A Glucuronic Acid-Palmitoylethanolamide Conjugate (GLUPEA) Is an Innovative Drug Delivery System and a Potential Bioregulator
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Procedure for the Synthesis of the Conjugate between PEA and Glucuronic Acid—GLUPEA
2.2.1. Intermediate 1
2.2.2. Intermediate 2
2.2.3. GLUPEA
2.3. Cell Cultures
2.4. Analysis of PEA and 2-AG Levels by LC-APCI-MS
2.5. Detection of Intracellular Calcium Elevation
2.6. Poly-(I:C)-Induced ACD in HaCaT Cells
2.7. ELISA Assay
2.8. Animals
2.9. DNBS-Induced Colitis in Mice
2.10. Myeloperoxidase (MPO) Activity
2.11. Statistics
3. Results
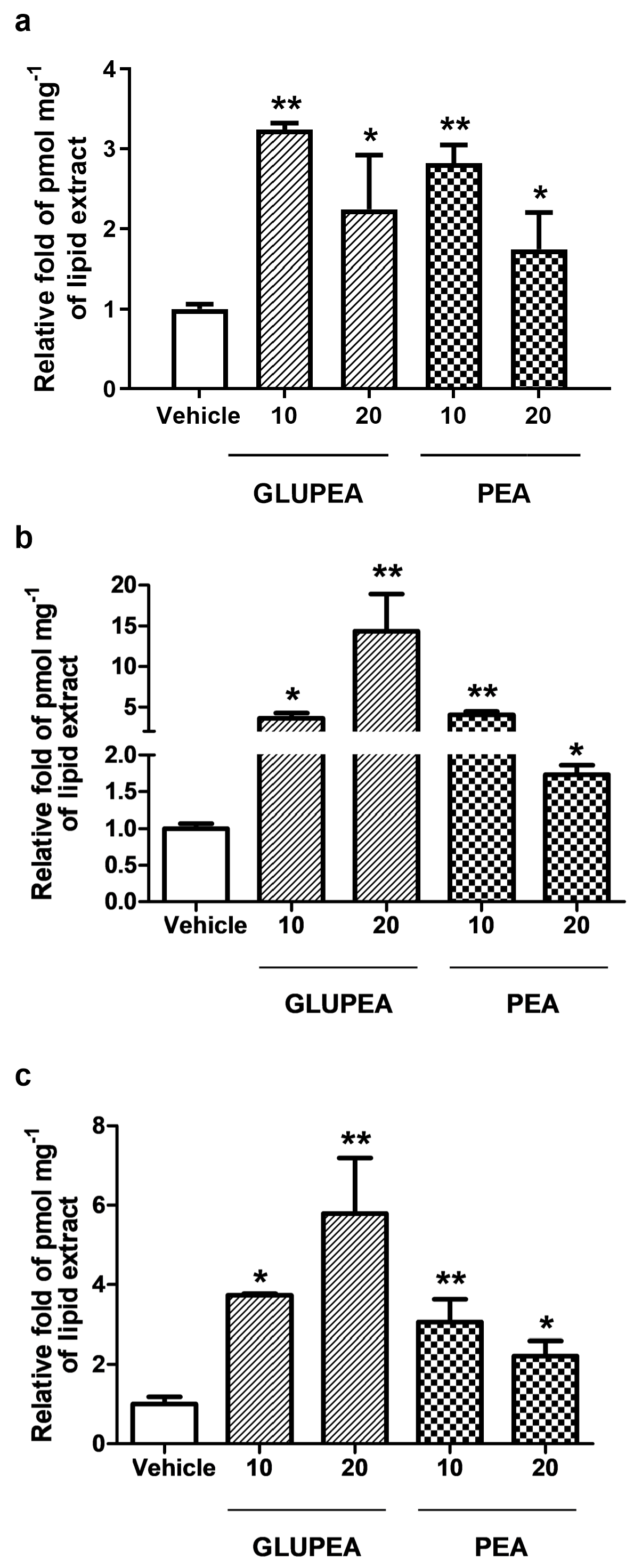
3.1. GLUPEA Releases PEA in HaCaT Keratinocytes
3.2. Glucuronic Acid Does Not Increase Endogenous PEA Levels in HaCaT Keratinocytes
3.3. GLUPEA Increases Endogenous 2-AG Levels in HaCaT Keratinocytes
3.4. GLUPEA Enhances 2-AG-Induced TRPV1 Channel Activation and Desensitization in HEK-293 Transfected with TRPV1 Channels
3.5. GLUPEA Releases PEA in HEK-TRPV1 Cells
3.6. GLUPEA Reduces MCP-2 Chemokine Levels in Poly-(I:C)-Stimulated HaCaT Keratinocytes
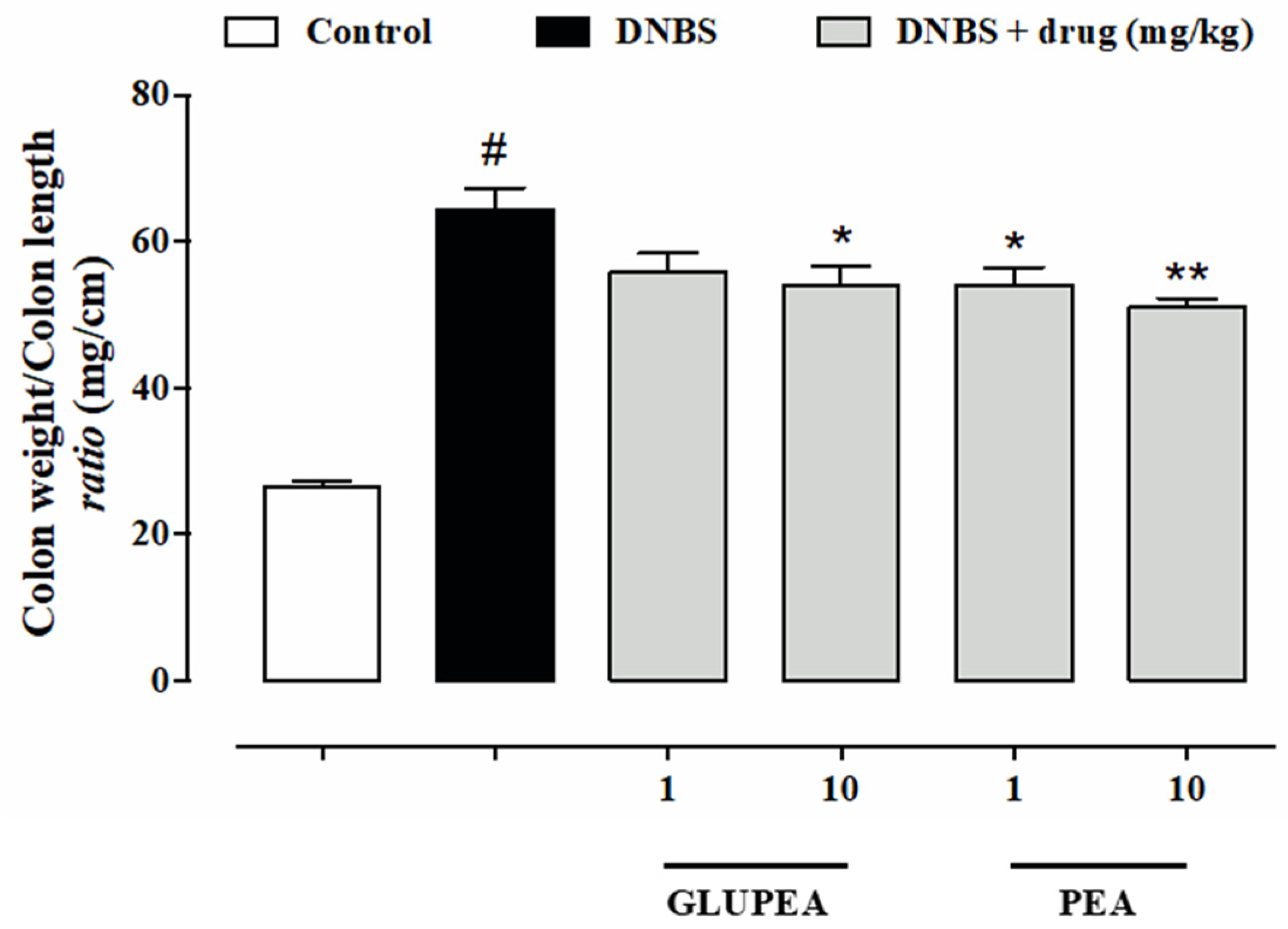
3.7. GLUPEA Reduces the Signs of Colitis in DNBS-Injected Mice
3.8. GLUPEA Reduces MPO Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Coburn, A.F.; Graham, C.E.; Haninger, J. The Effect of Egg Yolk in Diets on Anaphylactic Arthritis (Passive Arthus Phenomenon) in the Guinea Pig. J. Exp. Med. 1954, 100, 425–435. [Google Scholar] [CrossRef]
- Ganley, O.H.; Graessle, O.E.; Robinson, H.J. Anti-Inflammatory Activity on Compounds Obtained from Egg Yolk, Peanut Oil, and Soybean Lecithin. J. Lab. Clin. Med. 1958, 51, 709–714. [Google Scholar]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-Acylethanolamines in Human Reproductive Fluids. Chem. Phys. Lipids 2002, 121, 211–227. [Google Scholar] [CrossRef]
