Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space
Abstract
:1. Introduction
2. The CoV Budding Compartment
3. The Assembly and Exit Strategies of CoVs Are Not Unique
4. CoV Assembly at the IC Membranes
5. A Viral Perspective on IC Organization
6. Golgi Stack-Independent Secretion of CoVs
7. Mechanisms and Consequences of CoV-Induced Golgi Disassembly
8. Passage of Large Cargo across the Golgi Ribbon
9. Summary and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BFA | Brefeldin A |
| COPI | coatomer protein I |
| CoV | coronavirus |
| EM | electron microscopy |
| ER | endoplasmic reticulum |
| ERES | ER exit site |
| ERGIC | ER-Golgi intermediate compartment |
| GalNAc | N-acetyl-galactosamine |
| GM130 | Golgi matrix protein of 130 kD |
| GRASP65 | Golgi reassembly and stacking protein of 65 kD |
| HCV | hepatitis C virus |
| IBV | avian infectious bronchitis virus |
| IC | pre-Golgi intermediate compartment |
| KDEL-receptor | Lys-Asp-Glu-Leu tetrapeptide receptor |
| LAMP-1 | lysosome-associated membrane protein-1 |
| MHV | mouse hepatitis virus |
| MT | microtubule |
| PM | plasma membrane |
| Rab | rat brain |
| RE | recycling endosome |
| TANGO-1 | transport and Golgi organization protein-1 |
| TGN | trans-Golgi network |
| VLDL | very low density lipoprotein |
| VLP | virus-like particle |
| VTC | vesicular tubular cluster |
References
- Pettersson, R.F. Protein localization and virus assembly at intracellular membranes. Curr. Top. Microbiol. Immunol. 1991, 170, 67–106. [Google Scholar]
- Griffiths, G.; Rottier, P. Cell biology of viruses that assemble along the biosynthetic pathway. Semin. Cell Biol. 1992, 3, 367–381. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. The ins and outs of hepatitis C virus entry and assembly. Nat. Rev. Microbiol. 2013, 11, 688–700. [Google Scholar] [CrossRef] [Green Version]
- Ruch, T.R.; Machamer, C.E. The coronavirus E protein: Assembly and beyond. Viruses 2012, 4, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Sturman, L.S.; Holmes, K.V. The molecular biology of coronaviruses. Adv. Virus Res. 1983, 28, 35–112. [Google Scholar]
- Tooze, J.; Tooze, S.A.; Warren, G. Replication of coronavirus MHV-A59 in sac-cells: Determination of the first site of budding of progeny virions. Eur. J. Cell Biol. 1984, 33, 281–293. [Google Scholar]
- Palade, G. Intracellular aspects of the process of protein synthesis. Science 1975, 189, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Tooze, J.; Warren, G. Site of addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J. Cell Biol. 1988, 106, 1475–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tooze, J.; Tooze, S.A. Infection of AtT20 murine pituitary tumor cells by mouse hepatitis virus strain A59: Virus budding is restricted to the Golgi region. Eur. J. Cell Biol. 1985, 37, 203–212. [Google Scholar]
- Tooze, S. Biogenesis of the E1 Glycoprotein of MHV-A59: Characterization and Use as a Model for Intracellular Membrane Proteins. Ph.D Thesis, University of Heidelberg, Heidelberg, Germany, 1987. [Google Scholar]
- Saraste, J.; Kuismanen, E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell 1984, 38, 535–549. [Google Scholar] [CrossRef]
- Pelham, H.R. Control of protein exit from the endoplasmic reticulum. Annu. Rev. Cell Biol. 1989, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Saraste, J.; Marie, M. Intermediate compartment (IC): From pre-Golgi vacuoles to a semi-autonomous membrane system. Histochem. Cell Biol. 2018, 150, 407–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klumperman, J.; Locker, J.K.; Meijer, A.; Horzinek, M.C.; Geuze, H.J.; Rottier, P.J. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J. Virol. 1994, 68, 6523–6534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krijnse-Locker, J.; Ericsson, M.; Rottier, P.J.; Griffiths, G. Characterization of the budding compartment of mouse hepatitis virus: Evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J. Cell Biol. 1994, 124, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Salanueva, I.J.; Carrascosa, J.L.; Risco, C. Structural maturation of the transmissible gastroenteritis coronavirus. J. Virol. 1999, 73, 7952–7964. [Google Scholar] [CrossRef] [Green Version]
