Role of Gonadotropin-Releasing Hormone (GnRH) in Ovarian Cancer
Abstract
1. Introduction
2. Methods
3. Results and Discussion
3.1. Expression of GnRH and Its Receptor in Ovarian Cancer
3.2. Direct Effects of GnRH on Ovarian Cancer
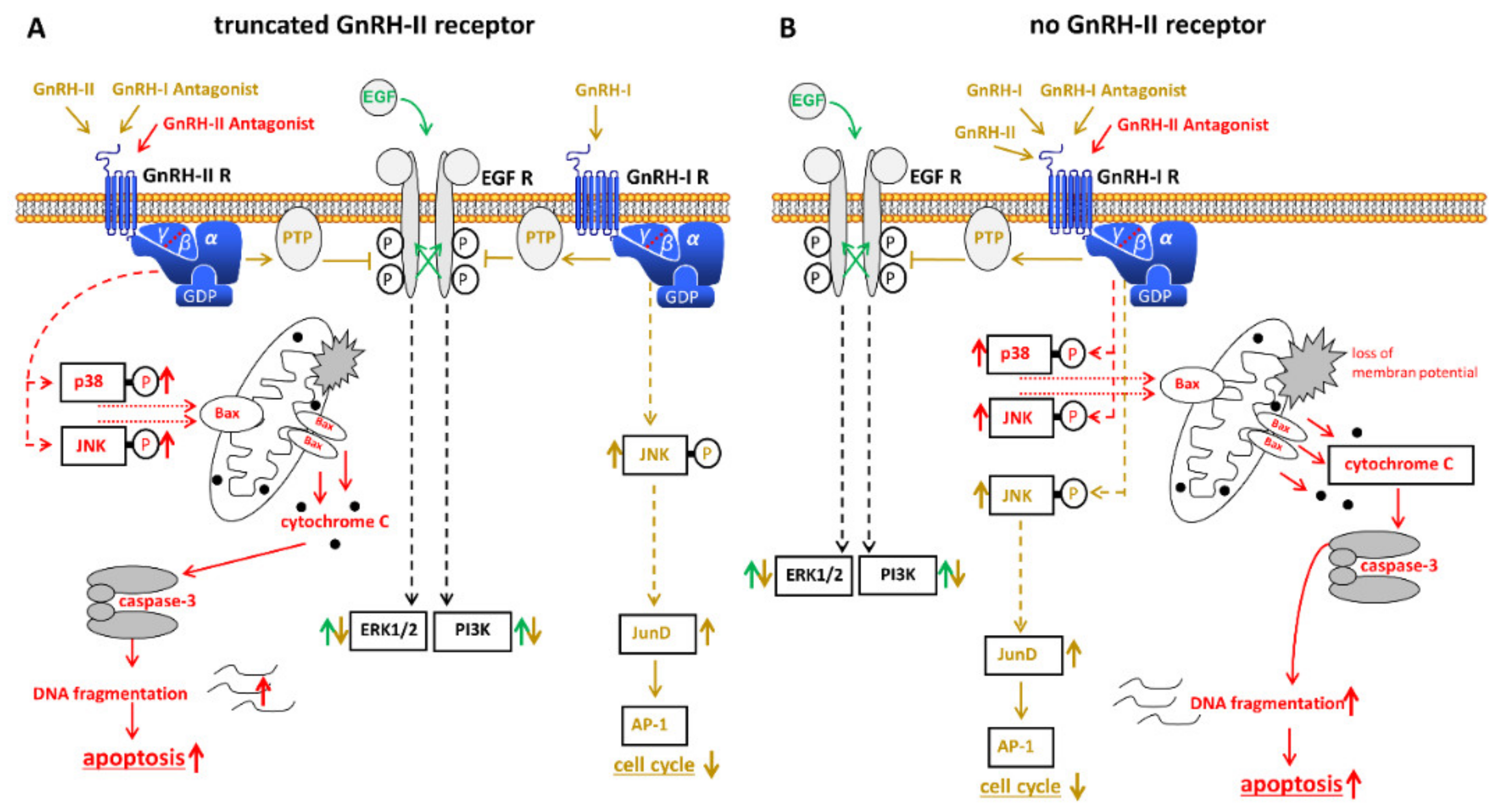
3.3. Expression of GnRH-II and Its Receptor in Ovarian Cancer
3.4. GnRH Analogs as a Possible Therapy for Ovarian Cancer
3.4.1. Suppression of Pituitary Gonadotropin Secretion by GnRH Analogs
3.4.2. Direct Treatment of Ovarian Cancer
3.4.3. GnRH Receptor Target Therapies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Mph, K.D.M.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Cho, K.R.; Shih, I.-M. Ovarian Cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 287–313. [Google Scholar] [CrossRef]
- Gajjar, K.; Ogden, G.; Mujahid, M.I.; Razvi, K. Symptoms and Risk Factors of Ovarian Cancer: A Survey in Primary Care. ISRN Obstet. Gynecol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Nezhat, F.R.; Apostol, R.; Nezhat, C.; Pejovic, T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am. J. Obstet. Gynecol. 2015, 213, 262–267. [Google Scholar] [CrossRef]
- Nezhat, F.R.; Pejovic, T.; Reis, F.M.; Guo, S.-W. The Link Between Endometriosis and Ovarian Cancer: Clinical Implications. Int. J. Gynecol. Cancer 2014, 24, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Cress, R.D.; Chen, Y.S.; Morris, C.R.; Petersen, M.; Leiserowitz, G.S. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet. Gynecol. 2015, 126, 491–497. [Google Scholar] [CrossRef]
- Falconer, H.; Yin, L.; Grönberg, H.; Altman, D. Ovarian Cancer Risk After Salpingectomy: A Nationwide Population-Based Study. J. Natl. Cancer Inst. 2014, 107, dju410. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Perets, R.; Drapkin, R. It’s Totally Tubular…Riding The New Wave of Ovarian Cancer Research. Cancer Res. 2016, 76, 10–17. [Google Scholar] [CrossRef]
- Langdon, S.; Crew, A.; Ritchie, A.; Muir, M.; Wakeling, A.; Smyth, J.; Miller, W. Growth inhibition of oestrogen receptor-positive human ovarian carcinoma by anti-oestrogens in vitro and in a xenograft model. Eur. J. Cancer 1994, 30, 682–686. [Google Scholar] [CrossRef]
- Langdon, S.; Hirst, G.; Miller, E.; Hawkins, R.; Tesdale, A.; Smyth, J.; Miller, W. The regulation of growth and protein expression by estrogen in vitro: A study of 8 human ovarian carcinoma cell lines. J. Steroid Biochem. Mol. Biol. 1994, 50, 131–135. [Google Scholar] [CrossRef]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://www.clinicaltrials.gov (accessed on 18 December 2020).
