Aging-Related Changes in the Ultrastructure of Hepatocytes and Cardiomyocytes of Elderly Mice Are Enhanced in ApoE-Deficient Animals
Abstract
1. Introduction
2. Materials and Methods
3. Results
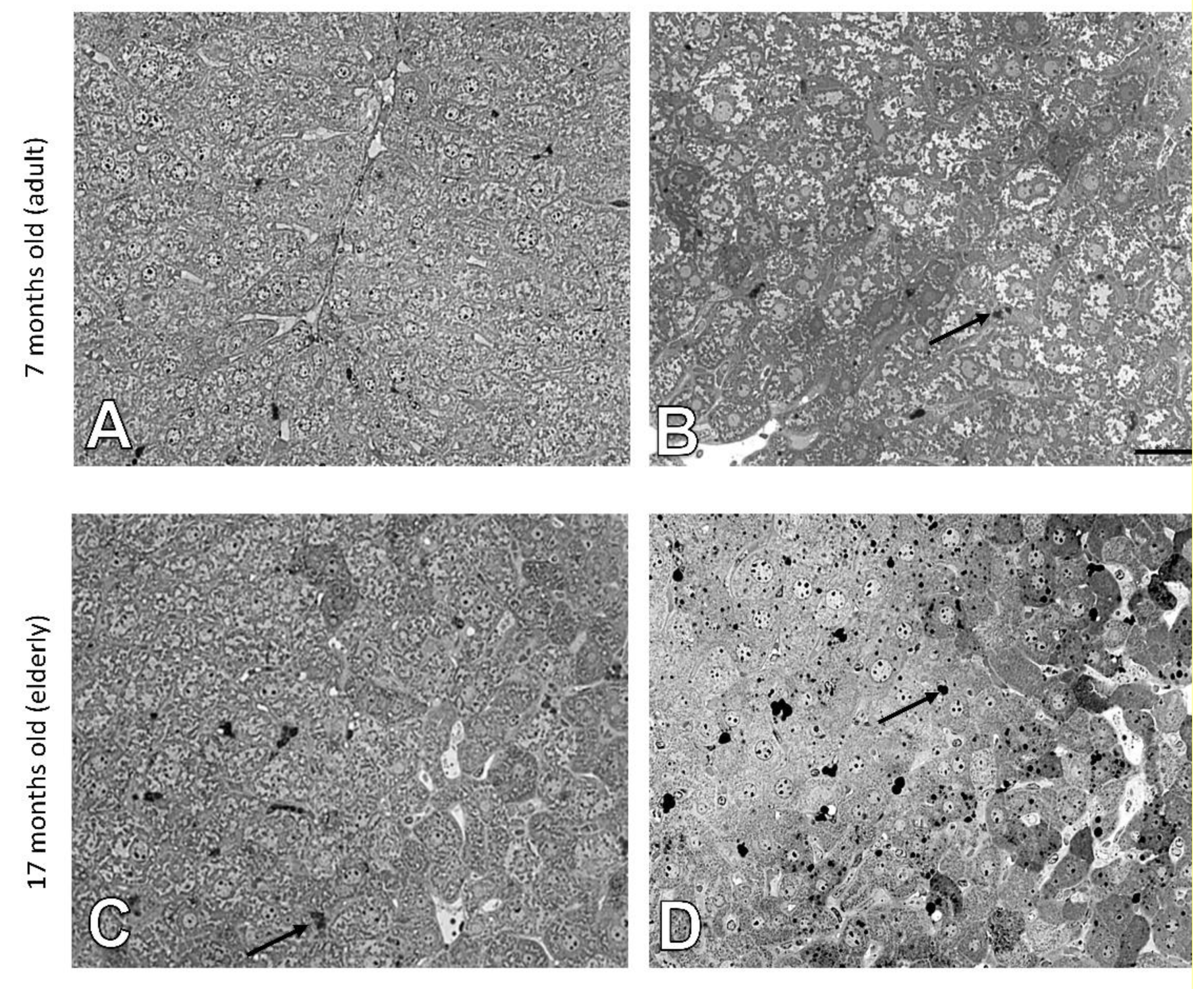
3.1. Age-Related Changes in the Structure of Hepatocytes
3.2. Age-Related Changes in the Structure of Cardiomyocytes
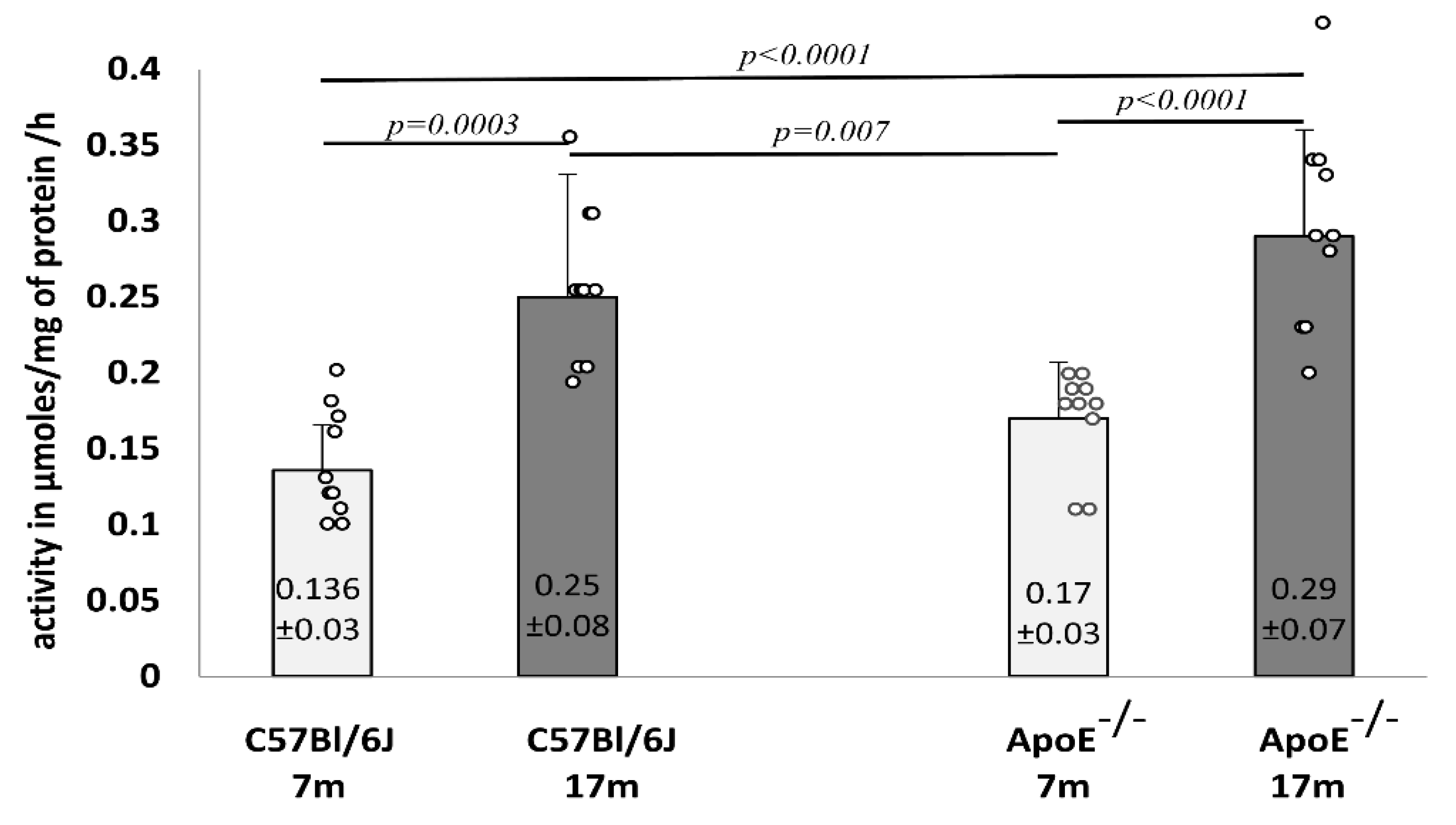
3.3. Age-Related Changes of Biochemical Markers
3.4. Analysis of Lysosomal Beta-Galactosidase
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregg, S.Q.; Gutiérrez, V.; Robinson, A.R.; Woodell, T.; Nakao, A.; Ross, M.A.; Michalopoulos, G.K.; Rigatti, L.; Rothermel, C.E.; Kamileri, I.; et al. A Mouse Model of Accelerated Liver Aging Due to a Defect in DNA Repair. Hepatology 2012, 55, 609–621. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Aggarwal, B.B.; Barreca, D.; Battino, M.; Belwal, T.; Horbańczuk, O.K.; Berindan-Neagoe, I.; Bishayee, A.; Daglia, M.; Devkota, H.P.; et al. Dietary Natural Products and Their Potential to Influence Health and Disease Including Animal Model Studies. Anim. Sci. Pap. Rep. 2019, 36, 345–358. [Google Scholar]
- Yen, W.-L.; Klionsky, D.J. How to Live Long and Prosper: Autophagy, Mitochondria, and Aging. Physiology (Bethesda) 2008, 23, 248–262. [Google Scholar] [CrossRef]
- Levine, B. Eating Oneself and Uninvited Guests: Autophagy-Related Pathways in Cellular Defense. Cell 2005, 120, 159–162. [Google Scholar] [CrossRef]
- Son, H.G.; Altintas, O.; Kim, E.J.E.; Kwon, S.; Lee, S.-J.V. Age-Dependent Changes and Biomarkers of Aging in Caenorhabditis Elegans. Aging Cell 2019, 18, e12853. [Google Scholar] [CrossRef]
- Khraiwesh, H.; López-Domínguez, J.A.; del Río, L.F.; Gutierrez-Casado, E.; López-Lluch, G.; Navas, P.; de Cabo, R.; Ramsey, J.J.; Burón, M.I.; Villalba, J.M.; et al. Mitochondrial Ultrastructure and Markers of Dynamics in Hepatocytes from Aged, Calorie Restricted Mice Fed with Different Dietary Fats. Exp. Gerontol. 2014, 56, 77–88. [Google Scholar] [CrossRef]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef]
- Brandt, T.; Mourier, A.; Tain, L.S.; Partridge, L.; Larsson, N.-G.; Kühlbrandt, W. Changes of Mitochondrial Ultrastructure and Function during Ageing in Mice and Drosophila. eLife 2017, 6, e24662. