Inducible Pluripotent Stem Cells as a Potential Cure for Diabetes
Abstract
:1. Insulin as a Treatment, Not a Cure
2. Novel Subcutaneous Insulin Delivery
3. Islets of Langerhans
3.1. Embryological Development and Structure
3.2. Function
4. Islet Cell Transplantation
Barriers to Islet Cell Transplant
5. The Promise and Future Challenges for Stem Cells
5.1. Stem Cell Source
5.2. Transplant Sites
5.3. Immunoreactivity
5.4. Scale out, Scale up, and Increased Culture Surface per Volume
5.4.1. Growth Medium
5.4.2. Extracellular Matrix
5.4.3. Environment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Williams, P. Notes on diabetes treated with extract and by grafts of sheep’s pancreas. BMJ 1894, 2, 1303. [Google Scholar]
- Banting, F.G. Nobel Lecture. Available online: https://www.nobelprize.org/prizes/medicine/1923/banting/lecture/ (accessed on 25 May 2020).
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Latres, E.; Finan, D.A.; Greenstein, J.L.; Kowalski, A.; Kieffer, T.J. Navigating Two Roads to Glucose Normalization in Diabetes: Automated Insulin Delivery Devices and Cell Therapy. Cell Metab. 2019, 29, 545–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am. J. Med. 1991, 90, 450–459. [Google Scholar] [CrossRef]
- Ruan, Y.; Thabit, H.; Leelarathna, L.; Hartnell, S.; Willinska, M.E.; Dellweg, S.; Benesch, C.; Mader, J.K.; Holzer, M.; Kojzar, H.; et al. Variability of Insulin Requirements Over 12 Weeks of Closed-Loop Insulin Delivery in Adults With Type 1 Diabetes. Diabetes Care 2016, 39, 830. [Google Scholar] [CrossRef] [Green Version]
- Pedersen-Bjergaard, U.; Pramming, S.; Heller, S.R.; Wallace, T.M.; Rasmussen, A.K.; Jorgensen, H.V.; Matthews, D.R.; Hougaard, P.; Thorsteinsson, B. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: Influence of risk markers and selection. Diabetes Metab Res. Rev. 2004, 20, 479–486. [Google Scholar] [CrossRef]
- Ter Braak, E.W.; Appelman, A.M.; van de Laak, M.; Stolk, R.P.; van Haeften, T.W.; Erkelens, D.W. Clinical characteristics of type 1 diabetic patients with and without severe hypoglycemia. Diabetes Care 2000, 23, 1467–1471. [Google Scholar] [CrossRef] [Green Version]
- Weinstock, R.S.; DuBose, S.N.; Bergenstal, R.M.; Chaytor, N.S.; Peterson, C.; Olson, B.A.; Munshi, M.N.; Perrin, A.J.; Miller, K.M.; Beck, R.W.; et al. Risk Factors Associated With Severe Hypoglycemia in Older Adults With Type 1 Diabetes. Diabetes Care 2016, 39, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.M.J. State of the Art of Clinical Islet Transplantation and Novel Protocols of Immunosuppression. Curr. Diabetes Rep. 2011, 11, 345. [Google Scholar] [CrossRef]
- Mullen, D.M.; Bergenstal, R.; Criego, A.; Arnold, K.C.; Goland, R.; Richter, S. Time Savings Using a Standardized Glucose Reporting System and Ambulatory Glucose Profile. J. Diabetes Sci. Technol. 2018, 12, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Schnell, O.; Barnard, K.; Bergenstal, R.; Bosi, E.; Garg, S.; Guerci, B.; Haak, T.; Hirsch, I.B.; Ji, L.; Joshi, S.R.; et al. Role of Continuous Glucose Monitoring in Clinical Trials: Recommendations on Reporting. Diabetes Technol. Ther. 2017, 19, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, H.C.; Brown, T.T.; Maruthur, N.; Ranasinghe, P.; Berger, Z.; Suh, Y.D.; Wilson, L.M.; Haberl, E.B.; Brick, J.; Bass, E.B.; et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Bekiari, E.; Kitsios, K.; Thabit, H.; Tauschmann, M.; Athanasiadou, E.; Karagiannis, T.; Haidich, A.-B.; Hovorka, R.; Tsapas, A. Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-analysis. BMJ 2018, 361, k1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: Cluster randomised trial (REPOSE). BMJ 2017, 356, j1285. [Google Scholar] [CrossRef] [Green Version]
- Schmid, V.; Hohberg, C.; Borchert, M.; Forst, T.; Pfützner, A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J. Diabetes Sci. Technol. 2010, 4, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Thethi, T.K.; Rao, A.; Kawji, H.; Mallik, T.; Yau, C.L.; Christians, U.; Fonseca, V. Consequences of delayed pump infusion line change in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion. J. Diabetes Complicat. 2010, 24, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Pickup, J.C.; Yemane, N.; Brackenridge, A.; Pender, S. Nonmetabolic complications of continuous subcutaneous insulin infusion: A patient survey. Diabetes Technol. Ther. 2014, 16, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, L.; Krinelke, L. Insulin infusion set: The Achilles heel of continuous subcutaneous insulin infusion. J. Diabetes Sci. Technol. 2012, 6, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.J.; Benasi, K.; Ferrari, G.; Evans, M.G.; Shanmugham, S.; Wilson, D.M.; Buckingham, B.A. Randomized trial of infusion set function: Steel versus teflon. Diabetes Technol. Ther. 2014, 16, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Mecklenburg, R.S.; Guinn, T.S.; Sannar, C.A.; Blumenstein, B.A. Malfunction of continuous subcutaneous insulin infusion systems: A one-year prospective study of 127 patients. Diabetes Care 1986, 9, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Peden, N.R.; Braaten, J.T.; McKendry, J.B. Diabetic ketoacidosis during long-term treatment with continuous subcutaneous insulin infusion. Diabetes Care 1984, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- McVey, E.; Keith, S.; Herr, J.K.; Sutter, D.; Pettis, R.J. Evaluation of Intradermal and Subcutaneous Infusion Set Performance Under 24-Hour Basal and Bolus Conditions. J. Diabetes Sci. Technol. 2015, 9, 1282–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanenbaum, M.L.; Hanes, S.J.; Miller, K.M.; Naranjo, D.; Bensen, R.; Hood, K.K. Diabetes Device Use in Adults With Type 1 Diabetes: Barriers to Uptake and Potential Intervention Targets. Diabetes Care 2017, 40, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Englert, K.; Ruedy, K.; Coffey, J.; Caswell, K.; Steffen, A.; Levandoski, L. Skin and adhesive issues with continuous glucose monitors: A sticky situation. J. Diabetes Sci. Technol. 2014, 8, 745–751. [Google Scholar] [CrossRef]