- Hauri, H.P.; Kappeler, F.; Andersson, H.; Appenzeller, C. ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 2000, 113, 587–596. [Google Scholar]
- Klaus, J.P.; Eisenhauer, P.; Russo, J.; Mason, A.B.; Do, D.; King, B.; Taatjes, D.; Cornillez-Ty, C.; Boyson, J.E.; Thali, M.; et al. The intracellular cargo receptor ERGIC-53 is required for the production of infectious arenavirus, coronavirus and filovirus particles. Cell Host Microbe 2013, 14, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, G.; Ericsson, M.; Krijnse-Locker, J.; Nilsson, T.; Goud, B.; Söling, H.D.; Tang, B.L.; Wong, S.H.; Hong, W. Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J. Cell Biol. 1994, 127, 1557–1574. [Google Scholar] [CrossRef]
- Plutner, H.; Cox, A.D.; Pind, S.; Khosravi-Far, R.; Bourne, J.R.; Schwaninger, R.; Der, C.J.; Balch, W.E. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J. Cell Biol. 1991, 115, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Saraste, J.; Lahtinen, U.; Goud, B. Localization of the small GTP-binding protein Rab1 to early compartments of the secretory pathway. Cell Sci. 1995, 108, 1541–1552. [Google Scholar]
- Saraste, J. Spatial and functional aspects of ER-Golgi Rabs and tethers. Front. Cell Dev. Biol. 2016, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Goud, B.; Liu, S.; Storrie, B. Rab proteins as major determinants of the Golgi complex structure. Small GTPases 2018, 9, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Stertz, S.; Reichelt, M.; Spiegel, M.; Kuri, T.; Martinez-Sobrido, L.; Garcia-Sastre, A.; Weber, F.; Kochs, G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology 2007, 361, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Sodeik, B.; Krijnse-Locker, J. Assembly of vaccinia virus revisited: De novo membrane synthesis or acquisition from the host? Trends Microbiol. 2002, 10, 15–24. [Google Scholar] [CrossRef]
- Risco, C.; Rodríguez, J.R.; López-Iglesias, C.; Carrascosa, J.L.; Esteban, M.; Rodríguez, D. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J. Virol. 2002, 76, 1839–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenner, H.L.; Yoshimura, S.-I.; Barr, F.; Crump, C.M. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus-1 secondary envelopment. J. Virol. 2011, 85, 8012–8021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huovila, A.P.; Eder, A.M.; Fuller, S.D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Biol. 1992, 118, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Zeyen, L.; Döring, T.; Prange, R. Hepatitis B virus exploits ERGIC-53 in conjunction with COPII to exit cells. Cells 2020, 9, 1889. [Google Scholar] [CrossRef] [PubMed]
- Hobman, T.C. Targeting of viral glycoproteins to the Golgi complex. Trends Microbiol. 1993, 1, 124–130. [Google Scholar] [CrossRef]
- Kuismanen, E.; Hedman, K.; Saraste, J.; Pettersson, R.F. Uukuniemi virus maturation: Accumulation of virus particles and viral antigens in the Golgi complex. Mol. Cell Biol. 1982, 2, 1444–1458. [Google Scholar] [CrossRef]
- Shi, X.; Elliot, R.M. Golgi localization of Hantaan virus glycoproteins requires co-expression of G1 and G2. Virology 2002, 300, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Salanueva, I.J.; Novoa, R.R.; Cabezas, P.; López-Iglesisas, C.; Carrascosa, J.L.; Elliot, R.M.; Risco, C. Polymorphism and structural maturation of bunyamwera virus in Golgi and post-Golgi compartments. J. Virol. 2003, 77, 1368–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, Y.; Chen, S.Y.; Compans, R.W. Bunyavirus protein transport and assembly. Curr. Top. Microbiol. Immunol. 1991, 169, 161–179. [Google Scholar]
