Abstract
Glioblastoma (GBM) is the most common primary central nervous system tumor and one of the most lethal cancers worldwide, with morbidity of 5.26 per 100,000 population per year. These tumors are often associated with poor prognosis and terrible quality of life. Extracellular vesicles (EVs) are membrane-bound nanoparticles secreted by cells and contain lipid, protein, DNA, mRNA, miRNA and other bioactive substances. EVs perform biological functions by binding or horizontal transfer of bioactive substances to target cell receptors. In recent years, EVs have been considered as possible targets for GBM therapy. A great many types of research demonstrated that EVs played a vital role in the GBM microenvironment, development, progression, angiogenesis, invasion, and even the diagnosis of GBM. Nevertheless, the exact molecular mechanisms and roles of EVs in these processes are unclear. It can provide the basis for GBM treatment in the future that clarifying the regulatory mechanism and related signal pathways of EVs derived from GBM and their clinical value in GBM diagnosis and treatment. In this paper, the research progress and clinical application prospects of GBM-derived EVs are reviewed and discussed.
1. Introduction
Gliomas are primary tumors of the brain produced by glial stem cells or progenitors [1]. Gliomas are generally classified into four grades adopts the World Health Organization (WHO) classification system, with grade I and II being benign and III and IV being malignant. Grade IV glioma is also known as glioblastoma (GBM). GBM is the most deadly glioma type [2], accounting for 70–75% of all diffuse gliomas, with a median overall survival of only 14–17 months [3].
Many studies indicated that few available therapies could significantly improve survival chances of patients with GBM [4,5,6]. The histopathological definition of GBM is based essentially on the presence of tumor cells considered to be of neuroglial origin and on the examination of neovascularization and necrosis. At the molecular level, GBM is typically characterized by a lack of isocitrate dehydrogenase 1 or 2 (IDH1 or IDH2) mutations and mutations in genes regulating RTK/RAS/PI3K, p53, and RB. Amplifications and mutations of the epidermal growth factor receptor gene (EGFR) are found in 40% of the GBM [1,7,8,9]. GBMs are usually also stratified by the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation, as well as TERT (telomerase reverse transcriptase) promoter mutations, mutations/deletions in PTEN (phosphatase and tensin homolog) and nowadays, many efforts are made using whole-genome methylation data [10,11,12].
GBM’s fast deterioration, drug resistance and high recurrence rate attribute to several kinds of elements, including its rapid proliferation, extensive invasion, and genetic heterogeneity within the tumor. Moreover, the poor prognosis of GBM patients also partly results from a lack of understanding of molecular mechanisms and timely diagnosis and sensitive therapeutic monitoring tools [13]. Conventional therapies, including surgery, radiotherapy and typically chemotherapy with temozolomide, have not been conducive to major improvements in the survival outcomes of patients with GBM [14,15,16].
In the past several years, an increasing number of studies have indicated that extracellular vesicles (EVs) and the contents of EVs played an important part in the initiation, progression and diagnosis of GBM [17,18,19,20]. At present, attention has been focused on how EVs playing a role in mediating cellular communication in the tumor microenvironment of various cancers, including GBM. The field of EVs has attracted increasing attention in recent years. Many reviews have been published, which have contributed to the development of this field and attracted more researchers to devote themselves to this field [21,22]. In order for more researchers and those who are new to this field to have a quick and concise understanding, we have carried out this review, which mainly includes the formation, release, uptake, structure, function, isolation and purification of EVs; GBM-derived EVs play a variety of roles in GBM tumorigenesis, microenvironment, angiogenesis, immune response, metastasis, invasion, subtype and chemotherapy resistance.
2. Formation, Release and Uptake of EVs
EVs were first discovered in 1983 in mature sheep reticulocytes. They were originally named “exosomes” and thought to be cell debris in 1989 [23]. More than a decade later, it was reported that B lymphocytes and dendritic cells could secrete EVs, indicating that EVs had potential immunomodulatory effects and were regarded as carriers of antitumor immunity [24,25]. The production of EVs involves the double entrainment of the plasma membrane and the formation of intracellular MVBs [26].
Early EVs are originally formed by phagocytosis of cell membranes [27]. They gather molecular substances, such as target proteins, RNA and DNA along the motion path, furthermore, processing, embellishing and classifying these substances. The formation of EVs is primarily divided into endosomal sorting complex required for transport-dependent (ESCRT)-dependent and ESCRT-independent pathways [28,29]. Beyond that, carrying out numerous biologic functions, especially in intercellular communication, accumulated evidence manifested that a few biological substances in EVs like proteins, microRNAs and lncRNAs were bound up with the nosogenesis of many malignant tumors. Furthermore, EVs could be used as promising biomarkers for tumor diagnosis, treatment and the prognosis of survival [30,31,32].
It has been confirmed that all cell types secrete EVs, which play a vital role in cell–cell communication under physiological and pathological conditions [33]. EVs participate in the interchange of genetic information and materials in cell-to-cell communication [34]. They play an important part in sustaining many of the significant physiological processes, such as cell growth, development, differentiation, and apoptosis [35]. More and more research indicated that abnormal secretion and dysfunction of EVs had a significant effect on the genesis, development and therapy of human malignant tumors [36,37,38].
3. Structure and Function of EVs
EVs is a collective term that covers the various subtypes of membrane structures released by cells. EVs include species of exosomes with a diameter of 30–150 nm, microvesicles with a diameter of 100–1000 nm and apoptotic bodies with a diameter of 800–5000 nm [39]. EVs carry lipids, proteins, RNAs (including microRNAs, mRNAs and long non-coding RNAs) and DNAs, which transfer these contents from donor cells to recipient cells, causing changes in the tumor microenvironment (TME) [40].
There is growing evidence that EVs play a critical role in cancer development, including tumorigenesis and metastasis [41]. The continuous expansion, invasion and metastasis of tumor cells rest with the communication between cells in the microenvironment. This communication consists primarily of soluble factors secreted by the cancer cell and stromal cells in the tumor microenvironment, and these stromal cells export particles containing regulatory molecules, which facilitate intercellular communication [42,43]. EVs could be precipitated when the centrifugal force was 100,000 g, or the sucrose density gradient was 1.13–1.19 g/mL [44,45]. EVs are spherical in shape, “cup” or “dish” in the transmission electron microscope and have a clear lipid bilayer structure under electron microscope [25,46]. EVs are released not directly from cell membranes by exocytosis but from multivesicular bodies (MVBs) [47,48]. In the process, EVs carry all kinds of bioactive cargo, including protein, lipids, enzymes, DNA and RNA (mRNA, microRNA and lncRNA) [49]. These EVs are involved in homeostasis, intercellular communication, the mediation of the immune system and inflammation and the delivery of genetic information [50,51]. Furthermore, EVs influence the pathological state of many diseases, such as the occurrence, progression, metastasis of cancer, neurodegenerative diseases, infectious diseases and autoimmune diseases [37,49] (Figure 1). EVs play a vital role in the development, progression and drug resistance of various tumors, including GBM [52,53]. Moreover, EVs contribute to maintaining the differentiation of glioma stem cells and influencing angiogenesis, TME and the immune defense system of GBM [54,55]. Studies have shown that EVs increase cell invasiveness, proliferation, migration potential, invasiveness and therapeutic resistance [56,57,58].