- Barnard, K.; Crabtree, V.; Adolfsson, P.; Davies, M.; Kerr, D.; Kraus, A.; Gianferante, D.; Bevilacqua, E.; Serbedzija, G. Impact of Type 1 Diabetes Technology on Family Members/Significant Others of People With Diabetes. J. Diabetes Sci. Technol. 2016, 10, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Ionescu-Tirgoviste, C.; Gagniuc, P.A.; Gubceac, E.; Mardare, L.; Popescu, I.; Dima, S.; Militaru, M. A 3D map of the islet routes throughout the healthy human pancreas. Sci. Rep. 2015, 5, 14634. [Google Scholar] [CrossRef] [Green Version]
- Rezania, A.; Bruin, J.E.; Riedel, M.J.; Mojibian, M.; Asadi, A.; Xu, J.; Gauvin, R.; Narayan, K.; Karanu, F.; O’Neil, J.J.; et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012, 61, 2016–2029. [Google Scholar] [CrossRef] [Green Version]
- Hogrebe, N.J.; Augsornworawat, P.; Maxwell, K.G.; Velazco-Cruz, L.; Millman, J.R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nature Biotechnol. 2020, 38, 460–470. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Shook, D.; Keller, R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech. Dev. 2003, 120, 1351–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Y.; Hu, X.; Ma, M.; Wang, X.; Liang, H.; Liu, D. Effect of Wnt Signaling on the Differentiation of Islet β-Cells from Adipose-Derived Stem Cells. Biomed. Res. Int. 2017, 2017, 2501578. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Vincent, S.D.; Dunn, N.R.; Hayashi, S.; Norris, D.P.; Robertson, E.J. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003, 17, 1646–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, J.; Lu, C.C.; Norris, D.P.; Rodriguez, T.A.; Beddington, R.S.; Robertson, E.J. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 2001, 411, 965–969. [Google Scholar] [CrossRef]
- Lowe, L.A.; Yamada, S.; Kuehn, M.R. Genetic dissection of nodal function in patterning the mouse embryo. Development 2001, 128, 1831. [Google Scholar]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef]
- de Caestecker, M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004, 15, 1–11. [Google Scholar] [CrossRef]
- Kubo, A.; Shinozaki, K.; Shannon, J.M.; Kouskoff, V.; Kennedy, M.; Woo, S.; Fehling, H.J.; Keller, G. Development of definitive endoderm from embryonic stem cells in culture. Development 2004, 131, 1651–1662. [Google Scholar] [CrossRef] [Green Version]
- Sui, L.; Leibel, R.L.; Egli, D. Pancreatic Beta Cell Differentiation From Human Pluripotent Stem Cells. Curr. Protoc. Hum. Genet. 2018, 99, e68. [Google Scholar] [CrossRef]
- Chen, J.K.; Taipale, J.; Young, K.E.; Maiti, T.; Beachy, P.A. Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA 2002, 99, 14071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mfopou, J.K.; Chen, B.; Mateizel, I.; Sermon, K.; Bouwens, L. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology 2010, 138, 2233–2245. [Google Scholar] [CrossRef]
- Hart, A.; Papadopoulou, S.; Edlund, H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev. Dyn. 2003, 228, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Johansson, K.A.; Dursun, U.; Jordan, N.; Gu, G.; Beermann, F.; Gradwohl, G.; Grapin-Botton, A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell 2007, 12, 457–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamidi, A.; Prawiro, C.; Seymour, P.A.; de Lichtenberg, K.H.; Jackson, A.; Serup, P.; Semb, H. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors. Nature 2018, 564, 114–118. [Google Scholar] [CrossRef]
- Yabe, S.G.; Fukuda, S.; Takeda, F.; Nashiro, K.; Shimoda, M.; Okochi, H. Efficient generation of functional pancreatic β-cells from human induced pluripotent stem cells. J. Diabetes 2017, 9, 168–179. [Google Scholar] [CrossRef]
- Rukstalis, J.M.; Habener, J.F. Neurogenin3: A master regulator of pancreatic islet differentiation and regeneration. Islets 2009, 1, 177–184. [Google Scholar] [CrossRef]
- Suzuki, T.; Dai, P.; Hatakeyama, T.; Harada, Y.; Tanaka, H.; Yoshimura, N.; Takamatsu, T. TGF-β Signaling Regulates Pancreatic β-Cell Proliferation through Control of Cell Cycle Regulator p27 Expression. Acta Histochem. Cytochem. 2013, 46, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Borowiak, M.; Fox, J.L.; Maehr, R.; Osafune, K.; Davidow, L.; Lam, K.; Peng, L.F.; Schreiber, S.L.; Rubin, L.L.; et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 2009, 5, 258–265. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Xu, J.; Narayan, K.; Fox, J.K.; O’Neil, J.J.; Kieffer, T.J. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. STEM CELLS 2013, 31, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Mazzucato, C.; Zavacki, A.M.; Marinelarena, A.; Hollister-Lock, J.; El Khattabi, I.; Marsili, A.; Weir, G.C.; Sharma, A.; Larsen, P.R.; Bonner-Weir, S. Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 2013, 62, 1569–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorens, B. Neural regulation of pancreatic islet cell mass and function. Diabetes Obes. Metab. 2014, 16, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Gilon, P.; Henquin, J.C. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr. Rev. 2001, 22, 565–604. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Takei, M.; Ishii, H.; Sato, Y. Glucose-stimulated insulin secretion: A newer perspective. J. Diabetes Investig. 2013, 4, 511–516. [Google Scholar] [CrossRef]
- Seino, S.; Shibasaki, T. PKA-Dependent and PKA-Independent Pathways for cAMP-Regulated Exocytosis. Physiol. Rev. 2005, 85, 1303–1342. [Google Scholar] [CrossRef]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef]
- Capozzi, M.E.; Svendsen, B.; Encisco, S.E.; Lewandowski, S.L.; Martin, M.D.; Lin, H.; Jaffe, J.L.; Coch, R.W.; Haldeman, J.M.; MacDonald, P.E.; et al. beta Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Li, N.; Yang, Z.; Li, Q.; Yu, Z.; Chen, X.; Li, J.-C.; Li, B.; Ning, S.-L.; Cui, M.; Sun, J.-P.; et al. Ablation of somatostatin cells leads to impaired pancreatic islet function and neonatal death in rodents. Cell Death Dis. 2018, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.; Vijayasarathy, K.; Talukdar, R.; Sasikala, M.; Nageshwar Reddy, D. Reduced pancreatic polypeptide response is associated with early alteration of glycemic control in chronic pancreatitis. Diabetes Res. Clin. Pract. 2020, 160, 107993. [Google Scholar] [CrossRef]