- Jäntti, J.; Hildén, P.; Rönkä, H.; Mäkiranta, V.; Keränen, S.; Kuismanen, E. Immunocytochemical analysis of Uukuniemi virus budding compartments: Role of the intermediate compartment and the Golgi stack in virus maturation. J. Virol. 1997, 71, 1162–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, H.N.; Chung, D.H.; Plane, S.J.; Sztul, E.; Chu, Y.K.; Guttieri, M.C.; McDowell, M.; Ali, G.; Jonsson, C.B. Dynein-dependent transport of the hantaan virus nucleocapsid protein to the endoplasmic reticulum-Golgi intermediate compartment. J. Virol. 2007, 81, 8634–8647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sager, G.; Gabaglio, S.; Stzul, E.; Belov, G.A. Role of host cell secretory machinery in Zika virus life cycle. Viruses 2018, 10, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wang, C.; Liang, W.; Zhang, J.; Zhang, L.; Lv, H.; Dong, W.; Zhang, Y. Rab1A is required for assembly of classical swine fever virus particle. Virology 2018, 514, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Grandadam, M.; Kwok, K.; Lagache, T.; Siu, Y.L.; Zhang, J.S.; Sayteng, K.; Kudelko, M.; Qin, C.F.; Olivo-Marin, J.C.; et al. KDEL receptors assist Dengue virus exit from the endoplasmic reticulum. Cell Rep. 2015, 10, 1496–1507. [Google Scholar] [CrossRef] [Green Version]
- Chwetzoff, S.; Trugnan, G. Rotavirus assembly: An alternative model that utilizes an atypical trafficking pathway. Curr. Top. Microbiol. Immunol. 2006, 309, 245–261. [Google Scholar]
- Cuadras, M.A.; Bordier, B.B.; Zambrano, J.L.; Ludert, J.E.; Greenberg, H.B. Dissecting rotavirus particle-raft interaction with small interfering RNAs: Insights into rotavirus transit through the secretory pathway. J. Virol. 2006, 80, 3935–3946. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, J.M.; Westaway, E.G. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J. Virol. 2001, 75, 10787–10799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bárcena, M.; Oostergetel, G.T.; Bartelink, W.; Faas, F.G.A.; Verkleij, A.; Rottier, P.J.; Koster, A.J.; Bosch, B.J. Cryo-electron tomography of mouse hepatitis virus: Insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. USA 2009, 106, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Cortese, M.; Winter, S.L.; Wachsmuth-Melm, M.; Neufeldt, C.J.; Cerikan, B.; Stanifer, M.L.; Boulant, S.; Bartenschlager, R.; Chlanda, P. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun. 2020, 11, 5885. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1281, 1–23. [Google Scholar]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020, 295, 12910–12934. [Google Scholar] [CrossRef]
- Vennema, H.; Godeke, G.J.; Rossen, J.W.; Voorhout, W.F.; Horzinek, M.C.; Opstelten, D.J.; Rottier, P.J. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 1996, 15, 2020–2028. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Boulan, E.; Sabatini, D.D. Asymmetric budding of viruses in epithelial monolayers: A model system for study of epithelial polarity. Proc. Natl. Acad. Sci. USA 1978, 75, 5071–5075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iserman, C.; Roden, C.A.; Boerneke, M.A.; Sealfon, R.S.G.; McLaughlin, G.A.; Jungreis, I.; Fritch, E.J.; Hou, Y.J.; Ekena, J.; Weidmann, C.A.; et al. Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol. Cell 2020, 80, 1078–1091. [Google Scholar] [CrossRef]
- Lu, S.; Ye, Q.; Singh, D.; Cao, Y.; Diedrich, J.K.; Yates, J.R., 3rd; Villa, E.; Cleveland, D.W.; Corbett, K.D. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat. Commun. 2021, 12, 502. [Google Scholar] [CrossRef]
- de Haan, C.A.M.; Rottier, P.J.M. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005, 64, 165–230. [Google Scholar]
- Hogue, B.G.; Machamer, C.E. Coronavirus structural proteins and virus assembly. In Nidoviruses; Perlman, S., Gallagher, T., Snijder, E.J., Eds.; ASM Press: Washington, DC, USA, 2008; pp. 179–200. [Google Scholar]
- Ujike, M.; Taguchi, F. Incorporation of spike and membrane glycoproteins into coronavirus virions. Viruses 2015, 7, 1700–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Shi, M.; Li, J.; Song, P.; Li, N. Construction of SARS-CoV-2 virus-like particles by mammalian expression system. Front. Bioeng. Biotechnol. 2020, 8, 862. [Google Scholar] [CrossRef]
- Machamer, C.E.; Rose, J.K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J. Cell Biol. 1987, 105, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machamer, C.E.; Mentone, S.A.; Rose, J.K.; Farquhar, M.G. The E1 glycoprotein of an avian coronavirus is targeted to the cis-Golgi complex. Proc. Natl. Acad. Sci. USA 1990, 87, 6944–6948. [Google Scholar] [CrossRef] [Green Version]
- Krijnse-Locker, J.; Griffiths, G.; Hornizek, M.K.; Rottier, P.J.M. O-glycosylation of the coronavirus M protein. Differential localization of sialyltransferases in N- and O-linked glycosylation. J. Biol. Chem. 1992, 267, 14094–14101. [Google Scholar] [CrossRef]
