adjuvant Checkpoint Inhibitors in Patients with Resected Primary Renal Cell Carcinoma (RCC) at High or Intermediate Risk of Relapse. Contemp. Clin. Trials 2021, 108, 106482. [Google Scholar] [CrossRef] [PubMed]
- Ornstein, M.C.; Zabell, J.; Wood, L.S.; Hobbs, B.; Devonshire, S.; Martin, A.; Allman, K.D.; Rao, A.; Gilligan, T.D.; Campbell, S.; et al. A Phase Ib Trial of Neoadjuvant/Adjuvant Durvalumab +/- Tremelimumab in Locally Advanced Renal Cell Carcinoma (RCC). J. Clin. Oncol. 2020, 38, 5021. [Google Scholar] [CrossRef]
- Atkins, M.B.; Jegede, O.; Haas, N.B.; McDermott, D.F.; Bilen, M.A.; Drake, C.G.; Sosman, J.A.; Alter, R.S.; Plimack, E.R.; Rini, B.I.; et al. Phase II Study of Nivolumab and Salvage Nivolumab + Ipilimumab in Treatment-Naïve Patients (Pts) with Advanced Renal Cell Carcinoma (RCC) (HCRN GU16-260). J. Clin. Oncol. 2020, 38, 5006. [Google Scholar] [CrossRef]
- Grimm, M.-O.; Esteban, E.; Barthélémy, P.; Schmidinger, M.; Busch, J.; Valderrama, B.P.; Schmitz, M.; Schumacher, U.; Baretton, G.B.; Duran, I.; et al. Efficacy of Nivolumab/Ipilimumab in Patients with Initial or Late Progression with Nivolumab: Updated Analysis of a Tailored Approach in Advanced Renal Cell Carcinoma (TITAN-RCC). J. Clin. Oncol. 2021, 39, 4576. [Google Scholar] [CrossRef]
- McKay, R.R.; McGregor, B.A.; Xie, W.; Braun, D.A.; Wei, X.; Kyriakopoulos, C.E.; Zakharia, Y.; Maughan, B.L.; Rose, T.L.; Stadler, W.M.; et al. Optimized Management of Nivolumab and Ipilimumab in Advanced Renal Cell Carcinoma: A Response-Based Phase II Study (OMNIVORE). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4240–4248. [Google Scholar] [CrossRef]
- Gedye, C.; Pook, D.W.; Krieger, L.E.M.; Harris, C.A.; Goh, J.C.; Kichenadasse, G.; Gurney, H.; Underhill, C.; Parnis, F.; Joshua, A.M.; et al. UNISON: Nivolumab Then Ipilimumab + Nivolumab in Advanced Nonclear Cell Renal Cell Carcinoma (ANZUP 1602). J. Clin. Oncol. 2020, 38, TPS768. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Kluger, H.M.; George, S.; Tykodi, S.S.; Kuzel, T.M.; Perets, R.; Nair, S.; Procopio, G.; Carducci, M.A.; Castonguay, V.; et al. FRACTION-RCC: Innovative, High-Throughput Assessment of Nivolumab + Ipilimumab for Treatment-Refractory Advanced Renal Cell Carcinoma (ARCC). J. Clin. Oncol. 2020, 38, 5007. [Google Scholar] [CrossRef]
- Hong, D.S.; Schoffski, P.; Calvo, A.; Sarantopoulos, J.; Ochoa De Olza, M.; Carvajal, R.D.; Prawira, A.; Kyi, C.; Esaki, T.; Akerley, W.L.; et al. Phase I/II Study of LAG525 ± Spartalizumab (PDR001) in Patients (Pts) with Advanced Malignancies. J. Clin. Oncol. 2018, 36, 3012. [Google Scholar] [CrossRef]
- Abstract CT183: Phase (Ph) I/II Study of MBG453± Spartalizumab (PDR001) in Patients (Pts) with Advanced Malignancies|Cancer Research. Available online: https://cancerres.aacrjournals.org/content/79/13_Supplement/CT183 (accessed on 2 October 2021).
