MiRNAs in Canine and Human Osteosarcoma: A Highlight Review on Comparative Biomolecular Aspects
Abstract
:1. Introduction
2. Osteosarcoma
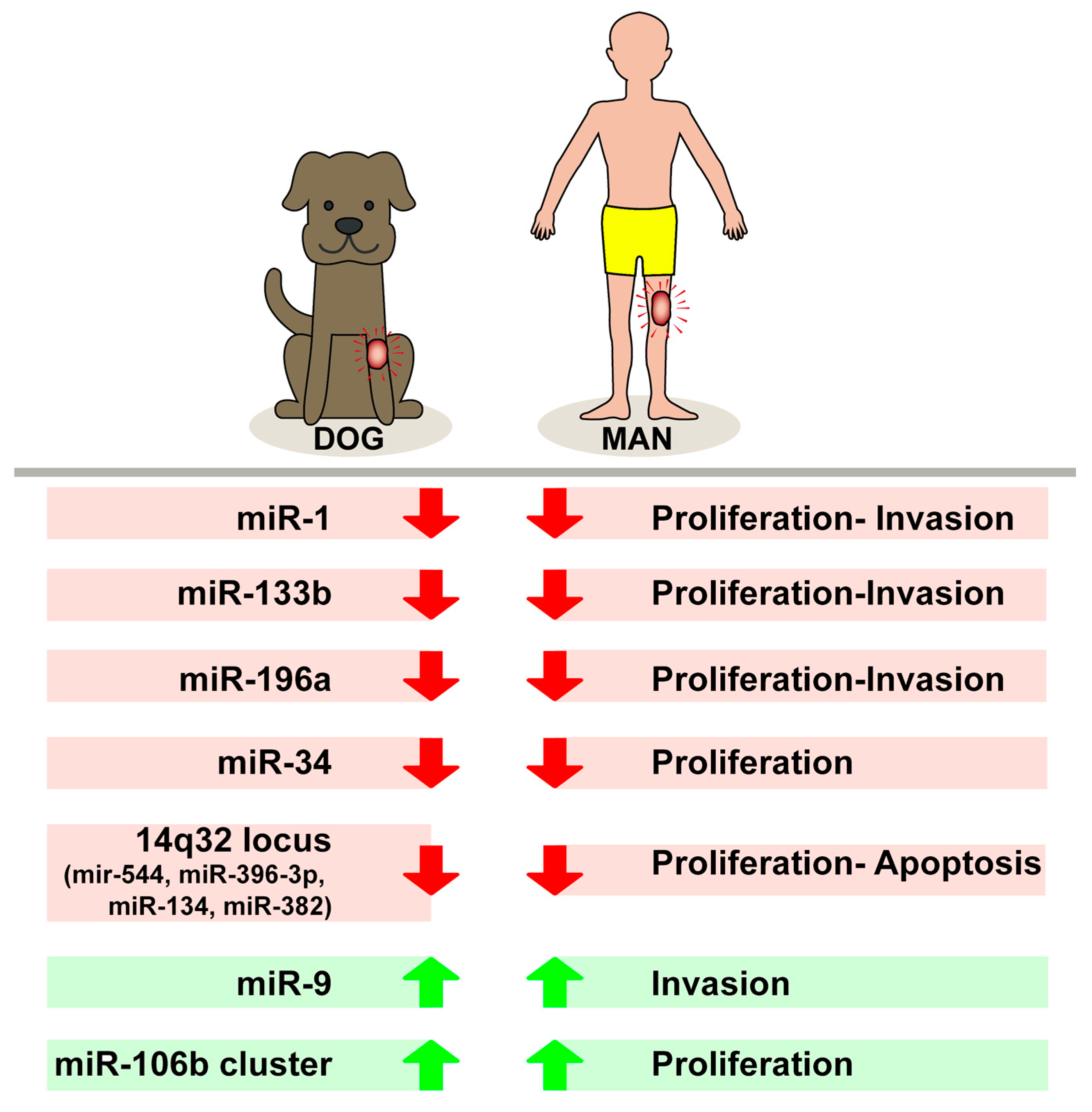
3. MiRNA in Canine and Human Osteosarcoma: Comparative Features
4. Circulating MiRNAs
5. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegre, F.; Ormonde, A. A genetically engineered microRNA-34a prodrug demonstrates anti-tumor activity in a canine model of osteosarcoma. PLoS ONE 2018, 13, e0209941. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef]
- Picci, P. “Osteosarcomas”, in Diagnosis of Musculoskeletal Tumors and Tumor-Like Conditions, 2nd ed.; Picci, P., Manfrini, M., Donati, D.M., Gambarotti, M., Righi, A., Vanel, D., Dei Tos, A.P., Eds.; Springer: Cham, Switzerland, 2020; pp. 185–212. [Google Scholar]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciriano, I.C.; Lee, J.J.; Xi, R.; Jain, D.; Jung, Y.L.; Yang, L.; Gordeni, D.; Klimcizak, L.J.; Zhang, C.Z.; Pellman, D.S.; et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat. Genet. 2020, 52, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonavolt, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; et al. Bone sarcomas: ESMO-PaedCan-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv79–iv95. [Google Scholar] [CrossRef]
- Gougelet, A.; Pissaloux, D.; Besse, A.; Perez, J.; Duc, A.; Dutour, A.; Blay, J.V.; Alberti, L. Micro-RNA profiles in osteosarcoma as a predictive tool for ifosfamide response. Int. J. Cancer 2011, 129, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.B.; Salah, Z.; Del Mare, S.; Galasso, M.; Gaudio, E.; Nuovo, G.J.; Lovat, F.; LeBlanc, K.; Palatini, J.; Randal, R.L.; et al. miRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 2012, 72, 1865–1877. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Satow, R.; Ono, M.; Masuda, M.; Honda, K.; Sakuma, T.; Toyama, Y.; Yamada, T. MicroRNA expression and functional profiles of osteosarcoma. Oncology 2014, 86, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Wu, S.; Peng, Z.; Tania, M.; Zhang, C. MicroRNAs in osteosarcoma: Diagnostic and therapeutic aspects. Tumour. Biol. 2013, 34, 2093–2098. [Google Scholar] [CrossRef]
- Lozano Calderón, S.A.; Garbutt, C.; Kim, J.; Lietz, C.E.; Chen, Y.L.; Bernstein, K.; Chebib, I.; Nielsen, G.P.; Dehspande, V.; Rubio, R.; et al. Clinical and Molecular Analysis of Pathologic Fracture-associated Osteosarcoma: MicroRNA profile Is Different and Correlates with Prognosis. Clin. Orthop. Relat. Res. 2019, 477, 2114–2126. [Google Scholar] [CrossRef]
- Heishima, K.; Meuten, T.; Yoshida, K.; Mori, T.; Thamm, D.H. Prognostic significance of circulating microRNA-214 and -126 in dogs with appendicular osteosarcoma receiving amputation and chemotherapy. BMC Vet Res. 2019, 15, 39. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lee, K.H.; Nam, A.R.; Cho, J.Y. Genome-Wide Methylation Profiling in Canine Mammary Tumor Reveals miRNA Candidates Associated with Human Breast Cancer. Cancers 2019, 11, 1466. [Google Scholar] [CrossRef] [Green Version]