- Isberg, V.; De Graaf, C.; Bortolato, A.; Cherezov, V.; Katritch, V.; Marshall, F.H.; Mordalski, S.; Pin, J.-P.; Stevens, R.C.; Vriend, G.; et al. Generic GPCR residue numbers—aligning topology maps while minding the gaps. Trends Pharmacol. Sci. 2015, 36, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N.; Rong, H.; Dou, Q.; Kipersztok, S.; Williams, R.S. Gonadotropin-releasing hormone (GnRH) and GnRH receptor gene expression in human myometrium and leiomyomata and the direct action of GnRH analogs on myometrial smooth muscle cells and interaction with ovarian steroids in vitro. J. Clin. Endocrinol. Metab. 1996, 81, 3215–3221. [Google Scholar] [CrossRef][Green Version]
- Minaretzis, D.; Jakubowski, M.; Mortola, J.F.; Pavlou, S.N. Gonadotropin-releasing hormone receptor gene expression in human ovary and granulosa-lutein cells. J. Clin. Endocrinol. Metab. 1995, 80, 430–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Emons, G.; Weiss, S.; Ortmann, O.; Gründker, C.; Schulz, K.D. LHRH might act as a negative autocrine regulator of proliferation of human ovarian cancer. Eur. J. Endocrinol. 2000, 142, 665–670. [Google Scholar] [CrossRef][Green Version]
- Gründker, C.; Günthert, A.R.; Westphalen, S.; Emons, G. Biology of the gonadotropin-releasing hormone system in gynecological cancers. Eur. J. Endocrinol. 2002, 146, 1–14. [Google Scholar] [CrossRef]
- Emons, G.; Grundker, C.; Gunthert, A.R.; Westphalen, S.; Kavanagh, J.; Verschraegen, C. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr. Relat. Cancer 2003, 10, 291–299. [Google Scholar] [CrossRef]
- Emons, G.; Ortmann, O.; Schulz, K.-D.; Schally, A.V. Growth-inhibitory actions of analogues of Luteinizing Hormone Releasing Hormone on tumor cells. Trends Endocrinol. Metab. 1997, 8, 355–362. [Google Scholar] [CrossRef]
- Volker, P.; Grundker, C.; Schmidt, O.; Schulz, K.D.; Emons, G. Expression of receptors for luteinizing hor-mone-releasing hormone in human ovarian and endometrial cancers: Frequency, autoregulation, and corre-lation with direct antiproliferative activity of luteinizing hormone-releasing hormone analogues. Am. J. Obstet. Gynecol. 2002, 186, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wentao, Y.; Bi, R.; Xiaojun, C.; Chen, X.; Yang, W.; Wu, X. A clinically applicable molecular classification for high-grade serous ovarian cancer based on hormone receptor expression. Sci. Rep. 2016, 6, 25408. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wen, H.; Ju, X.; Bi, R.; Chen, X.; Yang, W.; Wu, X. Expression of hypothalamic-pituitary-gonadal axis-related hormone receptors in low-grade serous ovarian cancer (LGSC). J. Ovarian Res. 2017, 10, 7. [Google Scholar] [CrossRef]
- Emons, G.; Schally, A.V. The use of luteinizing hormone releasing hormone agonists and antagonists in gynaecological cancers. Hum. Reprod. 1994, 9, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Loop, S.M.; Gorder, C.A.; Lewis, S.M.; Saiers, J.H.; Drivdahl, R.H.; Ostenson, R.C. Growth inhibition of hu-man prostate tumor cells by an agonist of gonadotrophin-releasing hormone. Prostate 1995, 26, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Eidne, K.A.; Flanagan, C.A.; Harris, N.S.; Millar, R.P. Gonadotropin-releasing hormone (GnRH)-binding sites in human breast cancer cell lines and inhibitory effects of GnRH antagonists. J. Clin. Endocrinol. Metab. 1987, 64, 425–432. [Google Scholar] [CrossRef]
- Kakar, S.S.; Musgrove, L.C.; Devor, D.C.; Sellers, J.C.; Neill, J.D. Cloning, sequencing, and expression of hu-man gonadotropin releasing hormone (GnRH) receptor. Biochem. Biophys. Res. Commun. 1992, 189, 289–295. [Google Scholar] [CrossRef]
- Imai, A.; Ohno, T.; Iida, K.; Fuseya, T.; Furui, T.; Tamaya, T. Gonadotropin-releasing hormone receptor in gynecologic tumors. Frequent expression in adenocarcinoma histologic types. Cancer 1994, 74, 2555–2561. [Google Scholar] [CrossRef]
- Imai, A.; Ohno, T.; Iida, K.; Fuseya, T.; Furui, T.; Tamaya, T. Presence of Gonadotropin-Releasing Hormone Receptor and Its Messenger Ribonucleic Acid in Endometrial Carcinoma and Endometrium. Gynecol. Oncol. 1994, 55, 144–148. [Google Scholar] [CrossRef]
- Imai, A.; Ohno, T.; Ohsuye, K.; Tamaya, T. Expression of Gonadotropin-Releasing Hormone Receptor in Human Epithelial Ovarian Carcinoma. Ann. Clin. Biochem. Int. J. Lab. Med. 1994, 31, 550–555. [Google Scholar] [CrossRef]
- Irmer, G.; Bürger, C.; Müller, R.; Ortmann, O.; Peter, U.; Kakar, S.S.; Neill, J.D.; Schulz, K.D.; Emons, G. Ex-pression of the messenger RNAs for luteinizing hormone-releasing hormone (LHRH) and its receptor in human ovarian epithelial carcinoma. Cancer Res. 1995, 55, 817–822. [Google Scholar] [PubMed]
- Emons, G.; Ortmann, O.; Becker, M.; Irmer, G.; Springer, B.; Laun, R.; Hölzel, F.; Schulz, K.D.; Schally, A.V. High affinity binding and direct antiproliferative effects of LHRH analogues in human ovarian cancer cell lines. Cancer Res. 1993, 53, 5439–5446. [Google Scholar] [PubMed]
- Emons, G.; Schröder, B.; Ortmann, O.; Westphalen, S.; Schulz, K.D.; Schally, A.V. High affinity binding and direct antiproliferative effects of luteinizing hormone-releasing hormone analogs in human endometrial cancer cell lines. J. Clin. Endocrinol. Metab. 1993, 77, 1458–1464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohno, T.; Imai, A.; Furui, T.; Takahashi, K.; Tamaya, T. Presence of gonadotropin-releasing hormone and its messenger ribonucleic acid in human ovarian epithelial carcinoma. Am. J. Obstet. Gynecol. 1993, 169, 605–610. [Google Scholar] [CrossRef]
- Irmer, G.; Bürger, C.; Ortmann, O.; Schulz, K.D.; Emons, G. Expression of luteinizing hormone releasing hormone and its mRNA in human endometrial cancer cell lines. J. Clin. Endocrinol. Metab. 1994, 79, 916–919. [Google Scholar] [CrossRef]
