Genetic Influence on the Peripheral Differentiation Signature of Vδ2+ γδ and CD4+ αβ T Cells in Adults
Abstract
:1. Introduction
2. Material and Methods
2.1. Cohort
2.2. Flow Cytometry
2.3. Statistics
3. Results
3.1. Definition of a T Cell Differentiation Signature
3.2. Consideration of CMV as Confounder for T Cell Differentiation Signature Analyses
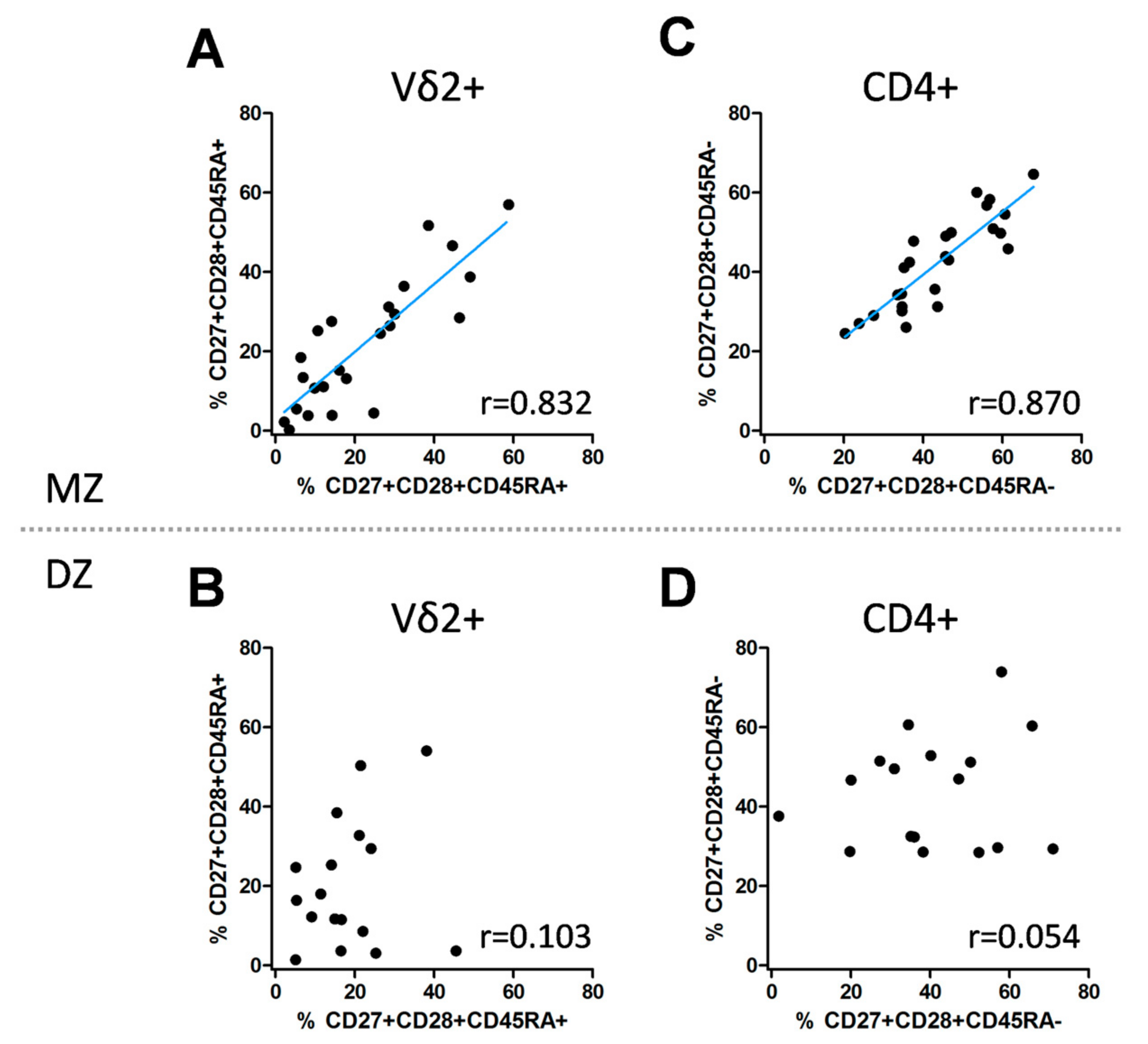
3.3. Genetic Impact on the Differentiation Signature of Vδ2+ and CD4+ T Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Pawelec, G. The human immunosenescence phenotype: Does it exist? Semin. Immunopathol. 2020, 42, 537–544. [Google Scholar] [CrossRef]
- Linton, P.J.; Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G. Human T Cell Aging and the Impact of Persistent Viral Infections. Front. Immunol. 2013, 4, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Wistuba-Hamprecht, K.; Haehnel, K.; Janssen, N.; Demuth, I.; Pawelec, G. Peripheral blood T-cell signatures from high-resolution immune phenotyping of gamma delta and alpha beta T-cells in younger and older subjects in the Berlin Aging Study II. Immun. Ageing 2015, 12, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deusch, K.; Luling, F.; Reich, K.; Classen, M.; Wagner, H.; Pfeffer, K. A Major Fraction of Human Intraepithelial Lymphocytes Simultaneously Expresses the Gamma/Delta T-Cell Receptor, the Cd8 Accessory Molecule and Preferentially Uses the V-Delta-1 Gene Segment. Eur. J. Immunol. 1991, 21, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.; Mavaddat, N.; Litzenberger, C.; Ehrich, E.W.; Davis, M.M.; Bluestone, J.A.; Matis, L.; Draper, R.K.; Chien, Y.H. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell 1994, 76, 29–37. [Google Scholar] [CrossRef]
- Marlin, R.; Pappalardo, A.; Kaminski, H.; Willcox, C.R.; Pitard, V.; Netzer, S.; Khairallah, C.; Lomenech, A.M.; Harly, C.; Bonneville, M.; et al. Sensing of cell stress by human gamma delta TCR-dependent recognition of annexin A2. Proc. Natl. Acad. Sci. USA 2017, 114, 3163–3168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Santos, B.; Schamel, W.W.A.; Fisch, P.; Eberl, M. Gamma delta T-cell conference 2012: Close encounters for the fifth time. Eur. J. Immunol. 2012, 42, 3101–3105. [Google Scholar] [CrossRef]
- Hayday, A.C. Gamma delta cells: A right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 2000, 18, 975–1026. [Google Scholar] [CrossRef]
- Holtmeier, W.; Kabelitz, D. Gamma delta T cells link innate and adaptive immune responses. Chem. Immunol. Allergy 2005, 86, 151–183. [Google Scholar]