- Voss, M.H.; Azad, A.A.; Hansen, A.R.; Gray, J.E.; Welsh, S.J.; Achour, I.; Hu, H.; Lewis, L.; Walcott, F.L.; Oosting, S.F. Results from a Randomised Phase I/II Trial Evaluating the Safety and Antitumour Activity of Anti-PD-1 (MEDI0680)/Anti-PD-L1 (Durvalumab) vs Anti-PD-1 (Nivolumab) Alone in Metastatic Clear Cell Renal Cell Carcinoma (CcRCC). Ann. Oncol. 2019, 30, v516. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Sudarshan, N.S.; Adurthi, S.; Ramachandra, R.K.; Samiulla, D.S.; Lakshminarasimhan, A.; Ramanathan, A.; Chandrasekhar, T.; Dhudashiya, A.A.; Talapati, S.R.; et al. PD-1 Derived CA-170 Is an Oral Immune Checkpoint Inhibitor That Exhibits Preclinical Anti-Tumor Efficacy. Commun. Biol. 2021, 4, 1–12. [Google Scholar] [CrossRef]
- Lara, P.; Parikh, M.; Robles, D.; Lara, F.; Meyers, F.J.; Pan, C. Pilot Trial of Ibrutinib plus Nivolumab in Patients (Pts) with Metastatic Renal Cell Cancer (MRCC): Results from a Dose-Finding Cohort. J. Clin. Oncol. 2018, 36, 600. [Google Scholar] [CrossRef]
- Karam, J.A.; Msaouel, P.; Matin, S.F.; Campbell, M.T.; Zurita, A.J.; Shah, A.Y.; Wistuba, I.I.; Haymaker, C.L.; Marmonti, E.; Duose, D.Y.; et al. A Phase II Study of Sitravatinib (Sitra) in Combination with Nivolumab (Nivo) in Patients (Pts) Undergoing Nephrectomy for Locally-Advanced Clear Cell Renal Cell Carcinoma (AccRCC). J. Clin. Oncol. 2021, 39, 312. [Google Scholar] [CrossRef]
- Pal, S.K.; McGregor, B.; Suárez, C.; Tsao, C.-K.; Kelly, W.; Vaishampayan, U.; Pagliaro, L.; Maughan, B.L.; Loriot, Y.; Castellano, D.; et al. Cabozantinib in Combination With Atezolizumab for Advanced Renal Cell Carcinoma: Results From the COSMIC-021 Study. J. Clin. Oncol. 2021, 39, 3725–3736. [Google Scholar] [CrossRef]
- Apolo, A.B.; Nadal, R.; Girardi, D.M.; Niglio, S.A.; Ley, L.; Cordes, L.M.; Steinberg, S.M.; Sierra Ortiz, O.; Cadena, J.; Diaz, C.; et al. Phase I Study of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced or Metastatic Urothelial Carcinoma and Other Genitourinary Tumors. J. Clin. Oncol. 2020, 38, 3672–3684. [Google Scholar] [CrossRef]
- Kessler, E.R.; Hu, J.; Srivastava, G.; Kemme, D.J.; Iruku, P.; Rana, V.; Schuster, S.R.; Amirault, M.; Callihan, E.; Flaig, T.W.; et al. Phase I/II Trial of Pembrolizumab and Cabozantinib in the Treatment of Metastatic Renal Cell Carcinoma (MRCC). J. Clin. Oncol. 2021, 39, 4544. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Albiges, L.; Powles, T.; Scheffold, C.; Wang, F.; Motzer, R.J. A Phase III Study (COSMIC-313) of Cabozantinib (C) in Combination with Nivolumab (N) and Ipilimumab (I) in Patients (Pts) with Previously Untreated Advanced Renal Cell Carcinoma (ARCC) of Intermediate or Poor Risk. J. Clin. Oncol. 2020, 38, TPS767. [Google Scholar] [CrossRef]
- Zhang, T.; Ballman, K.V.; Choudhury, A.D.; Chen, R.C.; Watt, C.; Wen, Y.; Shergill, A.; Zemla, T.J.; Emamekhoo, H.; Vaishampayan, U.N.; et al. PDIGREE: An Adaptive Phase III Trial of PD-Inhibitor Nivolumab and Ipilimumab (IPI-NIVO) with VEGF TKI Cabozantinib (CABO) in Metastatic Untreated Renal Cell Cancer (Alliance A031704). J. Clin. Oncol. 2020, 38, TPS5100. [Google Scholar] [CrossRef]