- Kakar, S.S.; Grizzle, W.E.; Neill, J.D. The nucleotide sequences of human GnRH receptors in breast and ovarian tumors are identical with that found in pituitary. Mol. Cell. Endocrinol. 1994, 106, 145–149. [Google Scholar] [CrossRef]
- Harris, N.; Dutlow, C.; Eidne, K.; Dong, K.W.; Roberts, J.; Millar, R. Gonadotropin-releasing hormone gene expression in MDA-MB-231 and ZR-75-1 breast carcinoma cell lines. Cancer Res. 1991, 51, 2577–2581. [Google Scholar]
- Dondi, D.; Limonta, P.; Moretti, R.M.; Marelli, M.M.; Garattini, E.; Motta, M. Antiproliferative effects of lu-teinizing hormone-releasing hormone (LHRH) agonists on human androgen-independent prostate cancer cell line DU 145: Evidence for an autocrine-inhibitory LHRH loop. Cancer Res. 1994, 54, 4091–4095. [Google Scholar]
- Limonta, P.; Dondi, D.; Moretti, R.M.; Maggi, R.; Motta, M. Antiproliferative effects of luteinizing hor-mone-releasing hormone agonists on the human prostatic cancer cell line LNCaP. J. Clin. Endocrinol. Metab. 1992, 75, 207–212. [Google Scholar]
- Limonta, P.; Moretti, R.M.; Dondi, D.; Marelli, M.M.; Motta, M. Androgen-dependent prostatic tumors: Bio-synthesis and possible actions of LHRH. J. Steroid Biochem. Mol. Biol. 1994, 49, 347–350. [Google Scholar] [CrossRef]
- Cheng, K.W.; Leung, P.C. The expression, regulation and signal transduction pathways of the mammalian gonadotropin-releasing hormone receptor. Can. J. Physiol. Pharm. 2000, 78, 1029–1052. [Google Scholar] [CrossRef]
- Naor, Z. Signal Transduction Mechanisms of Ca 2+ Mobilizing Hormones: The Case of Gonadotropin-Releasing Hormone. Endocr. Rev. 1990, 11, 326–353. [Google Scholar] [CrossRef]
- Kraus, S.; Naor, Z.; Seger, R. Intracellular Signaling Pathways Mediated by the Gonadotropin-Releasing Hormone (GnRH) Receptor. Arch. Med. Res. 2001, 32, 499–509. [Google Scholar] [CrossRef]
- McArdle, C.A.; Franklin, J.; Green, L.; Hislop, J.N. Signalling, cycling and desensitisation of gonadotrophin-releasing hormone receptors. J. Endocrinol. 2002, 173, 1–11. [Google Scholar] [CrossRef]
- Ruf, F.; Fink, M.Y.; Sealfon, S.C. Structure of the GnRH receptor-stimulated signaling network: Insights from genomics. Front. Neuroendocr. 2003, 24, 181–199. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nat. Cell Biol. 1993, 361, 315–325. [Google Scholar] [CrossRef]
- Keizer, J.; Li, Y.X.; Stojilković, S.; Rinzel, J. InsP3-induced Ca2+ excitability of the endoplasmic reticulum. Mol. Biol. Cell 1995, 6, 945–951. [Google Scholar] [CrossRef]
- Harris, D.; Bonfil, D.; Chuderland, D.; Kraus, S.; Seger, R.; Naor, Z. Activation of MAPK Cascades by GnRH: ERK and Jun N-Terminal Kinase Are Involved in Basal and GnRH-Stimulated Activity of the Glycoprotein Hormone LHβ-Subunit Promoter. Endocrinology 2002, 143, 1018–1025. [Google Scholar] [CrossRef]
- Zhang, T.; Roberson, M.S. Role of MAP kinase phosphatases in GnRH-dependent activation of MAP kinases. J. Mol. Endocrinol. 2006, 36, 41–50. [Google Scholar] [CrossRef]
- Bonfil, D.; Chuderland, D.; Kraus, S.; Shahbazian, D.; Friedberg, I.; Seger, R.; Naor, Z. Extracellular Signal-Regulated Kinase, Jun N-Terminal Kinase, p38, and c-Src Are Involved in Gonadotropin-Releasing Hormone-Stimulated Activity of the Glycoprotein Hormone Follicle-Stimulating Hormone β-Subunit Promoter. Endocrinology 2004, 145, 2228–2244. [Google Scholar] [CrossRef]
- Levi, N.L.; Hanoch, T.; Benard, O.; Rozenblat, M.; Harris, D.; Reiss, N.; Naor, Z.; Seger, R. Stimulation of Jun N-Terminal Kinase (JNK) by Gonadotropin-Releasing Hormone in Pituitary αT3–1 Cell Line Is Mediated by Protein Kinase C, c-Src, and CDC42. Mol. Endocrinol. 1998, 12, 815–824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberson, M.S.; Zhang, T.; Li, H.L.; Mulvaney, J.M. Activation of the p38 Mitogen-Activated Protein Kinase Pathway by Gonadotropin-Releasing Hormone. Endocrinology 1999, 140, 1310–1318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gründker, C.; Völker, P.; Emons, G. Antiproliferative signaling of luteinizing hormone-releasing hormone in human endometrial and ovarian cancer cells through G protein alpha(I)-mediated activation of phosphotyrosine phosphatase. Endocrinology 2001, 142, 2369–2380. [Google Scholar] [CrossRef]
- Limonta, P.; Moretti, R.M.; Marelli, M.M.; Dondi, D.; Parenti, M.; Motta, M. The luteinizing hormone-releasing hormone receptor in human prostate cancer cells: Messenger ribonucleic acid expression, molecular size, and signal transduction pathway. Endocrinology 1999, 140, 5250–5256. [Google Scholar] [CrossRef] [PubMed]
- Dobkin-Bekman, M.; Naidich, M.; Pawson, A.J.; Millar, R.P.; Seger, R.; Naor, Z. Activation of mitogen-activated protein kinase (MAPK) by GnRH is cell-context dependent. Mol. Cell Endocrinol. 2006, 252, 184–190. [Google Scholar] [CrossRef]
- Lee, M.T.; Liebow, C.; Kamer, A.R.; Schally, A.V. Effects of epidermal growth factor and analogues of lute-inizing hormone-releasing hormone and somatostatin on phosphorylation and dephosphorylation of tyro-sine residues of specific protein substrates in various tumors. Proc. Natl. Acad. Sci. USA 1991, 88, 1656–1660. [Google Scholar] [CrossRef]
- Furui, T.; Imai, A.; Takagi, H.; Horibe, S.; Fuseya, T.; Tamaya, T. Phosphotyrosine Phosphatase-Activity in Membranes from Endometrial Carcinoma. Oncol. Rep. 1995, 2, 1055–1057. [Google Scholar] [CrossRef]
- Imai, A.; Takagi, H.; Horibe, S.; Fuseya, T.; Tamaya, T. Coupling of gonadotropin-releasing hormone recep-tor to Gi protein in human reproductive tract tumors. J. Clin. Endocrinol. Metab. 1996, 81, 3249–3253. [Google Scholar] [PubMed]
- Imai, A.; Takagi, H.; Furui, T.; Horibe, S.; Fuseya, T.; Tamaya, T. Evidence for coupling of phosphotyrosine phosphatase to gonadotropin-releasing hormone receptor in ovarian carcinoma membrane. Cancer 1996, 77, 132–137. [Google Scholar] [CrossRef]