- Rabiee, A.; Galiatsatos, P.; Salas-Carrillo, R.; Thompson, M.J.; Andersen, D.K.; Elahi, D. Pancreatic polypeptide administration enhances insulin sensitivity and reduces the insulin requirement of patients on insulin pump therapy. J. Diabetes Sci. Technol. 2011, 5, 1521–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadheech, N.; James Shapiro, A.M. Human Induced Pluripotent Stem Cells in the Curative Treatment of Diabetes and Potential Impediments Ahead. Adv. Exp. Med. Biol 2019, 1144, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Lakey, J.R.T.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Bruni, A.; Shapiro, A.M.J. Clinical islet transplantation: Is the future finally now? Curr. Opin. Organ. Transplant. 2018, 23, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Pokrywczynska, M.; Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Holmes-Walker, D.J.; Gunton, J.E.; Hawthorne, W.; Payk, M.; Anderson, P.; Donath, S.; Loudovaris, T.; Ward, G.M.; Kay, T.W.; O’Connell, P.J. Islet Transplantation Provides Superior Glycemic Control With Less Hypoglycemia Compared With Continuous Subcutaneous Insulin Infusion or Multiple Daily Insulin Injections. Transplantation 2017, 101, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G.; et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.M.; Meloche, M.; Ao, Z.; Paty, B.; Keown, P.; Shapiro, R.J.; Ho, S.; Worsley, D.; Fung, M.; Meneilly, G.; et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation 2011, 91, 373–378. [Google Scholar] [CrossRef]
- Warnock, G.L.; Thompson, D.M.; Meloche, R.M.; Shapiro, R.J.; Ao, Z.; Keown, P.; Johnson, J.D.; Verchere, C.B.; Partovi, N.; Begg, I.S.; et al. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation 2008, 86, 1762–1766. [Google Scholar] [CrossRef]
- Venturini, M.; Fiorina, P.; Maffi, P.; Losio, C.; Vergani, A.; Secchi, A.; Del Maschio, A. Early increase of retinal arterial and venous blood flow velocities at color Doppler imaging in brittle type 1 diabetes after islet transplant alone. Transplantation 2006, 81, 1274–1277. [Google Scholar] [CrossRef]
- Del Carro, U.; Fiorina, P.; Amadio, S.; De Toni Franceschini, L.; Petrelli, A.; Menini, S.; Martinelli Boneschi, F.; Ferrari, S.; Pugliese, G.; Maffi, P.; et al. Evaluation of polyneuropathy markers in type 1 diabetic kidney transplant patients and effects of islet transplantation: Neurophysiological and skin biopsy longitudinal analysis. Diabetes Care 2007, 30, 3063–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Horiguchi, A.; Ito, M.; Nagata, H.; Ichii, H.; Ricordi, C.; Miyakawa, S. Quality control for clinical islet transplantation: Organ procurement and preservation, the islet processing facility, isolation, and potency tests. J. Hepatobiliary Pancreat Surg. 2009, 16, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ricordi, C.; Goldstein, J.S.; Balamurugan, A.N.; Szot, G.L.; Kin, T.; Liu, C.; Czarniecki, C.W.; Barbaro, B.; Bridges, N.D.; Cano, J.; et al. National Institutes of Health–Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities. Diabetes 2016, 65, 3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovitch, A.; Suarez-Pinzon, W.L.; Strynadka, K.; Schulz, R.; Lakey, J.R.; Warnock, G.L.; Rajotte, R.V. Human pancreatic islet beta-cell destruction by cytokines is independent of nitric oxide production. J. Clin. Endocrinol. Metab. 1994, 79, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.O.; Fraga, D.W.; Callicutt, C.S.; Gerling, I.C.; Sabek, O.M.; Kotb, M.Y. Improved in vivo pancreatic islet function after prolonged in vitro islet culture. Transplantation 2001, 72, 1730–1736. [Google Scholar] [CrossRef]
- Berney, T. Islet culture and counter-culture. Transplant. Int. 2009, 22, 531–533. [Google Scholar] [CrossRef]
- Hering, B.J.; Kandaswamy, R.; Harmon, J.V.; Ansite, J.D.; Clemmings, S.M.; Sakai, T.; Paraskevas, S.; Eckman, P.M.; Sageshima, J.; Nakano, M.; et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2004, 4, 390–401. [Google Scholar] [CrossRef]
- Bellin, M.D.; Barton, F.B.; Heitman, A.; Harmon, J.V.; Kandaswamy, R.; Balamurugan, A.N.; Sutherland, D.E.; Alejandro, R.; Hering, B.J. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2012, 12, 1576–1583. [Google Scholar] [CrossRef] [Green Version]
- Naziruddin, B.; Kanak, M.A.; Chang, C.A.; Takita, M.; Lawrence, M.C.; Dennison, A.R.; Onaca, N.; Levy, M.F. Improved outcomes of islet autotransplant after total pancreatectomy by combined blockade of IL-1β and TNFα. Am. J. Transplant. 2018, 18, 2322–2329. [Google Scholar] [CrossRef] [Green Version]
- Rabinovitch, A.; Baquerizo, H.; Sumoski, W. Cytotoxic effects of cytokines on islet beta-cells: Evidence for involvement of eicosanoids. Endocrinology 1990, 126, 67–71. [Google Scholar] [CrossRef]
- McCall, M.; Pawlick, R.; Kin, T.; Shapiro, A.M. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2012, 12, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Bruni, A.; Pepper, A.R.; Gala-Lopez, B.; Pawlick, R.; Abualhassan, N.; Crapo, J.D.; Piganelli, J.D.; Shapiro, A.M. A novel redox-active metalloporphyrin reduces reactive oxygen species and inflammatory markers but does not improve marginal mass engraftment in a murine donation after circulatory death islet transplantation model. Islets 2016, 8, e1190058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, A.; Pepper, A.R.; Pawlick, R.L.; Gala-Lopez, B.; Gamble, A.; Kin, T.; Malcolm, A.J.; Jones, C.; Piganelli, J.D.; Crapo, J.D.; et al. BMX-001, a novel redox-active metalloporphyrin, improves islet function and engraftment in a murine transplant model. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2018, 18, 1879–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; Senior, P.; Salam, A.; Kin, T.; Imes, S.; Dinyari, P.; Malcolm, A.; Toso, C.; Nilsson, B.; Korsgren, O.; et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation 2010, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Lukinius, A.; Moberg, L.; Lundgren, T.; Berne, C.; Foss, A.; Felldin, M.; Källen, R.; Salmela, K.; Tibell, A.; et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 2005, 54, 1755–1762. [Google Scholar] [CrossRef] [Green Version]
- Toso, C.; McCall, M.; Emamaullee, J.; Merani, S.; Davis, J.; Edgar, R.; Pawlick, R.; Kin, T.; Knudsen, L.B.; Shapiro, A.M. Liraglutide, a long-acting human glucagon-like peptide 1 analogue, improves human islet survival in culture. Transpl. Int. 2010, 23, 259–265. [Google Scholar] [CrossRef]
- McCall, M.; Toso, C.; Emamaullee, J.; Pawlick, R.; Edgar, R.; Davis, J.; Maciver, A.; Kin, T.; Arch, R.; Shapiro, A.M. The caspase inhibitor IDN-6556 (PF3491390) improves marginal mass engraftment after islet transplantation in mice. Surgery 2011, 150, 48–55. [Google Scholar] [CrossRef]