- Venables, B.J.; Waggoner, C.A.; Chapman, K.D. N-Acylethanolamines in Seeds of Selected Legumes. Phytochemistry 2005, 66, 1913–1918. [Google Scholar] [CrossRef]
- Kilaru, A.; Blancaflor, E.B.; Venables, B.J.; Tripathy, S.; Mysore, K.S.; Chapman, K.D. The N-Acylethanolamine-Mediated Regulatory Pathway in Plants. Chem. Biodivers. 2007, 4, 1933–1955. [Google Scholar] [CrossRef]
- Gouveia-Figueira, S.; Nording, M.L. Development and Validation of a Sensitive UPLC-ESI-MS/MS Method for the Simultaneous Quantification of 15 Endocannabinoids and Related Compounds in Milk and Other Biofluids. Anal. Chem. 2014, 86, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Di Marzo, V. The Pharmacology of Palmitoylethanolamide and First Data on the Therapeutic Efficacy of Some of Its New Formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Epps, D.E.; Schmid, P.C.; Natarajan, V.; Schmid, H.H. N-Acylethanolamine Accumulation in Infarcted Myocardium. Biochem. Biophys. Res. Commun. 1979, 90, 628–633. [Google Scholar] [CrossRef]
- Berdyshev, E.V.; Schmid, P.C.; Dong, Z.; Schmid, H.H. Stress-Induced Generation of N-Acylethanolamines in Mouse Epidermal JB6 P+ Cells. Biochem. J. 2000, 346 Pt 2, 369–374. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Croxford, J.L.; Brown, P.; Pertwee, R.G.; Makriyannis, A.; Khanolkar, A.; Layward, L.; Fezza, F.; Bisogno, T.; et al. Endocannabinoids Control Spasticity in a Multiple Sclerosis Model. FASEB J. 2001, 15, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Izzo, A.A.; Fezza, F.; Pinto, A.; Capasso, F.; Mascolo, N.; Di Marzo, V. Inhibitory Effect of Palmitoylethanolamide on Gastrointestinal Motility in Mice. Br. J. Pharmacol. 2001, 134, 945–950. [Google Scholar] [CrossRef]
- Walter, L.; Franklin, A.; Witting, A.; Moller, T.; Stella, N. Astrocytes in Culture Produce Anandamide and Other Acylethanolamides. J. Biol. Chem. 2002, 277, 20869–20876. [Google Scholar] [CrossRef]