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Jóźwik, A.; Łysek-Gładysińska, M.; Grzybek, W.; Adamus-Białek, W.; Bicki, J.; Strzałkowska, N.; Kamińska, A.; Horbańczuk, O.K.; Atanasov, A.G. Fenugreek (Trigonella Foenum-Graecum L.) Seeds Dietary Supplementation Regulates Liver Antioxidant Defense Systems in Aging Mice. Nutrients 2020, 12, 2552. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Orhan, I.E.; Aggarwal, B.B.; Battino, M.; Belwal, T.; Bishayee, A.; Daglia, M.; Devkota, H.P.; El-Demerdash, A.; Balacheva, A.A.; et al. Berberine, a Popular Dietary Supplement for Human and Animal Health: Quantitative Research Literature Analysis—A Review. Anim. Sci. Pap. Rep. 2020, 38, 5–19. [Google Scholar]
- Hunt, N.J.; Kang, S.W.S.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Bakala, H.; Delaval, E.; Hamelin, M.; Bismuth, J.; Borot-Laloi, C.; Corman, B.; Friguet, B. Changes in Rat Liver Mitochondria with Aging. Eur. J. Biochem. 2003, 270, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Howlett, S.E. Age-Related Deficit Accumulation and the Diseases of Ageing. Mech. Ageing Dev. 2019, 180, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.; Correia-Gomes, C. Long Live the Liver: Immunohistochemical and Stereological Study of Hepatocytes, Liver Sinusoidal Endothelial Cells, Kupffer Cells and Hepatic Stellate Cells of Male and Female Rats throughout Ageing. Cell Tissue Res. 2016, 366, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Premoli, A.; Paschetta, E.; Hvalryg, M.; Spandre, M.; Bo, S.; Durazzo, M. Characteristics of Liver Diseases in the Elderly: A Review. Minerva Gastroenterol. Dietol. 2009, 55, 71–78. [Google Scholar]
- Gan, L.; Chitturi, S.; Farrell, G.C. Mechanisms and Implications of Age-Related Changes in the Liver: Nonalcoholic Fatty Liver Disease in the Elderly. Curr. Gerontol. Geriatr. Res. 2011, 2011, 831536. [Google Scholar] [CrossRef] [PubMed]
- Sheedfar, F.; Biase, S.D.; Koonen, D.; Vinciguerra, M. Liver Diseases and Aging: Friends or Foes? Aging Cell 2013, 12, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Strait, J.B.; Lakatta, E.G. Aging-Associated Cardiovascular Changes and Their Relationship to Heart Failure. Heart Fail. Clin. 2012, 8, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Tandler, B.; Dunlap, M.; Hoppel, C.L.; Hassan, M. Giant Mitochondria in a Cardiomyopathic Heart. Ultrastruct. Pathol. 2002, 26, 177–183. [Google Scholar] [CrossRef]
- Hoppel, C.L.; Tandler, B.; Fujioka, H.; Riva, A. Dynamic Organization of Mitochondria in Human Heart and in Myocardial Disease. Int. J. Biochem. Cell Biol. 2009, 41, 1949–1956. [Google Scholar] [CrossRef]
