Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Monitoring Chemical-Induced Intracellular Calcium Ion Levels in F11 Cells
2.2. Measurement of Neurite Outgrowth of F11 Cells in the Presence of Paclitaxel
2.3. Establishment of the Mouse Model of Paclitaxel-Induced Neuropathic Pain
2.4. Determination of the Effect of Decursin in the Mouse Model of Paclitaxel-Induced Neuropathic Pain
2.5. Prediction of Target Proteins Using Swisstargetprediction
2.6. Monitoring Transient Receptor Potential Vanilloid 1 (TRPV1)-Mediated Ca2+ in Human TRPV1 Stable Cell Line-HEK293
2.7. Immunoblotting
2.8. Statistical Analysis
3. Results
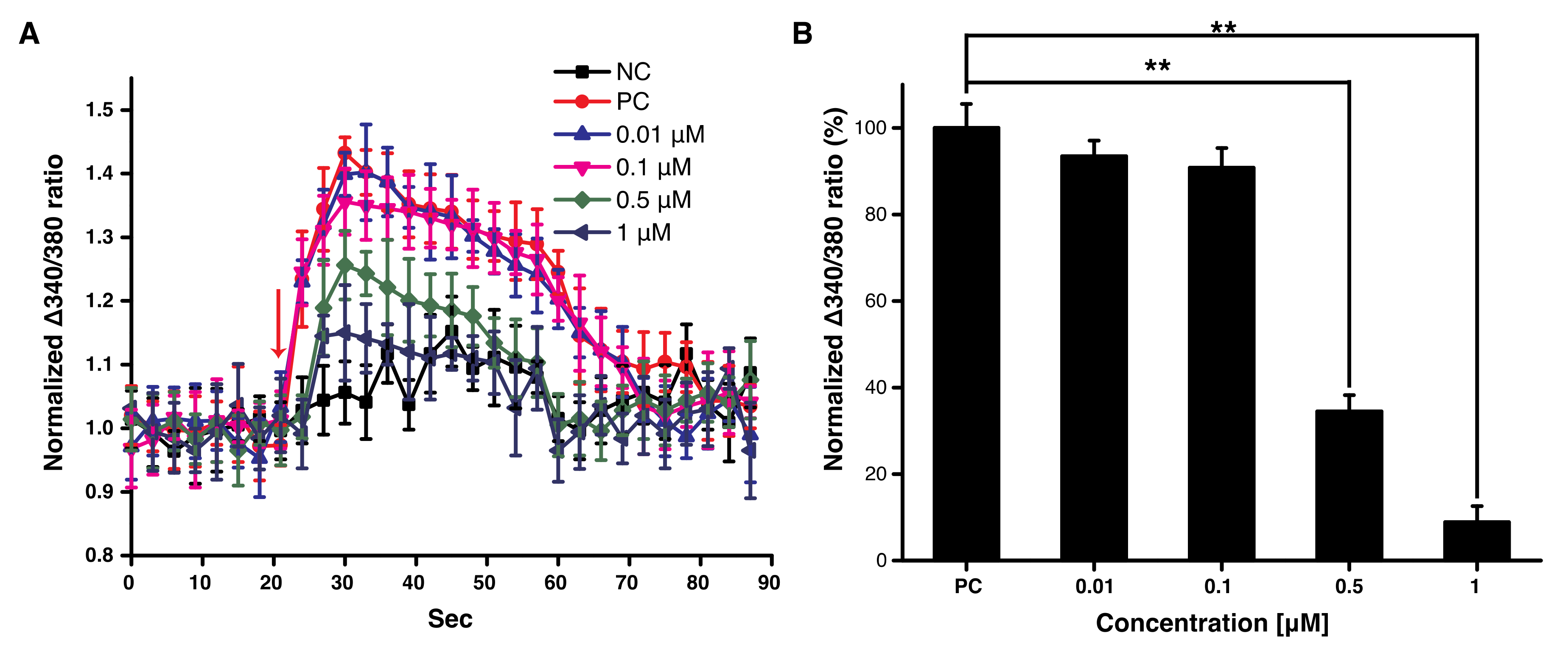
3.1. Decursin Reduced the Sudden Increase of Intracellular Ca2+ in F11 Cells
3.2. Decursin Induced Neuronal Differentiation of F11 Cells by Increasing Neurite Outgrowth
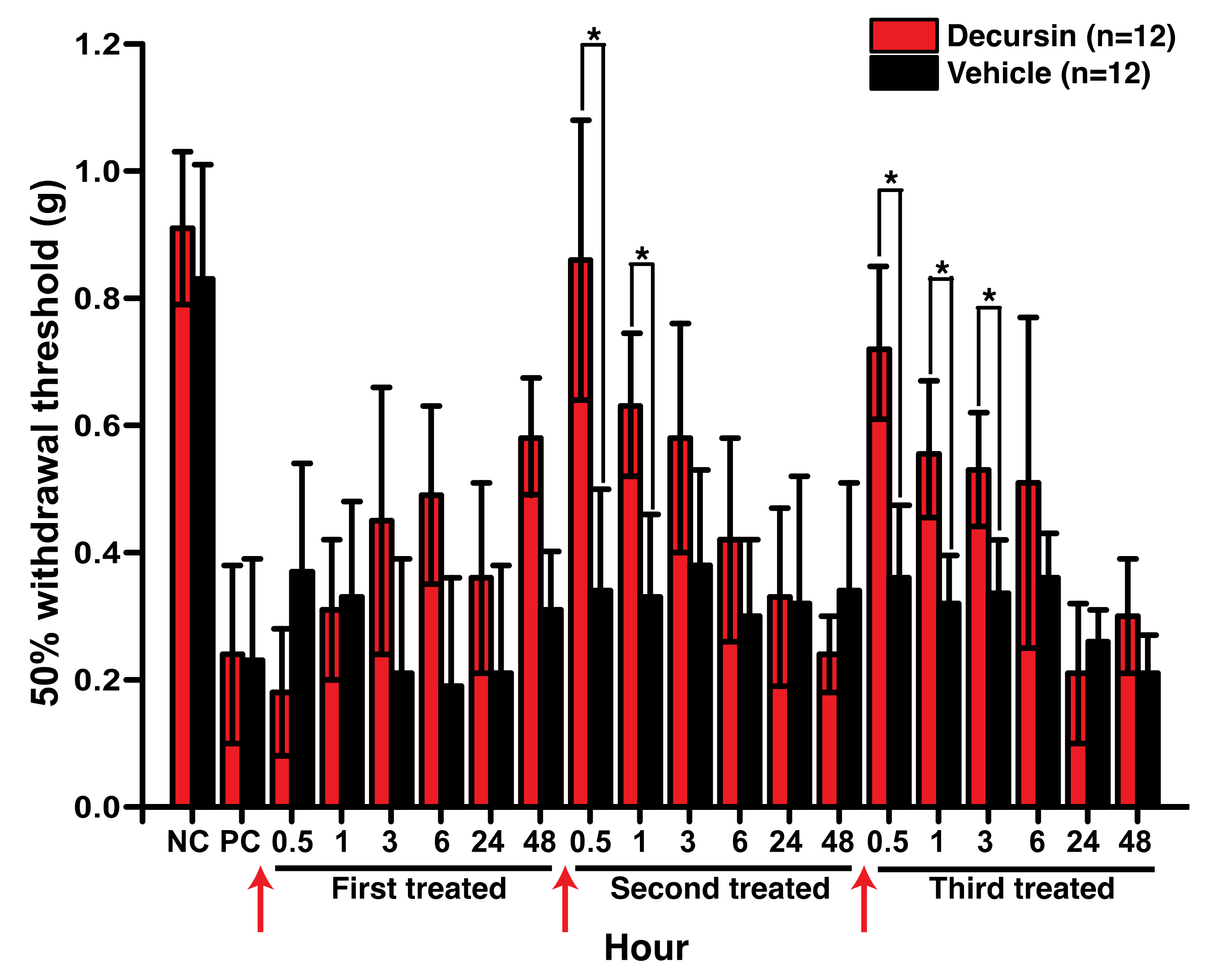
3.3. Decursin Alleviated Mechanical Allodynia in the Mouse Model of Paclitaxel-Induced Peripheral Neuropathy
3.4. Decursin Modulated Intracellular Ca2+ via TRPV1 Inhibition
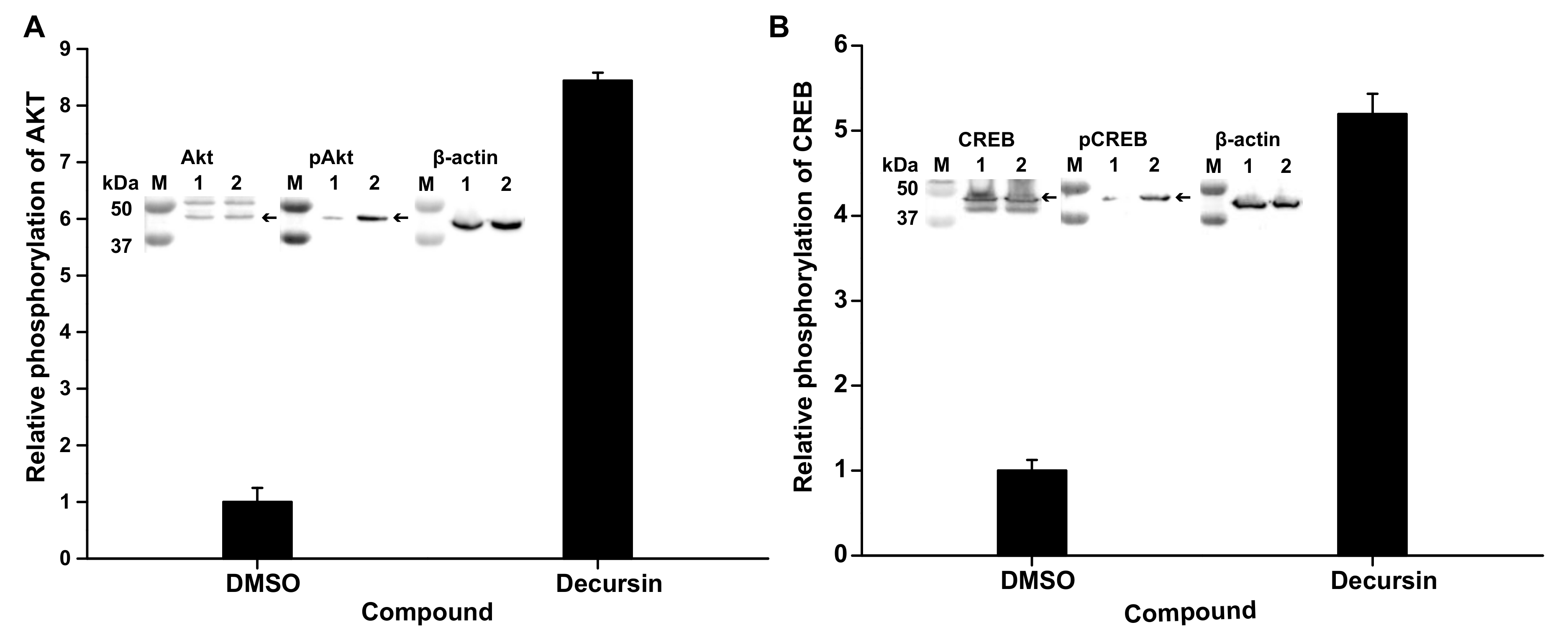
3.5. Decursin Can Phosphorylate Protein Kinase B and cAMP-Response Element-Binding Protein, Related to Neurite Outgrowth
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Zajaczkowską, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huang, K.M.; Adams, E.J.; Loprinzi, C.L.; Lustberg, M.B. Recent Developments of Novel Pharmacologic Therapeutics for Prevention of Chemotherapy-Induced Peripheral Neuropathy. Clin. Cancer Res. 2019, 25, 6295–6301. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Angelica Gigas Nakai Root: A Review. Sci. Pharm. 2017, 85, 21. [Google Scholar] [CrossRef]
