Treatment with Cyclic AMP Activators Reduces Glioblastoma Growth and Invasion as Assessed by Two-Photon Microscopy
Abstract
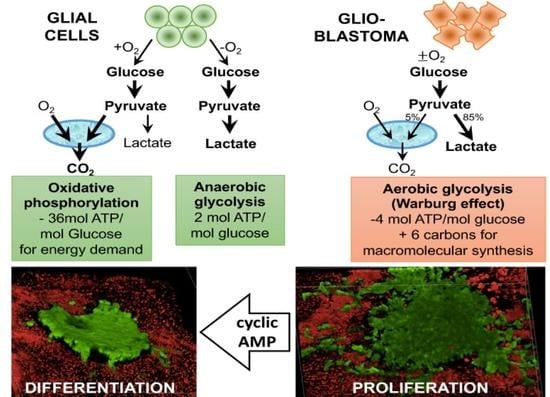
:1. Introduction
2. Materials and Methods
2.1. Organotypic Brain Slice Cultures
2.2. U87 Glioblastoma Cell Line Culture and Transplantation
2.3. Assessment of Cell Viability with Propidium Iodide
2.4. Treatment of the Glioblastoma Cell Line U87 Co-Cultured with Organotypic Brain Slices
2.5. Biochemical Analysis
2.6. Quantification of Tumor Area
2.7. Quantification of Tumor Volume by Two-Photon Fluorescence Microscopy
2.8. Statistical Analysis
3. Results
3.1. Metabolic Effects on the Glioblastoma Cell Line U87 Co-Cultured with Organotypic Brain Slices
3.2. Reduced Tumor Area Following Differentiation Therapy
3.3. Visualization of 3D Growth Pattern with Two-Photon Fluorescence Microscopy
4. Discussion
4.1. Experimental Model
4.2. Metabolic Effects of Differentiation Therapy in Glioblastoma
4.3. Effect of a Differentiation Therapy on Glioblastoma Growth and Invasion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Graber, J.J. Overview of prognostic factors in adult gliomas. Ann. Palliat. Med. 2020, 10, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Guzman, S.; Herrada-Pineda, T.; Revilla-Pacheco, F. Surgical Management of Glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, F.; Luan, Y.; Cai, J.; Wu, S.; Mai, J.; Gu, J.; Zhang, H.; Li, K.; Lin, Y.; Xiao, X.; et al. The Anti-Warburg Effect Elicited by the cAMP-PGC1alpha Pathway Drives Differentiation of Glioblastoma Cells into Astrocytes. Cell Rep. 2017, 18, 468–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leszczyniecka, M.; Roberts, T.; Dent, P.; Grant, S.; Fisher, P.B. Differentiation therapy of human cancer: Basic science and clinical applications. Pharmacol. Ther. 2001, 90, 105–156. [Google Scholar] [CrossRef]
- Jiao, B.; Ren, Z.H.; Liu, P.; Chen, L.J.; Shi, J.Y.; Dong, Y.; Ablain, J.; Shi, L.; Gao, L.; Hu, J.P.; et al. 8-CPT-cAMP/all-trans retinoic acid targets t(11;17) acute promyelocytic leukemia through enhanced cell differentiation and PLZF/RARalpha degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 3495–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, C.; Foster, K.; Corley, J.E.; Dimri, M.; Brady, M.F. Biochemistry, cAMP; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Warrington, N.M.; Gianino, S.M.; Jackson, E.; Goldhoff, P.; Garbow, J.R.; Piwnica-Worms, D.; Gutmann, D.H.; Rubin, J.B. Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res. 2010, 70, 5717–5727. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Jackson, E.; Woerner, B.M.; Perry, A.; Piwnica-Worms, D.; Rubin, J.B. Blocking CXCR4-mediated cyclic AMP suppression inhibits brain tumor growth in vivo. Cancer Res. 2007, 67, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Humpel, C. Organotypic brain slice cultures: A review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Marques-Torrejon, M.A.; Gangoso, E.; Pollard, S.M. Modelling glioblastoma tumour-host cell interactions using adult brain organotypic slice co-culture. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, N.; Maeda, Y.; Shibao, S.; Arima, Y.; Ohka, F.; Kondo, Y.; Maruyama, K.; Kusuhara, M.; Sasayama, T.; Kohmura, E.; et al. Organotypic brain explant culture as a drug evaluation system for malignant brain tumors. Cancer Med. 2017, 6, 2635–2645. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, H.; Ohnishi, T.; Kanemura, Y.; Maruno, M.; Yoshimine, T. Quantitative analysis of glioma cell invasion by confocal laser scanning microscopy in a novel brain slice model. Biochem. Biophys. Res. Commun. 2000, 269, 513–520. [Google Scholar] [CrossRef]
- Ghoochani, A.; Yakubov, E.; Sehm, T.; Fan, Z.; Hock, S.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N.E. A versatile ex vivo technique for assaying tumor angiogenesis and microglia in the brain. Oncotarget 2016, 7, 1838–1853. [Google Scholar] [CrossRef]
- Gage, F.H. Neurogenesis in the adult brain. J. Neurosci. 2002, 22, 612–613. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Schmid, B.; Tripal, P.; Fraass, T.; Kersten, C.; Ruder, B.; Gruneboom, A.; Huisken, J.; Palmisano, R. 3Dscript: Animating 3D/4D microscopy data using a natural-language-based syntax. Nat. Methods 2019, 16, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Hodges, T.; Arko, L.; Shen, M.; Dello Iacono, D.; McNabb, A.; Olsen Bailey, N.; Kreisl, T.N.; Iwamoto, F.M.; Sul, J.; et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J. Clin. Oncol. 2010, 28, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet. Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Kathagen, A.; Schulte, A.; Balcke, G.; Phillips, H.S.; Martens, T.; Matschke, J.; Gunther, H.S.; Soriano, R.; Modrusan, Z.; Sandmann, T.; et al. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol. 2013, 126, 763–780. [Google Scholar] [CrossRef]
- Seidel, S.; Garvalov, B.K.; Wirta, V.; von Stechow, L.; Schanzer, A.; Meletis, K.; Wolter, M.; Sommerlad, D.; Henze, A.T.; Nister, M.; et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain J. Neurol. 2010, 133, 983–995. [Google Scholar] [CrossRef] [Green Version]
- Megele, R.; Riemenschneider, M.J.; Dodoo-Schittko, F.; Feyrer, M.; Kleindienst, A. Intra-tumoral treatment with oxygen-ozone in glioblastoma: A systematic literature search and results of a case series. Oncol. Lett. 2018, 16, 5813–5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmann-Zwerenz, A.; Leidgens, V.; Feliciello, G.; Klein, C.A.; Hau, P. Tumor Cell Invasion in Glioblastoma. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [Green Version]
- Marksteiner, J.; Humpel, C. Beta-amyloid expression, release and extracellular deposition in aged rat brain slices. Mol. Psychiatry 2008, 13, 939–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lossi, L.; Alasia, S.; Salio, C.; Merighi, A. Cell death and proliferation in acute slices and organotypic cultures of mammalian CNS. Prog. Neurobiol. 2009, 88, 221–245. [Google Scholar] [CrossRef]