- Gründker, C.; Völker, P.; Schulz, K.-D.; Emons, G. Luteinizing Hormone—Releasing Hormone Agonist Triptorelin and Antagonist Cetrorelix Inhibit EGF-Induced c-fos Expression in Human Gynecological Cancers. Gynecol. Oncol. 2000, 78, 194–202. [Google Scholar] [CrossRef]
- Miller, W.R.; Scott, W.N.; Morris, R.; Fraser, H.M.; Sharpe, R.M. Growth of human breast cancer cells inhib-ited by a luteinizing hormone-releasing hormone agonist. Nature 1985, 313, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Pinski, J.; Halmos, G.; Szepesházi, K.; Groot, K.; Schally, A.V. Inhibition of growth of OV-1063 human epithelial ovarian cancer xenografts in nude mice by treatment with luteinizing hormone-releasing hormone antagonist SB-75. Proc. Natl. Acad. Sci. 1994, 91, 7090–7094. [Google Scholar] [CrossRef]
- Moretti, R.M.; Marelli, M.M.; Dondi, D.; Poletti, A.; Martini, L.; Motta, M.; Limonta, P. Luteinizing hormone-releasing hormone agonists interfere with the stimulatory actions of epidermal growth factor in human prostatic cancer cell lines, LNCaP and DU 145. J. Clin. Endocrinol. Metab. 1996, 81, 3930–3937. [Google Scholar] [CrossRef] [PubMed]
- Shirahige, Y.; Cook, C.; Pinski, J.; Halmos, G.; Nair, R.; Schally, A. Treatment with Luteinizing-Hormone-Releasing Hormone Antagonist sb-75 Decreases Levels of Epidermal Growth-Factor Receptor and its Messenger-RNA in ov-1063 Human Epithelial Ovarian-Cancer Xenografts in Nude-Mice. Int. J. Oncol. 1994, 5, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Kéri, G.; Balogh, Á.; Szöke, B.; Teplán, I.; Csuka, O. Gonadotropin-Releasing Hormone Analogues Inhibit Cell Proliferation and Activate Signal Transduction Pathways in MDA-MB-231 Human Breast Cancer Cell Line. Tumor Biol. 1991, 12, 61–67. [Google Scholar] [CrossRef]
- Liebow, C.; Lee, M.T.; Kamer, A.R.; Schally, A.V. Regulation of luteinizing hormone-releasing hormone re-ceptor binding by heterologous and autologous receptor-stimulated tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA 1991, 88, 2244–2248. [Google Scholar] [CrossRef]
- Hershkovitz, E.; Marbach, M.; Bosin, E.; Levy, J.; Roberts, C.T., Jr.; LeRoith, D.; Schally, A.V.; Sharoni, Y. Lu-teinizing hormone-releasing hormone antagonists interfere with autocrine and paracrine growth stimula-tion of MCF-7 mammary cancer cells by insulin-like growth factors. J. Clin. Endocrinol. Metab. 1993, 77, 963–968. [Google Scholar]
- Fuseya, T.; Imai, A.; Horibe, S.; Takagi, A.; Tamaya, T. Evidence for common signalling pathways of GnRH receptor and Fas in tumors. Oncol. Rep. 1996, 3, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Horibe, S.; Takagi, A.; Ohno, T.; Tamaya, T. Frequent expression of Fas in gonadotropin-releasing hormone receptor-bearing tumors. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 74, 73–78. [Google Scholar] [CrossRef]
- Imai, A.; Takagi, A.; Horibe, S.; Takagi, H.; Tamaya, T. Fas and Fas ligand system may mediate antiproliferative activity of gonadotropin-releasing hormone receptor in endometrial cancer cells. Int. J. Oncol. 1998, 13, 97–197. [Google Scholar] [CrossRef]
- Imai, A.; Takagi, A.; Horibe, S.; Takagi, H.; Tamaya, T. Evidence for Tight Coupling of Gonadotropin-Releasing Hormone Receptor to Stimulated Fas Ligand Expression in Reproductive Tract Tumors: Possible Mechanism for Hormonal Control of Apoptotic Cell Death 1. J. Clin. Endocrinol. Metab. 1998, 83, 427–431. [Google Scholar] [CrossRef]
- Gunthert, A.R.; Grundker, C.; Bottcher, B.; Emons, G. Luteinizing hormone-releasing hormone (LHRH) in-hibits apoptosis induced by cytotoxic agent and UV-light but not apoptosis mediated through CD95 in hu-man ovarian and endometrial cancer cells. Anticancer Res. 2004, 24, 1727–1732. [Google Scholar]
- Gründker, C.; Schulz, K.; Günthert, A.R.; Emons, G. Luteinizing Hormone-Releasing Hormone Induces Nuclear Factorκ B-Activation and Inhibits Apoptosis in Ovarian Cancer Cells. J. Clin. Endocrinol. Metab. 2000, 85, 3815–3820. [Google Scholar] [CrossRef][Green Version]
- Grundker, C.; Schlotawa, L.; Viereck, V.; Emons, G. Protein kinase C-independent stimulation of activator protein-1 and c-Jun N-terminal kinase activity in human endometrial cancer cells by the LHRH agonist triptorelin. Eur. J. Endocrinol. 2001, 145, 651–658. [Google Scholar] [CrossRef][Green Version]
- Günthert, A.R.; Gründker, C.; Hollmann, K.; Emons, G. Luteinizing hormone-releasing hormone induces JunD—DNA binding and extends cell cycle in human ovarian cancer cells. Biochem. Biophys. Res. Commun. 2002, 294, 11–15. [Google Scholar] [CrossRef]
- Bonapace, I.M.; Addeo, R.; Altucci, L.; Cicatiello, L.; Bifulco, M.; Laezza, C.; Salzano, S.; Sica, V.; Bresciani, F.; Weisz, A. 17 beta-Estradiol overcomes a G1 block induced by HMG-CoA reductase inhibitors and fosters cell cycle progression without inducing ERK-1 and -2 MAP kinases activation. Oncogene 1996, 12, 753–763. [Google Scholar] [PubMed]
- Doucas, V.; Spyrou, G.; Yaniv, M. Unregulated expression of c-Jun or c-Fos proteins but not Jun D inhibits oestrogen receptor activity in human breast cancer derived cells. EMBO J. 1991, 10, 2237–2245. [Google Scholar] [CrossRef]
- Duan, R.; Porter, W.; Safe, S. Estrogen-Induced c-fos Protooncogene Expression in MCF-7 Human Breast Cancer Cells: Role of Estrogen Receptor Sp1 Complex Formation. Endocrinology 1998, 139, 1981–1990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Der Burg, B.; De Groot, R.P.; Isbrücker, L.; Kruijer, W.; De Laat, S.W. Stimulation of TPA-Responsive Element Activity by a Cooperative Action of Insulin and Estrogen in Human Breast Cancer Cells. Mol. Endocrinol. 1990, 4, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Van der Burg, B.; de Groot, R.P.; Isbrucker, L.; Kruijer, W.; de Laat, S.W. Oestrogen directly stimulates growth factor signal transduction pathways in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 1991, 40, 215–221. [Google Scholar] [CrossRef]