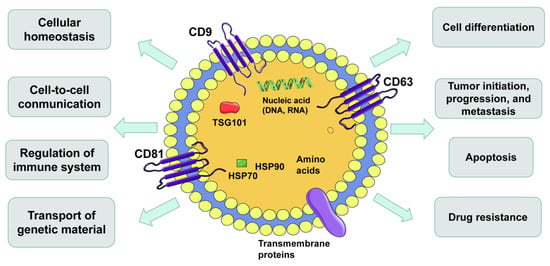
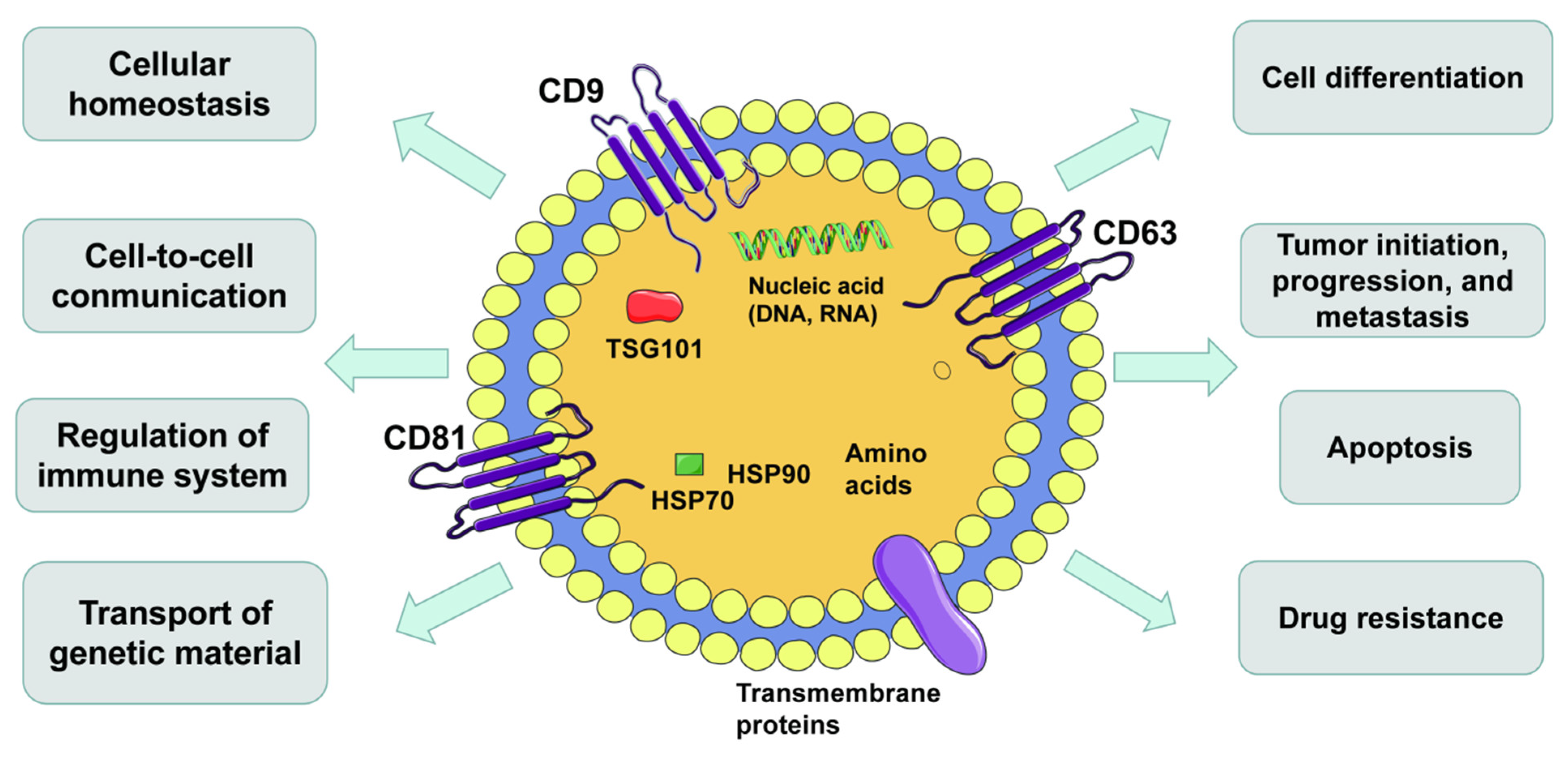
Figure 1.
Hallmarks of extracellular vesicles (EVs). EVs are secreted by all kinds of cells and carry DNAs, RNAs, proteins, lipids and metabolites. EVs are important mediators of intercellular communication and affect all aspects of cell biology.
4. Isolation and Purification of EVs
To explore the function and clinical application of EVs, the first and foremost is to isolate EVs from a large number of cells. The techniques used for EVs separation should be efficient and able to separate EVs from a wide variety of samples [59,60,61]. To check the characteristics of separated EVs, the size, morphological characteristics, distribution, number and components of EVs are measured using optical and non-optical technologies [62,63,64]. Several techniques put forward the enrichment of EVs, such as differential centrifugation, density-gradient ultracentrifugation, size-exclusion chromatography, gel filtration, flow field fractionation, commercial kits using polymer-based precipitation and immunoaffinity-based capture to achieve the purification of tumor-derived exosomes [65,66].
5. Glioblastoma-Derived EVs
A great amount of evidence indicates that EVs have significant effects on the formation and metastasis of GBM. Moreover, the substances in EVs are also used for clinical diagnosis and treatment. GBM-derived EVs are severely involved in tumorigenesis, microenvironment, angiogenesis, immune response, invasion, subtype and chemoresistance by transferring oncogenic proteins and nucleic acids.
5.1. Tumorigenesis
The important characteristic of GBM is its strong infiltration ability, which leads to the formation of satellite tumors in healthy brain parenchyma. However, it is not able to be surgically removed, which is one of the factors for the early recurrence of GBM patients [67]. Tumor-derived EVs are conducive to the proliferation, migration and invasion of GBM cells [68]. EVs are important carriers of oncogenic factors involved in the development of GBM [19,69].
A recent study has suggested that overexpression of miR-34a in EVs derived from human bone marrow mesenchymal stem cells (hBMSC) inhibited the proliferation and invasion of GBM cells [70]. The EVs secreted by GBM-associated macrophages (GAMs) contained a large amount of miR-21, which was a well-known cancer-promoting microRNA. Moreover, pacritinib, an oral tyrosine kinase inhibitor, could inhibit the occurrence of GBM by regulating signal transducers and activators of transcription 3 (STAT3))/miR-21/programmed cell death 4 (PDCD4) signaling [71].
One research showed that glioma-derived EVs affected M2 macrophage polarization under hypoxia, thus promoting the immunosuppressive microenvironment formation. In addition, researchers found that miR-1246 was abundant in cerebrospinal fluid (CSF) of GBM patients, which may serve as a new biomarker for diagnosis, treatment with targeted miR-1246 may be helpful for therapy of GBM [72]. MiRNA transport regulated by EVs from CD133+ U87 cells performed a vital part in adjusting pro-angiogenic responses and cell proliferation [73].
The serum EVs miR-301a level in glioma expressed its cancer biology and pathological variations; what is more, it could be a new biomarker and prognostic indicator for glioma diagnosis [17,74]. One recent study suggested that miR-301a in exosomes secreted by hypoxic glioma cells activated the Wnt/β-catenin signal pathway by targeting TCEAL7 and improved radiotherapy resistance and that TCEAL7 was a confirmed GBM suppressor gene [75].