- Merani, S.; Truong, W.; Emamaullee, J.A.; Toso, C.; Knudsen, L.B.; Shapiro, A.M. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology 2008, 149, 4322–4328. [Google Scholar] [CrossRef]
- Emamaullee, J.A.; Davis, J.; Pawlick, R.; Toso, C.; Merani, S.; Cai, S.X.; Tseng, B.; Shapiro, A.M. The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes 2008, 57, 1556–1566. [Google Scholar] [CrossRef] [Green Version]
- Krzystyniak, A.; Gołąb, K.; Witkowski, P.; Trzonkowski, P. Islet cell transplant and the incorporation of Tregs. Curr. Opin. Organ Transplant. 2014, 19, 610–615. [Google Scholar] [CrossRef]
- Lee, K.; Nguyen, V.; Lee, K.M.; Kang, S.M.; Tang, Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2014, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J. Islet transplantation in type 1 diabetes: Ongoing challenges, refined procedures, and long-term outcome. Rev. Diabet Stud. 2012, 9, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Shapiro, A.M.J. Risks and side effects of islet transplantation. Curr. Diabetes Rep. 2004, 4, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Raval, M.; Lam, A.; Cervera, C.; Senior, P.; Shapiro, J.; Kabbani, D. 1093. Infectious Complications after Pancreatic Islet Transplantation. Open Forum Infect. Dis. 2020, 7, S576. [Google Scholar] [CrossRef]
- Borda, B.; Lengyel, C.; Várkonyi, T.; Kemény, E.; Ottlakán, A.; Kubik, A.; Keresztes, C.; Lázár, G. Side effects of the calcineurin inhibitor, such as new-onset diabetes after kidney transplantation. Acta Physiol. Hung. 2014, 101, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef]
- Collaborative Islet Transplant Registry. CITR 9th Annual Report Chapter 7 Adverse Events; The Emmes Corporation: Rockville, MD, USA, 2015. [Google Scholar]
- Korsgren, O.; Lundgren, T.; Felldin, M.; Foss, A.; Isaksson, B.; Permert, J.; Persson, N.H.; Rafael, E.; Rydén, M.; Salmela, K.; et al. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia 2008, 51, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, B.; Ekdahl, K.N.; Korsgren, O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr. Opin. Organ. Transplant. 2011, 16, 620–629. [Google Scholar] [CrossRef]
- Fuenmayor, V.; Chavez, C.; Baidal, D.; Alvarez, A.M.; Padilla, N.; Ricordi, C.; Alejandro, R. 118-OR: HLA Matching and Clinical Outcomes in Islet Transplantation. Diabetes 2020, 69, 118-OR. [Google Scholar] [CrossRef]
- Henry, R.R.; Pettus, J.; Wilensky, J.O.N.; Shapiro, A.M.J.; Senior, P.A.; Roep, B.; Wang, R.; Kroon, E.J.; Scott, M.; Amour, K.; et al. Initial Clinical Evaluation of VC-01TM Combination Product—A Stem Cell–Derived Islet Replacement for Type 1 Diabetes (T1D). Diabetes 2018, 67, 138-OR. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Ismail, A.M.; Khater, S.M.; Ashamallah, S.A.; Azzam, M.M.; Ghoneim, M.A. Insulin-producing Cells from Adult Human Bone Marrow Mesenchymal Stromal Cells Could Control Chemically Induced Diabetes in Dogs: A Preliminary Study. Cell Transplant. 2018, 27, 937–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruin, J.E.; Rezania, A.; Xu, J.; Narayan, K.; Fox, J.K.; O’Neil, J.J.; Kieffer, T.J. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 2013, 56, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- Kenneth Ward, W. A review of the foreign-body response to subcutaneously-implanted devices: The role of macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2008, 2, 768–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Volpatti, L.R.; Thiono, D.; Yesilyurt, V.; McGladrigan, C.; Tang, Y.; Facklam, A.; Wang, A.; Jhunjhunwala, S.; Veiseh, O.; et al. A retrievable implant for the long-term encapsulation and survival of therapeutic xenogeneic cells. Nat. Biomed. Eng. 2020, 4, 814–826. [Google Scholar] [CrossRef]
- Vegas, A.J.; Veiseh, O.; Gürtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016, 22, 306–311. [Google Scholar] [CrossRef]
- Bochenek, M.A.; Veiseh, O.; Vegas, A.J.; McGarrigle, J.J.; Qi, M.; Marchese, E.; Omami, M.; Doloff, J.C.; Mendoza-Elias, J.; Nourmohammadzadeh, M.; et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2018, 2, 810–821. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013, 494, 100–104. [Google Scholar] [CrossRef]
- Guha, P.; Morgan, J.W.; Mostoslavsky, G.; Rodrigues, N.P.; Boyd, A.S. Lack of Immune Response to Differentiated Cells Derived from Syngeneic Induced Pluripotent Stem Cells. Cell Stem Cell 2013, 12, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, S.; Yamanaka, S. To Be Immunogenic, or Not to Be: That’s the iPSC Question. Cell Stem Cell 2013, 12, 385–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldner, F.; Jaenisch, R. Medicine. iPSC disease modeling. Science 2012, 338, 1155–1156. [Google Scholar] [CrossRef]
- Hockemeyer, D.; Jaenisch, R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell 2016, 18, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, P.J.; Cowan, P.J.; Hawthorne, W.J.; Yi, S.; Lew, A.M. Transplantation of xenogeneic islets: Are we there yet? Curr. Diab. Rep. 2013, 13, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Bottino, R.; Wijkstrom, M.; van der Windt, D.J.; Hara, H.; Ezzelarab, M.; Murase, N.; Bertera, S.; He, J.; Phelps, C.; Ayares, D.; et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2014, 14, 2275–2287. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.S.; Kim, J.M.; Kim, J.S.; Min, B.H.; Kim, Y.H.; Kim, H.J.; Jang, J.Y.; Yoon, I.H.; Kang, H.J.; Kim, J.; et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2015, 15, 2837–2850. [Google Scholar] [CrossRef]
- Dufrane, D.; Goebbels, R.M.; Gianello, P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation 2010, 90, 1054–1062. [Google Scholar] [CrossRef]
- Yang, L.; Güell, M.; Niu, D.; George, H.; Lesha, E.; Grishin, D.; Aach, J.; Shrock, E.; Xu, W.; Poci, J.; et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 2015, 350, 1101–1104. [Google Scholar] [CrossRef] [Green Version]
- Anazawa, T.; Okajima, H.; Masui, T.; Uemoto, S. Current state and future evolution of pancreatic islet transplantation. Ann. Gastroenterol. Surg. 2019, 3, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.; Tan, P.; Baker, J.; Durbin, K.; Tomiya, M.; Azuma, K.; Doi, M.; Elliott, R.B. Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant. Proc. 2014, 46, 1992–1995. [Google Scholar] [CrossRef]