- Van Der Burg, B.; Van Selm-Miltenburg, A.J.; De Laat, S.W.; Van Zoelen, E.J. Direct effects of estrogen on c-fos and c-myc protooncogene expression and cellular proliferation in human breast cancer cells. Mol. Cell. Endocrinol. 1989, 64, 223–228. [Google Scholar] [CrossRef]
- Weisz, A.; Bresciani, F. Estrogen regulation of proto-oncogenes coding for nuclear proteins. Crit. Rev. Oncog. 1993, 4, 361–388. [Google Scholar]
- Wilding, G.; Lippman, M.E.; Gelmann, E.P. Effects of steroid hormones and peptide growth factors on pro-tooncogene c-fos expression in human breast cancer cells. Cancer Res. 1988, 48, 802–805. [Google Scholar]
- Duan, R.; Xie, W.; Burghardt, R.C.; Safe, S. Estrogen Receptor-mediated Activation of the Serum Response Element in MCF-7 Cells through MAPK-dependent Phosphorylation of Elk-1. J. Biol. Chem. 2001, 276, 11590–11598. [Google Scholar] [CrossRef]
- Duan, R.; Xie, W.; Li, X.; McDougal, A.; Safe, S. Estrogen regulation of c-fos gene expression through phosphatidylinositol-3-kinase-dependent activation of serum response factor in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 294, 384–394. [Google Scholar] [CrossRef]
- Grundker, C.; Gunthert, A.R.; Hellriegel, M.; Emons, G. Gonadotropin-releasing hormone (GnRH) agonist triptorelin inhibits estradiol-induced serum response element (SRE) activation and c-fos expression in hu-man endometrial, ovarian and breast cancer cells. Eur. J. Endocrinol. 2004, 151, 619–628. [Google Scholar] [CrossRef]
- Girgert, R.; Emons, G.; Gründker, C. Inactivation of GPR30 reduces growth of triple-negative breast cancer cells: Possible application in targeted therapy. Breast Cancer Res. Treat. 2012, 134, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Girgert, R.; Emons, G.; Gründker, C. Inhibition of GPR30 by estriol prevents growth stimulation of triple-negative breast cancer cells by 17β-estradiol. BMC Cancer 2014, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Girgert, R.; Emons, G.; Gründker, C. 17β-estradiol-induced growth of triple-negative breast cancer cells is prevented by the reduction of GPER expression after treatment with gefitinib. Oncol. Rep. 2016, 37, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, L.M.; Daaka, Y.; Lefkowitz, R.J. Regulation of tyrosine kinase cascades by G-protein-coupled recep-tors. Curr. Opin. Cell Biol. 1999, 11, 177–183. [Google Scholar] [CrossRef]
- Günthert, A.R.; Gründker, C.; Olota, A.; Läsche, J.; Eicke, N.; Emons, G. Analogs of GnRH-I and GnRH-II inhibit epidermal growth factor-induced signal transduction and resensitize resistant human breast cancer cells to 4OH-tamoxifen. Eur. J. Endocrinol. 2005, 153, 613–625. [Google Scholar] [CrossRef][Green Version]
- Von Alten, J.; Fister, S.; Schulz, H.; Viereck, V.; Frosch, K.-H.; Emons, G.; Gründker, C. GnRH analogs reduce invasiveness of human breast cancer cells. Breast Cancer Res. Treat. 2006, 100, 13–21. [Google Scholar] [CrossRef]
- Ziegler, E.; Hansen, M.-T.; Haase, M.; Emons, G.; Gründker, C. Generation of MCF-7 cells with aggressive metastatic potential in vitro and in vivo. Breast Cancer Res. Treat. 2014, 148, 269–277. [Google Scholar] [CrossRef]
- Olbrich, T.; Ziegler, E.; Türk, G.; Schubert, A.; Emons, G.; Gründker, C. Kisspeptin-10 inhibits bone-directed migration of GPR54-positive breast cancer cells: Evidence for a dose-window effect. Gynecol. Oncol. 2010, 119, 571–578. [Google Scholar] [CrossRef]
- Magliocco, A.; Egan, C. Breast Cancer Metastasis: Advances Trough the Use of In Vitro Co-Culture Model Systems. In Breast Cancer-Focusing Tumor Micriinvironment, Stem Cells and Metastasis; Gunduz, M., Gunduz, E., Eds.; InTech: Rijeka, Croatia, 2011; Volume 1. [Google Scholar]
- Hellinger, J.W.; Hüchel, S.; Goetz, L.; Bauerschmitz, G.; Emons, G.; Gründker, C. Inhibition of CYR61-S100A4 Axis Limits Breast Cancer Invasion. Front. Oncol. 2019, 9, 1074. [Google Scholar] [CrossRef]
- Millar, R.; Lowe, S.; Conklin, D.; Pawson, A.; Maudsley, S.; Troskie, B.; Ott, T.; Millar, M.; Lincoln, G.; Sellar, R.; et al. A novel mammalian receptor for the evolutionarily conserved type II GnRH. Proc. Natl. Acad. Sci. 2001, 98, 9636–9641. [Google Scholar] [CrossRef] [PubMed]
- Neill, J.D.; Duck, L.; Sellers, J.C.; Musgrove, L.C. A Gonadotropin-Releasing Hormone (GnRH) Receptor Specific for GnRH II in Primates. Biochem. Biophys. Res. Commun. 2001, 282, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Neill, J.D.; Musgrove, L.C.; Duck, L.W. Newly recognized GnRH receptors: Function and relative role. Trends Endocrinol. Metab. TEM 2004, 15, 383–392. [Google Scholar] [CrossRef]
- Stewart, A.J.; Katz, A.A.; Millar, R.P.; Morgan, K. Retention and Silencing of Prepro-GnRH-II and Type II GnRH Receptor Genes in Mammals. Neuroendocrinology 2009, 90, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Gründker, C.; Föst, C.; Fister, S.; Nolte, N.; Günthert, A.R.; Emons, G. Gonadotropin-releasing hormone type II antagonist induces apoptosis in MCF-7 and triple-negative MDA-MB-231 human breast cancer cells in vitro and in vivo. Breast Cancer Res. 2010, 12, R49. [Google Scholar] [CrossRef] [PubMed]
- Gründker, C.; Günthert, A.R.; Millar, R.P.; Emons, G. Expression of Gonadotropin-Releasing Hormone II (GnRH-II) Receptor in Human Endometrial and Ovarian Cancer Cells and Effects of GnRH-II on Tumor Cell Proliferation. J. Clin. Endocrinol. Metab. 2002, 87, 1427–1430. [Google Scholar] [CrossRef]
- Millar, R.P. GnRH II and type II GnRH receptors. Trends Endocrinol. Metab. 2003, 14, 35–43. [Google Scholar] [CrossRef]
- Millar, R.; Conklin, D.; Lofton-Day, C.; Hutchinson, E.; Troskie, B.; Illing, N.; Sealfon, S.C.; Hapgood, J. A novel human GnRH receptor homolog gene: Abundant and wide tissue distribution of the antisense tran-script. J. Endocrinol. 1999, 162, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Wang, P.; Zhao, J.; Wu, Y.L.; Cheng, Z.J.; Wu, G.X.; Hu, W.; Ma, L.; Pei, G. Five-transmembrane do-mains appear sufficient for a G protein-coupled receptor: Functional five-transmembrane domain chemo-kine receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 7922–7927. [Google Scholar] [CrossRef]