- Rampoldi, F.; Ullrich, L.; Prinz, I. Revisiting the Interaction of gamma delta T-Cells and B-Cells. Cells 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, R.R.; Mackay, C.R.; Moser, B.; Eberl, M. IL-21 enhances the potential of human gamma delta T cells to provide B-cell help. Eur. J. Immunol. 2012, 42, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Brandes, M.; Willimann, K.; Moser, B. Professional antigen-presentation function by human gamma delta T cells. Science 2005, 309, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Braakman, E.; van de Winkel, J.G.; van Krimpen, B.A.; Jansze, M.; Bolhuis, R.L. CD16 on human gamma delta T lymphocytes: Expression, function, and specificity for mouse IgG isotypes. Cell Immunol. 1992, 143, 97–107. [Google Scholar] [CrossRef]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(-) subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Joyce, S.P.; Ladell, K.; Kasatskaya, S.A.; McLaren, J.E.; Hunter, S.; Salim, M.; Mohammed, F.; Price, D.A.; et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat. Commun. 2017, 8, 14760. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Mensurado, S.; Coffelt, S.B. Gammadelta T cells: Pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 2019, 19, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Wistuba-Hamprecht, K.; Pawelec, G.; Derhovanessian, E. OMIP-020: Phenotypic characterization of human gammadelta T-cells by multicolor flow cytometry. Cytometry A 2014, 85, 522–524. [Google Scholar] [CrossRef]
- Wistuba-Hamprecht, K.; Frasca, D.; Blomberg, B.; Pawelec, G.; Derhovanessian, E. Age-associated alterations in gammadelta T-cells are present predominantly in individuals infected with Cytomegalovirus. Immun. Ageing 2013, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Wistuba-Hamprecht, K.; Martens, A.; Haehnel, K.; Geukes Foppen, M.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; et al. Proportions of blood-borne Vdelta1+ and Vdelta2+ T-cells are associated with overall survival of melanoma patients treated with ipilimumab. Eur. J. Cancer 2016, 64, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallemeijn, M.J.; Boots, A.M.H.; Van Der Klift, M.Y.; Brouwer, E.; Abdulahad, W.; Wayel, H.; Verhaar, J.A.N.; Van Dongen, J.J.M.; Langerak, A.W. Ageing and latent CMV infection impact on maturation, differentiation and exhaustion profiles of T-cell receptor gammadelta T-cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Mourin, G.; Larsen, M.; Fastenackels, S.; Urrutia, A.; Gorochov, G.; Autran, B.; Donner, C.; Sidi, D.; Sibony-Prat, J.; et al. Differential impact of age and cytomegalovirus infection on the gammadelta T cell compartment. J. Immunol. 2013, 191, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Larbi, A. Markers of T Cell Senescence in Humans. Int. J. Mol. Sci. 2017, 18, 1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravens, S.; Fichtner, A.S.; Willers, M.; Torkornoo, D.; Pirr, S.; Schoning, J.; Deseke, M.; Sandrock, I.; Bubke, A.; Wilharm, A.; et al. Microbial exposure drives polyclonal expansion of innate gammadelta T cells immediately after birth. Proc. Natl. Acad. Sci. USA 2020, 117, 18649–18660. [Google Scholar] [CrossRef]
