Effect of Reducing Ataxia-Telangiectasia Mutated (ATM) in Experimental Autosomal Dominant Polycystic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Cell Lines
2.3. Three-Dimensional (3D) In Vitro Cyst Models
2.4. Assessment of Cell Viability, Cytotoxicity, and Proliferation
2.5. Mice
2.6. In Vivo Mouse Experiments
2.7. Histology, Immunohistochemistry, and Histological Quantification
2.8. Western Blot of Renal p53
2.9. Statistics
3. Results
3.1. Pharmacological Inhibition of ATR or ATM Reduces Cyst Growth In Vitro
3.1.1. Effect of VE-821 and KU-60019 on MDCK Cyst Model
3.1.2. Effect of AZD1390 and AZD0156 on MDCK Cyst Model
3.1.3. Effect of AZD0156 on Human ADPKD Cysts
3.2. AZD0156 Reduces Renal Cell Proliferation and Increases p53 in Pkd1RC/RC Mice
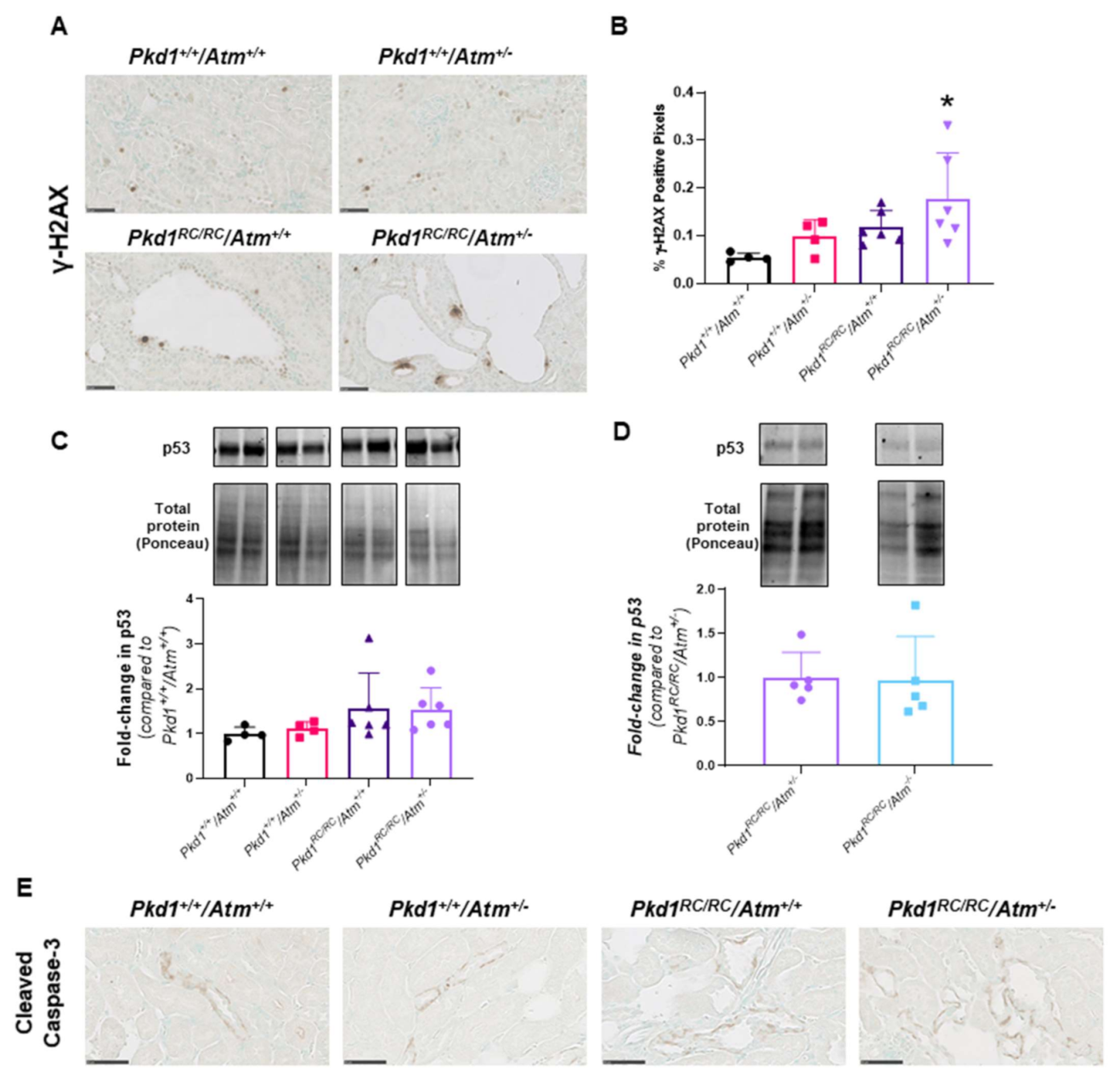
3.3. Progression of Cystic Kidney Disease Is Not Altered in Pkd1RC/RC/Atm+/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groopman, E.E.; Marasa, M.; Cameron-Christie, S.; Petrovski, S.; Aggarwal, V.S.; Milo-Rasouly, H.; Li, Y.; Zhang, J.; Nestor, J.; Krithivasan, P.; et al. Diagnostic Utility of Exome Sequencing for Kidney Disease. N. Engl. J. Med. 2019, 380, 142–151. [Google Scholar] [CrossRef]
- Gall, E.C.-L.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef]
- Grantham, J.J.; Geiser, J.L.; Evan, A.P. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987, 31, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Ong, A.C.M.; Harris, P.C. Molecular pathogenesis of ADPKD: The polycystin complex gets complex. Kidney Int. 2005, 67, 1234–1247. [Google Scholar] [CrossRef] [Green Version]
- Rangan, G.K.; Tchan, M.C.; Tong, A.; Wong, A.T.Y.; Nankivell, B.J. Recent advances in autosomal-dominant polycystic kidney disease. Intern. Med. J. 2016, 46, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Rangan, G.K.; Raghubanshi, A.; Chaitarvornkit, A.; Chandra, A.N.; Gardos, R.; Munt, A.; Read, M.N.; Saravanabavan, S.; Zhang, J.Q.; Wong, A.T. Current and emerging treatment options to prevent renal failure due to autosomal dominant polycystic kidney disease. Expert Opin. Orphan Drugs 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Seeger-Nukpezah, T.; Geynisman, D.M.; Nikonova, A.S.; Benzing, T.; Golemis, E.A. The hallmarks of cancer: Relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 515–534. [Google Scholar] [CrossRef] [Green Version]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Battini, L.; Macip, S.; Fedorova, E.; Dikman, S.; Somlo, S.; Montagna, C.; Gusella, G.L. Loss of polycystin-1 causes centrosome amplification and genomic instability. Hum. Mol. Genet. 