- Pal, S.K.; Albiges, L.; Suarez Rodriguez, C.; Liu, B.; Doss, J.; Khurana, S.; Scheffold, C.; Voss, M.H.; Choueiri, T.K. CONTACT-03: Randomized, Open-Label Phase III Study of Atezolizumab plus Cabozantinib versus Cabozantinib Monotherapy Following Progression on/after Immune Checkpoint Inhibitor (ICI) Treatment in Patients with Advanced/Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2021, 39, TPS370. [Google Scholar] [CrossRef]
- Suarez Rodriguez, C.; Larkin, J.; Patel, P.M.; Valderrama, B.P.; Rodriguez-Vida, A.; Glen, H.; Thistlethwaite, F.; Ralph, C.; Srinivasan, G.; Mendez-Vidal, M.J.; et al. Clinical Activity of Durvalumab and Savolitinib in MET-Driven, Metastatic Papillary Renal Cancer. J. Clin. Oncol. 2021, 39, 4511. [Google Scholar] [CrossRef]
- Lee, C.-H.; Shah, A.Y.; Rasco, D.; Rao, A.; Taylor, M.H.; Di Simone, C.; Hsieh, J.J.; Pinto, A.; Shaffer, D.R.; Girones Sarrio, R.; et al. Lenvatinib plus Pembrolizumab in Patients with Either Treatment-Naive or Previously Treated Metastatic Renal Cell Carcinoma (Study 111/KEYNOTE-146): A Phase 1b/2 Study. Lancet Oncol. 2021, 22, 946–958. [Google Scholar] [CrossRef]
- Choi, W.S.W.; Boland, J.; Lin, J. Hypoxia-Inducible Factor-2α as a Novel Target in Renal Cell Carcinoma. J. Kidney Cancer VHL 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Rini, B.I.; Appleman, L.J.; Figlin, R.A.; Plimack, E.R.; Merchan, J.R.; Wang, K.; Thamake, S.; Zojwalla, N.J.; Choueiri, T.K.; McDermott, D.F. Results from a Phase I Expansion Cohort of the First-in-Class Oral HIF-2α Inhibitor PT2385 in Combination with Nivolumab in Patients with Previously Treated Advanced RCC. J. Clin. Oncol. 2019, 37, 558. [Google Scholar] [CrossRef]
- Wong, T.W.; Shrimali, R.; Contreras, C.; Cheng, T.; Czerwinski, R.M.; Dixon, D.D.; Du, X.; Fett, C.; Goree, J.; Grina, J.A.; et al. Abstract B140: PT2977, a Novel HIF-2a Antagonist, Has Potent Antitumor Activity and Remodels the Immunosuppressive Tumor Microenvironment in Clear Cell Renal Cell Cancer. Mol. Cancer Ther. 2018, 17, B140. [Google Scholar] [CrossRef] [Green Version]
- Lamers, C.H.J.; Klaver, Y.; Gratama, J.W.; Sleijfer, S.; Debets, R. Treatment of Metastatic Renal Cell Carcinoma (MRCC) with CAIX CAR-Engineered T-Cells-a Completed Study Overview. Biochem. Soc. Trans. 2016, 44, 951–959. [Google Scholar] [CrossRef]
- Rini, B.I.; Stenzl, A.; Zdrojowy, R.; Kogan, M.; Shkolnik, M.; Oudard, S.; Weikert, S.; Bracarda, S.; Crabb, S.J.; Bedke, J.; et al. IMA901, a Multipeptide Cancer Vaccine, plus Sunitinib versus Sunitinib Alone, as First-Line Therapy for Advanced or Metastatic Renal Cell Carcinoma (IMPRINT): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2016, 17, 1599–1611. [Google Scholar] [CrossRef]
- Amin, A.; Dudek, A.Z.; Logan, T.F.; Lance, R.S.; Holzbeierlein, J.M.; Knox, J.J.; Master, V.A.; Pal, S.K.; Miller, W.H.; Karsh, L.I.; et al. Survival with AGS-003, an Autologous Dendritic Cell-Based Immunotherapy, in Combination with Sunitinib in Unfavorable Risk Patients with Advanced Renal Cell Carcinoma (RCC): Phase 2 Study Results. J. Immunother. Cancer 2015, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Figlin, R.A.; Tannir, N.M.; Uzzo, R.G.; Tykodi, S.S.; Chen, D.Y.T.; Master, V.; Kapoor, A.; Vaena, D.; Lowrance, W.; Bratslavsky, G.; et al. Results of the ADAPT Phase 3 Study of Rocapuldencel-T in Combination with Sunitinib as First-Line Therapy in Patients with Metastatic Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2327–2336. [Google Scholar] [CrossRef] [Green Version]