- Morgan, K.; Conklin, D.; Pawson, A.J.; Sellar, R.; Ott, T.R.; Millar, R.P. A Transcriptionally Active Human Type II Gonadotropin-Releasing Hormone Receptor Gene Homolog Overlaps Two Genes in the Antisense Orientation on Chromosome 1q.12. Endocrinology 2003, 144, 423–436. [Google Scholar] [CrossRef]
- Eicke, N.; Günthert, A.R.; Viereck, V.; Siebold, D.; Béhé, M.; Becker, T.; Emons, G.; Gründker, C. GnRH-II receptor-like antigenicity in human placenta and in cancers of the human reproductive organs. Eur. J. Endocrinol. 2005, 153, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Müller, V.; Ortmann, O.; Schulz, K.-D. Effects of LHRH-analogues on mitogenic signal transduction in cancer cells. J. Steroid Biochem. Mol. Biol. 1998, 65, 199–206. [Google Scholar] [CrossRef]
- Fister, S.; Günthert, A.R.; Emons, G.; Gründker, C. Gonadotropin-Releasing Hormone Type II Antagonists Induce Apoptotic Cell Death in Human Endometrial and Ovarian Cancer Cells In vitro and In vivo. Cancer Res. 2007, 67, 1750–1756. [Google Scholar] [CrossRef]
- Fister, S.; Günthert, A.R.; Aicher, B.; Paulini, K.W.; Emons, G.; Gründker, C. GnRH-II Antagonists Induce Apoptosis in Human Endometrial, Ovarian, and Breast Cancer Cells via Activation of Stress-Induced MAPKs p38 and JNK and Proapoptotic Protein Bax. Cancer Res. 2009, 69, 6473–6481. [Google Scholar] [CrossRef]
- Gründker, C.; Schlotawa, L.; Viereck, V.; Eicke, N.; Horst, A.; Kairies, B.; Emons, G. Antiproliferative effects of the GnRH antagonist cetrorelix and of GnRH-II on human endometrial and ovarian cancer cells are not mediated through the GnRH type I receptor. Eur. J. Endocrinol. 2004, 151, 141–149. [Google Scholar] [CrossRef][Green Version]
- Enomoto, M.; Endo, D.; Kawashima, S.; Park, M.K. Human Type II GnRH Receptor Mediates Effects of GnRH on Cell Proliferation. Zoöl. Sci. 2004, 21, 763–770. [Google Scholar] [CrossRef][Green Version]
- Choi, K.-C.; Auersperg, N.; Leung, P.C.K. Expression and Antiproliferative Effect of a Second Form of Gonadotropin-Releasing Hormone in Normal and Neoplastic Ovarian Surface Epithelial Cells. J. Clin. Endocrinol. Metab. 2001, 86, 5075. [Google Scholar] [CrossRef] [PubMed]
- Mangia, A.; Tommasi, S.; Reshkin, S.J.; Simone, G.; Stea, B.; Schittulli, F.; Paradiso, A. Gonadotropin releas-ing hormone receptor expression in primary breast cancer: Comparison of immunohistochemical, radiolig-and and Western blot analyses. Oncol. Rep. 2002, 9, 1127–1132. [Google Scholar] [PubMed]
- Kim, K.Y.; Choi, K.C.; Auersperg, N.; Leung, P.C. Mechanism of gonadotropin-releasing hormone (GnRH)-I and -II-induced cell growth inhibition in ovarian cancer cells: Role of the GnRH-I receptor and protein ki-nase C pathway. Endocr. Relat. Cancer 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Parmar, H.; Phillips, R.H.; Rustin, G.; Lightman, S.L.; Schally, A.V. Therapy of advanced ovarian cancer with D-Trp-6-LH-RH (decapeptyl) microcapsules. Biomed. Pharmacother. 1988, 42, 531–538. [Google Scholar]
- Parmar, H.; Rustin, G.; Lightman, S.L.; Phillips, R.H.; Hanham, I.W.; Schally, A.V. Response to D-Trp-6-luteinising hormone releasing hormone (Decapeptyl) microcapsules in advanced ovarian cancer. Br. Med J. Clin. Res. Ed. 1988, 296, 1229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruckner, H.W.; Motwani, B.T. Treatment of advanced refractory ovarian carcinoma with a gonadotropin-releasing hormone analogue. Am. J. Obstet. Gynecol. 1989, 161, 1216–1218. [Google Scholar] [CrossRef]
- Jäger, W.; Wildt, L.; Lang, N. Some observations on the effect of a GnRH analog in ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 1989, 32, 137–148. [Google Scholar] [CrossRef]
- Kavanagh, J.J.; Roberts, W.; Townsend, P.; Hewitt, S. Leuprolide acetate in the treatment of refractory or persistent epithelial ovarian cancer. J. Clin. Oncol. 1989, 7, 115–118. [Google Scholar] [CrossRef]
- Vavra, N.; Barrada, M.; Fitz, R.; Sevelda, P.; Baur, M.; Dittrich, C. Goserelin—A new form of hormone therapy in ovarian cancer. Gynakol. Rundsch. 1990, 30, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Ortmann, O.; Teichert, H.M.; Fassl, H.; Lohrs, U.; Kullander, S.; Kauppila, A.; Ayalon, D.; Schally, A.; Oberheuser, F. Luteinizing hormone-releasing hormone agonist triptorelin in combination with cytotox-ic chemotherapy in patients with advanced ovarian carcinoma. A prospective double blind randomized tri-al. Decapeptyl Ovarian Cancer Study Group. Cancer 1996, 78, 1452–1460. [Google Scholar] [CrossRef]
- Hasan, J.; Ton, N.; Mullamitha, S.; Clamp, A.; McNeilly, A.; Marshall, E.; Jayson, G.C. Phase II trial of tamoxifen and goserelin in recurrent epithelial ovarian cancer. Br. J. Cancer 2005, 93, 647–651. [Google Scholar] [CrossRef][Green Version]
- Lind, M.; Cantwell, B.; Millward, M.; Robinson, A.; Proctor, M.; Simmons, D.; Carmichael, J.; Harris, A. A phase II trial of goserelin (Zoladex) in relapsed epithelial ovarian cancer. Br. J. Cancer 1992, 65, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Brady, M.F.; Barrett, R.J. A Phase II Trial of Leuprolide Acetate in Patients with Advanced Epithelial Ovarian Carcinoma. Am. J. Clin. Oncol. 1992, 15, 125–128. [Google Scholar] [CrossRef]
- Carnino, F.; Iskra, L.; Fuda, G.; Foglia, G.; Odicino, F.; Bruzzone, M.; Chiara, S.; Gadducci, A.; Ragni, N. The treatment of progressive ovarian carcinoma with D-Trp-LHRH (decapeptyl). Eur. J. Cancer 1994, 30, 1903–1904. [Google Scholar] [CrossRef]