- Goldeck, D.; Larsen, L.A.; Christiansen, L.; Derhovanessian, E.; Christensen, K.; Hamprecht, K.; Pawelec, G. Genetic Influence on the Peripheral Blood CD4 + T-cell Differentiation Status in CMV Infection. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 71, 1537–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, D.A.; Larsen, L.A.; Nygaard, M.; Mengel-From, J.; McGue, M.; Dalgård, C.; Hvidberg, L.; Hjelmborg, J.B.; Skytthe, A.; Holm, N.V.; et al. The Danish Twin Registry: An Updated Overview. Twin Res. Hum. Genet. 2019, 22, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.; Frederiksen, H.; Schousboe, K.; Skytthe, A.; von Wurmb-Schwark, N.; Christensen, K.; Kyvik, K. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res. 2003, 6, 275–278. [Google Scholar] [CrossRef]
- Chidrawar, S.; Khan, N.; Wei, W.; McLarnon, A.; Smith, N.; Nayak, L.; Moss, P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 2009, 155, 423–432. [Google Scholar] [CrossRef]
- Derhovanessian, E.; Maier, A.B.; Hähnel, K.; Beck, R.; De Craen, A.J.M.; Slagboom, E.P.; Westendorp, R.G.J.; Pawelec, G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J. Gen. Virol. 2011, 92, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Almanzar, G.; Schwaiger, S.; Jenewein, B.; Keller, M.; Herndler-Brandstetter, D.; Würzner, R.; Schönitzer, D.; Grubeck-Loebenstein, B. Long-Term Cytomegalovirus Infection Leads to Significant Changes in the Composition of the CD8+ T-Cell Repertoire, Which May Be the Basis for an Imbalance in the Cytokine Production Profile in Elderly Persons. J. Virol. 2005, 79, 3675–3683. [Google Scholar] [CrossRef] [Green Version]
- Derhovanessian, E.; Maier, A.B.; Beck, R.; Jahn, G.; Hähnel, K.; Slagboom, P.E.; De Craen, A.J.M.; Westendorp, R.G.J.; Pawelec, G. Hallmark Features of Immunosenescence Are Absent in Familial Longevity. J. Immunol. 2010, 185, 4618–4624. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.; Dimova, T.; Shey, M.; Briel, L.; Veldtsman, H.; Khomba, N.; Africa, H.; Steyn, M.; Hanekom, W.A.; Scriba, T.J.; et al. Fetal public Vgamma9Vdelta2 T cells expand and gain potent cytotoxic functions early after birth. Proc. Natl. Acad. Sci. USA 2020, 117, 18638–18648. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, A.S.; Ravens, S.; Prinz, I. Human gammadelta TCR Repertoires in Health and Disease. Cells 2020, 9, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudev, A.; Ying, C.T.; Ayyadhury, S.; Puan, K.J.; Andiappan, A.K.; Nyunt, M.S.; Shadan, N.B.; Mustafa, S.; Low, I.; Rotzschke, O.; et al. Gamma/delta T cell subsets in human aging using the classical alpha/beta T cell model. J. Leukoc Biol. 2014, 96, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.T.; Wistuba-Hamprecht, K.; Xu, W.; Nyunt, M.S.; Vasudev, A.; Lee, B.T.; Pawelec, G.; Puan, K.J.; Rotzschke, O.; Ng, T.P.; et al. Vdelta2+ and alpha/ss T cells show divergent trajectories during human aging. Oncotarget 2016, 7, 44906–44918. [Google Scholar] [CrossRef]