- Laurell, A.; Lönnemark, M.; Brekkan, E.; Magnusson, A.; Tolf, A.; Wallgren, A.C.; Andersson, B.; Adamson, L.; Kiessling, R.; Karlsson-Parra, A. Intratumorally Injected Pro-Inflammatory Allogeneic Dendritic Cells as Immune Enhancers: A First-in-Human Study in Unfavourable Risk Patients with Metastatic Renal Cell Carcinoma. J. Immunother. Cancer 2017, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Lindskog, M.; Laurell, A.; Kjellman, A.; Melichar, B.; Niezabitowski, J.; Maroto, P.; Zieliński, H.; Villacampa, F.; Bigot, P.; Bajory, Z.; et al. A Randomized Phase II Study with Ilixadencel, a Cell-Based Immune Primer, plus Sunitinib versus Sunitinib Alone in Synchronous Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2020, 38, 11. [Google Scholar] [CrossRef]
- Cohen, R.B.; Twardowski, P.; Johnson, M.L.; Gillison, M.L.; Stein, M.N.; Vaishampayan, U.N.; McNeil, L.; Shainheit, M.; DeOliveira, D.; Jain, M.; et al. GEN-009, a Neoantigen Vaccine Containing ATLAS Selected Neoantigens, to Generate Broad Sustained Immunity against Immunogenic Tumor Mutations and Avoid Inhibitory Peptides. J. Clin. Oncol. 2020, 38, 3107. [Google Scholar] [CrossRef]
- Krauss, J.; Krackhardt, A.; Jager, E.; Williams, A.; Wold, H.; Gerner, L.; Sekelja, M.; Schjetne, K.; Fredriksen, A.B.; Axelsen, M. Abstract CT217: An Open-Label, Phase I/IIa Study of VB10.NEO (DIRECT-01) in Combination with Checkpoint Blockade in Patients with Locally Advanced or Metastatic Solid Tumors Including Melanoma, NSCLC, Renal Cell Carcinoma, Urothelial Cancer or SSCHN. Cancer Res. 2019, 79, CT217. [Google Scholar] [CrossRef]
- Rha, S.Y.; Merchan, J.; Oh, S.Y.; Kim, C.; Bae, W.K.; Lee, H.W.; Dillon, M.; Deering, R.; Kroog, G.; Ha, K.S. Abstract CT121: A Phase Ib Study of Recombinant Vaccinia Virus in Combination with Immune Checkpoint Inhibition (ICI) in Advanced Renal Cell Carcinoma (RCC). Cancer Res. 2020, 80, CT121. [Google Scholar] [CrossRef]
- Slingluff, C.L.; Dasilva, D.; Schwarzenberger, P.; Ricciardi, T.; Macri, M.J.; Ryan, A.; Venhaus, R.R.; Bhardwaj, N. Phase 1/2 Study of in Situ Vaccination with Tremelimumab + Intravenous (IV) Durvalumab + Poly-ICLC in Patients with Select Relapsed, Advanced Cancers with Measurable, Biopsy-Accessible Tumors. J. Clin. Oncol. 2017, 35, TPS3106. [Google Scholar] [CrossRef]
- Chatzkel, J.A.; Swank, J.; Ludlow, S.; Lombardi, K.; Croft, C.; Artigas, Y.; Rodriguez, Y.; Terraciano, T.; Hart, S.; Rembisz, J.; et al. Overall Responses with Coordinated Pembrolizumab and High Dose IL-2 (5-in-a-Row Schedule) for Therapy of Metastatic Clear Cell Renal Cancer: A Single Center, Single Arm Trial. J. Clin. Oncol. 2019, 37, 657. [Google Scholar] [CrossRef]
- Yentz, S.; Alva, A. Phase Ib/II Trial of Interleukin-2 (IL-2) and Nivolumab in Metastatic Clear Cell Renal Cell Cancer (RCC). Ann. Oncol. 2017, 28, v328–v329. [Google Scholar] [CrossRef]
- Diab, A.; Tannir, N.M.; Bentebibel, S.-E.; Hwu, P.; Papadimitrakopoulou, V.; Haymaker, C.; Kluger, H.M.; Gettinger, S.N.; Sznol, M.; Tykodi, S.S.; et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 2020, 10, 1158–1173. [Google Scholar] [CrossRef]