2008, 17, 2819–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Qin, S.; Wang, L.; Zhou, J. Genomic instability in patients with autosomal-dominant polycystic kidney disease. J. Int. Med. Res. 2013, 41, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.C.; Lin, J.-R.; Vannier, J.-B.; Slaats, G.G.; Kile, A.C.; Paulsen, R.D.; Manning, D.K.; Beier, D.R.; Giles, R.H.; Boulton, S.J.; et al. NEK8 Links the ATR-Regulated Replication Stress Response and S Phase CDK Activity to Renal Ciliopathies. Mol. Cell 2013, 51, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Ta, M.H.T.; Schwensen, K.G.; Liuwantara, D.; Huso, D.L.; Watnick, T.; Rangan, G.K. Constitutive renal Rel/nuclear factor-κB expression in Lewis polycystic kidney disease rats. World J. Nephrol. 2016, 5, 339. [Google Scholar] [CrossRef]
- Cassini, M.F.; Kakade, V.R.; Kurtz, E.; Sulkowski, P.; Glazer, P.; Torres, R.; Somlo, S.; Cantley, L.G. Mcp1 Promotes Macrophage-Dependent Cyst Expansion in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 2471–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Conduit, S.; Davies, E.M.; Ooms, L.M.; Gurung, R.; McGrath, M.J.; Hakim, S.; Cottle, D.L.; Smyth, I.M.; Dyson, J.M.; A. Mitchell, C. AKT signaling promotes DNA damage accumulation and proliferation in polycystic kidney disease. Hum. Mol. Genet. 2019, 29, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.J.; Burgess, J.; Stepanova, D.; Saravanabavan, S.; Wong, A.T.Y.; Kaldis, P.; Rangan, G.K. Role of cyclin-dependent kinase 2 in the progression of mouse juvenile cystic kidney disease. Lab. Investig. 2020, 100, 696–711. [Google Scholar] [CrossRef]