EVs isolated from the serum of patients with GBM were injected with normal epithelial cells, causing gliomas in mice. When EVs isolated from the serum of patients with GBM were injected simultaneously with normal epithelial cells, the mice developed gliomas, indicating an underlying mechanism for EVs in GBM tumorigenesis [76]. We summarized EVs’ microRNAs that played a role in GBM tumorigenesis (Table 1).

Table 1.
Tumorigenesis in EVs’ microRNAs with a role in glioblastoma (GBM).
5.2. Role of EVs in GBM Microenvironment
It is well-known that the tumor microenvironment (TME) plays a pivotal role in all aspects of GBM development. A recent study suggested that exosomes participated in the disease course of GBM and even played an important role in the reconstruction of TME [77,78]. EVs acted as a key mediator for tumor development and maintenance by regulating TME [79]. EVs mediated the communication between GBM cells and stromal cells, including monocytes, macrophages, mast cells, microglia, T cells, astrocytes and oligodendrocytes [80]. Hypoxia and GBM influenced the polarization of M2 macrophages through EVs, thereby promoting the formation of an immunosuppressive microenvironment [72].GBM tumor cell-derived EVs modulated different cellular and extracellular components of the tumor microenvironment to promote the development and progression of GBM [81]. MiR-301a in EVs secreted by hypoxic GBM cells could be transferred to normal oxygen culture cells to change the cell microenvironment, eventually leading to increased radiation resistance [75]. EVs secreted by GBM gave rise to an immunosuppressive response. STAT3 was a component of GDE and mediated the immunosuppressive switch. The data showed that glioblastoma stem cell-derived exosomes (GDEs) were factors released by GBM and effective regulators of the GBM immunosuppressive microenvironment [82]. Therefore, EVs secreted by GBM played an important role in the microenvironment regulation of GBM.
As an information carrier, glioblastoma stem cells (GSC) EVs mediated the dedifferentiation of non-GSC glioma cells into GSCs by activating Notch1 signal to transmit Notch1 protein, thus enhancing the stemness and tumorigenesis of non-GSC glioma cells [83]. The research described that Semaphorin 7A (SEMA7A) was exposed to the surface of patient-derived glioma-associated stem cells (GASC) EVs and increased the activity of GSC through the interaction between integrin β1 and GSC in the microenvironment [84].
GBM cells derived cancer-causing lncRNA-SBF2-AS1 EVs to reconstruct tumor microenvironment and accelerate tumor chemotherapeutic resistance [85]. EVs secreted by glioma cells activated glycolysis of hBMSCs leading to the transformation of tumor phenotypes. This indicated that disturbing the mutual effect between exosomes and hBMSCs in the tumor microenvironment may be a therapy for glioma [86]. A study elucidated a potential mechanism for glioma recurrence in which normal glioma-associated astrocytes protected MGMT negative glioma cells from temozolomide (TMZ)-induced apoptosis by transferring EVs MGMT mRNA [87].
5.3. Role of EVs in GBM Angiogenesis
Angiogenesis, the generation of new blood vessels from existing ones, is essential for the growth and maintenance of tumors [88]. The proliferation, migration, differentiation of endothelial cells and the generation of new blood vessels require the pro-angiogenic factors, anti-angiogenic factors and microRNAs in the EVs secreted by GBM (Table 2). It has been suggested that the targeting of vascular endothelial growth factor (VEGF) from EVs to brain endothelial cells might affect their function to make new blood vessels. Therefore, GBM-derived EVs cargo may be an important part of tumor-induced angiogenesis [89].

Table 2.
Angiogenic microRNAs in EVs with a role in GBM.
5.4. EVs and Immune Response of GBM
EVs released by GBM were taken up by microglial cells, which were associated with increased miRNA level, decreased target mRNA and coding protein, leading to increased proliferation of GBM cells and enhanced immunosuppression [94]. GBM regulated the immune system to affect monocytes, macrophages and microglia, leading to GBM’s invasiveness [76]. Interactions between programmed cell death 1 ligand 1 (PDL1) and its receptor programmed cell death 1 (PD1) inhibited the T-cell response. Studies have shown that EVs expressing PDL1 could inhibit antitumor immune responses [95]. GBM EVs blocked T cell activation and proliferation response to T cell receptor stimulation. PD-L1 on GBM-derived EVs might inhibit antitumor immunity [96]. GSC-derived EVs penetrated the cytoplasm of monocytes and induced the recombination of the actin cytoskeleton, which inclined monocytes to the immunosuppressive M2 phenotype and increased the expression of PD-L1. This suggested that EVs were factors released by GBM and effective regulators of GBM-related immune response [82,95]. GBM-derived EVs could modify the phenotype of monocytic cell lineage, including monocytes, macrophages and microglia. EVs altered them to resemble the tumor-supporting phenotypes described by patients [97]. Dendritic cell (DC) vaccine immunotherapy for GBM has displayed significant benefits in animal and early clinical trials. EVs delivered DC vaccine to bring about activation and proliferation of tumor-specific cytotoxic T lymphocytes, destroy immune tolerance and improve immunosuppressive environment [98]. GBM cell-derived EVs LGALS9 in the cerebrospinal fluid played a major regulatory role in the progression of GBM by inhibiting DC antigen presentation and cytotoxic T cell activation [99]. Moreover, antigen-presenting cells could reduce the ability of the immune system, thereby preventing the production of specific immune responses [100]. The serum EVs and cytokines in peripheral blood of GBM patients played a systemic role through immune regulation, which exceeded the limits of the central nervous system [101].
5.5. EVs and GBM Invasion
Substantial invasive capacity is one of the key characteristics of GBM [102]. EVs can reshape the extracellular matrix and promote GBM invasion. It has been reported that GBM-delivered EVs promoted GBM invasion offering the necessary stimulation for radiating GBM cells [103,104]. GSCs-derived EVs significantly increased proliferation, neurosphere formation, invasiveness and tumorigenicity of non-GSC glioma cells [83].
Molecules involved in cell migration, including neurotrophic tyrosine kinase receptor type 1 (TrkA), paxillin, focal adhesion kinase (FAK) and the oncogene tyrosine-protein kinase Src. GBM-derived EVs contribute to the activation of these factors [105]. Hypoxic-derived EVs bring about changes in GBM cell biosynthesis and ion regulation channels. Moreover, EVs secreted by hypoxic GBM cells can also promote intercellular communication, induce significant changes in gene expression in adjacent normal oxygen tumor cells, many of which take part in the process of cancer invasion and therapeutic resistance [106]. The novel data suggest that EVs deliver immunoglobulin superfamily protein L1CAM (L1, CD171) to promote GBM cell proliferation and invasion, thereby influencing GBM cell behavior [107]. In conclusion, the above researches suggest that EVs-mediated cell–cell communication may be involved in important mechanisms of GBM invasion.
5.6. The role of EVs in GBM Subtype
GBM subtype-specific gene markers are vital to the research of tumor heterogeneity [108]. In high-grade glioma (HGG), different levels of cell populations are derived from different glioma stem cell-like (GSC) subpopulations. It was found that EVs protein transport between different tumor cell subsets provided a means of dynamic transformation and was correlated with HGG heterogeneity [57]. MiR-128-dependent transcriptome on EVs could predict subtypes and prognosis in patients with GBM [109].