- Tannir, N.M.; Agarwal, N.; Pal, S.K.; Cho, D.C.; Formiga, M.; Guo, J.; George, D.J.; Tagliaferri, M.A.; Singel, S.M.; O’Keeffe, B.A.; et al. PIVOT-09: A Phase III Randomized Open-Label Study of Bempegaldesleukin (NKTR-214) plus Nivolumab versus Sunitinib or Cabozantinib (Investigator’s Choice) in Patients with Previously Untreated Advanced Renal Cell Carcinoma (RCC). J. Clin. Oncol. 2020, 38, TPS763. [Google Scholar] [CrossRef]
- Voss, M.H.; Bhatt, R.S.; Plimack, E.R.; Rini, B.I.; Alter, R.S.; Beck, J.T.; Wilson, D.; Zhang, X.; Mutyaba, M.; Glasser, C.; et al. The DART Study: Results from the Dose-Escalation and Expansion Cohorts Evaluating the Combination of Dalantercept plus Axitinib in Advanced Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3557–3565. [Google Scholar] [CrossRef] [Green Version]
- Voss, M.H.; Bhatt, R.S.; Vogelzang, N.J.; Fishman, M.; Alter, R.S.; Rini, B.I.; Beck, J.T.; Joshi, M.; Hauke, R.; Atkins, M.B.; et al. A Phase 2, Randomized Trial Evaluating the Combination of Dalantercept plus Axitinib in Patients with Advanced Clear Cell Renal Cell Carcinoma. Cancer 2019, 125, 2400–2408. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Michaelson, M.D.; Posadas, E.M.; Sonpavde, G.P.; McDermott, D.F.; Nixon, A.B.; Liu, Y.; Yuan, Z.; Seon, B.K.; Walsh, M.; et al. An Open Label Phase Ib Dose Escalation Study of TRC105 (Anti-Endoglin Antibody) with Axitinib in Patients with Metastatic Renal Cell Carcinoma. Oncologist 2019, 24, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Pili, R.; Adra, N.; Damayanti, N.; Logan, T.F.; Narayan, V.; Monk, P.; Dropcho, S.; Sego, L.M.; Liu, H.; Althouse, S.K.; et al. Immunomodulation by HDAC Inhibition: Results from a Phase I Study with Entinostat in Combination with Atezolizumab and Bevacizumab in Metastatic Renal Cell Carcinoma Patients. J. Clin. Oncol. 2020, 38, 5064. [Google Scholar] [CrossRef]
- Pili, R.; Quinn, D.I.; Hammers, H.J.; Monk, P.; George, S.; Dorff, T.B.; Olencki, T.; Shen, L.; Orillion, A.; Lamonica, D.; et al. Immunomodulation by Entinostat in Renal Cell Carcinoma Patients Receiving High-Dose Interleukin 2: A Multicenter, Single-Arm, Phase I/II Trial (NCI-CTEP#7870). Clin. Cancer Res. 2017, 23, 7199–7208. [Google Scholar] [CrossRef] [Green Version]
- Gabrail, N.Y.; Bessudo, A.; Hamilton, E.P.; Sachdev, J.C.; Patel, M.R.; Rodon Ahnert, J.; Evilevitch, L.; Duncan, M.; Guo, W.; Lu, S.; et al. IOLite: Multipart, Phase 1b, Dose-Finding Study of the PD-1 Inhibitor Dostarlimab in Combination with the PARP Inhibitor Niraparib ± Bevacizumab (Bev), or with Platinum-Based Chemotherapy ± Bev for Advanced Cancer. J. Clin. Oncol. 2019, 37, 2560. [Google Scholar] [CrossRef]
- Fong, L.; Forde, P.M.; Powderly, J.D.; Goldman, J.W.; Nemunaitis, J.J.; Luke, J.J.; Hellmann, M.D.; Kummar, S.; Doebele, R.C.; Mahadevan, D.; et al. Safety and Clinical Activity of Adenosine A2a Receptor (A2aR) Antagonist, CPI-444, in Anti-PD1/PDL1 Treatment-Refractory Renal Cell (RCC) and Non-Small Cell Lung Cancer (NSCLC) Patients. J. Clin. Oncol. 2017, 35, 3004. [Google Scholar] [CrossRef]
- Hotson, A.; Willingham, S.; Fong, L.; Powderly, J., II; Luke, J.; Sznol, M.; George, S.; Choueiri, T.; Giannakis, M.; Rini, B.; et al. P54: Adenosine Signature Genes Associate with Tumor Regression in Renal Cell Carcinoma (RCC) Patients Treated with the Adenosine A2A Receptor (A2AR) Antagonist, CPI-444. Available online: https://www.corvuspharma.com/file.cfm/23/docs/SITC2018_%20Hotson.pdf (accessed on 1 November 2021).