- Zhang, J.Q.J.; Saravanabavan, S.; Munt, A.; Wong, A.T.Y.; Harris, D.C.; Harris, P.C.; Wang, Y.; Rangan, G.K. The role of DNA damage as a therapeutic target in autosomal dominant polycystic kidney disease. Expert Rev. Mol. Med. 2019, 21, e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Saravanabavan, S.; Chandra, A.N.; Munt, A.; Wong, A.T.; Harris, P.C.; Harris, D.C.; McKenzie, P.; Wang, Y.; Rangan, G.K. Up-regulation of DNA Damage Response Signaling in Autosomal Dominant Polycystic Kidney Disease. Am. J. Pathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.-H.; Mattis, V.B.; Wang, N.; Al-Ramahi, I.; Berg, N.V.D.; Fratantoni, S.A.; Waldvogel, H.; Greiner, E.; Osmand, A.; ElZein, K.; et al. Targeting ATM ameliorates mutant Huntingtin toxicity in cell and animal models of Huntington’s disease. Sci. Transl. Med. 2014, 6, 268ra178. [Google Scholar] [CrossRef] [PubMed]
- Oberle, C.; Blattner, C. Regulation of the DNA Damage Response to DSBs by Post-Translational Modifications. Curr. Genom. 2010, 11, 184–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giglia-Mari, G.; Zotter, A.; Vermeulen, W. DNA Damage Response. Cold Spring Harb. Perspect. Biol. 2010, 3, a000745. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, P.; Foiani, M.; Kumar, A. ATM and ATR signaling at a glance. J. Cell Sci. 2015, 128, 4255–4262. [Google Scholar] [CrossRef] [Green Version]
- Cortez, D.; Guntuku, S.; Qin, J.; Elledge, S.J. ATR and ATRIP: Partners in Checkpoint Signaling. Science 2001, 294, 1713–1716. [Google Scholar] [CrossRef]
- Canman, E.C. Replication checkpoint: Preventing mitotic catastrophe. Curr. Biol. 2001, 11, R121–R124. [Google Scholar] [CrossRef] [Green Version]
- Barlow, C.; Hirotsune, S.; Paylor, R.; Liyanage, M.; Eckhaus, M.; Collins, F.; Shiloh, Y.; Crawley, J.N.; Ried, T.; Tagle, D.; et al. Atm-Deficient Mice: A Paradigm of Ataxia Telangiectasia. Cell 1996, 86, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Menon, V.R.; Peterson, E.J.; Valerie, K.; Farrell, N.P.; Povirk, L.F. Ligand modulation of a dinuclear platinum compound leads to mechanistic differences in cell cycle progression and arrest. Biochem. Pharmacol. 2013, 86, 1708–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecchio, D.; Daga, A.; Carra, E.; Marubbi, D.; Raso, A.; Mascelli, S.; Nozza, P.; Garrè, M.L.; Pitto, F.; Ravetti, J.L.; et al. Pharmacokinetics, pharmacodynamics and efficacy on pediatric tumors of the glioma radiosensitizer KU60019. Int. J. Cancer 2015, 136, 1445–1457. [Google Scholar] [CrossRef]