EVs cargo is different between GBM subtypes, including neurological neoplasms, neoplasms, classical neoplasms and mesenchymal neoplasms [110]. EVs are carriers of molecular and oncogenic signals present in tumor cell subsets and tumor-associated stroma, as well as mediators of intercellular communication. EVs may also reflect and influence balance at the stem cell level, including oncogenic drivers and regulatory microenvironments [111]. The role of EVs as biomarkers and mediators for GBM may depend on the molecular subtypes and functional status of donor tumor cells, including GSCs [81].
5.7. GBM EVs Induce Chemoresistance
An important reason for the poor GBM survival rate is chemoresistance. GBM-derived EVs play an important role in chemotherapy resistance. In GBM, EVs secreted by ptPRZ1-MET fused cells lead to a tumorigenic phenotype and temozolomide resistance [69]. Polymerase I and transcriptional release factor (PTRF) known as Cavin1 in serum EVs contributed to the detection of gliomas were hopeful biomarkers and may be a treatment target point for GBM [112].
Furthermore, it was found that lncRNA SBF2-AS1 in EVs was increased in TMZ-resistant GBM cells, while overexpression of SBF2-AS1 could promote TMZ drug resistance. On the contrary, inhibition of SBF2-AS1 made TMZ-resistant GBM cells sensitive to TMZ [113]. The TMZ-resistant GBM cells were related to miRNAs and EVs [16]. MiR-1238 was incorporated into EVs by TMZ-resistant GBM cells, resulting in a high-level of miR-1238 in EVs, which was absorbed by TMZ sensitive cells and spread TMZ resistance [114].
GBM cells transported miR-93 and miR-193 through EVs to target cyclin D1 to achieve TMZ-induced drug resistance [115]. TMZ-resistant GBM cell-derived EVs delivered miR-151a in an independent manner, so that receptor TMZ sensitive cells developed TMZ chemotherapeutic resistance [116]. Mesenchymal stem cell-derived EVs transport synthetic anti-miR-9 and reverse chemotherapeutic resistance of GBM cells [117].
6. Conclusions and Future Perspectives
As a new intercellular communication mechanism, EVs enable GBM cells to acquire different characteristics of tumoral recurrence and progression [118,119]. GBM-derived EVs contain various components, including DNAs, miRNAs, lncRNAs, mRNAs, enzymes, ligands and receptors [20,120]. EVs play an irreplaceable role in maintaining the stability of the human body [121]. Compared with other tumor markers in tissues or body fluids, EVs have higher stability and content, so they have more advantages and significant potential for clinical application in the early diagnosis and therapy of GBM [122]. The ability of EVs to cross the blood–brain barrier (BBB) is an important factor in considering their success in delivering drugs to TME. GBM-derived specific EVs can cross the BBB and circulate in body fluids, so they can be used as non-invasive biomarkers for early diagnosis of GBM [123,124]. GBM-derived EVs could enhance BBB permeability [125].
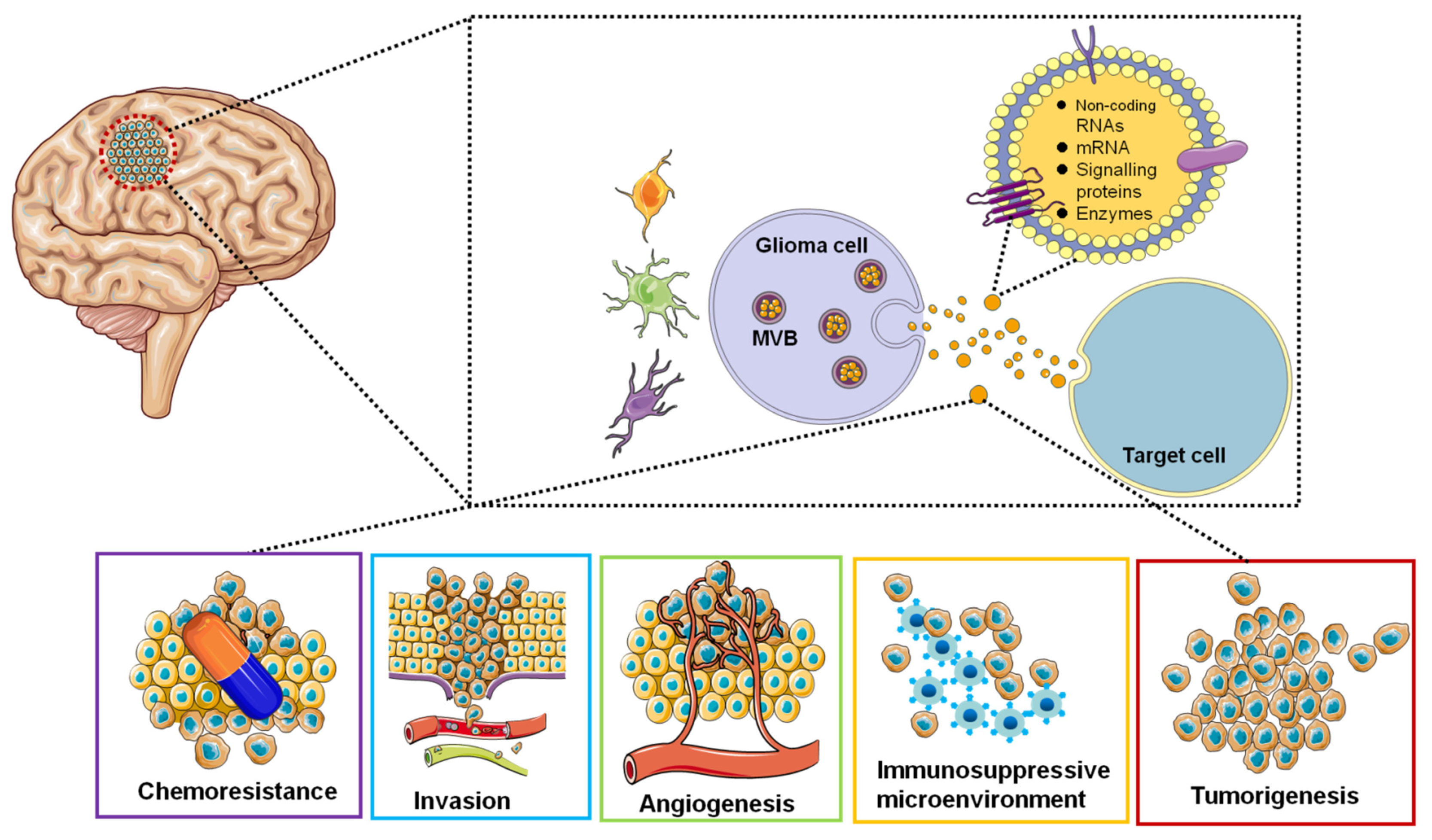
Research interests in the EVs area are focused on the use of EVs-based non-invasive biomarkers for cancer diagnosis and surveillance and the use of EVs to deliver targeted drugs for GBM treatment. EVs secreted by GBM tumor cells can be detected in the blood, cerebrospinal fluid, and urine of cancer patients [126]. Therefore, EVs can be used as diagnostic and therapeutic monitoring tools for GBM. EV isolated from glioblastoma cell lines contained tumor-specific mRNAs and miRNAs [127,128]. We represent the roles of EVs in GBM in a simplified diagram (Figure 2).