- Kent, M.S.; Zwingenberger, A.; Westropp, J.L.; Barrett, L.E.; Durbin-Johnson, B.P.; Ghosh, P.; Vinall, R.L. MicroRNA profiling of dogs with transitional cell carcinoma of the bladder using blood and urine samples. BMC Vet Res. 2017, 13, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahabi, K.; Selvarajah, G.T.; Abdullah, R.; Cheah, Y.K.; Tan, G.C. Comparative aspects of microRNA expression in canine and human cancers. J. Vet. Sci. 2018, 19, 162–171. [Google Scholar] [CrossRef]
- Lutful Kabir, F.M.; De Innocentes, P.; Bird, R.C. Altered microRNA expression profiles and regulation of INK4A/CDKN2A tumor suppressor genes in canine breast cancer models. J. Cell Biochem. 2015, 116, 2956–2969. [Google Scholar] [CrossRef]
- Thayanithy, V.; Park, C.; Sarver, A.L.; Kartha, R.V.; Korpela, D.M.; Graef, A.J.; Steer, C.J.; Modiano, J.F.; Subramanian, S. Combinatorial treatment of DNA and chromatin-modifying drugs cause cell death in human and canine osteosarcoma cell lines. PLoS ONE 2012, 7, e43720. [Google Scholar] [CrossRef]
- Sarver, A.L.; Thayanithy, V.; Scott, M.C.; Cleton-Jansen, A.M.; Hogendoorn, P.C.; Modiano, J.F.; Subramanian, S. MicroRNAs at the human 14q32 locus have prognostic significance in osteosarcoma. Orphanet. J. Rare Dis. 2013, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. HOX genes and their role in the development of human cancers. J. Mol. Med. 2014, 92, 811–823. [Google Scholar] [CrossRef]
- Y Yang, G.; Han, D.; Chen, X.; Zhang, D.; Wang, L.; Shi, C.; Zhang, W.; Li, C.; Zhang, D.; Kang, J.; et al. MiR-196a exerts its oncogenic effect in glioblastoma multiforme by inhibition of IκBα both in vitro and in vivo. Neuro Oncol. 2014, 16, 652–661. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Lin, X.; Tian, M.; Chang, W. microRNA-196b promotes cell migration and invasion by targeting FOXP2 in hepatocellular carcinoma. Oncol. Rep. 2018, 39, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Wang, C.; Liu, Z. miR-196a-5p promotes metastasis of colorectal cancer via targeting IκBα. BMC Cancer 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Binqui, W.; Dawei, W.; Zhenggang, B. miR-196 acts as a tumor suppressor in osteosarcoma by targeting HOXA. Int. J. Clin. Exp. Pathol. 2018, 11, 4579–4584. [Google Scholar]
- Pazzaglia, L.; Leonardi, L.; Conti, A.; Novello, C.; Quattrini, I.; Montanini, L.; Roperto, F.; Del Piero, F.; Di Guardo, G.; Piro, F.; et al. miR-196a expression in human and canine osteosarcomas: A comparative study. Res. Vet. Sci. 2015, 99, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Fenger, J.M.; Bear, M.D.; Volinia, S.; Lin, T.Y.; Harrington, B.K.; London, C.A.; Kisseberth, W.C. Overexpression of miR-9 in mast cells is associated with invasive behavior and spontaneous metastasis. BMC Cancer 2014, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qi, T.; Yang, D.; Qi, M.; Li, D.; Xiang, X.; Huang, K.; Tong, Q. microRNA-9 suppresses the proliferation, invasion and metastasis of gastric cancer cells through targeting cyclin D1 and Ets1. PLoS ONE 2013, 8, e55719. [Google Scholar] [CrossRef] [PubMed]