- Marinaccio, M.; D’Addario, V.; Serratì, A.; Pinto, V.; Cagnazzo, G. Leuprolide acetate as a salvage-therapy in relapsed epithelial ovarian cancer. Eur. J. Gynaecol. Oncol. 1996, 17, 286–288. [Google Scholar]
- Ron, I.G.; Wigler, N.; Merimsky, O.; Inbar, M.J.; Chaitchik, S. A Phase II Trial of D-Trp-6-LHRH (Decapeptyl) in Pretreated Patients with Advanced Epithelial Ovarian Cancer. Cancer Investig. 1995, 13, 272–275. [Google Scholar] [CrossRef]
- Duffaud, F.; El Van Der Burg, M.; Namer, M.; Vergote, I.; Willemse, P.B.; Huinink, W.T.B.; Guastalla, J.P.; Nooij, M.A.; Kerbrat, P.; Piccart, M.; et al. D-TRP-6-LHRH (Triptorelin) is not effective in ovarian carcinoma: An EORTC Gynaecological Cancer Co-operative Group Study. Anticancer Drugs 2001, 12, 159–162. [Google Scholar] [CrossRef]
- Paskeviciute, L.; Roed, H.; Engelholm, S.A. No rules without exception: Long-term complete remission observed in a study using a LH-RH agonist in platinum-refractory ovarian cancer. Gynecol. Oncol. 2002, 86, 297–301. [Google Scholar] [CrossRef]
- Du Bois, A.; Meier, W.; Lück, H.J.; Emons, G.; Moebus, V.; Schroeder, W.; Costa, S.; Bauknecht, T.; Olbricht, S.; Jackisch, C.; et al. Chemotherapy versus hormonal treatment in platinum- and paclitaxel-refractory ovarian cancer: A randomised trial of the German Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) Study Group Ovarian Cancer. Ann. Oncol. 2002, 13, 251–257. [Google Scholar] [CrossRef]
- Balbi, G.; Piano, L.D.; Cardone, A.; Cirelli, G. Second-line therapy of advanced ovarian cancer with GnRH analogs. Int. J. Gynecol. Cancer 2004, 14, 799–803. [Google Scholar] [CrossRef]
- Sevelda, P.; Vavra, N.; Fitz, R.; Barrada, M.; Salzer, H.; Baur, M.; Dittrich, C. Goserelin a GnRH-analogue as third-line therapy of refractory epithelial ovarian cancer. Int. J. Gynecol. Cancer 1992, 2, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.; Bukulmez, O. Biochemistry, molecular biology and cell biology of gonadotropin-releasing hormone antagonists. Curr. Opin. Obstet. Gynecol. 2011, 23, 238–244. [Google Scholar] [CrossRef]
- Verschraegen, C.F.; Westphalen, S.; Hu, W.; Loyer, E.; Kudelka, A.; Völker, P.; Kavanagh, J.; Steger, M.; Schulz, K.-D.; Emons, G. Phase II study of cetrorelix, a luteinizing hormone-releasing hormone antagonist in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2003, 90, 552–559. [Google Scholar] [CrossRef]
- Cho, N.; Harada, M.; Imaeda, T.; Imada, T.; Matsumoto, H.; Hayase, Y.; Sasaki, S.; Furuya, S.; Suzuki, N.; Okubo, S.; et al. Discovery of a Novel, Potent, and Orally Active Nonpeptide Antagonist of the Human Luteinizing Hormone-Releasing Hormone (LHRH) Receptor. J. Med. Chem. 1998, 41, 4190–4195. [Google Scholar] [CrossRef]
- Hara, T.; Araki, H.; Kusaka, M.; Harada, M.; Cho, N.; Suzuki, N.; Furuya, S.; Fujino, M. Suppression of a Pituitary-Ovarian Axis by Chronic Oral Administration of a Novel Nonpeptide Gonadotropin-Releasing Hormone Antagonist, TAK-013, in Cynomolgus Monkeys. J. Clin. Endocrinol. Metab. 2003, 88, 1697–1704. [Google Scholar] [CrossRef]
- Millar, R.P.; Zhu, Y.-F.; Struthers, R.S. Progress towards the development of non-peptide orally-active gonadotropin-releasing hormone (GnRH) antagonists: Therapeutic implications. Br. Med. Bull. 2000, 56, 761–772. [Google Scholar] [CrossRef]
- Miwa, K.; Hitaka, T.; Imada, T.; Sasaki, S.; Yoshimatsu, M.; Kusaka, M.; Tanaka, A.; Nakata, D.; Furuya, S.; Endo, S.; et al. Discovery of 1-{4-[1-(2,6-Difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385) as a Potent, Orally Active, Non-Peptide Antagonist of the Human Gonadotropin-Releasing Hormone Receptor. J. Med. Chem. 2011, 54, 4998–5012. [Google Scholar] [CrossRef] [PubMed]
- Nakata, D.; Masaki, T.; Tanaka, A.; Yoshimatsu, M.; Akinaga, Y.; Asada, M.; Sasada, R.; Takeyama, M.; Miwa, K.; Watanabe, T.; et al. Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: Studies in human GnRH receptor knock-in mice. Eur. J. Pharmacol. 2014, 723, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.P.; Newton, C.L. Current and future applications of GnRH, kisspeptin and neurokinin B analogues. Nat. Rev. Endocrinol. 2013, 9, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Tukun, F.-L.; Olberg, D.E.; Riss, P.J.; Haraldsen, I.; Kaass, A.; Klaveness, J. Recent Development of Non-Peptide GnRH Antagonists. Molecules 2017, 22, 2188. [Google Scholar] [CrossRef] [PubMed]
- Grundker, C.; Volker, P.; Griesinger, F.; Ramaswamy, A.; Nagy, A.; Schally, A.V.; Emons, G. Antitumor ef-fects of the cytotoxic luteinizing hormone-releasing hormone analog AN-152 on human endometrial and ovarian cancers xenografted into nude mice. Am. J. Obstet. Gynecol. 2002, 187, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Nagy, A. Cancer chemotherapy based on targeting of cytotoxic peptide conjugates to their re-ceptors on tumors. Eur. J. Endocrinol. 1999, 141, 1–14. [Google Scholar] [CrossRef]
- Westphalen, S.; Kotulla, G.; Kaiser, F.; Krauss, W.; Werning, G.; Elsasser, H.P.; Nagy, A.; Schulz, K.D.; Gründker, C.; Schally, A.V.; et al. Receptor mediated antiproliferative effects of the cytotoxic LHRH agonist AN-152 in human ovarian and endometrial cancer cell lines. Int. J. Oncol. 2000, 17, 1063–1069. [Google Scholar] [CrossRef]
- Günthert, A.R.; Gründker, C.; Bongertz, T.; Schlott, T.; Nagy, A.; Schally, A.V.; Emons, G. Internalization of cytotoxic analog AN-152 of luteinizing hormone-releasing hormone induces apoptosis in human endometrial and ovarian cancer cell lines independent of multidrug resistance-1 (MDR-1) system. Am. J. Obstet. Gynecol. 2004, 191, 1164–1172. [Google Scholar] [CrossRef]
- Engel, J.B.; Schally, A.V.; Dietl, J.; Rieger, L.; Hönig, A. Targeted Therapy of Breast and Gynecological Cancers with Cytotoxic Analogues of Peptide Hormones. Mol. Pharm. 2007, 4, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Gumlnthert, A.R.; Grumlndker, C.; Bongertz, T.; Nagy, A.; Schally, A.V.; Emons, G. Induction of apoptosis by AN-152, a cytotoxic analog of luteinizing hormone-releasing hormone (LHRH), in LHRH-R positive human breast cancer cells is independent of multidrug resistance-1 (MDR-1) system. Breast Cancer Res. Treat. 2004, 87, 255–264. [Google Scholar] [CrossRef]