- Xu, W.; Monaco, G.; Wong, E.H.; Tan, W.L.W.; Kared, H.; Simoni, Y.; Tan, S.W.; How, W.Z.Y.; Tan, C.T.Y.; Lee, B.T.K.; et al. Mapping of gamma/delta T cells reveals Vdelta2+ T cells resistance to senescence. EBioMedicine 2018, 39, 44–58. [Google Scholar] [CrossRef] [Green Version]
- Ryan, P.L.; Sumaria, N.; Holland, C.J.; Bradford, C.M.; Izotova, N.; Grandjean, C.L.; Jawad, A.S.; Bergmeier, L.A.; Pennington, D.J. Heterogeneous yet stable V delta 2((+)) T-cell profiles define distinct cytotoxic effector potentials in healthy human individuals. Proc. Natl. Acad. Sci. USA 2016, 113, 14378–14383. [Google Scholar] [CrossRef] [Green Version]
- Dieli, F.; Poccia, F.; Lipp, M.; Sireci, G.; Caccamo, N.; Di Sano, C.; Salerno, A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J. Exp. Med. 2003, 198, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Pitard, V.; Roumanes, D.; Lafarge, X.; Couzi, L.; Garrigue, I.; Lafon, M.E.; Merville, P.; Moreau, J.F.; Dechanet-Merville, J. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 2008, 112, 1317–1324. [Google Scholar] [CrossRef] [Green Version]
- Ravens, S.; Schultze-Florey, C.; Raha, S.; Sandrock, I.; Drenker, M.; Oberdorfer, L.; Reinhardt, A.; Ravens, I.; Beck, M.; Geffers, R.; et al. Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 2017, 18, 393–401. [Google Scholar] [CrossRef]
- Brodin, P.; Jojic, V.; Gao, T.; Bhattacharya, S.; Angel, C.J.L.; Furman, D.; Shen-Orr, S.; Dekker, C.L.; Swan, G.E.; Butte, A.J.; et al. Variation in the Human Immune System Is Largely Driven by Non-Heritable Influences. Cell 2015, 160, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangino, M.; Roederer, M.; Beddall, M.H.; Nestle, F.O.; Spector, T.D. Innate and adaptive immune traits are differentially affected by genetic and environmental factors. Nat. Commun. 2017, 8, 13850. [Google Scholar] [CrossRef] [Green Version]
- Wistuba-Hamprecht, K.; Martens, A.; Weide, B.; Teng, K.W.W.; Zelba, H.; Guffart, E.; Chen, J.; Garbe, C.; Newell, E.W.; Larbi, A.; et al. Establishing High Dimensional Immune Signatures from Peripheral Blood via Mass Cytometry in a Discovery Cohort of Stage IV Melanoma Patients. J. Immunol. 2016, 198, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Wistuba-Hamprecht, K.; Martens, A.; Heubach, F.; Romano, E.; Foppen, M.G.; Yuan, J.; Postow, M.; Wong, P.; Mallardo, D.; Schilling, B.; et al. Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients. Eur. J. Cancer 2017, 73, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur. J. Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Zippelius, A.; Kurth, I.; Pittet, M.J.; Touvrey, C.; Iancu, E.M.; Corthesy, P.; Devevre, E.; Speiser, D.E.; Rufer, N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 2007, 178, 4112–4119. [Google Scholar] [CrossRef] [Green Version]