- Smida, M.; De La Cruz, F.F.; Kerzendorfer, C.; Uras, I.Z.; Mair, B.; Mazouzi, A.; Suchankova, T.; Konopka, T.; Katz, A.M.; Paz, K.; et al. MEK inhibitors block growth of lung tumours with mutations in ataxia–telangiectasia mutated. Nat. Commun. 2016, 7, 13701. [Google Scholar] [CrossRef] [PubMed]
- Durant, S.T.; Zheng, L.; Wang, Y.; Chen, K.; Zhang, L.; Zhang, T.; Yang, Z.; Riches, L.; Trinidad, A.G.; Fok, J.H.L.; et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci. Adv. 2018, 4, eaat1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riches, L.C.; Trinidad, A.G.; Hughes, G.; Jones, G.N.; Hughes, A.M.; Thomason, A.G.; Gavine, P.; Cui, A.; Ling, S.; Stott, J.; et al. Pharmacology of the ATM inhibitor AZD0156: Potentiation of irradiation and olaparib responses pre-clinically. Mol. Cancer Ther. 2020, 19, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilley, R.L.; Verma, P.; Cho, N.W.; Winters, H.D.; Wondisford, A.R.; Greenberg, R.A. Break-induced telomere synthesis underlies alternative telomere maintenance. Nat. Cell Biol. 2016, 539, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, A.K.; Jodkowska, K.; Teloni, F.; Bizard, A.H.; Zellweger, R.; Herrador, R.; Ortega, S.; Hickson, I.D.; Altmeyer, M.; Mendez, J.; et al. A short G1 phase imposes constitutive replication stress and fork remodelling in mouse embryonic stem cells. Nat. Commun. 2016, 7, 10660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorthi, A.; Romero, J.C.; Loranc, E.; Cao, L.; Lawrence, L.A.; Goodale, E.; Iniguez, A.B.; Bernard, X.; Masamsetti, V.P.; Roston, S.; et al. EWS–FLI1 increases transcription to cause R-loops and block BRCA1 repair in Ewing sarcoma. Nat. Cell Biol. 2018, 555, 387–391. [Google Scholar] [CrossRef]
- Pike, K.G.; Barlaam, B.; Cadogan, E.; Campbell, A.; Chen, Y.; Colclough, N.; Davies, N.L.; De-Almeida, C.; Degorce, S.L.; Didelot, M.; et al. The Identification of Potent, Selective, and Orally Available Inhibitors of Ataxia Telangiectasia Mutated (ATM) Kinase: The Discovery of AZD0156 (8-{6-[3-(Dimethylamino)propoxy]pyridin-3-yl}-3-methyl-1-(tetrahydro-2H-pyran-4-yl)-1,3-dihydro-2H-imidazo[4, 5-c]quinolin-2-one). J. Med. Chem. 2018, 61, 3823–3841. [Google Scholar] [CrossRef]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager, R.A.; Torok-Storb, B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994, 45, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loghman-Adham, M.; Nauli, S.M.; Soto, C.E.; Kariuki, B.; Zhou, J. Immortalized epithelial cells from human autosomal dominant polycystic kidney cysts. Am. J. Physiol. Physiol. 