- Petrosino, S.; Palazzo, E.; de Novellis, V.; Bisogno, T.; Rossi, F.; Maione, S.; Di Marzo, V. Changes in Spinal and Supraspinal Endocannabinoid Levels in Neuropathic Rats. Neuropharmacology 2007, 52, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Stella, N. Microglia Produce and Hydrolyze Palmitoylethanolamide. Neuropharmacology 2008, 54, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tüting, T.; Bisogno, T.; De Filippis, D.; D’Amico, A.; Saturnino, C.; et al. Protective Role of Palmitoylethanolamide in Contact Allergic Dermatitis. Allergy 2010, 65, 698–711. [Google Scholar] [CrossRef]
- De Filippis, D.; D’Amico, A.; Cipriano, M.; Petrosino, S.; Orlando, P.; Di Marzo, V.; Iuvone, T. Levels of Endocannabinoids and Palmitoylethanolamide and Their Pharmacological Manipulation in Chronic Granulomatous Inflammation in Rats. Pharmacol. Res. 2010, 61, 321–328. [Google Scholar] [CrossRef]
- Abramo, F.; Campora, L.; Albanese, F.; della Valle, M.F.; Cristino, L.; Petrosino, S.; Di Marzo, V.; Miragliotta, V. Increased Levels of Palmitoylethanolamide and Other Bioactive Lipid Mediators and Enhanced Local Mast Cell Proliferation in Canine Atopic Dermatitis. BMC Vet. Res. 2014, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and Endocannabinoid-Related Mediators: Targets, Metabolism and Role in Neurological Disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Schiano Moriello, A. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries-A Systematic Review. Int. J. Mol. Sci. 2020, 21, 9526. [Google Scholar] [CrossRef]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular Characterization of a Phospholipase D Generating Anandamide and Its Congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular Characterization of an Enzyme That Degrades Neuromodulatory Fatty-Acid Amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Ueda, N.; Yamanaka, K.; Yamamoto, S. Purification and