- Bonomini, F.; Rodella, L.F.; Moghadasian, M.; Lonati, C.; Rezzani, R. Apolipoprotein E Deficiency and a Mouse Model of Accelerated Liver Aging. Biogerontology 2013, 14, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Lysek-Gladysinska, M.; Wieczorek, A.; Walaszczyk, A.; Jelonek, K.; Jozwik, A.; Pietrowska, M.; Dörr, W.; Gabrys, D.; Widlak, P. Long-Term Effects of Low-Dose Mouse Liver Irradiation Involve Ultrastructural and Biochemical Changes in Hepatocytes That Depend on Lipid Metabolism. Radiat. Environ. Biophys. 2018, 57, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.; Lysek-Gladysinska, M.; Walaszczyk, A.; Jelonek, K.; Smolarz, M.; Pietrowska, M.; Gabrys, D.; Kulik, R.; Widlak, P. Changes in Activity and Structure of Lysosomes from Liver of Mouse Irradiated in Vivo. Int. J. Radiat. Biol. 2018, 94, 443–453. [Google Scholar] [CrossRef]
- Hoare, M.; Das, T.; Alexander, G. Ageing, Telomeres, Senescence, and Liver Injury. J. Hepatol. 2010, 53, 950–961. [Google Scholar] [CrossRef]
- Shah, S.; Sass, D. Cardiac Hepatopathy. A review of Liver Dysfunction in Heart Failure. Liver Res. Open J. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Marzella, L.; Glaumann, H. Increased degradation in rat liver induced by vinblastine. II. Morphologic characterization. Lab. Investig. J. Tech. Methods Pathol. 1980, 42, 18–19. [Google Scholar]
- Marzella, M.; Glaumann, G. Increased Degradation in Rat Liver Induced by Vinblastine. I. Biochemical Characterization. Lab. Investig. 1980, 42, 8–17. [Google Scholar]
- Barrett, A.J.; Heath, M.F. Lysosomal enzymes. In Lysosomes Laboratory Handbook; Dingle, J.T., Ed.; North Holland Publishing Company: Amsterdam, The Netherlands, 1977; pp. 19–145. [Google Scholar]
- Kirschke, H.; Wiederanders, B. Methods for Determining Proteinases Activity; Congress and Conference Reports of the Martin-Luther University; Halle-Wittenberg: Halle, Germany, 1984; pp. 11–17. (In German) [Google Scholar]
- Anantharaju, A.; Feller, A.; Chedid, A. Aging Liver. Gerontology 2002, 48, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Huda, N.; Liu, G.; Hong, H.; Yan, S.; Khambu, B.; Yin, X.-M. Hepatic Senescence, the Good and the Bad. World J. Gastroenterol. 2019, 25, 5069–5081. [Google Scholar] [CrossRef]
- Alvarez, A.M.; Mukherjee, D. Liver Abnormalities in Cardiac Diseases and Heart Failure. Int. J. Angiol. 2011, 20, 135–142. [Google Scholar] [CrossRef]