- Cho, J.H.; Kwon, J.E.; Cho, Y.; Kim, I.; Kang, S.C. Anti-Inflammatory Effect of Angelica Gigas via Heme Oxygenase (HO)-1 Expression. Nutrients 2015, 7, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Mahat, B.; Chae, J.W.; Baek, I.H.; Song, G.Y.; Song, J.S.; Ma, J.Y.; Kwon, K.-I. Biopharmaceutical Characterization of Decursin and Their Derivatives for Drug Discovery. Drug Dev. Ind. Pharm. 2013, 39, 1523–1530. [Google Scholar] [CrossRef]
- Han, J.; Jin, W.; Ho, N.A.; Hong, J.; Kim, Y.J.; Shin, Y.; Lee, H.; Suh, J.W. Decursin and Decursinol Angelate Improve Wound Healing by Upregulating Transcription of Genes Encoding Extracellular Matrix Remodeling Proteins, Inflammatory Cytokines, and Growth Factors in Human Keratinocytes. Biochem. Biophys. Res. Commun. 2018, 499, 979–984. [Google Scholar] [CrossRef]
- Martínez, A.L.; Brea, J.; Monroy, X.; Merlos, M.; Burgueño, J.; Loza, M.I. A New Model of Sensorial Neuron-Like Cells for HTS of Novel Analgesics for Neuropathic Pain. Slas Discov. 2019, 24, 158–168. [Google Scholar] [CrossRef]
- Hylden, J.L.K.; Wilcox, G.L. Intrathecal Morphine in Mice: A New Technique. Eur. J. Pharm. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative Assessment of Tactile Allodynia in the Rat Paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W3664. [Google Scholar] [CrossRef]
- Roh, J.; Hwang, S.M.; Lee, S.H.; Lee, K.; Kim, Y.H.; Park, C.K. Functional Expression of Piezo1 in Dorsal Root Ganglion (DRG) Neurons. Int. J. Mol. Sci. 2020, 21, 3834. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H. Unravelling the Mystery of Capsaicin: A Tool to Understand and Treat Pain. Pharmacol. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef]
- Seabrook, G.R.; Sutton, K.G.; Jarolimek, W.; Hollingworth, G.J.; Teague, S.; Webb, J.; Clark, N.; Boyce, S.; Kerby, J.; Ali, Z.; et al. Functional Properties of the High-Affinity TRPV1 (VR1) Vanilloid Receptor Antagonist (4-Hydroxy-5-Iodo-3-Methoxyphenylacetate Ester) Iodo-Resiniferatoxin. J. Pharmacol. Exp. Ther. 2002, 303, 1052–1060. [Google Scholar] [CrossRef]