- Matsumoto, S.; Abalovich, A.; Wechsler, C.; Wynyard, S.; Elliott, R.B. Clinical Benefit of Islet Xenotransplantation for the Treatment of Type 1 Diabetes. EBioMedicine 2016, 12, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepper, A.R.; Pawlick, R.; Bruni, A.; Wink, J.; Rafiei, Y.; O’Gorman, D.; Yan-Do, R.; Gala-Lopez, B.; Kin, T.; MacDonald, P.E.; et al. Transplantation of Human Pancreatic Endoderm Cells Reverses Diabetes Post Transplantation in a Prevascularized Subcutaneous Site. Stem Cell Rep. 2017, 8, 1689–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-I.; Yu, J.E.; Park, C.-G.; Kim, S.-J. Comparison of four pancreatic islet implantation sites. J. Korean Med. Sci. 2010, 25, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindal, R.M.; Sidner, R.A.; McDaniel, H.B.; Johnson, M.S.; Fineberg, S.E. Intraportal vs kidney subcapsular site for human pancreatic islet transplantation. Transplant. Proc. 1998, 30, 398–399. [Google Scholar] [CrossRef]
- Rajab, A.; Buss, J.; Diakoff, E.; Hadley, G.A.; Osei, K.; Ferguson, R.M. Comparison of the portal vein and kidney subcapsule as sites for primate islet autotransplantation. Cell Transplant. 2008, 17, 1015–1023. [Google Scholar] [CrossRef]
- Stice, M.J.; Dunn, T.B.; Bellin, M.D.; Skube, M.E.; Beilman, G.J. Omental Pouch Technique for Combined Site Islet Autotransplantation Following Total Pancreatectomy. Cell Transplant. 2018, 27, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Baidal, D.A.; Ricordi, C.; Berman, D.M.; Alvarez, A.; Padilla, N.; Ciancio, G.; Linetsky, E.; Pileggi, A.; Alejandro, R. Bioengineering of an Intraabdominal Endocrine Pancreas. N. Engl. J. Med. 2017, 376, 1887–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucher, P.; Mathe, Z.; Bosco, D.; Becker, C.; Kessler, L.; Greget, M.; Benhamou, P.Y.; Andres, A.; Oberholzer, J.; Buhler, L.; et al. Morbidity associated with intraportal islet transplantation. Transplant. Proc. 2004, 36, 1119–1120. [Google Scholar] [CrossRef]
- Villiger, P.; Ryan, E.A.; Owen, R.; O’Kelly, K.; Oberholzer, J.; Al Saif, F.; Kin, T.; Wang, H.; Larsen, I.; Blitz, S.L.; et al. Prevention of bleeding after islet transplantation: Lessons learned from a multivariate analysis of 132 cases at a single institution. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2005, 5, 2992–2998. [Google Scholar] [CrossRef]
- Liang, Q.; Monetti, C.; Shutova, M.V.; Neely, E.J.; Hacibekiroglu, S.; Yang, H.; Kim, C.; Zhang, P.; Li, C.; Nagy, K.; et al. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety. Nature 2018, 563, 701–704. [Google Scholar] [CrossRef]
- Di Stasi, A.; Tey, S.K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Chen, S.; Li, X.; Qin, L.; Huang, K.; Wang, L.; Huang, W.; Li, S.; Jia, B.; Zhong, M.; et al. Low Immunogenicity of Neural Progenitor Cells Differentiated from Induced Pluripotent Stem Cells Derived from Less Immunogenic Somatic Cells. PLoS ONE 2013, 8, e69617. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Muffat, J.; Cheng, A.W.; Orlando, D.A.; Lovén, J.; Kwok, S.M.; Feldman, D.A.; Bateup, H.S.; Gao, Q.; et al. Global transcriptional and translational repression in human-embryonic-stem-cell-derived Rett syndrome neurons. Cell Stem Cell 2013, 13, 446–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Wang, J.; Beyer, A.I.; Teque, F.; Cradick, T.J.; Qi, Z.; Chang, J.C.; Bao, G.; Muench, M.O.; Yu, J.; et al. Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Δ32 mutation confers resistance to HIV infection. Proc. Natl. Acad. Sci. USA 2014, 111, 9591–9596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhardt, P.; Schmid, B.; Burbulla, L.F.; Schöndorf, D.C.; Wagner, L.; Glatza, M.; Höing, S.; Hargus, G.; Heck, S.A.; Dhingra, A.; et al. Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell 2013, 12, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Schwank, G.; Koo, B.K.; Sasselli, V.; Dekkers, J.F.; Heo, I.; Demircan, T.; Sasaki, N.; Boymans, S.; Cuppen, E.; van der Ent, C.K.; et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013, 13, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Maetzel, D.; Sarkar, S.; Wang, H.; Abi-Mosleh, L.; Xu, P.; Cheng, A.W.; Gao, Q.; Mitalipova, M.; Jaenisch, R. Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick Type C patient-specific iPS cells. Stem Cell Reports 2014, 2, 866–880. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.R.; Tang, Z.H.; Zheng, J.; Shi, H.S.; Ding, J.; Qian, X.D.; Zhang, C.; Chen, J.L.; Wang, C.C.; Li, L.; et al. Effects of genetic correction on the differentiation of hair cell-like cells from iPSCs with MYO15A mutation. Cell Death Differ. 2016, 23, 1347–1357. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, S.; Sato, M.; Loisel-Meyer, S.; Matsuda, Y.; Oishi, H.; Guan, Z.; Saito, T.; Yeung, J.; Cypel, M.; Hwang, D.M.; et al. Lentivirus IL-10 gene therapy down-regulates IL-17 and attenuates mouse orthotopic lung allograft rejection. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2013, 13, 1586–1593. [Google Scholar] [CrossRef]
- Parker, D.G.; Coster, D.J.; Brereton, H.M.; Hart, P.H.; Koldej, R.; Anson, D.S.; Williams, K.A. Lentivirus-mediated gene transfer of interleukin 10 to the ovine and human cornea. Clin. Exp. Ophthalmol. 2010, 38, 405–413. [Google Scholar] [CrossRef]
- Niu, J.; Yue, W.; Song, Y.; Zhang, Y.; Qi, X.; Wang, Z.; Liu, B.; Shen, H.; Hu, X. Prevention of acute liver allograft rejection by IL-10-engineered mesenchymal stem cells. Clin. Exp. Immunol. 2014, 176, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Karabekian, Z.; Ding, H.; Stybayeva, G.; Ivanova, I.; Muselimyan, N.; Haque, A.; Toma, I.; Posnack, N.G.; Revzin, A.; Leitenberg, D.; et al. HLA Class I Depleted hESC as a Source of Hypoimmunogenic Cells for Tissue Engineering Applications. Tissue Eng. Part. A 2015, 21, 2559–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riolobos, L.; Hirata, R.K.; Turtle, C.J.; Wang, P.-R.; Gornalusse, G.G.; Zavajlevski, M.; Riddell, S.R.; Russell, D.W. HLA engineering of human pluripotent stem cells. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 1232–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Wang, M.; Duan, S.; Franco, P.J.; Kenty, J.H.; Hedrick, P.; Xia, Y.; Allen, A.; Ferreira, L.M.R.; Strominger, J.L.; et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2019, 116, 10441–10446. [Google Scholar] [CrossRef] [Green Version]