2003, 285, F397–F412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauli, S.M.; Rossetti, S.; Kolb, R.J.; Alenghat, F.J.; Consugar, M.B.; Harris, P.C.; Ingber, N.E.; Loghman-Adham, M.; Zhou, J. Loss of Polycystin-1 in Human Cyst-Lining Epithelia Leads to Ciliary Dysfunction. J. Am. Soc. Nephrol. 2006, 17, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Sonawane, N.D.; Zhao, D.; Somlo, S.; Verkman, A.S. Small-Molecule CFTR Inhibitors Slow Cyst Growth in Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2008, 19, 1300–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, C.M.; King, B.F.; Srai, K.S.; Unwin, R.J. Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am. J. Physiol. Physiol. 2007, 292, F15–F25. [Google Scholar] [CrossRef] [PubMed]
- Holditch, S.J.; Brown, C.N.; Atwood, D.J.; Lombardi, A.M.; Nguyen, K.N.; Toll, H.W.; Hopp, K.; Edelstein, C.L. A study of sirolimus and mTOR kinase inhibitor in a hypomorphic Pkd1 mouse model of autosomal dominant polycystic kidney disease. Am. J. Physiol. Physiol. 2019, 317, F187–F196. [Google Scholar] [CrossRef]
- Lannoy, M.; Valluru, M.K.; Chang, L.; Abdela-Ali, F.; Peters, D.J.; Streets, A.J.; Ong, A.C. The positive effect of selective prostaglandin E2 receptor EP2 and EP4 blockade on cystogenesis in vitro is counteracted by increased kidney inflammation in vivo. Kidney Int. 2020, 98, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hopp, K.; Ward, C.J.; Hommerding, C.J.; Nasr, S.H.; Tuan, H.-F.; Gainullin, V.G.; Rossetti, S.; Torres, V.E.; Harris, P.C. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J. Clin. Investig. 2012, 122, 4257–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genik, P.C.; Bielefeldt-Ohmann, H.; Liu, X.; Story, M.D.; Ding, L.; Bush, J.M.; Fallgren, C.M.; Weil, M.M. Strain Background Determines Lymphoma Incidence in Atm Knockout Mice. Neoplasia 2014, 16, 129–136, W6–W7. [Google Scholar] [CrossRef] [Green Version]