- Allen, L.A.; Felker, G.M.; Pocock, S.; McMurray, J.J.V.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; Granger, C.B.; et al. Liver Function Abnormalities and Outcome in Patients with Chronic Heart Failure: Data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Eur. J. Heart Fail. 2009, 11, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Pendse, A.A.; Arbones-Mainar, J.M.; Johnson, L.A.; Altenburg, M.K.; Maeda, N. Apolipoprotein E Knock-out and Knock-in Mice: Atherosclerosis, Metabolic Syndrome, and Beyond. J. Lipid Res. 2009, 50, S178–S182. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.M.; Yehia, R. Hepato-Cardiac Disorders. World J. Hepatol. 2014, 6, 41–54. [Google Scholar] [CrossRef]
- Schmucker, D.L. Aging and the Liver: An Update. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, B315–B320. [Google Scholar] [CrossRef]
- Wang, M.-J.; Chen, F.; Li, J.-X.; Liu, C.-C.; Zhang, H.-B.; Xia, Y.; Yu, B.; You, P.; Xiang, D.; Lu, L.; et al. Reversal of Hepatocyte Senescence after Continuous in Vivo Cell Proliferation. Hepatology 2014, 60, 349–361. [Google Scholar] [CrossRef]
- Trifunovic, A.; Larsson, N.-G. Mitochondrial Dysfunction as a Cause of Ageing. J. Intern. Med. 2008, 263, 167–178. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.-G. The Role of Mitochondria in Aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Kõks, S.; Dogan, S.; Tuna, B.G.; González-Navarro, H.; Potter, P.; Vandenbroucke, R.E. Mouse Models of Ageing and Their Relevance to Disease. Mech. Ageing Dev. 2016, 160, 41–53. [Google Scholar] [CrossRef]
- Stroikin, Y.; Dalen, H.; Brunk, U.T.; Terman, A. Testing the “Garbage” Accumulation Theory of Ageing: Mitotic Activity Protects Cells from Death Induced by Inhibition of Autophagy. Biogerontology 2005, 6, 39–47. [Google Scholar] [CrossRef]
- Spazzafumo, L.; Mensà, E.; Matacchione, G.; Galeazzi, T.; Zampini, L.; Recchioni, R.; Marcheselli, F.; Prattichizzo, F.; Testa, R.; Antonicelli, R.; et al. Age-Related Modulation of Plasmatic Beta-Galactosidase Activity in Healthy Subjects and in Patients Affected by T2DM. Oncotarget 2017, 8, 93338–93348. [Google Scholar] [CrossRef]