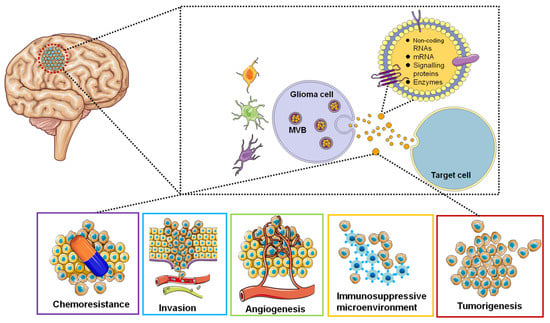
Figure 2.
Functions of glioblastoma-derived EVs. EVs are involved in tumorigenesis, microenvironment, angiogenesis, immune response, invasion and chemoresistance by transferring oncogenic proteins and nucleic acids.
In addition, we highlighted the GBM-derived EVs influencing tumor microenvironment, angiogenesis, microRNAs, tumorigenesis, immune response, subtype and chemoresistance. Moreover, EVs regulate the migration and invasion of GBM cells, which is expected to break new ground for GBM treatment. EVs are similar in size and function to synthetic nanoparticles and have many advantages that make them the most promising targeted drug or gene vectors [129]. EVs may be a more targeted material source for the discovery of biomarkers. Systematic EVs accumulate in the liver, kidney, and spleen. Several types of EVs secreted by cells exhibit target selection, including DCs, B-cells and macrophages [130,131]. Target EVs can be obtained by presenting target molecules, such as peptides or fragments of antibodies that recognize target antigens on the outer surface of EVs. However, the tendency of EVs secreted by most cells to specific cell types is limited, so future research requires targeting strategies for systemically delivered EVs. Moreover, future studies on EVs urgently need to understand the potential of EV-mediated targeted communication to enable EVs as a novel cancer therapy. There is an urgent need to improve the identification, isolation and purification techniques of EVs, and the molecular mechanism of action on diseases is still not very clear. Synthetic EV technology is not mature enough at present.
The field of GBM-derived EVs has attracted more attention in recent years. We hope that more researchers will pay attention to this field on the basis of GBM-derived EVs in future studies so as to jointly solve the important issue of the occurrence and development of GBM. We hope that a growing number of researchers will join us in the research area of EVs, which shows the importance of this area. In conclusion, there is still a long way to go to achieve clinical application before the above problems have been explored and verified, especially the research on GBM diagnosis and treatment is still in the experimental stage.
Author Contributions
Y.J. and Y.C. conceived of the presented idea. Y.J. and N.W. critically reviewed the manuscript. All authors contributed to and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No.81871415), Heilongjiang Touyan Innovation Team Program (to Yan Jin). General Support from China Postdoctoral Science Foundation (2018M631954); the National Natural Science Foundation of China (No.81802428) (to Nan Wu).Youth Innovation Fund Project of Harbin Medical University (2017JCZX01); Scientific Research Fund Project of Harbin Medical University-Daqing (DQXN201602) (to Yunping Chen).
Acknowledgments
We thank members of our research groups for technical advice and support and for critical reading of the manuscript.
Conflicts of Interest
The authors declare that no conflict of interest.
References
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Glioblastoma: From molecular pathology to targeted treatment. Annu. Rev. Pathol. 2014, 9, 1–25. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.Y.; Kim, W.K.; Lee, J.K.; Park, J.; et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef]
- Stupp, R.; Lukas, R.V.; Hegi, M.E. Improving survival in molecularly selected glioblastoma. Lancet 2019, 393, 615–617. [Google Scholar] [CrossRef]
- Willyard, C. The innovative therapies that could break the brain-cancer stalemate. Nature 2018, 561, S59–S61. [Google Scholar] [CrossRef] [PubMed]
- DeWeerdt, S. The genomics of brain cancer. Nature 2018, 561, S54–S55. [Google Scholar] [CrossRef]
- Gerardo Valadez, J.; Grover, V.K.; Carter, M.D.; Calcutt, M.W.; Abiria, S.A.; Lundberg, C.J.; Williams, T.V.; Cooper, M.K. Identification of Hedgehog pathway responsive glioblastomas by isocitrate dehydrogenase mutation. Cancer Lett. 2013, 328, 297–306. [Google Scholar] [CrossRef]
- Vaubel, R.A.; Tian, S.; Remonde, D.; Schroeder, M.A.; Mladek, A.C.; Kitange, G.J.; Caron, A.; Kollmeyer, T.M.; Grove, R.; Peng, S.; et al. Genomic and Phenotypic Characterization of a Broad Panel of Patient-Derived Xenografts Reflects the Diversity of Glioblastoma. Clin. Cancer Res. 2020, 26, 1094–1104. [Google Scholar] [CrossRef]
- Wu, S.; Wang, S.; Gao, F.; Li, L.; Zheng, S.; Yung, W.; Koul, D. Activation of WEE1 confers resistance to PI3K inhibition in glioblastoma. Neuro Oncol. 2018, 20, 78–91. [Google Scholar] [CrossRef]
- Draaisma, K.; Chatzipli, A.; Taphoorn, M.; Kerkhof, M.; Weyerbrock, A.; Sanson, M.; Hoeben, A.; Lukacova, S.; Lombardi, G.; Leenstra, S.; et al. Molecular Evolution of IDH Wild-Type Glioblastomas Treated With Standard of Care Affects Survival and Design of Precision Medicine Trials: A Report From the EORTC 1542 Study. J. Clin. Oncol. 2020, 38, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Azevedo, A.; Esteves, S.; Marques, A.R.; Martins, C.; Costa, I.; Mafra, M.; Bravo Marques, J.M.; Roque, L.; Pojo, M. Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer. 2019, 19, 968. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.M.; Quddusi, A.; Shamim, M.S. The significance of MGMT methylation in Glioblastoma Multiforme prognosis. J. Pak. Med. Assoc. 2018, 68, 1137–1139. [Google Scholar] [PubMed]
- Zhao, J.; Chen, A.X.; Gartrell, R.D.; Silverman, A.M.; Aparicio, L.; Chu, T.; Bordbar, D.; Shan, D.; Samanamud, J.; Mahajan, A.; et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019, 25, 462–469. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Garnier, D.; Meehan, B.; Kislinger, T.; Daniel, P.; Sinha, A.; Abdulkarim, B.; Nakano, I.; Rak, J. Divergent evolution of temozolomide resistance in glioblastoma stem cells is reflected in extracellular vesicles and coupled with radiosensitization. Neuro Oncol. 2018, 20, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Saadatpour, L.; Fadaee, E.; Fadaei, S.; Nassiri Mansour, R.; Mohammadi, M.; Mousavi, S.M.; Goodarzi, M.; Verdi, J.; Mirzaei, H. Glioblastoma: Exosome and microRNA as novel diagnosis biomarkers. Cancer Gene Ther. 2016, 23, 415–418. [Google Scholar] [CrossRef]