- Perrier, A.; Bonnin, A.; Desmarets, L.; Danneels, A.; Goffard, A.; Rouillé, Y.; Dubuisson, J.; Belouzard, S. The C-terminal domain of the MERS coronavirus M protein contains a trans-Golgi network localization signal. J. Biol. Chem. 2019, 294, 14406–14421. [Google Scholar] [CrossRef] [Green Version]
- Weisz, O.A.; Swift, M.A.; Machamer, C.E. Oligomerization of a membrane protein correlates with its retention in the Golgi complex. J. Cell Biol. 1993, 122, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Lontok, E.; Corse, E.; Machamer, C.E. Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site. J. Virol. 2004, 78, 5913–5922. [Google Scholar] [CrossRef] [Green Version]
- McBride, C.E.; Li, J.; Machamer, C.E. The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein. J. Virol. 2007, 81, 2418–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwegmann-Wessels, C.; Al-Falah, M.; Escors, D.; Wang, Z.; Zimmer, G.; Deng, H.; Enjuanes, L.; Naim, H.Y.; Herrler, G. A novel sorting signal for intracellular localization is present in the S protein of a porcine coronavirus but absent from severe acute respiratory syndrome-associated coronavirus. J. Biol. Chem. 2004, 279, 43661–43666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boson, B.; Legros, V.; Zhou, B.; Siret, E.; Mathieu, C.; Cosset, F.; Lavillette, D.; Denolly, S. The SARS-CoV-2 Envelope and Membrane proteins modulate maturation and retention of the Spike protein, allowing assembly of virus-like particles. J. Biol. Chem. 2020, 3, 100111. [Google Scholar]
- Raamsman, M.J.; Locker, J.K.; de Hooge, A.; de Vries, A.A.; Griffiths, G.; Vennema, H.; Rottier, P.J. Characterization of the coronavirus mouse hepatitis virus strain A59 small membrane protein E. J. Virol. 2000, 74, 2333–2342. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Torres, J.L.; Dediego, M.L.; Alvarez, E.; Jiménez-Guardeño, J.M.; Regla-Nava, J.A.; Llorente, M.; Kremer, L.; Shuo, S.; Enjuanes, L. Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology 2011, 415, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatagopalan, P.; Daskalova, S.M.; Lopez, L.A.; Dolezal, K.A.; Houge, B.G. Coronavirus envelope (E) protein remains at the site of assembly. Virology 2015, 478, 75–85. [Google Scholar] [CrossRef]
- Sicari, D.; Chatziioannou, A.; Koutsandreas, T.; Sitia, R.; Chevet, E. Role of the early secretory pathway in SARS-CoV-2 infection. J. Cell. Biol. 2020, 219, e202006005. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; O’Meara, M.J.; Guo, J.Z.; Swaney, D.L.; Tummino, T.A.; Hüttenhain, R.; et al. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. Nature 2020. [Google Scholar] [CrossRef]
- Cluett, E.B.; Kuismanen, E.; Machamer, C.E. Heterogeneous distribution of the unusual phospholipid semilysobisphosphatidic acid through the Golgi complex. Mol. Biol. Cell 1997, 8, 2233–2240. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fang, S.; Xiao, H.; Chen, B.; Tam, J.P.; Liu, D.X. Interaction of coronavirus infectious bronchitis virus membrane protein with β-actin and its implication in virion assembly and budding. PLoS ONE 2009, 4, e4908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruch, T.R.; Machamer, C.E. The hydrophobic domain of infectious bronchitis virus E protein alters the host secretory pathway and is important for release of infectious virus. J. Virol. 2011, 85, 675–685. [Google Scholar] [CrossRef] [Green Version]
- Westerbeck, J.W.; Machamer, C.E. A coronavirus E protein is present in two distinct pools with different effects on assembly and the secretory pathway. J. Virol. 2015, 89, 9313–9323. [Google Scholar] [CrossRef] [Green Version]
- Westerbeck, J.W.; Machamer, C.E. The infectious bronchitis coronavirus envelope protein alters Golgi pH to protect the spike protein and promote the release of infectious virus. J. Virol. 2019, 93, e00015-19. [Google Scholar] [CrossRef] [Green Version]
- Palokangas, H.; Ying, M.; Väänänen, K.; Saraste, J. Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol. Biol. Cell 1998, 9, 3561–3578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, M.; Flatmark, T.; Saraste, J. The p58-positive pre-Golgi intermediates consist of distinct subpopulations of particles that show differential binding of COPI and COPII coats and contain vacuolar H+-ATPase. J. Cell Sci. 2000, 113, 3623–3638. [Google Scholar] [PubMed]