- Clark, M.J.; Homer, N.; O’Connor, B.D.; Chen, Z.; Eskin, A.; Lee, H.; Merriman, B.; Nelson, S.F. U87MG decoded: The genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS Genet. 2010, 6, e1000832, Correction in 2018, 14, e1007392. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Bjerke, M.; Edlund, H.; Nelander, S.; Westermark, B. Origin of the U87MG glioma cell line: Good news and bad news. Sci. Transl. Med. 2016, 8, 354re353. [Google Scholar] [CrossRef]
- Yu, S.C.; Ping, Y.F.; Yi, L.; Zhou, Z.H.; Chen, J.H.; Yao, X.H.; Gao, L.; Wang, J.M.; Bian, X.W. Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 2008, 265, 124–134. [Google Scholar] [CrossRef]
- Hailer, N.P.; Vogt, C.; Korf, H.W.; Dehghani, F. Interleukin-1beta exacerbates and interleukin-1 receptor antagonist attenuates neuronal injury and microglial activation after excitotoxic damage in organotypic hippocampal slice cultures. Eur. J. Neurosci. 2005, 21, 2347–2360. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, K.A.; Kleindienst, A.; Muller, C.; Chen, T.; Muir, J.K.; Ellis, E.F. S100B protein is released by in vitro trauma and reduces delayed neuronal injury. J. Neurochem. 2004, 91, 1284–1291. [Google Scholar] [CrossRef]
- Whalen, M.J.; Dalkara, T.; You, Z.; Qiu, J.; Bermpohl, D.; Mehta, N.; Suter, B.; Bhide, P.G.; Lo, E.H.; Ericsson, M.; et al. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2008, 28, 490–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, O.; Lifshitz, J.; Povlishock, J.T. Mechanoporation induced by diffuse traumatic brain injury: An irreversible or reversible response to injury? J. Neurosci. 2006, 26, 3130–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogolla, N.; Galimberti, I.; DePaola, V.; Caroni, P. Long-term live imaging of neuronal circuits in organotypic hippocampal slice cultures. Nat. Protoc. 2006, 1, 1223–1226. [Google Scholar] [CrossRef]
- Bradberry, C.W.; Sprouse, J.S.; Sheldon, P.W.; Aghajanian, G.K.; Roth, R.H. In vitro microdialysis: A novel technique for stimulated neurotransmitter release measurements. J. Neurosci. Methods 1991, 36, 85–90. [Google Scholar] [CrossRef]
- Gramsbergen, J.B.; Leegsma-Vogt, G.; Venema, K.; Noraberg, J.; Korf, J. Quantitative on-line monitoring of hippocampus glucose and lactate metabolism in organotypic cultures using biosensor technology. J. Neurochem. 2003, 85, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Colquhoun, A. Cell biology-metabolic crosstalk in glioma. Int. J. Biochem. Cell Biol. 2017, 89, 171–181. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillered, L.; Valtysson, J.; Enblad, P.; Persson, L. Interstitial glycerol as a marker for membrane phospholipid degradation in the acutely injured human brain. J. Neurol. Neurosurg. Psychiatry 1998, 64, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Roslin, M.; Henriksson, R.; Bergstrom, P.; Ungerstedt, U.; Bergenheim, A.T. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J. Neuro Oncol. 2003, 61, 151–160. [Google Scholar] [CrossRef]
- Sugimoto, N.; Miwa, S.; Tsuchiya, H.; Hitomi, Y.; Nakamura, H.; Yachie, A.; Koizumi, S. Targeted activation of PKA and Epac promotes glioblastoma regression in vitro. Mol. Clin. Oncol. 2013, 1, 281–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Baseline | Treatment with dbcAMP or Forskolin | Statistics | |||
|---|---|---|---|---|---|