- Nitta, Y.; Tashiro, F.; Tokui, M.; Shimada, A.; Takei, I.; Tabayashi, K.; Miyazaki, J. Systemic delivery of interleukin 10 by intramuscular injection of expression plasmid DNA prevents autoimmune diabetes in nonobese diabetic mice. Hum. Gene Ther. 1998, 9, 1701–1707. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Pileggi, A.; Agarwal, A.; Molano, R.D.; Powers, M.; Brusko, T.; Wasserfall, C.; Goudy, K.; Zahr, E.; Poggioli, R.; et al. Adeno-Associated Virus-Mediated IL-10 Gene Therapy Inhibits Diabetes Recurrence in Syngeneic Islet Cell Transplantation of NOD Mice. Diabetes 2003, 52, 708. [Google Scholar] [CrossRef] [Green Version]
- Goudy, K.S.; Burkhardt, B.R.; Wasserfall, C.; Song, S.; Campbell-Thompson, M.L.; Brusko, T.; Powers, M.A.; Clare-Salzler, M.J.; Sobel, E.S.; Ellis, T.M.; et al. Systemic overexpression of IL-10 induces CD4+CD25+ cell populations in vivo and ameliorates type 1 diabetes in nonobese diabetic mice in a dose-dependent fashion. J. Immunol. 2003, 171, 2270–2278. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Zhu, W.; Li, T.; Li, X.; Cheng, J.; Li, C.; Yi, P.; Liu, L. Interleukin-10 gene transfer into insulin-producing β cells protects against diabetes in non-obese diabetic mice. Mol. Med. Rep. 2015, 12, 3881–3889. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yoshida, T.; Nakaki, F.; Hiai, H.; Okazaki, T.; Honjo, T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc. Natl. Acad. Sci. USA 2005, 102, 11823–11828. [Google Scholar] [CrossRef] [Green Version]
- Fife, B.T.; Guleria, I.; Gubbels Bupp, M.; Eagar, T.N.; Tang, Q.; Bour-Jordan, H.; Yagita, H.; Azuma, M.; Sayegh, M.H.; Bluestone, J.A. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 2006, 203, 2737–2747. [Google Scholar] [CrossRef]
- Paterson, A.M.; Brown, K.E.; Keir, M.E.; Vanguri, V.K.; Riella, L.V.; Chandraker, A.; Sayegh, M.H.; Blazar, B.R.; Freeman, G.J.; Sharpe, A.H. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J. Immunol. 2011, 187, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Fousteri, G. Role of the PD-1/PD-L1 Dyad in the Maintenance of Pancreatic Immune Tolerance for Prevention of Type 1 Diabetes. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Tejon, G.; Fuentes, C.; Hidalgo, Y.; Bono, M.R.; Maldonado, P.; Fernandez, R.; Wood, K.J.; Fierro, J.A.; Rosemblatt, M.; et al. Alloreactive regulatory T cells generated with retinoic acid prevent skin allograft rejection. Eur. J. Immunol. 2015, 45, 452–463. [Google Scholar] [CrossRef]
- Cheraï, M.; Hamel, Y.; Baillou, C.; Touil, S.; Guillot-Delost, M.; Charlotte, F.; Kossir, L.; Simonin, G.; Maury, S.; Cohen, J.L.; et al. Generation of Human Alloantigen-Specific Regulatory T Cells Under Good Manufacturing Practice-Compliant Conditions for Cell Therapy. Cell Transplant. 2015, 24, 2527–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voltarelli, J.C.; Couri, C.E.; Stracieri, A.B.; Oliveira, M.C.; Moraes, D.A.; Pieroni, F.; Coutinho, M.; Malmegrim, K.C.; Foss-Freitas, M.C.; Simões, B.P.; et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. Jama 2007, 297, 1568–1576. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, M.; Kumar, A.; Bhonde, R.R. Reversal of experimental diabetes by multiple bone marrow transplantation. Biochem. Biophys. Res. Commun. 2005, 328, 318–325. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Y.C.; Wolfe, S.; Valencia, V.; Qian, K.; Shen, L.; Tang, Y.L.; Hsu, W.H.; Atkinson, M.A.; Phillips, M.I. Combinatorial treatment of bone marrow stem cells and stromal cell-derived factor 1 improves glycemia and insulin production in diabetic mice. Mol. Cell. Endocrinol. 2011, 345, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Izumida, Y.; Aoki, T.; Yasuda, D.; Koizumi, T.; Suganuma, C.; Saito, K.; Murai, N.; Shimizu, Y.; Hayashi, K.; Odaira, M.; et al. Hepatocyte growth factor is constitutively produced by donor-derived bone marrow cells and promotes regeneration of pancreatic beta-cells. Biochem. Biophys. Res. Commun. 2005, 333, 273–282. [Google Scholar] [CrossRef]
- Li, F.X.; Zhu, J.W.; Tessem, J.S.; Beilke, J.; Varella-Garcia, M.; Jensen, J.; Hogan, C.J.; DeGregori, J. The development of diabetes in E2f1/E2f2 mutant mice reveals important roles for bone marrow-derived cells in preventing islet cell loss. Proc. Natl. Acad. Sci. USA 2003, 100, 12935–12940. [Google Scholar] [CrossRef] [Green Version]
- Than, S.; Ishida, H.; Inaba, M.; Fukuba, Y.; Seino, Y.; Adachi, M.; Imura, H.; Ikehara, S. Bone marrow transplantation as a strategy for treatment of non-insulin-dependent diabetes mellitus in KK-Ay mice. J. Exp. Med. 1992, 176, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, Y.; Ogihara, T.; Yamada, T.; Ishigaki, Y.; Imai, J.; Uno, K.; Gao, J.; Kaneko, K.; Ishihara, H.; Sasano, H.; et al. Bone Marrow (BM) Transplantation Promotes β-Cell Regeneration after Acute Injury through BM Cell Mobilization. Endocrinology 2007, 148, 2006–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couri, C.E.; Oliveira, M.C.; Stracieri, A.B.; Moraes, D.A.; Pieroni, F.; Barros, G.M.; Madeira, M.I.; Malmegrim, K.C.; Foss-Freitas, M.C.; Simões, B.P.; et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. Jama 2009, 301, 1573–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmegrim, K.C.; de Azevedo, J.T.; Arruda, L.C.; Abreu, J.R.; Couri, C.E.; de Oliveira, G.L.; Palma, P.V.; Scortegagna, G.T.; Stracieri, A.B.; Moraes, D.A.; et al. Immunological Balance Is Associated with Clinical Outcome after Autologous Hematopoietic Stem Cell Transplantation in Type 1 Diabetes. Front. Immunol. 2017, 8, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef]
- Wang, X.; Rivière, I. Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Mol. Ther. Oncolytics 2016, 3, 16015. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Mei, Y.; Cai, D.; Han, W. Standardizing CAR-T therapy: Getting it scaled up. Biotechnol. Adv. 2019, 37, 239–245. [Google Scholar] [CrossRef]
- Lambrechts, T. Bioreactors and Process Monitoring for Scale-Up of Stem Cell Production; KU Leuven: Kasteelpark, Arenberg, 2016. [Google Scholar]
- Nam, S.; Smith, J.; Yang, G. Driving the next wave of innovation in CAR T-cell therapies. McKinsey and Company. 2019. Available online: https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/driving-the-next-wave-of-innovation-in-car-t-cell-therapies (accessed on 25 May 2020).