- Vavassori, S.; Cortini, M.; Masui, S.; Sannino, S.; Anelli, T.; Caserta, I.R.; Fagioli, C.; Mossuto, M.F.; Fornili, A.; van Anken, E.; et al. A pH-regulated quality control cycle for surveillance of secretory protein assembly. Mol. Cell 2013, 50, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balch, W.E.; McCaffery, J.M.; Plutner, H.; Farquhar, M.G. Vesicular stomatitis virus is sorted and concentrated during export from the endoplasmic reticulum. Cell 1994, 76, 841–852. [Google Scholar] [CrossRef]
- Horstmann, H.; Ng, C.P.; Tang, B.L.; Hong, W. Ultrastructural characterization of endoplasmic reticulum-Golgi transport containers (EGTC). J. Cell Sci. 2002, 115, 4263–4273. [Google Scholar] [CrossRef] [Green Version]
- Ulasli, M.; Verheije, M.H.; de Haan, C.A.; Reggiori, F. Qualitative and quantitative ultrastructural analysis of the membrane rearrangements induced by coronavirus. Cell Microbiol. 2010, 12, 844–861. [Google Scholar] [CrossRef] [Green Version]
- Sannerud, R.; Marie, M.; Nizak, C.; Dale, H.A.; Pernet-Gallay, K.; Perez, F.; Goud, B.; Saraste, J. Rab1 defines a novel pathway connecting the pre-Golgi intermediate compartment with the cell periphery. Mol. Biol. Cell. 2006, 17, 1514–1526. [Google Scholar] [CrossRef] [Green Version]
- Marie, M.; Dale, H.A.; Sannerud, R.; Saraste, J. The function of the intermediate compartment in pre-Golgi trafficking involves its stable connection with the centrosome. Mol. Biol. Cell 2009, 20, 4458–4470. [Google Scholar] [CrossRef] [Green Version]
- Westrate, L.M.; Hoyer, M.J.; Nash, M.J.; Voeltz, G.K. Vesicular and uncoated Rab1-dependent cargo carriers facilitate ER to Golgi transport. J. Cell Sci. 2020, 133, jcs239814. [Google Scholar] [CrossRef]
- Presley, J.F.; Ward, T.H.; Pfeifer, A.C.; Siggia, E.D.; Phair, R.D.; Lippincott-Schwartz, J. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature 2002, 417, 187–193. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2015. [Google Scholar]
- Glick, B.S.; Luini, A. Models for Golgi traffic: A critical assessment. Cold Spring Harb. Perspect. Biol. 2011, 3, a005215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volchuk, A.; Amherdt, M.; Ravazzola, M.; Brugger, B.; Rivera, V.M.; Clackson, T.; Perrelet, A.; Söllner, T.; Rothman, J.E.; Orci, L. Megavesicles implicated in the rapid transport of intracisternal aggregates across the Golgi stack. Cell 2000, 102, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Lavieu, G.; Zheng, H.; Rothman, J.E. Stapled Golgi cisternae remain in place as cargo passes through the stack. Elife 2013, 2, e00558. [Google Scholar] [CrossRef]
- Tooze, J.; Tooze, S.A.; Fuller, S.D. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J. Cell Biol. 1987, 105, 1215–1226. [Google Scholar] [CrossRef] [Green Version]
- Ben-Tekaya, H.; Miura, K.; Pepperkok, R.; Hauri, H.P. Live imaging of bidirectional traffic from the ERGIC. J. Cell Sci. 2005, 118, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Marie, M.; Dale, H.A.; Kouprina, N.; Saraste, J. Division of the intermediate compartment at the onset of mitosis provides a mechanism for Golgi inheritance. J. Cell Sci. 2012, 125, 5403–5416. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, M.J.; Hanus, C. Architecture and dynamics of the neuronal secretory network. Annu. Rev. Cell Dev. Biol. 2019, 35, 543–566. [Google Scholar] [CrossRef] [PubMed]
- Tveit, H.; Akslen, L.K.; Fagereng, G.L.; Tranulis, M.A.; Prydz, K. A secretory Golgi bypass route to the apical surface domain of epithelial MDCK cells. Traffic 2009, 10, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Hanus, C.; Geptin, H.; Tushev, G.; Garg, S.; Alvarez-Castelao, B.; Sambandan, S.; Kochen, L.; Hafner, A.S.; Langer, J.D.; Schuman, E.M. Unconventional secretory processing diversifies neuronal ion channel properties. Elife 2016, 5, e20609. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.B.; Bourke, A.M.; Hiester, B.G.; Hanus, C.; Kennedy, M.J. Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. Elife 2017, 6, e27362. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.; Lin, X.; Wang, Y.; Li, Y.; Guo, Q.; Li, S.; Sun, Y.; Tao, X.; Zhang, D.; et al. A translocation pathway for vesicle-mediated unconventional protein secretion. Cell 2020, 181, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Marie, M.; Sannerud, R.; Dale, H.A.; Saraste, J. Take the ‘A’ train: On fast tracks to the cell surface. Cell Mol. Life Sci. 2008, 65, 2859–2874. [Google Scholar] [CrossRef]