- Cekaite, L.; Rantala, J.K.; Bruun, J.; Guriby, M.; Agesen, T.H.; Danielsen, S.A.; Lind, G.E.; Nesbakken, A.; Kallionemi, O.; Lothe, R.A.; et al. MiR-9, -31, and -182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia 2012, 14, 868–879. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, M.A.; Gu, J.; Lin, J.; Ye, Y.; Tan, W.; Tamboli, P.; Wood, G.C.; Wu, X. Hsa-miR-9 methylation status is associated with cancer development and metastatic recurrence in patients with clear cell renal cell carcinoma. Oncogene 2010, 29, 5724–5728. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.M.; Pu, Y.; Han, Z.; Liu, T.; Li, Y.X.; Liu, M.; Li, X.; Tang, H. MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-κB1. FEBS J. 2009, 276, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, X.; Han, L.; Shen, H.; Liu, L.; Shu, Y. Up-regulation of miR-9 expression as a poor prognostic biomarker in patients with non-small cell lung cancer. Clin. Transl. Oncol. 2013, 16, 469–475. [Google Scholar] [CrossRef]
- Ma, L.; Young, J.; Prabhala, H.; Pan, E.; Mestdagh, P.; Muth, D.; Teruya-Feldstein, J.; Reinhardt, F.; Onder, T.T.; Valastyan, S.; et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010, 12, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Shigehara, K.; Yokomuro, S.; Ishibashi, O.; Mizuguchi, Y.; Arima, Y.; Kawahigashi, Y.; Kanda, T.; Akagi, I.; Tajiri, T.; Yoshida, H.; et al. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLoS ONE 2011, 6, e23584. [Google Scholar] [CrossRef]
- Xu, S.H.; Yang, Y.L.; Han, S.M.; Wu, Z.H. MicroRNA-9 expression is a prognostic biomarker in patients with osteosarcoma. World J. Surg. Oncol. 2014, 12, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Wang, J.; Tian, S.; Luo, J. miR-9 depletion suppresses the proliferation of osteosarcoma cells by targeting p16. Int. J. Oncol. 2019, 54, 1921–1932. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.W.; Li, J.P.; Ma, X.L.; Ma, J.X.; Yang, Y.; Chen, Y.; Liu, W. miR-9 modulates osteosarcoma cell growth by targeting the GCIP tumor suppressor. Asian. Pac. J. Cancer Prev. 2015, 16, 4509–4513. [Google Scholar] [CrossRef]
- Gwak, J.M.; Kim, H.J.; Kim, E.J.; Chung, Y.R.; Yun, S.; Seo, A.N. MicroRNA-9 is associated with epithelial- mesenchymal transition, breast cancer stem cell phenotype, and tumor progression in breast cancer. Breast Cancer Res. Treat. 2014, 147, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Cai, X. Up-regulation of miR-9 expression predicate advanced clinicopathological features and poor prognosis in patients with hepatocellular carcinoma. Diagn. Pathol. 2014, 9, 1000. [Google Scholar] [CrossRef] [Green Version]
- Gourbault, O.; Llobat, L. MicroRNAs as Biomarkers in Canine Osteosarcoma: A New Future? Vet. Sci. 2020, 7, 146. [Google Scholar] [CrossRef]
- Leonardi, L.; Pazzaglia, L.; Benassi, M.S. miR-1 and miR-133b expression in canine osteosarcoma. Res. Vet. Sci. 2018, 117, 133–137. [Google Scholar] [CrossRef]
- Agostini, M.; Knight, R.A. miR-34: From bench to bedside. Oncotarget 2014, 5, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.Y.; Yu, Y.; Walsh, W.R.; Yang, J.L. microRNA-34 family and treatment of cancers with mutant or wild-type p53. Int. J. Oncol. 2011, 38, 1189–1195. [Google Scholar] [PubMed]