Characterization of an Acid Amidase Selective for N-Palmitoylethanolamine, a Putative Endogenous Anti-Inflammatory Substance. J. Biol. Chem. 2001, 276, 35552–35557. [Google Scholar] [CrossRef] [PubMed]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The Nuclear Receptor Peroxisome Proliferator-Activated Receptor-Alpha Mediates the Anti-Inflammatory Actions of Palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The Orphan Receptor GPR55 Is a Novel Cannabinoid Receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Ambrosino, P.; Soldovieri, M.V.; Russo, C.; Taglialatela, M. Activation and Desensitization of TRPV1 Channels in Sensory Neurons by the PPARα Agonist Palmitoylethanolamide. Br. J. Pharmacol. 2013, 168, 1430–1444. [Google Scholar] [CrossRef]
- Ambrosino, P.; Soldovieri, M.V.; De Maria, M.; Russo, C.; Taglialatela, M. Functional and Biochemical Interaction between PPARα Receptors and TRPV1 Channels: Potential Role in PPARα Agonists-Mediated Analgesia. Pharmacol. Res. 2014, 87, 113–122. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Boccella, S.; Giordano, M.E.; Romano, R.; Bellini, G.; Manzo, I.; Furiano, A.; Rizzo, A.; Imperatore, R.; et al. Palmitoylethanolamide Induces Microglia Changes Associated with Increased Migration and Phagocytic Activity: Involvement of the CB2 Receptor. Sci. Rep. 2017, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide Inhibits the Expression of Fatty Acid Amide Hydrolase and Enhances the Anti-Proliferative Effect of Anandamide in Human Breast Cancer Cells. Biochem. J. 2001, 358, 249–255. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Verde, R.; Allarà, M.; Imperatore, R.; Ligresti, A.; Mahmoud, A.M.; Peritore, A.F.; Iannotti, F.A.; Di Marzo, V. Palmitoylethanolamide Counteracts Substance P-Induced Mast Cell Activation in Vitro by Stimulating Diacylglycerol Lipase Activity. J. Neuroinflamm. 2019, 16, 274. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned CDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid Receptors on Sensory Nerves Mediate the Vasodilator Action of Anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Ermund, A.; Movahed, P.; Andersson, D.A.; Simonsen, C.; Jönsson, B.A.G.; Blomgren, A.; Birnir, B.; Bevan, S.; Eschalier, A.; et al. Monoacylglycerols Activate TRPV1--a Link between Phospholipase C and TRPV1. PLoS ONE 2013, 8, e81618. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Davis, J.B.; Di Marzo, V. Palmitoylethanolamide Enhances Anandamide Stimulation of Human Vanilloid VR1 Receptors. FEBS Lett. 2001, 506, 253–256. [Google Scholar] [CrossRef]
- Ho, W.-S.V.; Barrett, D.A.; Randall, M.D. “Entourage” Effects of N-Palmitoylethanolamide and N-Oleoylethanolamide on Vasorelaxation to Anandamide Occur through TRPV1 Receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The Anti-Inflammatory Mediator Palmitoylethanolamide Enhances the Levels of 2-Arachidonoyl-Glycerol and Potentiates Its Actions at TRPV1 Cation Channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-Hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Artamonov, M.; Zhukov, O.; Shuba, I.; Storozhuk, L.; Khmel, T.; Klimashevsky, V.; Mikosha, A.; Gula, N. Incorporation of Labelled N-Acylethanolamine (NAE) into Rat Brain Regions In Vivo and Adaptive Properties of Saturated NAE under X-ray Irradiation. Ukr. Biokhimicheskii Zhurnal 2005, 77, 51–62. [Google Scholar]
- Vacondio, F.; Bassi, M.; Silva, C.; Castelli, R.; Carmi, C.; Scalvini, L.; Lodola, A.; Vivo, V.; Flammini, L.; Barocelli, E.; et al. Amino Acid Derivatives as Palmitoylethanolamide Prodrugs: Synthesis, In Vitro Metabolism and In Vivo Plasma Profile in Rats. PLoS ONE 2015, 10, e0128699. [Google Scholar] [CrossRef]