- Prydz, K.; Tveit, T.; Vedeler, A.; Saraste, J. Arrivals and departures at the plasma membrane: Direct and indirect transport routes. J. Cell Tissue Res. 2013, 352, 5–20. [Google Scholar] [CrossRef]
- Saraste, J.; Prydz, K. A new look at the functional organization of the Golgi ribbon. Front. Cell Dev. Biol. 2019, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Kurokawa, K.; Inaba, R.; Hiramatsu, N.; Tago, T.; Nakamura, Y.; Nakano, A.; Satoh, T.; Satoh, A.K. Recycling endosomes attach to the trans-side of Golgi stacks in Drosophila and mammalian cells. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Ladinsky, M.S.; Mastronarde, D.N.; McIntosh, J.R.; Howell, K.E.; Staehelin, L.A. Golgi structure in three dimensions: Functional insights from the normal rat kidney cell. J. Cell Biol. 1999, 144, 1135–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheije, M.H.; Raaben, M.; Mari, M.; Te Lintelo, E.G.; Reggiori, F.; van Kuppeveld, F.J.; Rottier, P.J.; de Haan, C.A. Mouse hepatitis coronavirus RNA replication depends on GBF1-mediated ARF1 activation. PLoS Pathog. 2008, 4, e1000088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoops, K.; Swett-Tapia, C.; van den Worm, S.H.; Te Velthuis, A.J.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J.; Kikkert, M. Integrity of the early secretory pathway promotes, but is not required for, severe acute respiratory syndrome coronavirus RNA synthesis and virus-induced remodeling of endoplasmic reticulum membranes. J. Virol. 2010, 84, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Risco, C.; Muntión, M.; Enjuanes, L.; Carrascosa, J.L. Two types of virus-related particles are found during transmissible gastroenteritis virus morphogenesis. J. Virol. 1998, 72, 4022–4031. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-coronavirus use lysosomes for egress instead of the biosynthetic secretory pathway. Cell 2020, 183, 1520–1535. [Google Scholar] [CrossRef]
- Carpp, L.N.; Rogers, R.S.; Moritz, R.L.; Aitchison, J.D. Quantitative proteomic analysis of host-virus interactions reveals a role for Golgi brefeldin A resistance factor 1 (GBF1) in dengue infection. Mol. Cell Proteomics 2014, 13, 2836–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, E.A.; Stuart, A.; McCaffrey, M.W.; Digard, P. Role of the Rab11 pathway in negative-strand virus assembly. Biochem. Soc. Trans. 2012, 40, 1409–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vale-Costa, S.; Amorim, M. Recycling endosomes and viral infection. Viruses 2016, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.K.; Suszko, J.W.; Pekosz, A. Roles for the recycling endosome, Rab8, and Rab11 in hantavirus release from epithelial cells. Virology 2008, 382, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Coller, K.E.; Heaton, N.S.; Berger, K.L.; Cooper, J.D.; Saunders, J.L.; Randall, G. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS Pathog. 2012, 8, e1002466. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Suzuki, T.; Kawaguchi, A.; Phongphaew, W.; Yoshii, K.; Iwano, T.; Harada, A.; Kariwa, H.; Orba, Y.; Sawa, H. Rab8b regulates transport of West Nile virus particles from recycling endosomes. J. Biol. Chem. 2016, 291, 6559–6568. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-K.; Jeng, K.-S.; Machida, K.; Lai, M.M.C. Hepatitis C virus egress and release depend on endosomal trafficking of core protein. J. Virol. 2010, 84, 11590–11598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer, K.; Banning, C.; Bruss, V.; Wiltzer-Bach, L.; Schindler, M. Hepatitis C virus is released via a non-canonical secretory route. J. Virol. 2016, 90, 10558–10573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavi, E.; Wang, Q.; Weiss, S.R.; Gonatas, N.K. Syncytia formation induced by coronavirus infection is associated with fragmentation and rearrangement of the Golgi apparatus. Virology 1996, 221, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Cortese, M.; Lee, J.Y.; Cerikan, B.; Neufeldt, C.J.; Oorschot, V.M.J.; Köhrer, S.; Hennies, J.; Schieber, N.L.; Ronchi, P.; Mizzon, G.; et al. Integrative imaging reveals SARS-CoV-2-induced reshaping of subcellular morphologies. Cell Host Microbe 2020, 28, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Machamer, C.E. Accomodation of large cargo within Golgi cisternae. Histochem. Cell Biol. 2013, 140, 261–269. [Google Scholar] [CrossRef]