- Ichii, H.; Sakuma, Y.; Pileggi, A.; Fraker, C.; Alvarez, A.; Montelongo, J.; Szust, J.; Khan, A.; Inverardi, L.; Naziruddin, B.; et al. Shipment of human islets for transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2007, 7, 1010–1020. [Google Scholar] [CrossRef]
- Goss, J.A.; Goodpastor, S.E.; Brunicardi, F.C.; Barth, M.H.; Soltes, G.D.; Garber, A.J.; Hamilton, D.J.; Alejandro, R.; Ricordi, C. Development of a human pancreatic islet-transplant program through a collaborative relationship with a remote islet-isolation center. Transplantation 2004, 77, 462–466. [Google Scholar] [CrossRef]
- Goss, J.A.; Schock, A.P.; Brunicardi, F.C.; Goodpastor, S.E.; Garber, A.J.; Soltes, G.; Barth, M.; Froud, T.; Alejandro, R.; Ricordi, C. Achievement of insulin independence in three consecutive type-1 diabetic patients via pancreatic islet transplantation using islets isolated at a remote islet isolation center. Transplantation 2002, 74, 1761–1766. [Google Scholar] [CrossRef]
- Simaria, A.S.; Hassan, S.; Varadaraju, H.; Rowley, J.; Warren, K.; Vanek, P.; Farid, S.S. Allogeneic cell therapy bioprocess economics and optimization: Single-use cell expansion technologies. Biotechnol. Bioeng. 2014, 111, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-H.; Ramachandran, K.; Stehno-Bittel, L. A replacement for islet equivalents with improved reliability and validity. Acta Diabetol. 2013, 50, 687–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.G.; Robey, P.G. Human pluripotent stem cell culture: Considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Diaz, L.G.; Ross, A.M.; Lahann, J.; Krebsbach, P.H. Concise review: The evolution of human pluripotent stem cell culture: From feeder cells to synthetic coatings. Stem Cells 2013, 31, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodin, S.; Domogatskaya, A.; Ström, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Futaki, S.; Hasegawa, K.; Kawasaki, M.; Sanzen, N.; Hayashi, M.; Kawase, E.; Sekiguchi, K.; Nakatsuji, N.; Suemori, H. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 375, 27–32. [Google Scholar] [CrossRef]