- Hopp, K.; Hommerding, C.J.; Wang, X.; Ye, H.; Harris, P.C.; Torres, V.E. Tolvaptan plus Pasireotide Shows Enhanced Efficacy in a PKD1 Model. J. Am. Soc. Nephrol. 2014, 26, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopp, K.; Wang, X.; Ye, H.; Irazabal, M.V.; Harris, P.C.; Torres, V.E. Effects of hydration in rats and mice with polycystic kidney disease. Am. J. Physiol. Physiol. 2015, 308, F261–F266. [Google Scholar] [CrossRef]
- Gürtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef]
- Taylor, S.C.; Berkelman, T.; Yadav, G.; Hammond, M. A Defined Methodology for Reliable Quantification of Western Blot Data. Mol. Biotechnol. 2013, 55, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.H.; Oh, D.-Y. ATM in DNA repair in cancer. Pharmacol. Ther. 2019, 203, 107391. [Google Scholar] [CrossRef] [PubMed]
- Banin, S.; Moyal, L.; Shieh, S.-Y.; Taya, Y.; Anderson, C.W.; Chessa, L.; Smorodinsky, N.I.; Prives, C.; Reiss, Y.; Shiloh, Y.; et al. Enhanced Phosphorylation of p53 by ATM in Response to DNA Damage. Science 1998, 281, 1674–1677. [Google Scholar] [CrossRef]
- Canman, C.E.; Lim, D.-S.; Cimprich, K.A.; Taya, Y.; Tamai, K.; Sakaguchi, K.; Appella, E.; Kastan, M.B.; Siliciano, J.D. Activation of the ATM Kinase by Ionizing Radiation and Phosphorylation of p53. Science 1998, 281, 1677–1679. [Google Scholar] [CrossRef]
- Burma, S.; Chen, B.P.; Murphy, M.; Kurimasa, A.; Chen, D.J. ATM Phosphorylates Histone H2AX in Response to DNA Double-strand Breaks. J. Biol. Chem. 2001, 276, 42462–42467. [Google Scholar] [CrossRef] [Green Version]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Grantham, J.J. Polycystic Kidney Disease: Neoplasia in Disguise. Am. J. Kidney Dis. 1990, 15, 110–116. [Google Scholar] [CrossRef]
- Harris, P.C.; Watson, M.L. Autosomal dominant polycystic kidney disease: Neoplasia in disguise? Nephrol. Dial. Transplant. 1997, 12, 1089–1090. [Google Scholar] [CrossRef] [Green Version]
- Pearl, L.H.; Schierz, A.C.; Ward, S.E.; Al-Lazikani, B.; Pearl, F.M.G. Therapeutic opportunities within the DNA damage response. Nat. Rev. Cancer 2015, 15, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Golding, S.E.; Rosenberg, E.; Valerie, N.; Hussaini, I.; Frigerio, M.; Cockcroft, X.F.; Chong, W.Y.; Hummersone, M.; Rigoreau, L.; Menear, K.A.; et al. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol. Cancer Ther. 2009, 8, 2894–2902. [Google Scholar] [CrossRef] [Green Version]