- Yamashiro, D.J.; Tycko, B.; Fluss, S.R.; Maxfield, F.R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell 1984, 37, 789–800. [Google Scholar] [CrossRef]
- Ritchie, G.; Harvey, D.J.; Feldmann, F.; Stroeher, U.; Feldmann, H.; Royle, L.; Dwek, R.A.; Rudd, P.M. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology 2010, 399, 257–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002, 3, 753–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millet, J.K.; Whittaker, G.R. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015, 202, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Röttger, S.; White, J.; Wandall, H.H.; Olivo, J.C.; Stark, A.; Bennett, E.P.; Whitehouse, C.; Berger, E.G.; Clausen, H.; Nilsson, T. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J. Cell Sci. 1998, 111, 45–60. [Google Scholar]
- Jarvela, T.; Linstedt, A.D. Irradiation-induced protein inactivation reveals Golgi enzyme cycling to cell periphery. J. Cell Sci. 2012, 125, 973–980. [Google Scholar] [CrossRef] [Green Version]
- Gill, D.J.; Chia, J.; Senewiratne, J.; Bard, F. Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J. Cell Biol. 2010, 189, 843–858. [Google Scholar] [CrossRef] [Green Version]
- Kellokumpu, S. Golgi pH, ion and redox homeostasis: How much do they really matter? Front. Cell Dev. Biol. 2019, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, D.J.; Tham, K.M.; Chia, J.; Wang, S.C.; Steentoft, C.; Clausen, H.; Bard-Chapeau, E.A.; Bard, F. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc. Natl. Acad. Sci. USA 2013, 110, E3152–E3161. [Google Scholar] [CrossRef] [Green Version]
- Mansbach, C.M.; Siddiqi, S. Control of chylomicron export from the intestine. Am. J. Physiol. 2015, 310, G659–G668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, V.; Erlmann, P.; Nogueira, C. Procollagen export from the endoplasmic reticulum. Biochem. Soc. Trans. 2015, 43, 104–107. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, J.; Stephens, D.J. ER-to-Golgi transport: A sizeable problem. Trends Cell Biol. 2019, 29, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Ehrenreich, J.H.; Bergeron, J.J.; Siekevitz, P.; Palade, G. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J. Cell Biol. 1973, 59, 45–72. [Google Scholar] [CrossRef] [Green Version]
- Sabesin, S.M.; Frase, S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J. Lipid Res. 1977, 18, 496–511. [Google Scholar] [CrossRef]
- Leblond, C.P. Synthesis and secretion of collagen by cells of connective tissue, bone, and dentin. Anat. Rec. 1989, 224, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Dahan, S.; Ahluwalia, J.P.; Wong, L.; Posner, B.I.; Bergeron, J.J. Concentration of intracellular hepatic apolipoprotein E in Golgi apparatus saccular distensions and endosomes. J. Cell Biol. 1994, 127, 1859–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfanti, L.; Mironov, A.A., Jr.; Martínez-Menárguez, J.A.; Martella, O.; Fusella, A.; Baldassarre, M.; Buccione, R.; Geuze, H.J.; Mironov, A.A.; Luini, A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: Evidence for cisternal maturation. Cell 1998, 95, 993–1003. [Google Scholar] [CrossRef]
- Takacs, C.N.; Andreo, U.; Dao Thi, V.L.; Wu, X.; Gleason, C.E.; Itano, M.S.; Spitz-Becker, G.S.; Belote, R.L.; Heldin, B.R.; Scull, M.A.; et al. Differential regulation of lipoprotein and hepatitis C virus secretion by rab1b. Cell Rep. 2017, 21, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.J.; Raote, I.; Scarpa, M.; Brouwers, N.; Malhotra, V. TANGO1 recruits ERGIC membranes to the endoplasmic reticulum for procollagen export. Elife 2015, 4, e10982. [Google Scholar] [CrossRef] [PubMed]