- Rasenack, N.; Müller, B.W. Micron-Size Drug Particles: Common and Novel Micronization Techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Campolo, M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. Effect of a New Formulation of Micronized and Ultramicronized N-Palmitoylethanolamine in a Tibia Fracture Mouse Model of Complex Regional Pain Syndrome. PLoS ONE 2017, 12, e0178553. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic Acid-Paclitaxel Conjugate Micelles: Synthesis, Characterization, and Antitumor Activity. Bioconjug. Chem. 2008, 19, 1319–1325. [Google Scholar] [CrossRef]
- Manju, S.; Sreenivasan, K. Conjugation of Curcumin onto Hyaluronic Acid Enhances Its Aqueous Solubility and Stability. J. Colloid Interface Sci. 2011, 359, 318–325. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Deepagan, V.G.; Jayakumar, R.; Park, J.H. Hyaluronic Acid-Based Conjugates for Tumor-Targeted Drug Delivery and Imaging. J. Biomed. Nanotechnol. 2014, 10, 17–31. [Google Scholar] [CrossRef]
- Clementi, C.; Miller, K.; Mero, A.; Satchi-Fainaro, R.; Pasut, G. Dendritic Poly(Ethylene Glycol) Bearing Paclitaxel and Alendronate for Targeting Bone Neoplasms. Mol. Pharm. 2011, 8, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.R.; Lee, H.J.; Kim, J.-D. Histidine-Conjugated Poly(Amino Acid) Derivatives for the Novel Endosomolytic Delivery Carrier of Doxorubicin. J. Control. Release 2006, 114, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, G.; Zhang, J.; Lin, W.; Ji, F.; Bernards, M.T.; Chen, S. Development of Zwitterionic Polymer-Based Doxorubicin Conjugates: Tuning the Surface Charge to Prolong the Circulation and Reduce Toxicity. Langmuir 2014, 30, 3764–3774. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Baik, H.J.; Oh, Y.T.; Oh, K.T.; Youn, Y.S.; Lee, E.S. A Smart Polysaccharide/Drug Conjugate for Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2011, 50, 1644–1647. [Google Scholar] [CrossRef]