- Emons, G.; Kaufmann, M.; Gorchev, G.; Tsekova, V.; Gründker, C.; Günthert, A.R.; Hanker, L.C.; Velikova, M.; Sindermann, H.; Engel, J.; et al. Dose escalation and pharmacokinetic study of AEZS-108 (AN-152), an LHRH agonist linked to doxorubicin, in women with LHRH receptor-positive tumors. Gynecol. Oncol. 2010, 119, 457–461. [Google Scholar] [CrossRef]
- Emons, G.; Gorchev, G.; Sehouli, J.; Wimberger, P.; Stähle, A.; Hanker, L.; Hilpert, F.; Sindermann, H.; Gründker, C.; Harter, P. Efficacy and safety of AEZS-108 (INN: Zoptarelin Doxorubicin Acetate) an LHRH agonist linked to doxorubicin in women with platinum refractory or resistant ovarian cancer expressing LHRH receptors: A multicenter Phase II trial of the ago-study group (AGO GYN 5). Gynecol. Oncol. 2014, 133, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Scambia, G.; Bondarenko, I.; Westermann, A.M.; Oaknin, A.; Oza, A.M.; Lisyanskaya, A.S.; Vergote, I.; Wenham, R.M.; Temkin, S.M.; et al. ZoptEC: Phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer (NCT01767155). J. Clin. Oncol. 2018, 36, 5503. [Google Scholar] [CrossRef]
- Gründker, C.; Nia, A.H.; Emons, G. Gonadotropin-releasing hormone receptor-targeted gene therapy of gynecologic cancers. Mol. Cancer Ther. 2005, 4, 225–231. [Google Scholar]
- Curtis, K.K.; Sarantopoulos, J.; Northfelt, D.W.; Weiss, G.J.; Barnhart, K.M.; Whisnant, J.K.; Leuschner, C.; Alila, H.; Borad, M.J.; Ramanathan, R.K. Novel LHRH-receptor-targeted cytolytic peptide, EP-100: First-in-human phase I study in patients with advanced LHRH-receptor-expressing solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Pradeep, S.; Villar-Prados, A.; Wen, Y.; Bayraktar, E.; Mangala, L.S.; Kim, M.S.; Wu, S.Y.; Hu, W.; Rodriguez-Aguayo, C.; et al. GnRH-R—Targeted Lytic Peptide Sensitizes BRCA Wild-type Ovarian Cancer to PARP Inhibition. Mol. Cancer Ther. 2019, 18, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, X.; Feng, W.; Pu, T.; Li, X.; Li, F.; Kang, Y.; Zhang, X.; Xu, C. Gonadotropin-Releasing Hormone Receptor-Targeted Near-Infrared Fluorescence Probe for Specific Recognition and Localization of Peritoneal Metastases of Ovarian Cancer. Front. Oncol. 2020, 10, 266. [Google Scholar] [CrossRef]

| Reference | Pat. | Age | Diagnosis | Drug | Results |
|---|---|---|---|---|---|
| [118] | 41 | 42–79 | advanced ovarian cancer | Triptorelin depot | PR: 6 SD: 5 |
| [120] | 5 | 50–64 | refractory ovarian cancer | Leuprolide acetate 1 mg/day | CR: 1 PR: 4 |
| [121] | 19 11 untreated | 38–73 | progressive ovarian cancer | Triptorelin 0.1 mg/day or 3.2 mg/month depot | SD: 12 |
| [122] | 18 | 21–68 | progressive ovarian cancer | Leuprolide acetate 1.0 mg/day | PR: 4 SD: 2 NE: 5 |
| [124] | 69 66 placebo | <44–>75 | stage III or IV epithelial ovarian cancer | Triptorelin 3.75 mg/month depot | No significant differences in progression free and overall survival |
| [125] | 26 | 49–79 | advanced ovarian cancer | Tamoxifen 20 mg/month Goserelin 3.6 mg/month | CR: 1 PR: 2 SD: 10 |
| [126] | 30 | 38–90 | relapsed epithelial ovarian cancer | Goserelin 3.6 mg/month | PR: 2 SD: 5 |
| [127] | 25 | median 65.5 | advanced epithelial ovarian cancer | Leuprolide acetate | PR: 4 SD:15 |
| [128] | 20 | average 60 | progressive ovarian cancer | Triptorelin 3.75 mg/4 weeks | SD: 14 |
| [129] | 32 | relapsed epithelial ovarian cancer | Leuprolide acetate 3.75 mg depot | PR: 4 SD: 5 | |
| [130] | 14 | 47–77 | advanced epithelial ovarian cancer | Triptorelin 3.2 mg/28 days | SD: 8 |
| [131] | 68 | 42–87 | relapsed ovarian cancer, mostly refractory to platinum | Triptorelin 3.75 mg/on days 1, 8 and 28 followed by 4-weekly | SD: 11 |
| [132] | 32 | 32–77 | platinum-refractory ovarian cancer | Leuprolide 3.75 mg/month | CR: 1 PR: 2 SD: 4 |
| [133] | 37 | 27–75 | platinum- and paclitaxel-refractory ovarian cancer | Leuprolide acetate 3.75 mg/4 weeks | SD: 4 |
| [134] | 12 | 45–68 | advanced ovarian cancer | Leuprolide acetate 3.75 mg on days 1, 8, 28 followed by 28-day intervals | PR: 1 SD: 3 |
| [135] | 23 | 44–72 | refractory ovarian cancer | Goserelin 3.6 mg/month | PR: 4 SD: 7 |
| Reference | Pat. | Age | Diagnosis | Drug | Treatment | Results |
|---|---|---|---|---|---|---|
| [151] | 4 | 55 +/− 11 | epithelial cancers of ovary, endometrium, or breast | Zoptarelin-Doxorubicin | 10, 20, 40, or 80 mg/m2 | maximum tolerated dose: 267 mg/m2 |
| 6 | 59 +/− 5 | 160 mg/m2 | ||||
| 7 | 48 +/− 11 | 267 mg/m2 | ||||
| [152] | 42 | 49 +/− 10 | epithelial ovarian, fallopian tube or primary peritoneal cancer | Zoptarelin-Doxorubicin | 267 mg/m2 | PR: 6 (14.3%) SD: 16 (38.1%) PD: 16 (38.1%) NE: 4 (9.5%) |
| [155] | 28 (F) 9 (M) | 59 (39–80) 61 (39–80) | ovarian, endometrial, breast and other cancers | EP-100 | Initial cohorts: 1–7, 0.6–7.8 mg/m2, n = 21 Later cohorts:: 7–11, 7.8–40 mg/m2, n = 16 | recommended phase II dose: 40 mg/m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gründker, C.; Emons, G. Role of Gonadotropin-Releasing Hormone (GnRH) in Ovarian Cancer. Cells 2021, 10, 437. https://doi.org/10.3390/cells10020437
Gründker C, Emons G. Role of Gonadotropin-Releasing Hormone (GnRH) in Ovarian Cancer. Cells. 2021; 10(2):437. https://doi.org/10.3390/cells10020437
Chicago/Turabian StyleGründker, Carsten, and Günter Emons. 2021. "Role of Gonadotropin-Releasing Hormone (GnRH) in Ovarian Cancer" Cells 10, no. 2: 437. https://doi.org/10.3390/cells10020437
APA StyleGründker, C., & Emons, G. (2021). Role of Gonadotropin-Releasing Hormone (GnRH) in Ovarian Cancer. Cells, 10(2), 437. https://doi.org/10.3390/cells10020437