- Yan, K.; Gao, J.; Yang, T.; Ma, Q.; Qiu, X.; Fan, Q.; Baoan, M. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PLoS ONE 2012, 7, e33778. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, M.J.; Wang, W.P.; Qiu, J.X.; Yu, A.X.; Yu, A.M. Genetically engineered pre-microRNA-34a prodrug suppresses orthotopic osteosarcoma xenograft tumor growth via the induction of apoptosis and cell cycle arrest. Sci. Rep. 2016, 6, 26611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, C.M.; Yu, P.Y.; Zhang, X.; Yilmaz, A.S.; London, C.A.; Fenger, J.M. MiR-34a regulates the invasive capacity of canine osteosarcoma cell lines. PLoS ONE 2018, 13, e0190086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanini, L.; Lasagna, L.; Barili, V.; Jonstrup, S.P.; Murgia, A.; Pazzaglia, L.; Conti, A.; Novello, C.; Kjems, J.; Perris, R.; et al. MicroRNA cloning and sequencing in osteosarcoma cell lines: Differential role of miR-93-5p. Cell Oncol. 2012, 35, 29–41. [Google Scholar] [CrossRef]
- Leonardi, L.; Benassi, M.S.; Pollino, S.; Locaputo, C.; Pazzaglia, L. miR-106B-25 Cluster expression: A comparative human and canine osteosarcoma study. Vet. Rec. Open. 2020, 7, e000379. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, F.; Zhu, L. Clinical significance and functions of microRNA 93/CDKN1A axis in human cervical cancer. Life Sci. 2018, 209, 242–248. [Google Scholar] [CrossRef]
- Kosela-Paterczyk, H.; Paziewska, A.; Kulecka, M.; Balabas, A.; Kluska, A.; Dabrowska, M.; Piatkowska, M.; Zeber-Lubecka, N.; Ambrozkiewicz, F.; Karczmarski, J.; et al. Signatures of circulating microRNA in four sarcoma subtypes. J. Cancer 2020, 11, 874–882. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Cai, H.; Lin, L.; Tang, M.; Cai, H. Upregulated expression of microRNA214 is linked to tumor progression and adverse prognosis in pediatric osteosarcoma. Pediatr. Blood Cancer 2014, 61, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Yu, K.L.; Liu, G.L.; Tian, D.H. MiR214 promotes osteosarcoma tumor growth and metastasis by decreasing the expression of PTEN. Mol. Med. Rep. 2015, 12, 6261–6266. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; He, A.; He, X.; Tao, C. MicroRNA-126 enhances the sensitivity of osteosarcoma cells to cisplatin and methotrexate. Oncol. Lett. 2015, 10, 3769–3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, L.; Scotlandi, K.; Pettinari, I.; Benassi, M.S.; Porcellato, I.; Pazzaglia, L. MiRNAs in Canine and Human Osteosarcoma: A Highlight Review on Comparative Biomolecular Aspects. Cells 2021, 10, 428. https://doi.org/10.3390/cells10020428
Leonardi L, Scotlandi K, Pettinari I, Benassi MS, Porcellato I, Pazzaglia L. MiRNAs in Canine and Human Osteosarcoma: A Highlight Review on Comparative Biomolecular Aspects. Cells. 2021; 10(2):428. https://doi.org/10.3390/cells10020428
Chicago/Turabian StyleLeonardi, Leonardo, Katia Scotlandi, Ilaria Pettinari, Maria Serena Benassi, Ilaria Porcellato, and Laura Pazzaglia. 2021. "MiRNAs in Canine and Human Osteosarcoma: A Highlight Review on Comparative Biomolecular Aspects" Cells 10, no. 2: 428. https://doi.org/10.3390/cells10020428
APA StyleLeonardi, L., Scotlandi, K., Pettinari, I., Benassi, M. S., Porcellato, I., & Pazzaglia, L. (2021). MiRNAs in Canine and Human Osteosarcoma: A Highlight Review on Comparative Biomolecular Aspects. Cells, 10(2), 428. https://doi.org/10.3390/cells10020428