- Raote, I.; Malhotra, V. Protein transport by vesicles and tunnels. J. Cell Biol. 2019, 218, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Feng, Z.; Ke, H.; Liu, Y.; Sun, T.; Dai, J.; Cui, W.; Pastor-Pareja, J.C. Tango1 spatially organizes ER exit sites to control ER export. J. Cell Biol. 2017, 216, 1035–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.J.; Nogueira, C.; Ortega-Bellido, M.; Malhotra, V. TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. J. Cell Biol. 2016, 213, 343–354. [Google Scholar] [CrossRef]
- Saito, K.; Maeda, M. Not just a cargo receptor for large cargoes; an emerging role of TANGO1 as an organizer of ER exit sites. J. Biochem. 2019, 166, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Lavieu, G.; Dunlop, M.H.; Lerich, A.; Zheng, H.; Bottanelli, F.; Rothman, J.E. The Golgi ribbon structure facilitates anterograde transport of large cargoes. Mol. Biol. Cell 2014, 25, 3028–3036. [Google Scholar] [CrossRef]
- Rink, J.; Ghigo, E.; Kalaidzidis, Y.; Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005, 122, 735–749. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, S. How the Golgi works: A cisternal progenitor model. Proc. Natl. Acad. Sci. USA 2010, 107, 19614–19618. [Google Scholar] [CrossRef] [Green Version]
- Lipatova, Z.; Hain, A.U.; Nazarko, V.Y.; Segev, N. Ypt/Rab GTPases: Principles learned from yeast. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.L.; Fromme, J.C. Extensive GTPase crosstalk regulates Golgi trafficking and maturation. Curr. Opin. Cell Biol. 2020, 65, 1–7. [Google Scholar] [CrossRef]
- Suda, Y.; Kurokawa, K.; Nakano, A. Regulation of ER-Golgi transport dynamics by GTPases in budding yeast. Front. Cell Dev. Biol. 2018, 5, 122. [Google Scholar] [CrossRef] [Green Version]
- Day, K.J.; Casler, J.C.; Glick, B.S. Budding yeast has a minimal endomembrane system. Dev. Cell. 2018, 44, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, W.; Whitney, J.A.; Mellman, I. Brefeldin A and the endocytic pathway. Possible implications for membrane traffic and sorting. FEBS Lett. 1992, 307, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Helenius, A.; Kartenbeck, J.; Simons, K.; Fries, E. On the entry of Semliki Forest virus into BHK-21 cells. J. Cell Biol. 1980, 84, 404–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, J.E.; Singer, S.J. Immunoelectron microscopic studies of the intracellular transport of the membrane glycoprotein (G) of vesicular stomatitis virus in infected chinese hamster ovary cells. J. Cell Biol. 1983, 97, 1777–1787. [Google Scholar] [CrossRef] [Green Version]
- Knipe, D.M.; Baltimore, D.; Lodish, H.F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J. Virol. 1977, 21, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, K.; Suzuki, K.; Simizu, B. Maturation defect of a temperature-sensitive mutant of Western Equine Encephalitis virus. Arch. Virol. 1977, 53, 209–219. [Google Scholar] [CrossRef]
- Saraste, J.; von Bonsdorff, C.H.; Hashimoto, K.; Kääriäinen, L.; Keränen, S. Semliki Forest virus mutants with temperature-sensitive transport defect of envelope proteins. Virology 1980, 100, 229–245. [Google Scholar] [CrossRef]
- Rossen, J.W.A.; Horzinek, M.C.; Rottier, P.J.M. Coronavirus infection in polarized epithelial cells. Trends Microbiol. 1995, 3, 486–490. [Google Scholar] [CrossRef]
- Cong, Y.; Ren, X. Coronavirus entry and release in polarized epithelial cells. Rev. Med. Virol. 2014, 24, 308–315. [Google Scholar] [CrossRef] [PubMed]
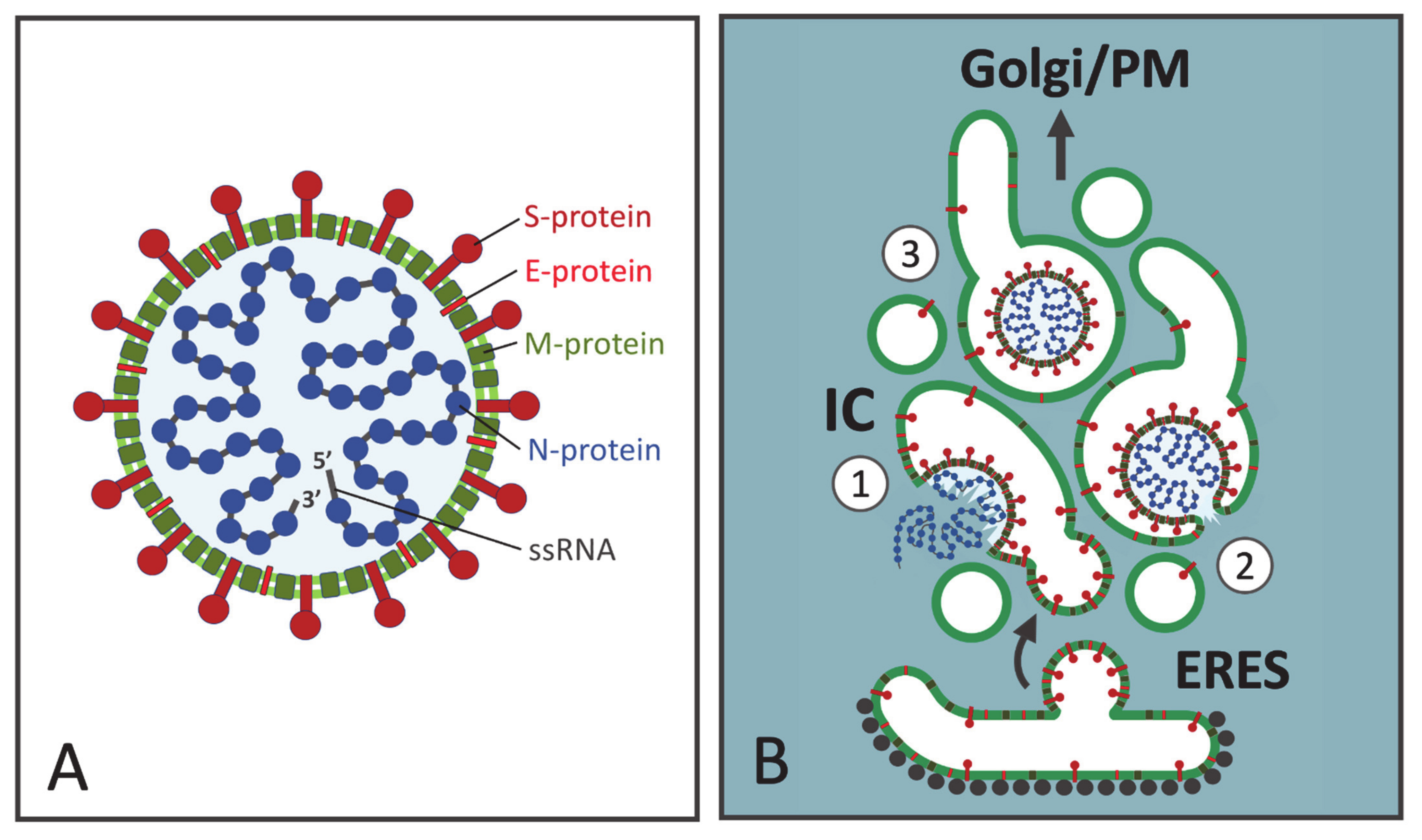
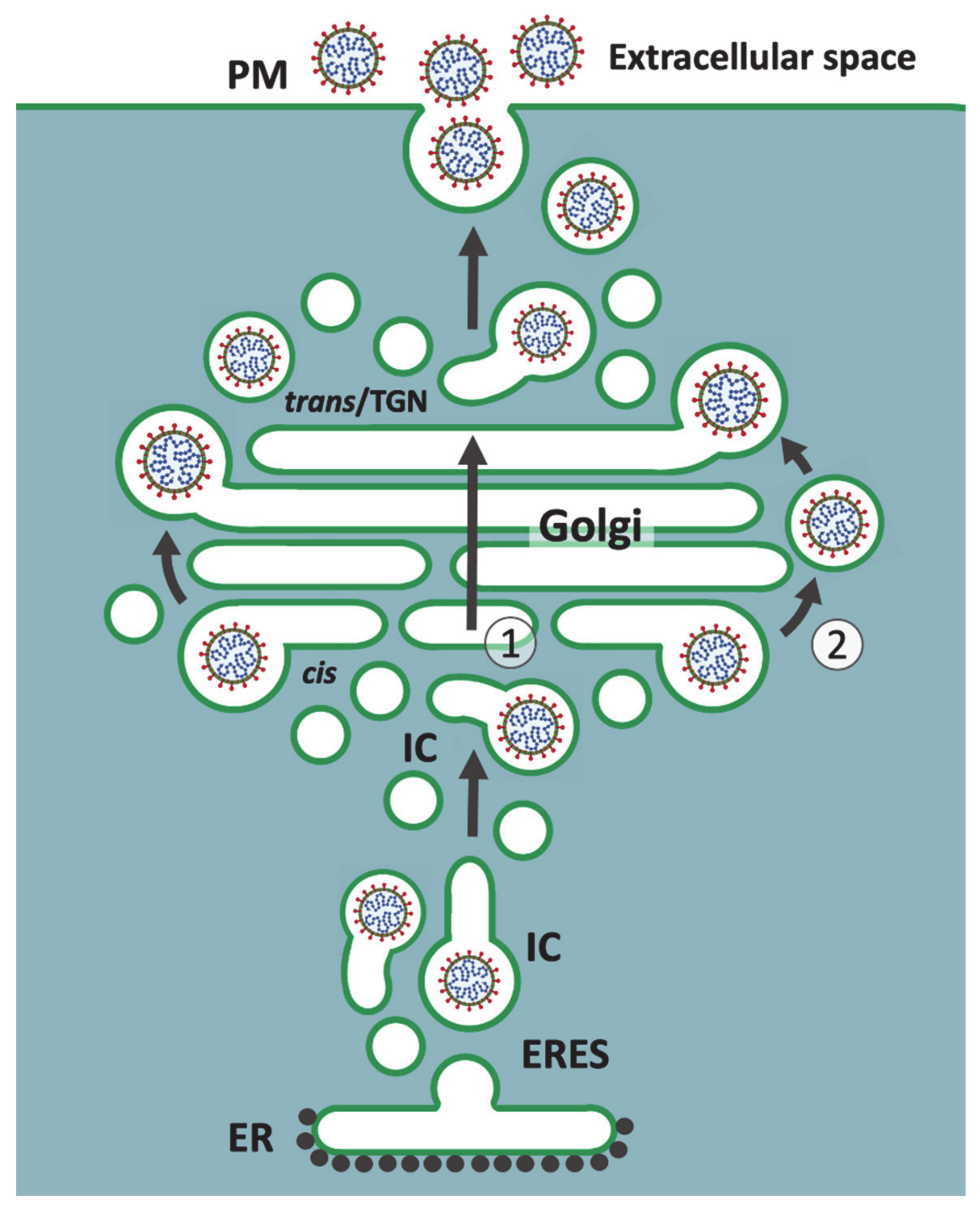
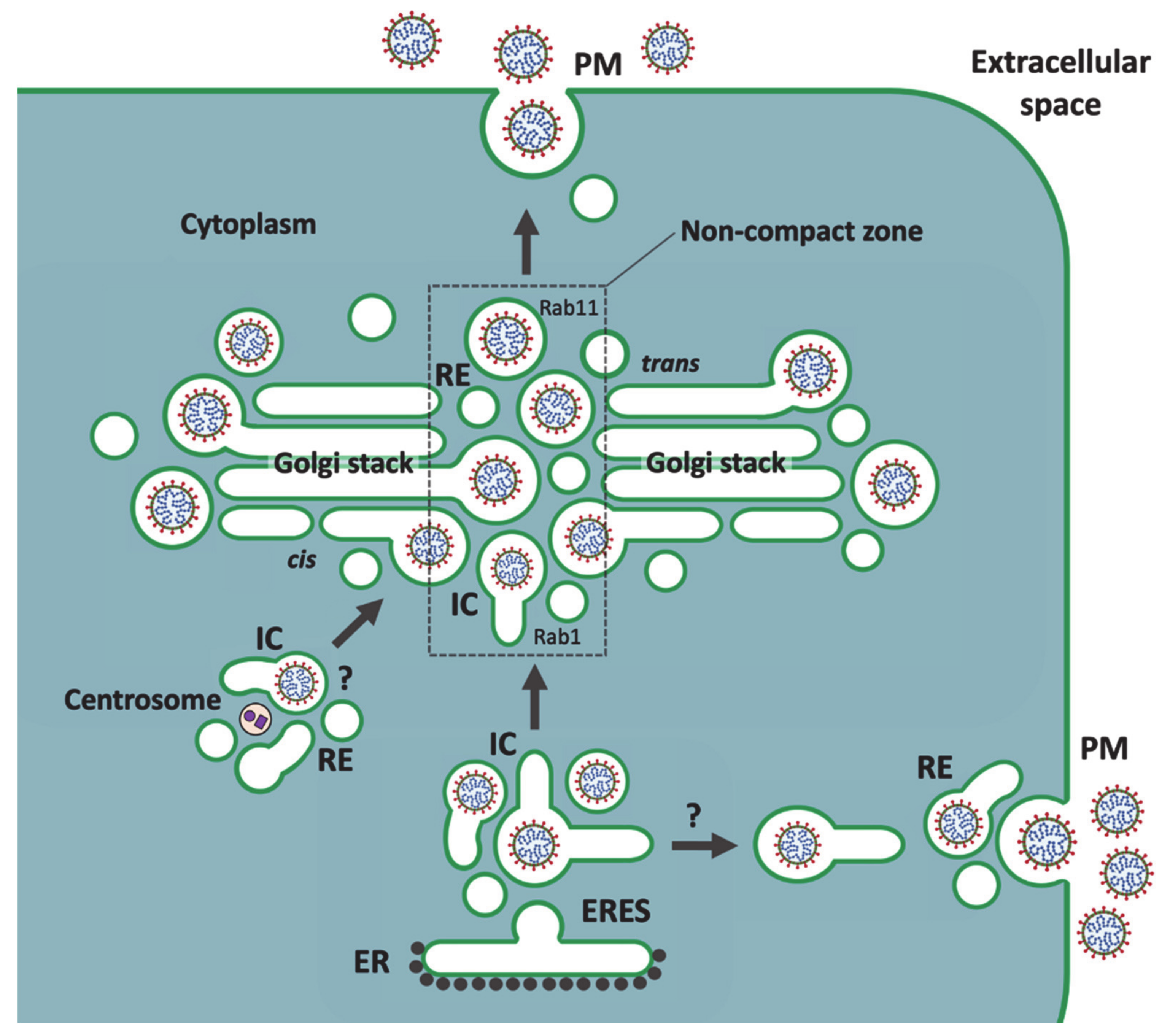
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraste, J.; Prydz, K. Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space. Cells 2021, 10, 503. https://doi.org/10.3390/cells10030503
Saraste J, Prydz K. Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space. Cells. 2021; 10(3):503. https://doi.org/10.3390/cells10030503
Chicago/Turabian StyleSaraste, Jaakko, and Kristian Prydz. 2021. "Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space" Cells 10, no. 3: 503. https://doi.org/10.3390/cells10030503
APA StyleSaraste, J., & Prydz, K. (2021). Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space. Cells, 10(3), 503. https://doi.org/10.3390/cells10030503