- Fraser, K.; Jo, A.; Giedt, J.; Vinegoni, C.; Yang, K.S.; Peruzzi, P.; Chiocca, E.A.; Breakefield, X.O.; Lee, H.; Weissleder, R. Characterization of single microvesicles in plasma from glioblastoma patients. Neuro Oncol. 2019, 21, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Ezrin, A.; Hadjipanayis, C. Small extracellular vesicles as tumor biomarkers for glioblastoma. Mol. Aspects Med. 2015, 45, 97–102. [Google Scholar] [CrossRef]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic Stem Cells-Derived Exosomes Endowed with Targeting Properties as Chemotherapeutics Delivery Vehicles for Glioblastoma Therapy. Adv. Sci. (Weinh). 2019, 6, 1801899. [Google Scholar] [CrossRef]
- Bălașa, A.; Șerban, G.; Chinezu, R.; Hurghiș, C.; Tămaș, F.; Manu, D. The Involvement of Exosomes in Glioblastoma Development, Diagnosis, Prognosis, and Treatment. Brain Sci. 2020, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, Y.; Yao, B.; Sun, P.; Hao, Y.; Piao, H.; Zhao, X. Role of Exosomes in the Progression, Diagnosis, and Treatment of Gliomas. Med. Sci. Monit. 2020, 26, e924023. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Christ, L.; Raiborg, C.; Wenzel, E.M.; Campsteijn, C.; Stenmark, H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem. Sci. 2017, 42, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.; Radulovic, M.; Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef]
- Casanova-Salas, I.; Athie, A.; Boutros, P.C.; Del Re, M.; Miyamoto, D.T.; Pienta, K.J.; Posadas, E.M.; Sowalsky, A.G.; Stenzl, A.; Wyatt, A.W.; et al. Quantitative and Qualitative Analysis of Blood-based Liquid Biopsies to Inform Clinical Decision-making in Prostate Cancer. Eur. Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, K.; Dwivedi, O.P.; Leparc, G.; Rolser, M.; Delic, D.; Forsblom, C.; Groop, P.H.; Groop, L.; Huber, T.B.; Puhka, M.; et al. Comparison of urinary extracellular vesicle isolation methods for transcriptomic biomarker research in diabetic kidney disease. J. Extracell. Vesicles. 2020, 10, e12038. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ge, X.; Shi, Z.; Yu, C.; Lu, C.; Wei, Y.; Zeng, A.; Wang, X.; Yan, W.; Zhang, J.; et al. Extracellular vesicles derived from hypoxic glioma stem-like cells confer temozolomide resistance on glioblastoma by delivering miR-30b-3p. Theranostics 2021, 11, 1763–1779. [Google Scholar] [CrossRef]
- Sung, B.H.; Weaver, A.M. Exosome secretion promotes chemotaxis of cancer cells. Cell Adh. Migr. 2017, 11, 187–195. [Google Scholar] [CrossRef]
- Yang, F.; Liao, X.; Tian, Y.; Li, G. Exosome separation using microfluidic systems: Size-based, immunoaffinity-based and dynamic methodologies. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef]
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 2019, 10, 4136. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I.I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Boriachek, K.; Islam, M.N.; Möller, A.; Salomon, C.; Nguyen, N.T.; Hossain, M.; Yamauchi, Y.; Shiddiky, M. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Acharyya, S. Understanding cachexia in the context of metastatic progression. Nat. Rev. Cancer 2020, 20, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Smith, J.A. Explicating Exosomes: Reclassifying the Rising Stars of Intercellular Communication. Cell 2019, 177, 225–227. [Google Scholar] [CrossRef] [PubMed]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Van Hoof, A.; Parker, R. The exosome: A proteasome for RNA? Cell 1999, 99, 347–350. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Invest. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Ajezi, S.; Avci, Ç.B.; Karimipour, M.; Geranmayeh, M.H.; Nourazarian, A.; Sokullu, E.; Rezabakhsh, A.; Rahbarghazi, R. Exosomes and their Application in Biomedical Field: Difficulties and Advantages. Mol. Neurobiol. 2018, 55, 3372–3393. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem. Cells Transl Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef]
- Raab-Traub, N.; Dittmer, D.P. Viral effects on the content and function of extracellular vesicles. Nat. Rev. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef]
- Sato, K.; Meng, F.; Glaser, S.; Alpini, G. Exosomes in liver pathology. J. Hepatol. 2016, 65, 213–221. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Koritzinsky, E.H.; Street, J.M.; Star, R.A.; Yuen, P.S. Quantification of Exosomes. J. Cell Physiol. 2017, 232, 1587–1590. [Google Scholar] [CrossRef]
- Graner, M.W. Roles of Extracellular Vesicles in High-Grade Gliomas: Tiny Particles with Outsized Influence. Annu. Rev. Genomics. Hum. Genet. 2019, 20, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef]
- Ricklefs, F.; Mineo, M.; Rooj, A.K.; Nakano, I.; Charest, A.; Weissleder, R.; Breakefield, X.O.; Chiocca, E.A.; Godlewski, J.; Bronisz, A. Extracellular Vesicles from High-Grade Glioma Exchange Diverse Pro-oncogenic Signals That Maintain Intratumoral Heterogeneity. Cancer Res. 2016, 76, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M.W.; Li, M.; Giovanazzi, A.; Fleming, R.L.; Tabet, E.I.; Nakano, I.; Würdinger, T.; Chiocca, E.A.; Tian, T.; Tannous, B.A. Extracellular Vesicles Induce Mesenchymal Transition and Therapeutic Resistance in Glioblastomas through NF-kappaB/STAT3 Signaling. Adv. Biosyst. 2020, 4, e1900312. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, B.; Li, M.; Mi, S. Secretome of Activated Fibroblasts Induced by Exosomes for the Discovery of Biomarkers in Non-Small Cell Lung Cancer. Small 2020, e2004750. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, Y.; Zhang, X.; Yang, Y.; Zheng, X.; Li, X.; Liu, Y.; Zhang, Y. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol. Cancer 2018, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Arbelaiz, A.; Azkargorta, M.; Krawczyk, M.; Santos-Laso, A.; Lapitz, A.; Perugorria, M.J.; Erice, O.; Gonzalez, E.; Jimenez-Agüero, R.; Lacasta, A.; et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017, 66, 1125–1143. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Khatun, Z.; Bhat, A.; Sharma, S.; Sharma, A. Elucidating diversity of exosomes: Biophysical and molecular characterization methods. Nanomedicine (Lond) 2016, 11, 2359–2377. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, R.; Gauthier, S.A.; Kumar, A.; Saito, M.; Saito, M.; Levy, E. A Method for Isolation of Extracellular Vesicles and Characterization of Exosomes from Brain Extracellular Space. Methods Mol. Biol. 2017, 1545, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chung, J.; Lee, K.; Balaj, L.; Min, C.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Lee, H.; Weissleder, R. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 2015, 6, 6999. [Google Scholar] [CrossRef] [PubMed]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef]