- Irwin, E.F.; Gupta, R.; Dashti, D.C.; Healy, K.E. Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials 2011, 32, 6912–6919. [Google Scholar] [CrossRef] [Green Version]
- Steiner, D.; Khaner, H.; Cohen, M.; Even-Ram, S.; Gil, Y.; Itsykson, P.; Turetsky, T.; Idelson, M.; Aizenman, E.; Ram, R.; et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nature Biotechnol. 2010, 28, 361–364. [Google Scholar] [CrossRef]
- Singh, H.; Mok, P.; Balakrishnan, T.; Rahmat, S.N.B.; Zweigerdt, R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res. 2010, 4, 165–179. [Google Scholar] [CrossRef] [Green Version]
- Olmer, R.; Haase, A.; Merkert, S.; Cui, W.; Paleček, J.; Ran, C.; Kirschning, A.; Scheper, T.; Glage, S.; Miller, K.; et al. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010, 5, 51–64. [Google Scholar] [CrossRef] [Green Version]
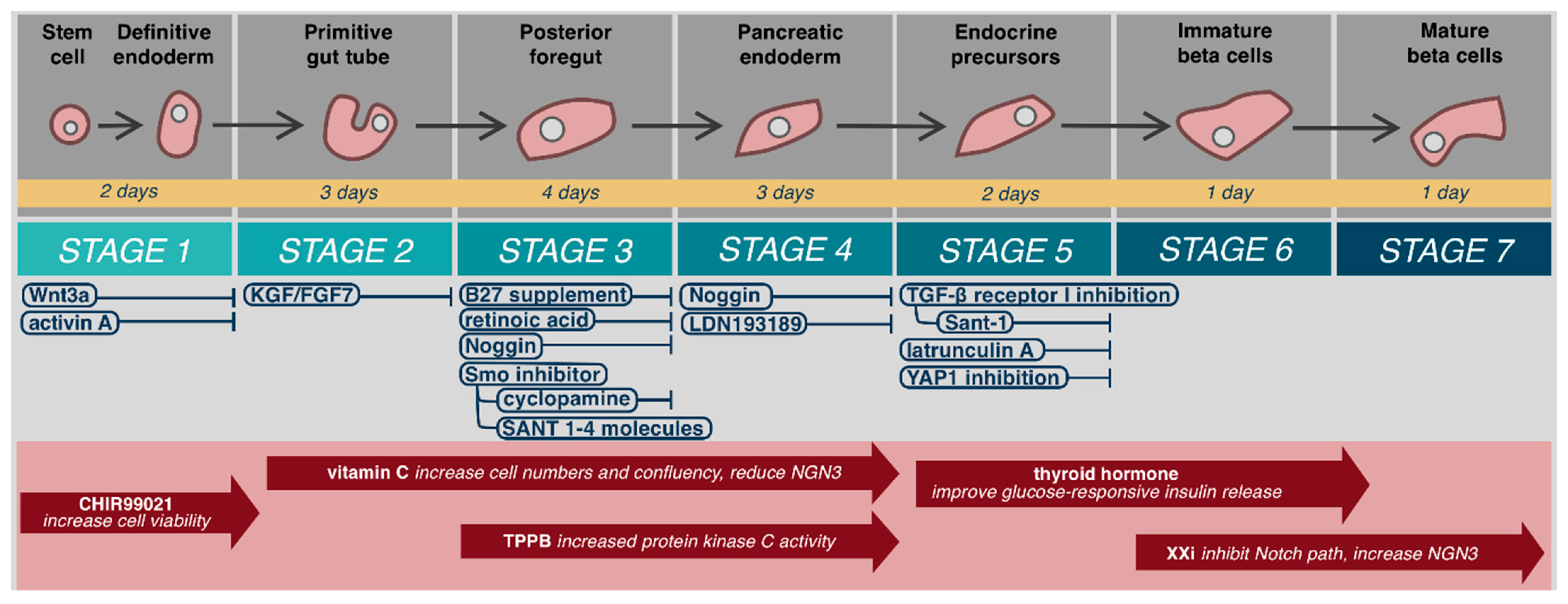


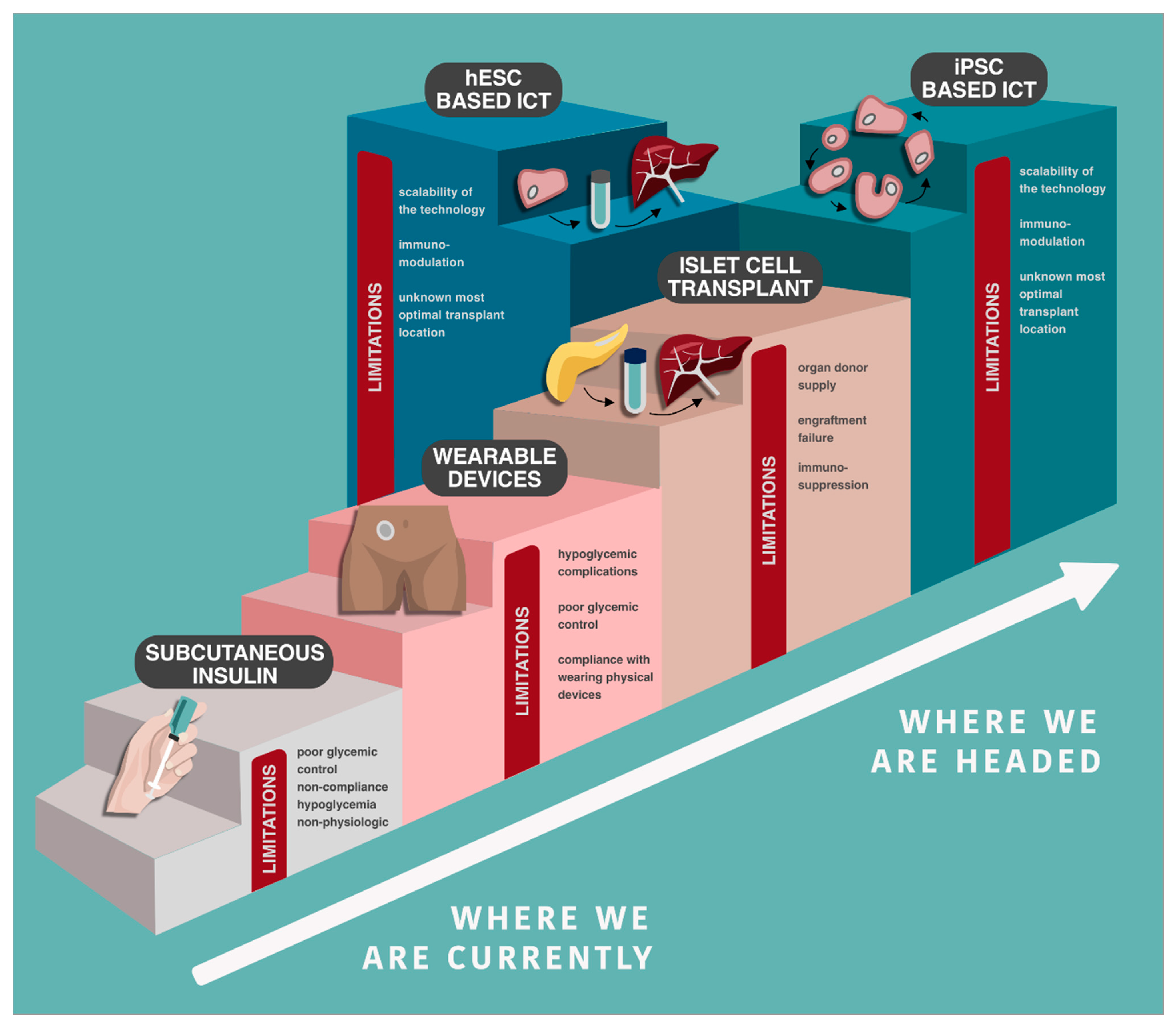

| Technology | Benefits | Drawbacks |
|---|---|---|
| Continuous glucose monitoring |
| |
| Continuous subcutaneous insulin infusion (i.e., insulin pump) |
|
|
| Closed loop, wearable insulin delivery device (i.e., artificial pancreas) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoeff, K.; Henschke, S.J.; Marfil-Garza, B.A.; Dadheech, N.; Shapiro, A.M.J. Inducible Pluripotent Stem Cells as a Potential Cure for Diabetes. Cells 2021, 10, 278. https://doi.org/10.3390/cells10020278
Verhoeff K, Henschke SJ, Marfil-Garza BA, Dadheech N, Shapiro AMJ. Inducible Pluripotent Stem Cells as a Potential Cure for Diabetes. Cells. 2021; 10(2):278. https://doi.org/10.3390/cells10020278
Chicago/Turabian StyleVerhoeff, Kevin, Sarah J. Henschke, Braulio A. Marfil-Garza, Nidheesh Dadheech, and Andrew Mark James Shapiro. 2021. "Inducible Pluripotent Stem Cells as a Potential Cure for Diabetes" Cells 10, no. 2: 278. https://doi.org/10.3390/cells10020278
APA StyleVerhoeff, K., Henschke, S. J., Marfil-Garza, B. A., Dadheech, N., & Shapiro, A. M. J. (2021). Inducible Pluripotent Stem Cells as a Potential Cure for Diabetes. Cells, 10(2), 278. https://doi.org/10.3390/cells10020278