- Ta, M.H.T.; Schwensen, K.G.; Foster, S.; Korgaonkar, M.; Ozimek-Kulik, J.E.; Phillips, J.K.; Peduto, A.; Rangan, G.K. Effects of TORC1 Inhibition during the Early and Established Phases of Polycystic Kidney Disease. PLoS ONE 2016, 11, e0164193. [Google Scholar] [CrossRef]
- Kurbegovic, A.; Trudel, M. The master regulators Myc and p53 cellular signaling and functions in polycystic kidney disease. Cell Signal. 2020, 71, 109594. [Google Scholar] [CrossRef] [PubMed]
- Menolfi, D.; Zha, S. ATM, ATR and DNA-PKcs kinases—the lessons from the mouse models: Inhibition ≠ deletion. Cell Biosci. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Callén, E.; Jankovic, M.; Wong, N.; Zha, S.; Chen, H.-T.; Difilippantonio, S.; Di Virgilio, M.; Heidkamp, G.; Alt, F.W.; Nussenzweig, A.; et al. Essential Role for DNA-PKcs in DNA Double-Strand Break Repair and Apoptosis in ATM-Deficient Lymphocytes. Mol. Cell 2009, 34, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiff, T.; O’Driscoll, M.; Rief, N.; Iwabuchi, K.; Löbrich, M.; Jeggo, P.A. ATM and DNA-PK Function Redundantly to Phosphorylate H2AX after Exposure to Ionizing Radiation. Cancer Res. 2004, 64, 2390–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, J.A.; Pellegrini, M.; Lee, B.-S.; Guo, Z.; Filsuf, D.; Belkina, N.V.; You, Z.; Paull, T.T.; Sleckman, B.P.; Feigenbaum, L.; et al. Loss of ATM kinase activity leads to embryonic lethality in mice. J. Cell Biol. 2012, 198, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Wang, Y.; Jiang, W.; Liu, X.; Dubois, R.L.; Lin, C.-S.; Ludwig, T.; Bakkenist, C.J.; Zha, S. Kinase-dead ATM protein causes genomic instability and early embryonic lethality in mice. J. Cell Biol. 2012, 198, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnitz, L.M.; Zou, L. Molecular Pathways: Targeting ATR in Cancer Therapy. Clin. Cancer Res. 2015, 21, 4780–4785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.M.; Ryan, A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol. Ther. 2015, 149, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Hutcherson, R.J.; Kemp, M.G. ATR kinase inhibition sensitizes quiescent human cells to the lethal effects of cisplatin but increases mutagenesis. Mutat. Res. Mol. Mech. Mutagen. 2019, 816–818, 111678. [Google Scholar] [CrossRef]