- Terman, A.; Gustafsson, B.; Brunk, U.T. Autophagy, Organelles and Ageing. J. Pathol. 2007, 211, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Gouras, P.; Ivert, L.; Neuringer, M.; Nagasaki, T. Mitochondrial Elongation in the Macular RPE of Aging Monkeys, Evidence of Metabolic Stress. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.A.; Broughton, K.M.; Firouzi, F.; Sussman, M.A. Cardiac Ageing: Extrinsic and Intrinsic Factors in Cellular Renewal and Senescence. Nat. Rev. Cardiol. 2018, 15, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Buzello, M.; Törnig, J.; Faulhaber, J.; Ehmke, H.; Ritz, E.; Amann, K. The Apolipoprotein e Knockout Mouse: A Model Documenting Accelerated Atherogenesis in Uremia. J. Am. Soc. Nephrol. 2003, 14, 311–316. [Google Scholar] [CrossRef]
- Kaneva, A.M.; Bojko, E.R.; Potolitsyna, N.N.; Odland, J.O. Plasma Levels of Apolipoprotein-E in Residents of the European North of Russia. Lipids Health Dis. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Leonova, E.I.; Sadovnikova, E.S.; Shaykhutdinova, E.R.; Galzitskaya, O.V.; Murashev, A.N.; Solonin, A.S. Hepatic and Aortic Arch Expression and Serum Levels of Syndecan-1 in ApoE−/− Mice. Open Biochem. J. 2017, 11, 77–93. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysek-Gładysińska, M.; Wieczorek, A.; Jóźwik, A.; Walaszczyk, A.; Jelonek, K.; Szczukiewicz-Markowska, G.; Horbańczuk, O.K.; Pietrowska, M.; Widłak, P.; Gabryś, D. Aging-Related Changes in the Ultrastructure of Hepatocytes and Cardiomyocytes of Elderly Mice Are Enhanced in ApoE-Deficient Animals. Cells 2021, 10, 502. https://doi.org/10.3390/cells10030502
Łysek-Gładysińska M, Wieczorek A, Jóźwik A, Walaszczyk A, Jelonek K, Szczukiewicz-Markowska G, Horbańczuk OK, Pietrowska M, Widłak P, Gabryś D. Aging-Related Changes in the Ultrastructure of Hepatocytes and Cardiomyocytes of Elderly Mice Are Enhanced in ApoE-Deficient Animals. Cells. 2021; 10(3):502. https://doi.org/10.3390/cells10030502
Chicago/Turabian StyleŁysek-Gładysińska, Małgorzata, Anna Wieczorek, Artur Jóźwik, Anna Walaszczyk, Karol Jelonek, Grażyna Szczukiewicz-Markowska, Olaf K. Horbańczuk, Monika Pietrowska, Piotr Widłak, and Dorota Gabryś. 2021. "Aging-Related Changes in the Ultrastructure of Hepatocytes and Cardiomyocytes of Elderly Mice Are Enhanced in ApoE-Deficient Animals" Cells 10, no. 3: 502. https://doi.org/10.3390/cells10030502
APA StyleŁysek-Gładysińska, M., Wieczorek, A., Jóźwik, A., Walaszczyk, A., Jelonek, K., Szczukiewicz-Markowska, G., Horbańczuk, O. K., Pietrowska, M., Widłak, P., & Gabryś, D. (2021). Aging-Related Changes in the Ultrastructure of Hepatocytes and Cardiomyocytes of Elderly Mice Are Enhanced in ApoE-Deficient Animals. Cells, 10(3), 502. https://doi.org/10.3390/cells10030502