- Jiao, Y.; Pang, X.; Zhai, G. Advances in Hyaluronic Acid-Based Drug Delivery Systems. Curr. Drug Targets 2016, 17, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Higuchi, Y.; Kitamura, H.; Murao, N.; Saitoh, R.; Morikawa, T.; Sato, H. Novel Hyaluronic Acid-Methotrexate Conjugate Suppresses Joint Inflammation in the Rat Knee: Efficacy and Safety Evaluation in Two Rat Arthritis Models. Arthritis Res. Ther. 2016, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.-S.; Chen, C.-W.; Lin, C.-A.; Yu, H.-P.; Lin, H.-Y.; Liao, M.-Y.; Wu, S.-H.; Lin, Y.-F.; Lai, P.-S. Hyaluronic Acid-Nimesulide Conjugates as Anticancer Drugs against CD44-Overexpressing HT-29 Colorectal Cancer in Vitro and in Vivo. Int. J. Nanomed. 2017, 12, 2315–2333. [Google Scholar] [CrossRef]
- Bisogno, T.; Maurelli, S.; Melck, D.; De Petrocellis, L.; Di Marzo, V. Biosynthesis, Uptake, and Degradation of Anandamide and Palmitoylethanolamide in Leukocytes. J. Biol. Chem. 1997, 272, 3315–3323. [Google Scholar] [CrossRef]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgänsberger, W.; et al. The Endogenous Cannabinoid System Controls Extinction of Aversive Memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef]
- Pagano, E.; Romano, B.; Iannotti, F.A.; Parisi, O.A.; D’Armiento, M.; Pignatiello, S.; Coretti, L.; Lucafò, M.; Venneri, T.; Stocco, G.; et al. The Non-Euphoric Phytocannabinoid Cannabidivarin Counteracts Intestinal Inflammation in Mice and Cytokine Expression in Biopsies from UC Pediatric Patients. Pharmacol. Res. 2019, 149, 104464. [Google Scholar] [CrossRef]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.-L.; Sibaev, A.; Storr, M.; Lutz, B. The Endogenous Cannabinoid System Protects against Colonic Inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide, a Naturally Occurring Lipid, Is an Orally Effective Intestinal Anti-Inflammatory Agent. Br. J. Pharmacol. 2015, 172, 142–158. [Google Scholar] [CrossRef] [PubMed]