- Zeng, A.L.; Yan, W.; Liu, Y.W.; Wang, Z.; Hu, Q.; Nie, E.; Zhou, X.; Li, R.; Wang, X.F.; Jiang, T.; et al. Tumour exosomes from cells harbouring PTPRZ1-MET fusion contribute to a malignant phenotype and temozolomide chemoresistance in glioblastoma. Oncogene 2017, 36, 5369–5381. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.H.; Lou, P.Y.; Chai, C.; Han, S.Y.; Ning, J.F.; Li, M. Human bone marrow-derived mesenchymal stem cell-secreted exosomes overexpressing microRNA-34a ameliorate glioblastoma development via down-regulating MYCN. Cell Oncol. (Dordr). 2019, 42, 783–799. [Google Scholar] [CrossRef]
- Chuang, H.Y.; Su, Y.K.; Liu, H.W.; Chen, C.H.; Chiu, S.C.; Cho, D.Y.; Lin, S.Z.; Chen, Y.S.; Lin, C.M. Preclinical Evidence of STAT3 Inhibitor Pacritinib Overcoming Temozolomide Resistance via Downregulating miR-21-Enriched Exosomes from M2 Glioblastoma-Associated Macrophages. J. Clin. Med. 2019, 8, 959. [Google Scholar] [CrossRef]
- Qian, M.; Wang, S.; Guo, X.; Wang, J.; Zhang, Z.; Qiu, W.; Gao, X.; Chen, Z.; Xu, J.; Zhao, R.; et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-kappaB pathways. Oncogene 2020, 39, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Y.; Cheng, S.; Wu, Z.; Liu, F.; Zhang, J. CD133 positive U87 glioblastoma cells-derived exosomal microRNAs in hypoxia- versus normoxia-microenviroment. J. Neurooncol. 2017, 135, 37–46. [Google Scholar] [CrossRef]
- Lan, F.; Qing, Q.; Pan, Q.; Hu, M.; Yu, H.; Yue, X. Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma. Cell Oncol. (Dordr). 2018, 41, 25–33. [Google Scholar] [CrossRef]
- Yue, X.; Lan, F.; Xia, T. Hypoxic Glioma Cell-Secreted Exosomal miR-301a Activates Wnt/beta-catenin Signaling and Promotes Radiation Resistance by Targeting TCEAL7. Mol. Ther. 2019, 27, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014, 26, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017, 13, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Moses, M.A. Brainwashed by extracellular vesicles: The role of extracellular vesicles in primary and metastatic brain tumour microenvironment. J. Extracell. Vesicles. 2019, 8, 1627164. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lam, E.W.; Sun, Y. Extracellular vesicles in the tumor microenvironment: Old stories, but new tales. Mol. Cancer 2019, 18, 59. [Google Scholar] [CrossRef]
- Spinelli, C.; Montermini, L.; Meehan, B.; Brisson, A.R.; Tan, S.; Choi, D.; Nakano, I.; Rak, J. Molecular subtypes and differentiation programmes of glioma stem cells as determinants of extracellular vesicle profiles and endothelial cell-stimulating activities. J. Extracell. Vesicles. 2018, 7, 1490144. [Google Scholar] [CrossRef]
- Gabrusiewicz, K.; Li, X.; Wei, J.; Hashimoto, Y.; Marisetty, A.L.; Ott, M.; Wang, F.; Hawke, D.; Yu, J.; Healy, L.M.; et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 2018, 7, e1412909. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, Y.; Dong, L.; Ma, W.; Lv, L.; Zhang, J.; Wang, X. Glioblastoma Stem Cell-Derived Exosomes Enhance Stemness and Tumorigenicity of Glioma Cells by Transferring Notch1 Protein. Cell Mol. Neurobiol. 2020, 40, 767–784. [Google Scholar] [CrossRef]
- Manini, I.; Ruaro, M.E.; Sgarra, R.; Bartolini, A.; Caponnetto, F.; Ius, T.; Skrap, M.; Di Loreto, C.; Beltrami, A.P.; Manfioletti, G.; et al. Semaphorin-7A on Exosomes: A Promigratory Signal in the Glioma Microenvironment. Cancers (Basel) 2019, 11, 758. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, J.; Lu, C.; Wei, Y.; Zeng, A.; You, Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 166. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cui, X.; Lu, L.; Chen, G.; Yang, Y.; Hu, Y.; Lu, Y.; Cao, Z.; Wang, Y.; Wang, X. Exosomes from glioma cells induce a tumor-like phenotype in mesenchymal stem cells by activating glycolysis. Stem. Cell Res. Ther. 2019, 10, 60. [Google Scholar] [CrossRef]
- Yu, T.; Wang, X.; Zhi, T.; Zhang, J.; Wang, Y.; Nie, E.; Zhou, F.; You, Y.; Liu, N. Delivery of MGMT mRNA to glioma cells by reactive astrocyte-derived exosomes confers a temozolomide resistance phenotype. Cancer Lett. 2018, 433, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.; Droppelmann, C.A.; Salsoso, R.; Westermeier, F.; Toledo, F.; Salomon, C.; Sanhueza, C.; Pardo, F.; Leiva, A.; Sobrevia, L. A Hypothesis for the Role of RECK in Angiogenesis. Curr. Vasc. Pharmacol. 2016, 14, 106–115. [Google Scholar] [CrossRef]
- Treps, L.; Perret, R.; Edmond, S.; Ricard, D.; Gavard, J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J. Extracell. Vesicles. 2017, 6, 1359479. [Google Scholar] [CrossRef]
- Thuringer, D.; Chanteloup, G.; Boucher, J.; Pernet, N.; Boudesco, C.; Jego, G.; Chatelier, A.; Bois, P.; Gobbo, J.; Cronier, L.; et al. Modulation of the inwardly rectifying potassium channel Kir4.1 by the pro-invasive miR-5096 in glioblastoma cells. Oncotarget 2017, 8, 37681–37693. [Google Scholar] [CrossRef]
- Yang, J.K.; Yang, J.P.; Tong, J.; Jing, S.Y.; Fan, B.; Wang, F.; Sun, G.Z.; Jiao, B.H. Exosomal miR-221 targets DNM3 to induce tumor progression and temozolomide resistance in glioma. J. Neurooncol. 2017, 131, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, X.; Wang, J.; Zhao, Y.; Wang, Y.; Bihl, J.C.; Chen, Y.; Jiang, C. Glioma stem cells-derived exosomes promote the angiogenic ability of endothelial cells through miR-21/VEGF signal. Oncotarget 2017, 8, 36137–36148. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Van der Vos, K.E.; Abels, E.R.; Zhang, X.; Lai, C.; Carrizosa, E.; Oakley, D.; Prabhakar, S.; Mardini, O.; Crommentuijn, M.H.; Skog, J.; et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol. 2016, 18, 58–69. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef]
- De Vrij, J.; Maas, S.L.; Kwappenberg, K.M.; Schnoor, R.; Kleijn, A.; Dekker, L.; Luider, T.M.; de Witte, L.D.; Litjens, M.; van Strien, M.E.; et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int. J. Cancer. 2015, 137, 1630–1642. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Liu, J.; Meng, H.; Zhang, R.; Ma, L.; Wu, L.; Yu, S.; Shi, F.; Li, Y.; et al. Co-delivery of tumor-derived exosomes with alpha-galactosylceramide on dendritic cell-based immunotherapy for glioblastoma. Cancer Lett. 2017, 411, 182–190. [Google Scholar] [CrossRef]
- Wang, M.; Cai, Y.; Peng, Y.; Xu, B.; Hui, W.; Jiang, Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis. 2020, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, A.C.; Badie, B. Microglia and macrophages in malignant gliomas: Recent discoveries and implications for promising therapies. Clin. Dev. Immunol. 2013, 2013, 264124. [Google Scholar] [CrossRef] [PubMed]