- Tolcher, A.W.; Sznol, M.; Hu-Lieskovan, S.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Gravio, D.D.; Huang, B.; Gambhire, D.; Chen, Y.; et al. Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 5349–5357. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.; Abdul-Karim, R.; Rizvi, N.; Rischen, D.; Hilton, J.; Li, Z.; Ott, P.; Karpinich-Fedoriw, N.; Yadavilli, S.; Wang, X.; et al. Abstract CT166: A Phase I/II, Open-Label, Two Part Study of GSK3359609 in Combination with Tremelimumab in Participants with Selected, Advanced Solid Tumors. Cancer Res. 2019, 79, CT166. [Google Scholar] [CrossRef]
- Zakharia, Y.; Singer, E.A.; Ross, R.; Joshi, M.; Abern, M.; Garje, R.; Park, J.J.; Kryczek, I.; Zou, W.; Alva, A.S. Phase Ib/II Study of Durvalumab and Guadecitabine in Advanced Kidney Cancer Big Ten Cancer Research Consortium BTCRC GU16-043. J. Clin. Oncol. 2021, 39, 328. [Google Scholar] [CrossRef]
- Masini, C.; Iotti, C.; De Giorgi, U.; Bellia, R.S.; Buti, S.; Salaroli, F.; Zampiva, I.; Mazzarotto, R.; Mucciarini, C.; Baldessari, C.; et al. Nivolumab (NIVO) in Combination with Stereotactic Body Radiotherapy (SBRT) in Pretreated Patients (Pts) with Metastatic Renal Cell Carcinoma (MRCC): First Results of Phase II NIVES Study. J. Clin. Oncol. 2020, 38, 613. [Google Scholar] [CrossRef]
- Vuong, L.; Kotecha, R.R.; Voss, M.H.; Hakimi, A.A. Tumor Microenvironment Dynamics in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2019, 9, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- Deleuze, A.; Saout, J.; Dugay, F.; Peyronnet, B.; Mathieu, R.; Verhoest, G.; Bensalah, K.; Crouzet, L.; Laguerre, B.; Belaud-Rotureau, M.-A.; et al. Immunotherapy in Renal Cell Carcinoma: The Future Is Now. Int. J. Mol. Sci. 2020, 21, 2532. [Google Scholar] [CrossRef] [Green Version]