- Bradbury, A.; Hall, S.; Curtin, N.; Drew, Y. Targeting ATR as Cancer Therapy: A new era for synthetic lethality and synergistic combinations? Pharmacol. Ther. 2020, 207, 107450. [Google Scholar] [CrossRef] [PubMed]
- Sans-Atxer, L.; Joly, D. Tolvaptan in the treatment of autosomal dominant polycystic kidney disease: Patient selection and special considerations. Int. J. Nephrol. Renov. Dis. 2018, 11, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Kou, P.; Wei, S.; Xiong, F. Recent Advances of mTOR Inhibitors Use in Autosomal Dominant Polycystic Kidney Disease: Is the Road Still Open? Curr. Med. Chem. 2019, 26, 2962–2973. [Google Scholar] [CrossRef] [PubMed]
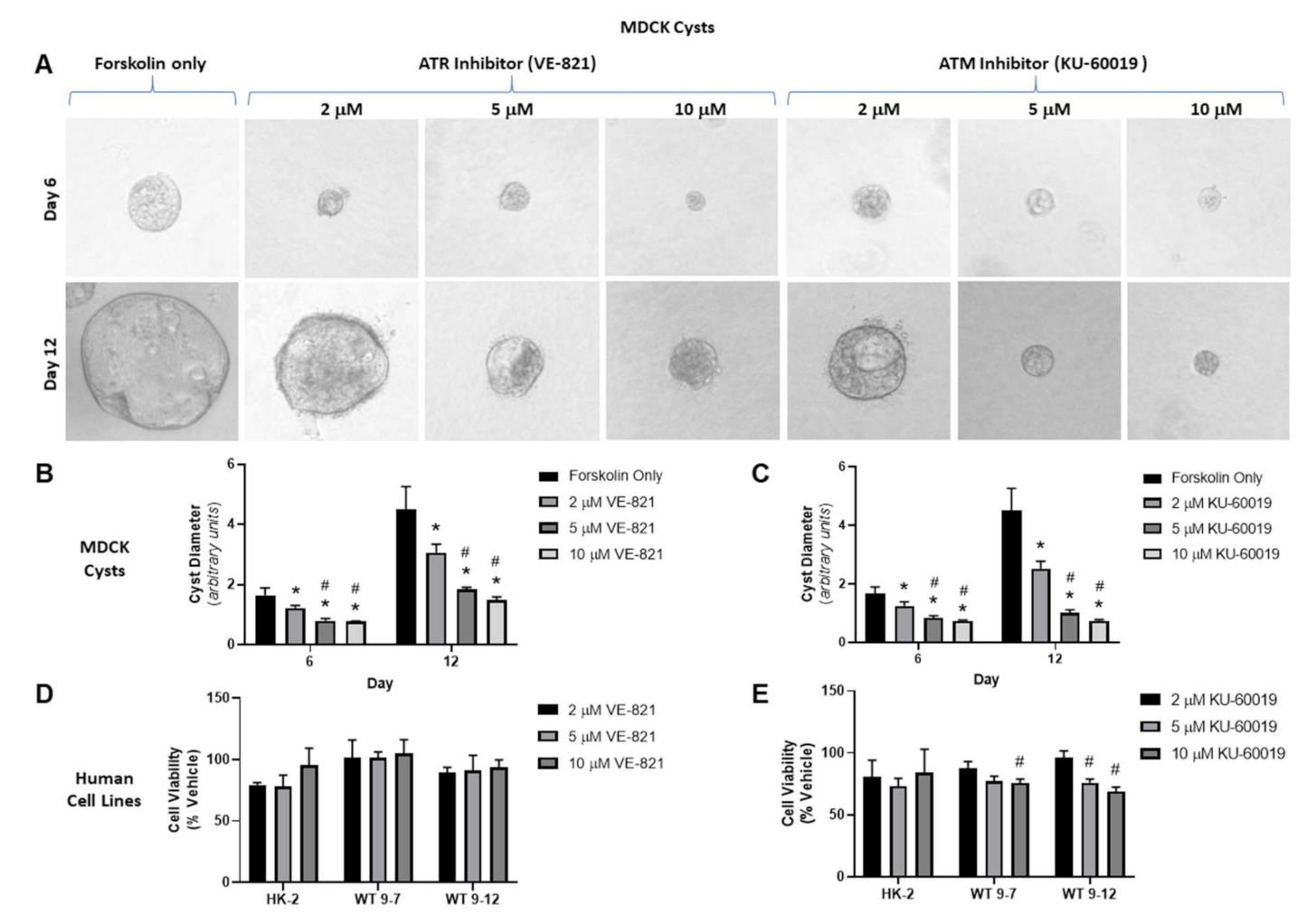

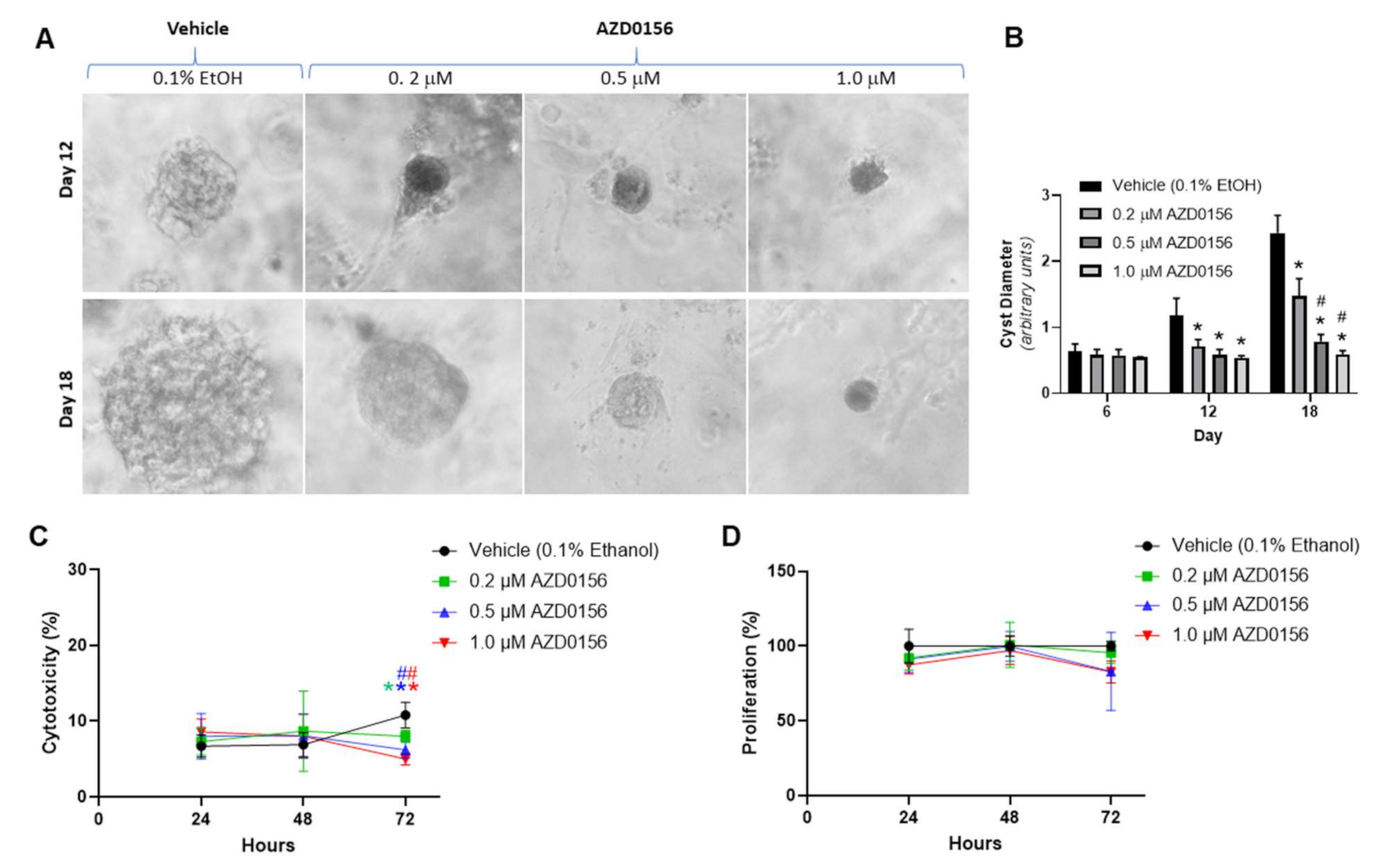

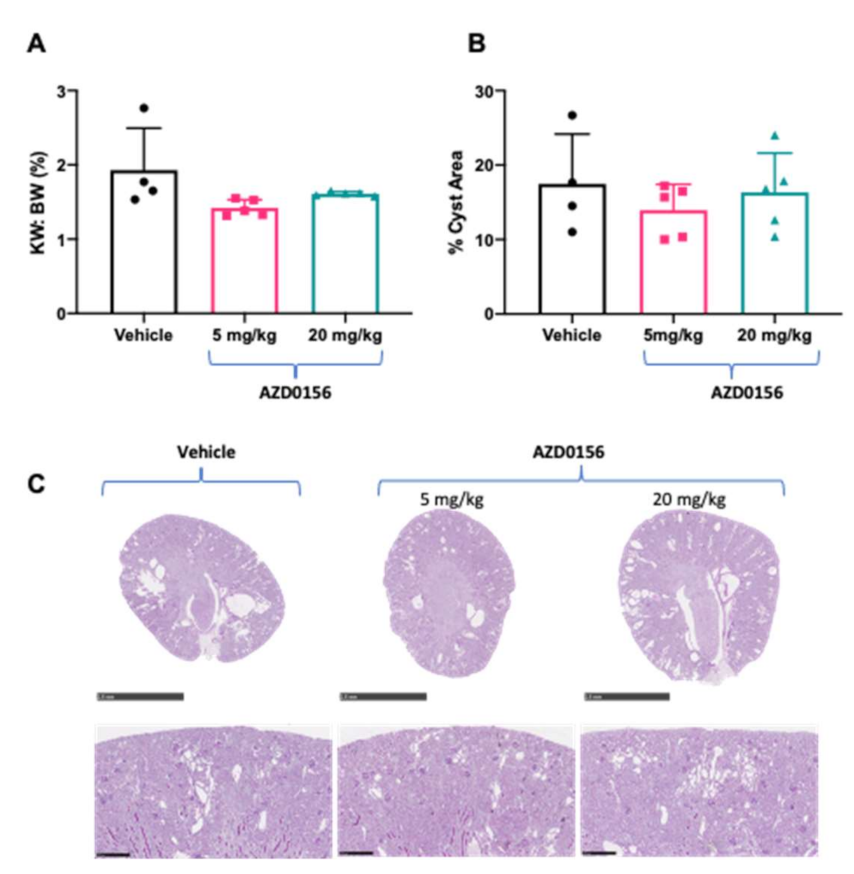


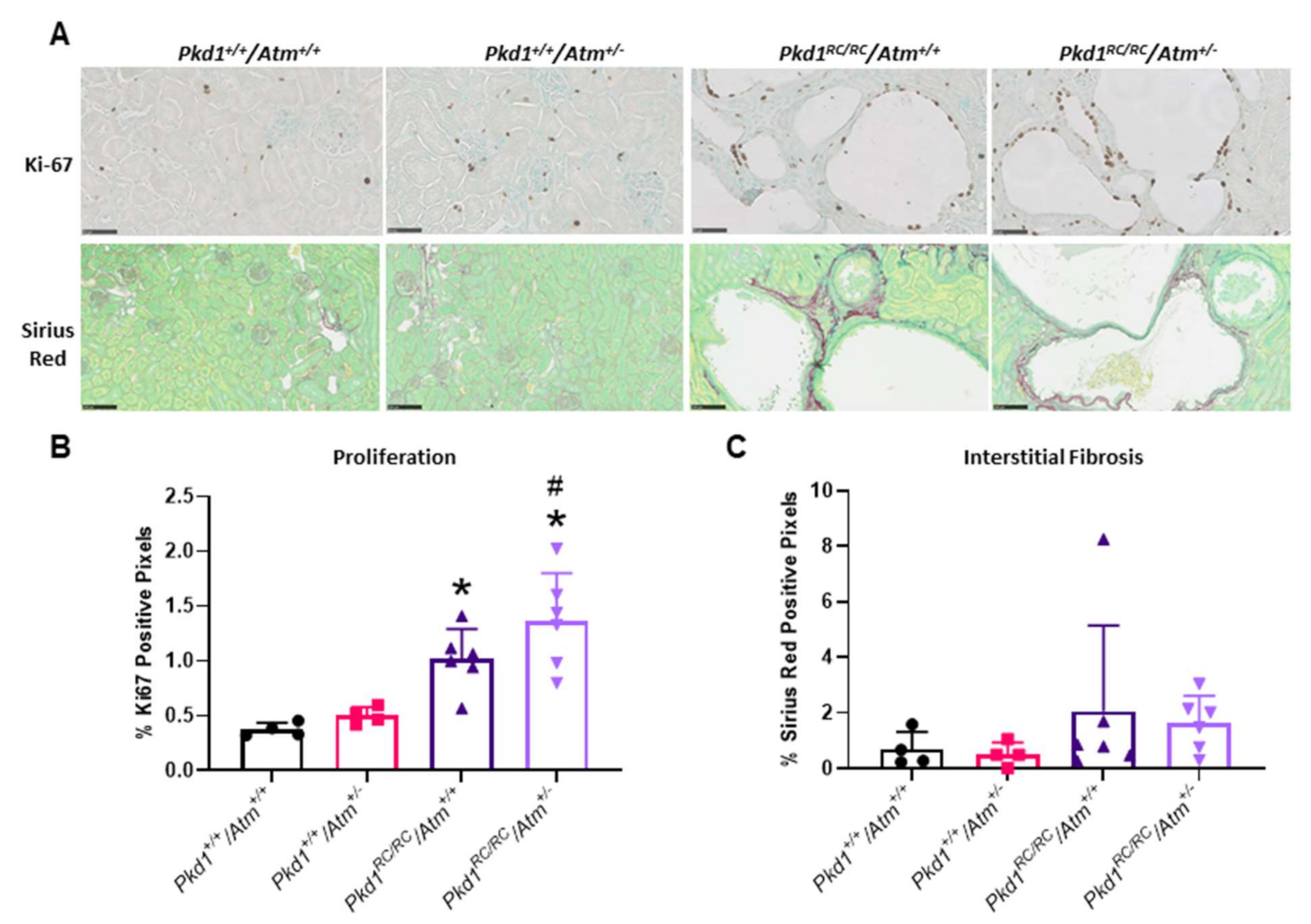
| Group | Dose | n | % BrdU Synthesis | % LDH Leakage |
|---|---|---|---|---|
| Vehicle | 8 | 100.0 ± 2.5 | 23.5 ± 0.96 | |
| VE-821 | 2 µM | 8 | 86.6 ± 8.2 * | 22.0 ± 1.4 |
| 5 µM | 8 | 68.2 ± 8.3 * | 21.7 ± 0.7 | |
| 10 µM | 8 | 51.9 ± 4.6 * | 22.6 ± 1.4 | |
| KU-60019 | 2 µM | 8 | 86.1 ± 3.6 * | 21.6 ± 1.0 * |
| 5 µM | 8 | 65.0 ± 4.0 * | 21.6 ± 1.34 * | |
| 10 µM | 8 | 13.5 ± 1.5 * | 21.3 ± 0.8 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.Q.J.; Saravanabavan, S.; Rangan, G.K. Effect of Reducing Ataxia-Telangiectasia Mutated (ATM) in Experimental Autosomal Dominant Polycystic Kidney Disease. Cells 2021, 10, 532. https://doi.org/10.3390/cells10030532
Zhang JQJ, Saravanabavan S, Rangan GK. Effect of Reducing Ataxia-Telangiectasia Mutated (ATM) in Experimental Autosomal Dominant Polycystic Kidney Disease. Cells. 2021; 10(3):532. https://doi.org/10.3390/cells10030532
Chicago/Turabian StyleZhang, Jennifer Q. J., Sayanthooran Saravanabavan, and Gopala K. Rangan. 2021. "Effect of Reducing Ataxia-Telangiectasia Mutated (ATM) in Experimental Autosomal Dominant Polycystic Kidney Disease" Cells 10, no. 3: 532. https://doi.org/10.3390/cells10030532
APA StyleZhang, J. Q. J., Saravanabavan, S., & Rangan, G. K. (2021). Effect of Reducing Ataxia-Telangiectasia Mutated (ATM) in Experimental Autosomal Dominant Polycystic Kidney Disease. Cells, 10(3), 532. https://doi.org/10.3390/cells10030532






