New Inhibitory Effects of Cilnidipine on Microglial P2X7 Receptors and IL-1β Release: An Involvement in its Alleviating Effect on Neuropathic Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures
2.3. Animals
2.4. Evaluation of rP2X7R Inhibition by High-Throughput Screening
2.5. Measurement of Ca2+ Responses
2.6. YO-PRO-1 Dye Uptake Assay
2.7. Western Blotting Analysis
2.8. ELISA Assay (Measurement of Interleukin-1β (IL-1β) Release)
2.9. Intrathecal Administration
2.10. Neuropathic Pain Model and Behavioral Test
2.11. Statistical Analysis
3. Results
3.1. Identification of Several Compounds That Inhibit P2X7R Function by High-Throughput Screening
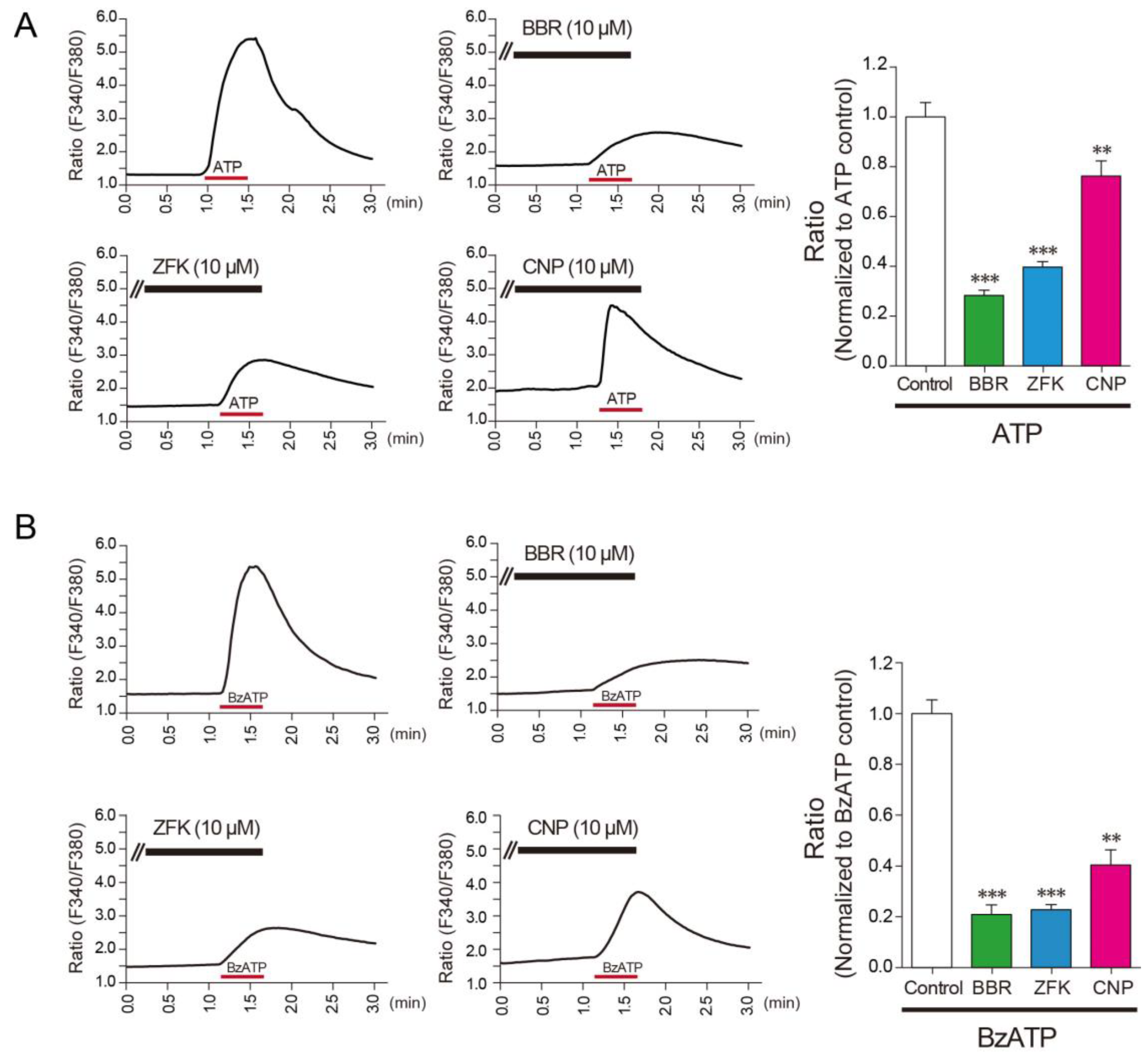
3.2. Benzbromarone, Zafirlukast, and Cilnidipine Inhibit the Responses of Rat Microglial Cells
3.3. Cilnidipine Alleviates Mechanical Pain Hypersensitivity in a Model of Neuropathic Pain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Guan, Z.; Kuhn, J.A.; Wang, X.; Colquitt, B.; Solorzano, C.; Vaman, S.; Guan, A.K.; Evans-Reinsch, Z.; Braz, J.; Devor, M.; et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 2016, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Biber, K.; Tsuda, M.; Tozaki-Saitoh, H.; Tsukamoto, K.; Toyomitsu, E.; Masuda, T.; Boddeke, H.; Inoue, K. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 2011, 30, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Kitano, J.; Kohro, Y.; Tozaki-Saitoh, H.; Inoue, K.; Tsuda, M. Temporal Kinetics of Microgliosis in the Spinal Dorsal Horn after Peripheral Nerve Injury in Rodents. Biol. Pharm. Bull. 2018, 41, 1096–1102. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Tsuda, M. P2 receptors, microglial cytokines and chemokines, and neuropathic pain. J. Neurosci. Res. 2017, 95, 1319–1329. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory disease s. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef]
- Monif, M.; Reid, C.A.; Powell, K.L.; Drummond, K.J.; O’Brien, T.J.; Williams, D.A. Interleukin-1beta has trophic effects in microglia and its release is mediated by P2X7R pore. J. Neuroinflamm. 2016, 13, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L.; et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef]
- He, W.J.; Cui, J.; Du, L.; Zhao, Y.D.; Burnstock, G.; Zhou, H.D.; Ruan, H.Z. Spinal P2X(7) receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav. Brain Res. 2012, 226, 163–170. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.M.; Zheng, B.J.; Wang, X.R. Electroacupuncture Relieves Nerve Injury-Induced Pain Hypersensitivity via the Inhibition of Spinal P2X7 Receptor-Positive Microglia. Anesth. Analg. 2016, 122, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.; Donnelly-Roberts, D.; Namovic, M.T.; Hsieh, G.; Zhu, C.Z.; Mikusa, J.P.; Hernandez, G.; Zhong, C.; Gauvin, D.M.; Chandran, P.; et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J. Pharmacol. Exp. Ther. 2006, 319, 1376–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Takahashi, E.; Miyagawa, Y.; Yamanaka, H.; Noguchi, K. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci. Lett. 2011, 504, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sperlagh, B.; Illes, P. P2X7 receptor: An emerging target in central nervous system diseases. Trends Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Moller, T.; Boddeke, H.W. Glial cells as drug targets: What does it take? Glia 2016, 64, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Biber, K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 2016, 64, 1772–1787. [Google Scholar] [CrossRef]