- Orru, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy (vol 52, 1036, 2020). Nat. Genet. 2020, 52, 1266. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M.; Quaye, L.; Mangino, M.; Beddall, M.H.; Mahnke, Y.; Chattopadhyay, P.; Tosi, I.; Napolitano, L.; Barberio, M.T.; Menni, C.; et al. The Genetic Architecture of the Human Immune System: A Bioresource for Autoimmunity and Disease Pathogenesis. Cell 2015, 161, 387–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Zygosity | Gender | CMV Serostatus | Age (Years) | |
|---|---|---|---|---|
| Positive | Negative | (Range; Median) | ||
| MZ n = 56 | ♀: n = 39 (69.6%) | n = 26 (66.7%) | n = 13 (33.3%) | 41–66; 46 |
| ♂: n = 17 (30.4%) | n = 12 (70.6%) | n = 5 (29.4%) | 42–64; 45 | |
| All | n = 38 (67.9%) | n = 18 (32.1%) | 41–66; 46 | |
| DZ n = 39 | ♀: n = 26 (66.7%) | n = 19 (73.1%) | n = 7 (26.9%) | 43–73; 49.5 |
| ♂: n = 13 (33.3%) | n = 10 (76.9%) | n = 3 (23.1%) | 43–77; 53 | |
| All | n = 29 (74.4%) | n = 10 (25.6%) | 43–77; 50 | |
| Subset | Twins | Spearman r | p-Value (Spearman) | 95% CI (Spearman) | ICC | p-Value (ICC) | 95% CI (ICC) |
|---|---|---|---|---|---|---|---|
| Vδ2+, CD27+CD28+CD45RA+ | MZ | 0.832 | <0.0001 | 0.638; 0.927 | 0.856 | <0.0001 | 0.699; 0.935 |
| Vδ2+, CD27+CD28+ CD45RA+ | DZ | 0.103 | 0.694 | −0.410; 0.567 | 0.207 | 0.200 | −0.279; 0.613 |
| CD4+, CD27+CD28+CD45RA– | MZ | 0.870 | <0.0001 | 0.718; 0.943 | 0.859 | <0.0001 | 0.709; 0.935 |
| CD4+, CD27+CD28+CD45RA- | DZ | 0.054 | 0.837 | −0.451; 0.532 | 0.154 | 0.266 | −0.328; 0.577 |
| Vδ2+, CD27+CD28+CD45RA+ | CD4+, CD27+CD28+CD45RA− | |
|---|---|---|
| Model 1 (not adjusted) | 0.831 (0.688; 0.916) | 0.897 (0.795; 0.952) |
| Model 2 (adjusted for age, gender) | 0.820 (0.668; 0.912) | 0.891 (0.782; 0.949) |
| Model 3 (adjusted for age, gender, CMV) | 0.800 (0.626; 0.905) | 0.887 (0.768; 0.949) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beucke, N.; Wingerter, S.; Hähnel, K.; Larsen, L.A.; Christensen, K.; Pawelec, G.; Wistuba-Hamprecht, K. Genetic Influence on the Peripheral Differentiation Signature of Vδ2+ γδ and CD4+ αβ T Cells in Adults. Cells 2021, 10, 373. https://doi.org/10.3390/cells10020373
Beucke N, Wingerter S, Hähnel K, Larsen LA, Christensen K, Pawelec G, Wistuba-Hamprecht K. Genetic Influence on the Peripheral Differentiation Signature of Vδ2+ γδ and CD4+ αβ T Cells in Adults. Cells. 2021; 10(2):373. https://doi.org/10.3390/cells10020373
Chicago/Turabian StyleBeucke, Nicola, Svenja Wingerter, Karin Hähnel, Lisbeth Aagaard Larsen, Kaare Christensen, Graham Pawelec, and Kilian Wistuba-Hamprecht. 2021. "Genetic Influence on the Peripheral Differentiation Signature of Vδ2+ γδ and CD4+ αβ T Cells in Adults" Cells 10, no. 2: 373. https://doi.org/10.3390/cells10020373
APA StyleBeucke, N., Wingerter, S., Hähnel, K., Larsen, L. A., Christensen, K., Pawelec, G., & Wistuba-Hamprecht, K. (2021). Genetic Influence on the Peripheral Differentiation Signature of Vδ2+ γδ and CD4+ αβ T Cells in Adults. Cells, 10(2), 373. https://doi.org/10.3390/cells10020373