- Heiser, J.H.; Schuwald, A.M.; Sillani, G.; Ye, L.; Müller, W.E.; Leuner, K. TRPC6 Channel-Mediated Neurite Outgrowth in PC12 Cells and Hippocampal Neurons Involves Activation of RAS/MEK/ERK, PI3K, and CAMKIV Signaling. J. Neurochem. 2013, 127, 303–313. [Google Scholar] [CrossRef]
- Jimenez-Andrade, J.M.; Peters, C.M.; Mejia, N.A.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Sensory Neurons and Their Supporting Cells Located in the Trigeminal, Thoracic and Lumbar Ganglia Differentially Express Markers of Injury Following Intravenous Administration of Paclitaxel in the Rat. Neurosci. Lett. 2006, 405, 62–67. [Google Scholar] [CrossRef]
- Liu, C.C.; Lu, N.; Cui, Y.; Yang, T.; Zhao, Z.Q.; Xin, W.J.; Liu, X.G. Prevention of Paclitaxel-Induced Allodynia by Minocycline: Effect on Loss of Peripheral Nerve Fibers and Infiltration of Macrophages in Rats. Mol. Pain 2010, 6, 76. [Google Scholar] [CrossRef]
- Da Costa, R.; Passos, G.F.; Quintão, N.L.M.; Fernandes, E.S.; Maia, J.R.L.C.B.; Campos, M.M.; Calixto, J.B. Taxane-Induced Neurotoxicity: Pathophysiology and Therapeutic Perspectives. Br. J. Pharmacol. 2020, 177, 3127–3146. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Xing, C.; Kim, S.H.; Lü, J. Single oral dose pharmacokinetics of decursin, decursinol angelate, and decursinol in rats. Planta Med. 2013, 79, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Shaik, A.A.; Zhang, Y.; Wang, L.; Xing, C.; Kim, S.H.; Lü, J. Quantitative determination of decursin, decursinol angelate, and decursinol in mouse plasma and tumor tissue using liquid-liquid extraction and HPLC. Planta Med. 2012, 78, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Liu, Z.; Lv, P.; Wang, H.; Zhu, Y.; Qi, Q.; Xu, J. Autophagy