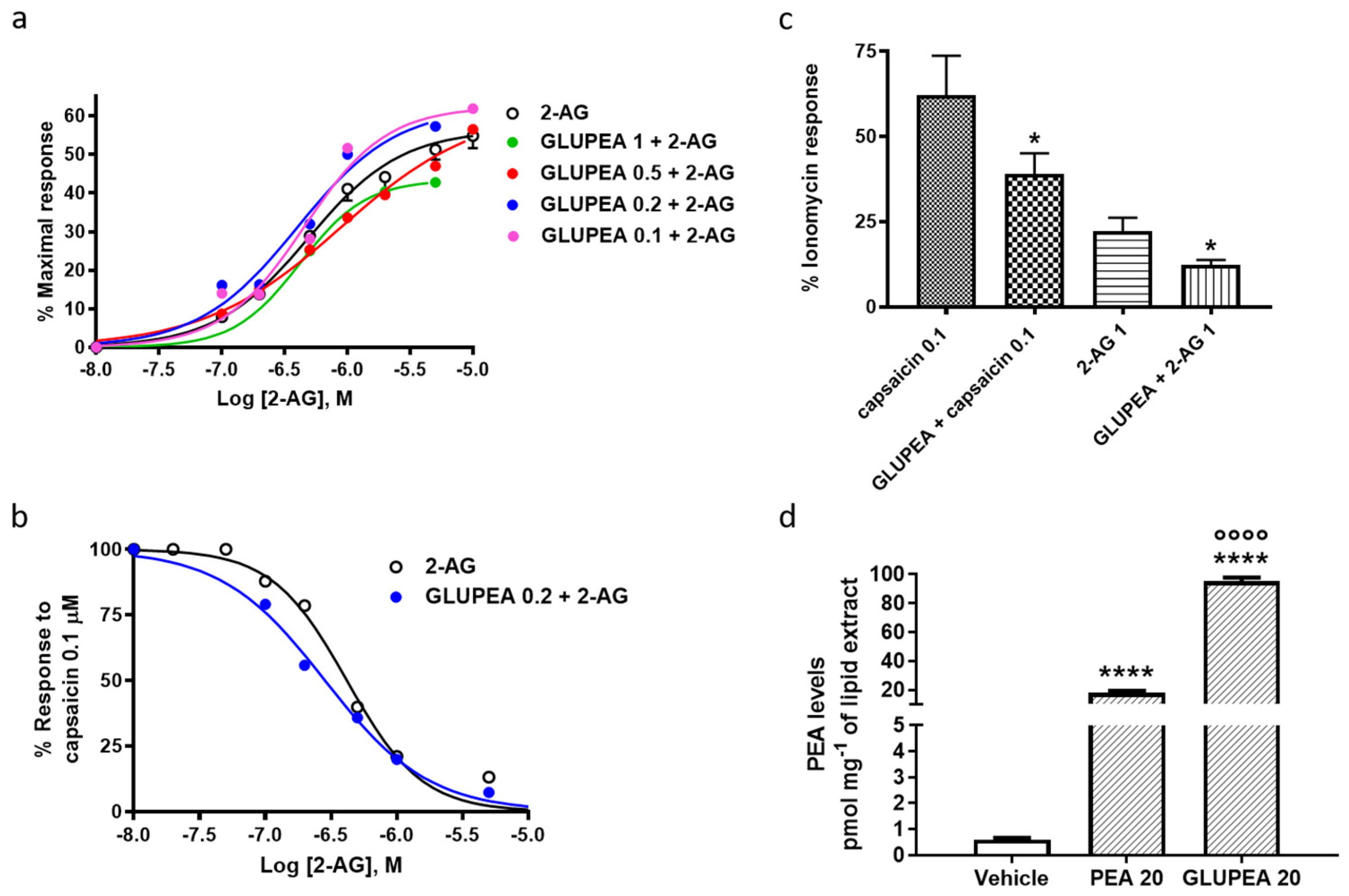

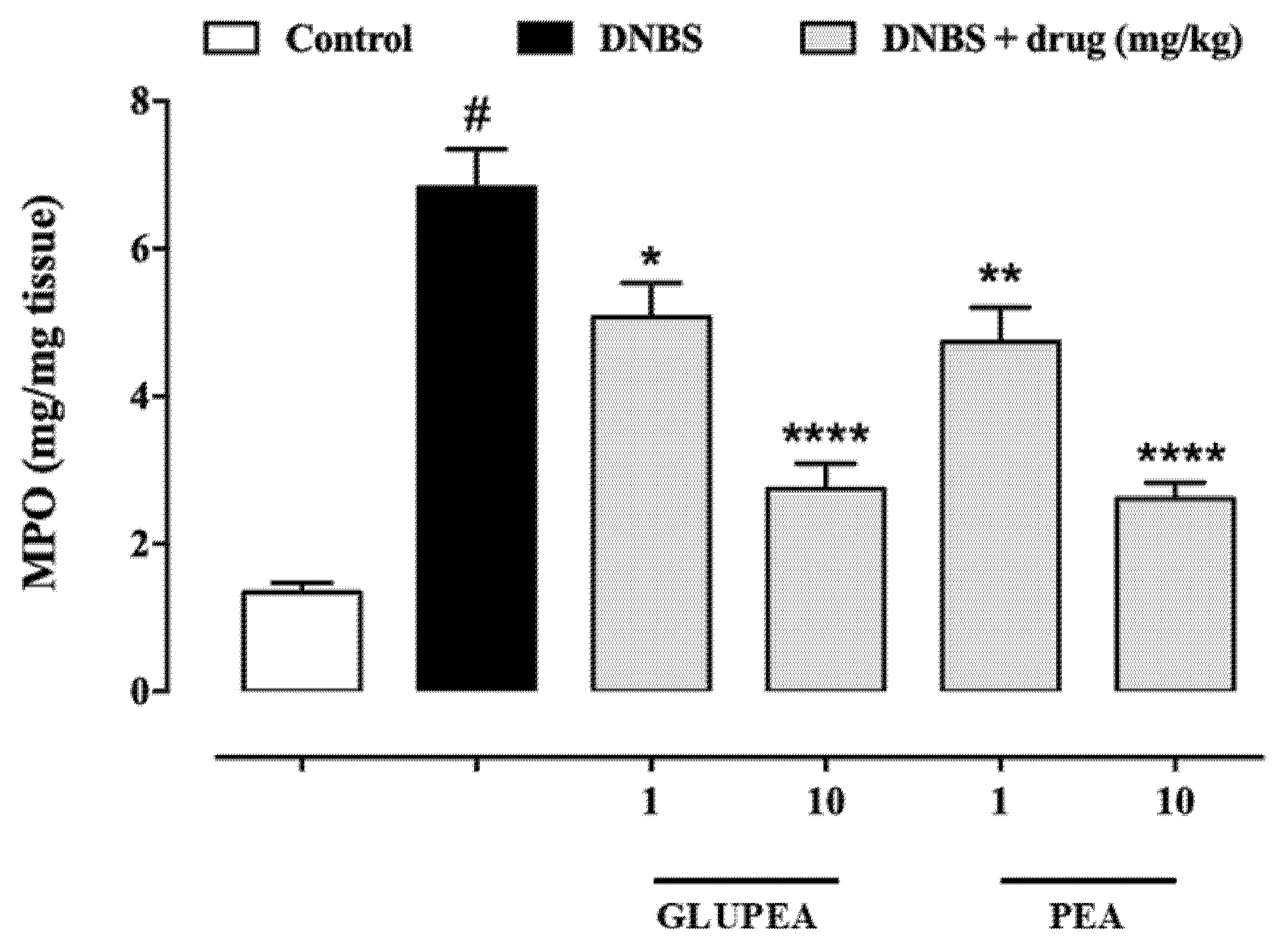
| EC50 | IC50 (vs. Capsaicin 0.1 μM) | % Efficacy (vs. Ionomycin 4 μM) | |
|---|---|---|---|
| 2-AG | 0.48 ± 0.04 | 0.42 ± 0.02 | 56.4 ± 1.0 |
| GLUPEA 1.0 μM + 2-AG | 0.42 ± 0.01 | 1.07 ± 0.06 | 43.4 ± 0.1 |
| GLUPEA 0.5 μM + 2-AG | 0.89 ± 0.14 | 0.43 ± 0.03 | 62.2 ± 3.1 |
| GLUPEA 0.2 μM + 2-AG | 0.40 ± 0.08 | 0.28 ± 0.01 ** | 62.3 ± 5.0 |
| GLUPEA 0.1 μM + 2-AG | 0.45 ± 0.05 | 0.38 ± 0.01 | 62.4 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzo, E.; Schiano Moriello, A.; Tinto, F.; Verde, R.; Allarà, M.; De Petrocellis, L.; Pagano, E.; Izzo, A.A.; Di Marzo, V.; Petrosino, S. A Glucuronic Acid-Palmitoylethanolamide Conjugate (GLUPEA) Is an Innovative Drug Delivery System and a Potential Bioregulator. Cells 2021, 10, 450. https://doi.org/10.3390/cells10020450
Manzo E, Schiano Moriello A, Tinto F, Verde R, Allarà M, De Petrocellis L, Pagano E, Izzo AA, Di Marzo V, Petrosino S. A Glucuronic Acid-Palmitoylethanolamide Conjugate (GLUPEA) Is an Innovative Drug Delivery System and a Potential Bioregulator. Cells. 2021; 10(2):450. https://doi.org/10.3390/cells10020450
Chicago/Turabian StyleManzo, Emiliano, Aniello Schiano Moriello, Francesco Tinto, Roberta Verde, Marco Allarà, Luciano De Petrocellis, Ester Pagano, Angelo A. Izzo, Vincenzo Di Marzo, and Stefania Petrosino. 2021. "A Glucuronic Acid-Palmitoylethanolamide Conjugate (GLUPEA) Is an Innovative Drug Delivery System and a Potential Bioregulator" Cells 10, no. 2: 450. https://doi.org/10.3390/cells10020450
APA StyleManzo, E., Schiano Moriello, A., Tinto, F., Verde, R., Allarà, M., De Petrocellis, L., Pagano, E., Izzo, A. A., Di Marzo, V., & Petrosino, S. (2021). A Glucuronic Acid-Palmitoylethanolamide Conjugate (GLUPEA) Is an Innovative Drug Delivery System and a Potential Bioregulator. Cells, 10(2), 450. https://doi.org/10.3390/cells10020450