- Harshyne, L.A.; Nasca, B.J.; Kenyon, L.C.; Andrews, D.W.; Hooper, D.C. Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro Oncol. 2016, 18, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Klein, E.; Neirinckx, V.; Knudsen, A.M.; Fabian, C.; Hau, A.C.; Dieterle, M.; Oudin, A.; Nazarov, P.V.; Golebiewska, A.; et al. AN1-type zinc finger protein 3 (ZFAND3) is a transcriptional regulator that drives Glioblastoma invasion. Nat. Commun. 2020, 11, 6366. [Google Scholar] [CrossRef]
- Cai, Q.; Zhu, A.; Gong, L. Exosomes of glioma cells deliver miR-148a to promote proliferation and metastasis of glioblastoma via targeting CADM1. Bull. Cancer 2018, 105, 643–651. [Google Scholar] [CrossRef]
- Naryzhny, S.; Volnitskiy, A.; Kopylov, A.; Zorina, E.; Kamyshinsky, R.; Bairamukov, V.; Garaeva, L.; Shlikht, A.; Shtam, T. Proteome of Glioblastoma-Derived Exosomes as a Source of Biomarkers. Biomedicines 2020, 8, 216. [Google Scholar] [CrossRef]
- Ryskalin, L.; Biagioni, F.; Lenzi, P.; Frati, A.; Fornai, F. mTOR Modulates Intercellular Signals for Enlargement and Infiltration in Glioblastoma Multiforme. Cancers 2020, 12, 2486. [Google Scholar] [CrossRef] [PubMed]
- Kore, R.A.; Edmondson, J.L.; Jenkins, S.V.; Jamshidi-Parsian, A.; Dings, R.; Reyna, N.S.; Griffin, R.J. Hypoxia-derived exosomes induce putative altered pathways in biosynthesis and ion regulatory channels in glioblastoma cells. Biochem. Biophys. Rep. 2018, 14, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Pace, K.R.; Dutt, R.; Galileo, D.S. Exosomal L1CAM Stimulates Glioblastoma Cell Motility, Proliferation, and Invasiveness. Int. J. Mol. Sci. 2019, 20, 3982. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Ferrer-Luna, R.; Rooj, A.K.; Mineo, M.; Ricklefs, F.; Takeda, Y.S.; Nowicki, M.O.; Salińska, E.; Nakano, I.; Lee, H.; et al. MicroRNA Signatures and Molecular Subtypes of Glioblastoma: The Role of Extracellular Transfer. Stem. Cell Rep. 2017, 8, 1497–1505. [Google Scholar] [CrossRef]
- Rooj, A.K.; Ricklefs, F.; Mineo, M.; Nakano, I.; Chiocca, E.A.; Bronisz, A.; Godlewski, J. MicroRNA-Mediated Dynamic Bidirectional Shift between the Subclasses of Glioblastoma Stem-like Cells. Cell Rep. 2017, 19, 2026–2032. [Google Scholar] [CrossRef]
- Lee, E.; Yong, R.L.; Paddison, P.; Zhu, J. Comparison of glioblastoma (GBM) molecular classification methods. Semin. Cancer Biol. 2018, 53, 201–211. [Google Scholar] [CrossRef]
- Nakano, I.; Garnier, D.; Minata, M.; Rak, J. Extracellular vesicles in the biology of brain tumour stem cells--Implications for inter-cellular communication, therapy and biomarker development. Semin. Cell Dev. Biol. 2015, 40, 17–26. [Google Scholar] [CrossRef]
- Huang, K.; Fang, C.; Yi, K.; Liu, X.; Qi, H.; Tan, Y.; Zhou, J.; Li, Y.; Liu, M.; Zhang, Y.; et al. The role of PTRF/Cavin1 as a biomarker in both glioma and serum exosomes. Theranostics 2018, 8, 1540–1557. [Google Scholar] [CrossRef]
- Towner, R.A.; Smith, N.; Saunders, D.; Brown, C.A.; Cai, X.; Ziegler, J.; Mallory, S.; Dozmorov, M.G.; Coutinho De Souza, P.; Wiley, G.; et al. OKN-007 Increases temozolomide (TMZ) Sensitivity and Suppresses TMZ-Resistant Glioblastoma (GBM) Tumor Growth. Transl. Oncol. 2019, 12, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zeng, A.; Zhang, Z.; Shi, Z.; Yan, W.; You, Y. Exosomal transfer of miR-1238 contributes to temozolomide-resistance in glioblastoma. EBioMedicine 2019, 42, 238–251. [Google Scholar] [CrossRef]
- Munoz, J.L.; Walker, N.D.; Mareedu, S.; Pamarthi, S.H.; Sinha, G.; Greco, S.J.; Rameshwar, P. Cycling Quiescence in Temozolomide Resistant Glioblastoma Cells Is Partly Explained by microRNA-93 and -193-Mediated Decrease of Cyclin, D. Front. Pharmacol. 2019, 10, 134. [Google Scholar] [CrossRef]
- Zeng, A.; Wei, Z.; Yan, W.; Yin, J.; Huang, X.; Zhou, X.; Li, R.; Shen, F.; Wu, W.; Wang, X.; et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018, 436, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic. Acids. 2013, 2, e126. [Google Scholar] [CrossRef]
- Peng, C.; Trojanowski, J.Q.; Lee, V.M. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 2020, 16, 199–212. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Madhusoodanan, J. The therapeutic potential of exosomes. Nature 2020, 581, S10–S11. [Google Scholar] [CrossRef]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Wu, S.; Pan, Y.; Dong, Y.; Zhu, S.; Yang, J.; Yin, Y.; Li, G. An Electrochemical Biosensor Designed by Using Zr-Based Metal-Organic Frameworks for the Detection of Glioblastoma-Derived Exosomes with Practical Application. Anal. Chem. 2020, 92, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, N.; Carrión-Navarro, J.; Esteban-Rubio, S.; Lázaro-Ibáñez, E.; Peris-Celda, M.; Alonso, M.M.; Guzmán-De-Villoria, J.; Fernández-Carballal, C.; de Mendivil, A.O.; García-Duque, S. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget 2017, 8, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, H.; Xiong, C.; Liu, Y. Hypoxic glioblastoma release exosomal VEGF-A induce the permeability of blood-brain barrier. Biochem. Biophys. Res. Commun. 2018, 502, 324–331. [Google Scholar] [CrossRef]
- Yekula, A.; Minciacchi, V.R.; Morello, M.; Shao, H.; Park, Y.; Zhang, X.; Muralidharan, K.; Freeman, M.R.; Weissleder, R.; Lee, H.; et al. Large and small extracellular vesicles released by glioma cells in vitro and in vivo. J. Extracell. Vesicles. 2020, 9, 1689784. [Google Scholar] [CrossRef]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Phillips, S.; Kaimal, V.; Mao, Y.; Hua, W.; Yang, I.; Fu, C.C.; Nolan, J.; et al. miRNA contents of cerebrospinal fluid extracellular vesicles in glioblastoma patients. J. Neurooncol. 2015, 123, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Montermini, L.; Kim, D.K.; Meehan, B.; Roth, F.P.; Rak, J. The Impact of Oncogenic EGFRvIII on the Proteome of Extracellular Vesicles Released from Glioblastoma Cells. Mol. Cell Proteomics. 2018, 17, 1948–1964. [Google Scholar] [CrossRef]
- Di, C.; Zhang, Q.; Wang, Y.; Wang, F.; Chen, Y.; Gan, L.; Zhou, R.; Sun, C.; Li, H.; Zhang, X.; et al. Exosomes as drug carriers for clinical application. Artif. Cells Nanomed Biotechnol. 2018, 46, S564–S570. [Google Scholar] [CrossRef]
- Nolte-’t Hoen, E.N.; Buschow, S.I.; Anderton, S.M.; Stoorvogel, W.; Wauben, M.H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 2009, 113, 1977–1981. [Google Scholar] [CrossRef]
- Saunderson, S.C.; Dunn, A.C.; Crocker, P.R.; McLellan, A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 2014, 123, 208–216. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).