and Akt/CREB Signalling Play an Important Role in the Neuroprotective Effect of Nimodipine in a Rat Model of Vascular Dementia. Behav. Brain Res. 2017, 325, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, H.; Zhang, X.; Chen, L.; Zhao, X.; Bai, X.; Zhang, J. Nobiletin Protects against Cerebral Ischemia via Activating the P-Akt, p-CREB, BDNF and Bcl-2 Pathway and Ameliorating BBB Permeability in Rat. Brain Res. Bull. 2013, 96, 45–53. [Google Scholar] [CrossRef]
- Simão, F.; Matté, A.; Pagnussat, A.S.; Netto, C.A.; Salbego, C.G. Resveratrol Prevents CA1 Neurons against Ischemic Injury by Parallel Modulation of Both GSK-3β and CREB through PI3-K/Akt Pathways. Eur. J. Neurosci. 2012, 36, 2899–2905. [Google Scholar] [CrossRef]
- Brazil, D.P.; Hemmings, B.A. Ten Years of Protein Kinase B Signalling: A Hard Akt to Follow. Trends Biochem. Sci. 2001, 26, 657–664. [Google Scholar] [CrossRef]
- Ishii, N.; Tsubouchi, H.; Miura, A.; Yanagi, S.; Ueno, H.; Shiomi, K.; Nakazato, M. Ghrelin Alleviates Paclitaxel-Induced Peripheral Neuropathy by Reducing Oxidative Stress and Enhancing Mitochondrial Anti-Oxidant Functions in Mice. Eur. J. Pharmacol. 2018, 819, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Kyung, E.J.; Suh, J.S.; Lee, H.S.; Lee, J.H.; Chae, S.I.; Park, E.S.; Chung, Y.H.; Bae, J.; Lee, T.J.; et al. Phosphatidylcholine Attenuated Docetaxel-Induced Peripheral Neurotoxicity in Rats. Drug Chem. Toxicol. 2018, 41, 476–485. [Google Scholar] [CrossRef]
- Li, Y.; Adamek, P.; Zhang, H.; Tatsui, C.E.; Rhines, L.D.; Mrozkova, P.; Li, Q.; Kosturakis, A.K.; Cassidy, R.M.; Harrison, D.S.; et al. The Cancer Chemotherapeutic Paclitaxel Increases Human and Rodent Sensory Neuron Responses to TRPV1 by Activation of TLR4. J. Neurosci. 2015, 35, 13487–13500. [Google Scholar] [CrossRef]