- Biber, K.; Moller, T.; Boddeke, E.; Prinz, M. Central nervous system myeloid cells as drug targets: Current status and translational challenges. Nat. Rev. Drug Discov. 2016, 15, 110–124. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Yoshida, K.; Ito, M.; Matsuoka, I. P2X7 receptor antagonist activity of the anti-allergic agent oxatomide. Eur. J. Pharmacol. 2015, 767, 41–51. [Google Scholar] [CrossRef]
- Dao-Ung, P.; Skarratt, K.K.; Fuller, S.J.; Stokes, L. Paroxetine suppresses recombinant human P2X7 responses. Purinergic Signal. 2015, 11, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaracharya, A.; Dao-Ung, P.; Jalilian, I.; Spildrejorde, M.; Skarratt, K.K.; Fuller, S.J.; Sluyter, R.; Stokes, L. Probenecid blocks human P2X7 receptor-induced dye uptake via a pannexin-1 independent mechanism. PLoS ONE 2014, 9, e93058. [Google Scholar] [CrossRef] [Green Version]
- Marques-da-Silva, C.; Chaves, M.M.; Castro, N.G.; Coutinho-Silva, R.; Guimaraes, M.Z. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: Implications for its therapeutic action. Br. J. Pharmacol. 2011, 163, 912–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Tsuda, M.; Tozaki-Saitoh, H.; Inoue, K. Lentiviral transduction of cultured microglia. Methods Mol. Biol. 2013, 1041, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Toyomitsu, E.; Tsuda, M.; Yamashita, T.; Tozaki-Saitoh, H.; Tanaka, Y.; Inoue, K. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 2012, 8, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, M.; Masuda, T.; Kitano, J.; Shimoyama, H.; Tozaki-Saitoh, H.; Inoue, K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc. Natl. Acad. Sci. USA 2009, 106, 8032–8037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, K.; Tozaki-Saitoh, H.; Kojima, C.; Masuda, T.; Tsuda, M.; Inoue, K.; Hoka, S. Chemokine (C-C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 2014, 120, 1491–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, T.; Yamamoto, S.; Zhang, J.; Kometani, M.; Tomiyama, D.; Kohno, K.; Tozaki-Saitoh, H.; Inoue, K.; Tsuda, M. Duloxetine Inhibits Microglial P2X4 Receptor Function and Alleviates Neuropathic Pain after Peripheral Nerve Injury. PLoS ONE 2016, 11, e0165189. [Google Scholar] [CrossRef] [Green Version]
- Suadicani, S.O.; Brosnan, C.F.; Scemes, E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 2006, 26, 1378–1385. [Google Scholar] [CrossRef] [Green Version]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.K.; Staniland, A.A.; Marchand, F.; Kaan, T.K.; McMahon, S.B.; Malcangio, M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. 2010, 30, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Giancotti, L.A.; Lauro, F.; Mufti, F.; Salvemini, D. Treatment of chronic neuropathic pain: Purine receptor modulation. Pain 2020, 161, 1425–1441. [Google Scholar] [CrossRef]
- Sorge, R.E.; Trang, T.; Dorfman, R.; Smith, S.B.; Beggs, S.; Ritchie, J.; Austin, J.S.; Zaykin, D.V.; Vander Meulen, H.; Costigan, M.; et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 2012, 18, 595–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, R.; Stokes, L.; Sluyter, R. The P2X7 receptor channel: Recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014, 66, 638–675. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, A.; Shimauchi, T.; Tanaka, T.; Shimoda, K.; Toyama, T.; Kitajima, N.; Ishikawa, T.; Shindo, N.; Numaga-Tomita, T.; Yasuda, S.; et al. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Jiang, W.; Yan, Y.; Gong, T.; Han, J.; Tian, Z.; Zhou, R. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat. Immunol. 2014, 15, 1126–1133. [Google Scholar] [CrossRef]