- Shirotake, S.; Kaneko, G.; Nagata, K.; Oyama, M.; Nishimoto, K. Histological Complete Response with Nivolumab for Renal Cell Carcinoma with Multiple Metastases: A Case Report. Mol. Clin. Oncol. 2019, 10, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallin, J.J.; Bendell, J.C.; Funke, R.; Sznol, M.; Korski, K.; Jones, S.; Hernandez, G.; Mier, J.; He, X.; Hodi, F.S.; et al. Atezolizumab in Combination with Bevacizumab Enhances Antigen-Specific T-Cell Migration in Metastatic Renal Cell Carcinoma. Nat. Commun. 2016, 7, 12624. [Google Scholar] [CrossRef]

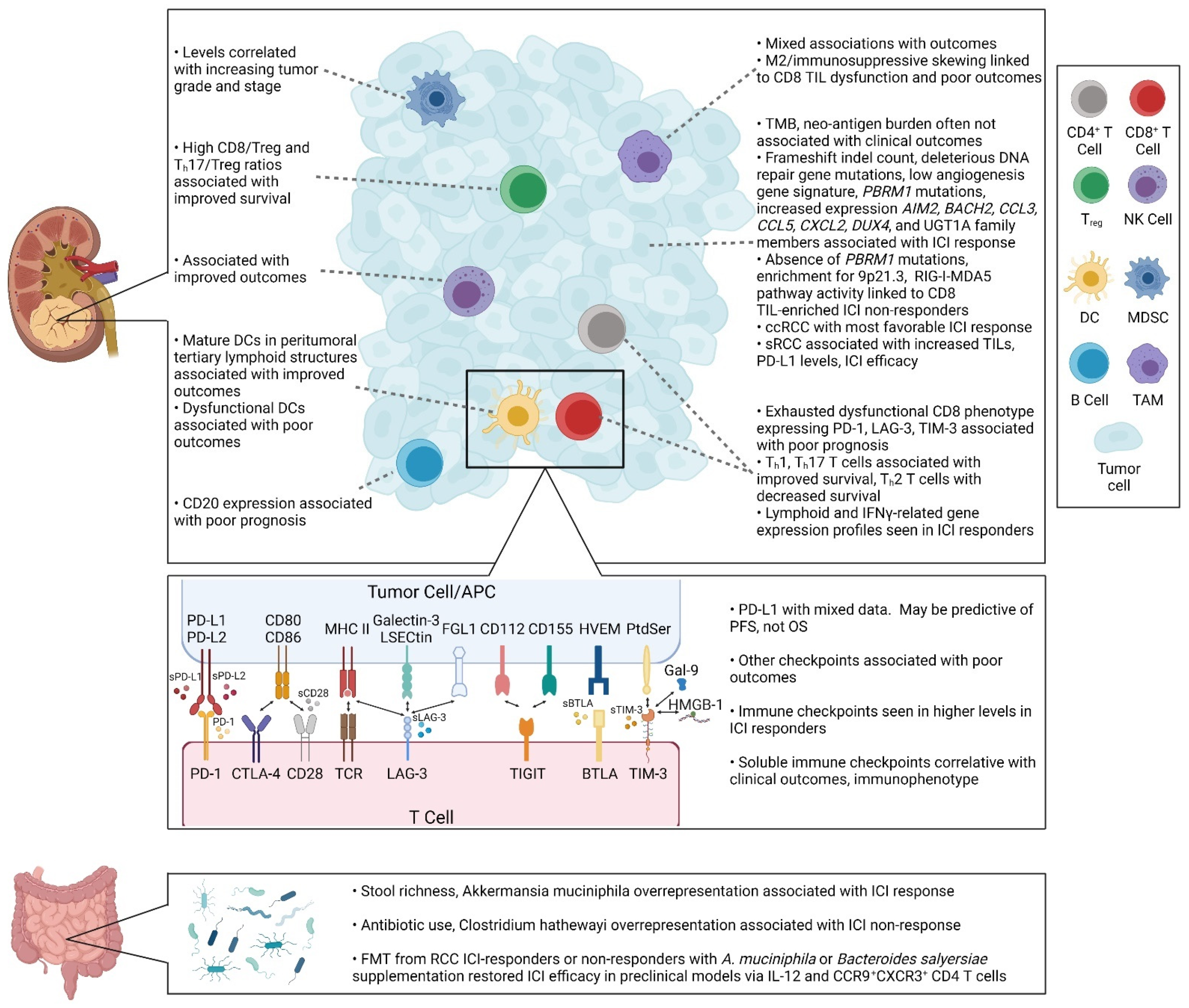
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anker, J.; Miller, J.; Taylor, N.; Kyprianou, N.; Tsao, C.-K. From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma. Cells 2021, 10, 3231. https://doi.org/10.3390/cells10113231
Anker J, Miller J, Taylor N, Kyprianou N, Tsao C-K. From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma. Cells. 2021; 10(11):3231. https://doi.org/10.3390/cells10113231
Chicago/Turabian StyleAnker, Jonathan, Justin Miller, Nicole Taylor, Natasha Kyprianou, and Che-Kai Tsao. 2021. "From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma" Cells 10, no. 11: 3231. https://doi.org/10.3390/cells10113231
APA StyleAnker, J., Miller, J., Taylor, N., Kyprianou, N., & Tsao, C.-K. (2021). From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma. Cells, 10(11), 3231. https://doi.org/10.3390/cells10113231