- Arsenault, P.; Chiche, D.; Brown, W.; Miller, J.; Treister, R.; Leff, R.; Walker, P.; Katz, N. NEO6860, Modality-Selective TRPV1 Antagonist: A Randomized, Controlled, Proof-of-Concept Trial in Patients with Osteoarthritis Knee Pain. Pain Rep. 2018, 3, e696. [Google Scholar] [CrossRef]
- Chukyo, A.; Chiba, T.; Kambe, T.; Yamamoto, K.; Kawakami, K.; Taguchi, K.; Abe, K. Oxaliplatin-Induced Changes in Expression of Transient Receptor Potential Channels in the Dorsal Root Ganglion as a Neuropathic Mechanism for Cold Hypersensitivity. Neuropeptides 2018, 67, 95–101. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.F.; Liao, M.F.; Yao, W.L.; Wang, J.; Wang, X.R. Blocking PAR2 Attenuates Oxaliplatin-Induced Neuropathic Pain via TRPV1 and Releases of Substance P and CGRP in Superficial Dorsal Horn of Spinal Cord. J. Neurol. Sci. 2015, 352, 62–67. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, D.B.; Choi, W.; Kim, M.; Go, E.J.; Jeong, D.; Park, C.-K.; Kim, Y.H.; Lee, H.; Suh, J.-W. Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model. Cells 2021, 10, 547. https://doi.org/10.3390/cells10030547
Son DB, Choi W, Kim M, Go EJ, Jeong D, Park C-K, Kim YH, Lee H, Suh J-W. Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model. Cells. 2021; 10(3):547. https://doi.org/10.3390/cells10030547
Chicago/Turabian StyleSon, Dang Bao, Woosik Choi, Mingu Kim, Eun Jin Go, Dabeen Jeong, Chul-Kyu Park, Yong Ho Kim, Hanki Lee, and Joo-Won Suh. 2021. "Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model" Cells 10, no. 3: 547. https://doi.org/10.3390/cells10030547
APA StyleSon, D. B., Choi, W., Kim, M., Go, E. J., Jeong, D., Park, C.-K., Kim, Y. H., Lee, H., & Suh, J.-W. (2021). Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model. Cells, 10(3), 547. https://doi.org/10.3390/cells10030547