- Park, S.; Won, J.H.; Hwang, I.; Hong, S.; Lee, H.K.; Yu, J.W. Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci. Rep. 2015, 5, 15489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef] [Green Version]
- Bourinet, E.; Altier, C.; Hildebrand, M.E.; Trang, T.; Salter, M.W.; Zamponi, G.W. Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014, 94, 81–140. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Nakagawasai, O.; Fujii, S.; Hosono, M.; Hozumi, S.; Esashi, A.; Taniguchi, R.; Okamura, T.; Suzuki, T.; Sasano, H.; et al. Antinociceptive effect of cilnidipine, a novel N-type calcium channel antagonist. Brain Res. 2000, 868, 123–127. [Google Scholar] [CrossRef]
- Yamamoto, S.; Suzuki, Y.; Ono, H.; Kume, K.; Ohsawa, M. N- and L-type calcium channels blocker cilnidipine ameliorates neuropathic pain. Eur. J. Pharmacol. 2016, 793, 66–75. [Google Scholar] [CrossRef]
- Ikeda, K.; Hosono, M.; Iida, H.; Ohnishi, H. Antihypertensive and Cardiovascular Profiles of a Newly Synthesized Dihydropyridine Derivative 2-Methoxyethyl(E)-3-phenyl-2-propen-1-yl(±)-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate (FRC-8653). Oyo Yakuri/Pharmacomet. 1992, 44, 433–442. [Google Scholar]
- Koganei, H.; Shoji, M.; Iwata, S. Suppression of formalin-induced nociception by cilnidipine, a voltage-dependent calcium channel blocker. Biol. Pharm. Bull. 2009, 32, 1695–1700. [Google Scholar] [CrossRef] [Green Version]
- Fujii, S.; Kameyama, K.; Hosono, M.; Hayashi, Y.; Kitamura, K. Effect of cilnidipine, a novel dihydropyridine Ca++-channel antagonist, on N-type Ca++ channel in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 1997, 280, 1184–1191. [Google Scholar]
- Brose, W.G.; Gutlove, D.P.; Luther, R.R.; Bowersox, S.S.; McGuire, D. Use of intrathecal SNX-111, a novel, N-type, voltage-sensitive, calcium channel blocker, in the management of intractable brachial plexus avulsion pain. Clin. J. Pain 1997, 13, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. P2X ion channel receptors and inflammation. Purinergic Signal. 2016, 12, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosli, S.; Kirby, F.J.; Lawlor, K.E.; Rainczuk, K.; Drummond, G.R.; Mansell, A.; Tate, M.D. Repurposing drugs targeting the P2X7 receptor to limit hyperinflammation and disease during influenza virus infection. Br. J. Pharmacol. 2019, 176, 3834–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, T.; Kamikaseda, S.; Tanaka, A.; Tozaki-Saitoh, H.; Caaveiro, J.M.M.; Inoue, K.; Tsuda, M. New Inhibitory Effects of Cilnidipine on Microglial P2X7 Receptors and IL-1β Release: An Involvement in its Alleviating Effect on Neuropathic Pain. Cells 2021, 10, 434. https://doi.org/10.3390/cells10020434
Yamashita T, Kamikaseda S, Tanaka A, Tozaki-Saitoh H, Caaveiro JMM, Inoue K, Tsuda M. New Inhibitory Effects of Cilnidipine on Microglial P2X7 Receptors and IL-1β Release: An Involvement in its Alleviating Effect on Neuropathic Pain. Cells. 2021; 10(2):434. https://doi.org/10.3390/cells10020434
Chicago/Turabian StyleYamashita, Tomohiro, Sawako Kamikaseda, Aya Tanaka, Hidetoshi Tozaki-Saitoh, Jose M. M. Caaveiro, Kazuhide Inoue, and Makoto Tsuda. 2021. "New Inhibitory Effects of Cilnidipine on Microglial P2X7 Receptors and IL-1β Release: An Involvement in its Alleviating Effect on Neuropathic Pain" Cells 10, no. 2: 434. https://doi.org/10.3390/cells10020434