| Extracellular Medium | U87 Trans-Plantation | Treatment | Treatment | Treatment | Two-Way ANOVA |
| 48 h | Day 7 | Day 14 | |||
| Glucose (mg/dL) | F (3,137) = 2.257 p = 0.0846 | ||||
| No tumor | 435.89 ± 50.7 | 482.05 ± 49.23 | 550.86 ± 111.5 | 549.34 ± 43.16 | |
| No treatment | 506.75 ± 47.7 | 490.45 ± 132.9 | 530.66 ± 99.9 | 571.16 ± 79.85 | |
| 1 mM dbcAMP | 480.6 ± 100.5 | 463.49 ± 104.9 | 504.95 ± 86.81 | 557.08 ± 99.58 | |
| 50 μM Forskolin | 452.75 ± 56.2 | 458.04 ± 44.90 | 539.66 ± 68.95 | 502.60 ± 73.60 | |
| Pyruvate (μM) | F (3,103) = 3.024 p = 0.0331 | ||||
| No tumor | 93.63 ± 56.23 | 85.37 ± 20.35 | 71.40 ± 19.99 | 53.48 ± 25.43 | |
| No treatment | 68.99 ± 32.78 | 53.75 ± 22.46 | 63.23 ± 20.99 | 120.23 ± 168.8 | |
| 1 mM dbcAMP | 68.46 ± 39.53 | 80.33 ± 20.31 | 65.50 ± 35.70 | 183.5 ± 137.5 *# | |
| 50 μM Forskolin | 71.12 ± 31.87 | 55.37 ± 20.75 | 39.85 ± 24.78 | 147.9 ± 155.0 *# | |
| Lactate (mM) | F (3,144) = 1.758 p = 0.1579 | ||||
| No tumor | 6.14 ± 2.42 | 5.56 ± 2.48 | 7.15 ± 3.01 | 5.35 ± 1.46 | |
| No treatment | 6.45 ± 2.60 | 5.43 ± 2.09 | 5.95 ± 2.40 | 4.94 ± 3.90 | |
| 1 mM dbcAMP | 7.33 ± 2.50 | 5.39 ± 2.36 | 4.76 ± 1.93 | 5.71 ± 4.07 | |
| 50 μM Forskolin | 6.81 ± 3.07 | 4.74 ± 2.05 | 4.56 ± 1.55 | 3.01 ± 0.80 | |
| Glycerol (μM) | F (3,107) = 3.758 p = 0.0131 | ||||
| No tumor | 217.12 ± 88.7 | 183.5 ± 76.15 | 230.86 ± 29.51 | 44.90 ± 63.99 | |
| No treatment | 227.3 ± 65.7 | 153.0 ± 73.2 | 145.9 ± 96.1 | 147.6 ± 131.0 | |
| 1 mM dbcAMP | 179.6 ± 99.2 | 175.2 ± 107.5 | 226.2 ± 28.8 * | 236.2 ± 154.3 $# | |
| 50 μM Forskolin | 191.1 ± 101.1 | 90.3 ± 77.3 | 75.8 ± 110.9 * | 46.8 ± 67.4 | |
| Glutamate (μM) | F (3,113) = 0.4309 p = 0.7313 | ||||
| No tumor | 123.12 ± 35.7 | 138.13 ± 14.90 | 108.71 ± 51.40 | 148.56 ± 24.67 | |
| No treatment | 127.6 ± 27.5 | 103.6 ± 44.8 | 106.6 ± 21.9 | 111.7 ± 31.0 | |
| 1 mM dbcAMP | 114.9 ± 51.1 | 140.9 ± 36.4 | 132.8 ± 38.4 | 133.1 ± 91.2 | |
| 50 μM Forskolin | 121.1 ± 47.1 | 134.3 ± 68.6 | 108.2 ± 57.5 | 83.0 ± 43.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wartchow, K.M.; Schmid, B.; Tripal, P.; Stadlbauer, A.; Buchfelder, M.; Gonçalves, C.-A.; Kleindienst, A. Treatment with Cyclic AMP Activators Reduces Glioblastoma Growth and Invasion as Assessed by Two-Photon Microscopy. Cells 2021, 10, 556. https://doi.org/10.3390/cells10030556
Wartchow KM, Schmid B, Tripal P, Stadlbauer A, Buchfelder M, Gonçalves C-A, Kleindienst A. Treatment with Cyclic AMP Activators Reduces Glioblastoma Growth and Invasion as Assessed by Two-Photon Microscopy. Cells. 2021; 10(3):556. https://doi.org/10.3390/cells10030556
Chicago/Turabian StyleWartchow, Krista Minéia, Benjamin Schmid, Philipp Tripal, Andreas Stadlbauer, Michael Buchfelder, Carlos-Alberto Gonçalves, and Andrea Kleindienst. 2021. "Treatment with Cyclic AMP Activators Reduces Glioblastoma Growth and Invasion as Assessed by Two-Photon Microscopy" Cells 10, no. 3: 556. https://doi.org/10.3390/cells10030556
APA StyleWartchow, K. M., Schmid, B., Tripal, P., Stadlbauer, A., Buchfelder, M., Gonçalves, C.-A., & Kleindienst, A. (2021). Treatment with Cyclic AMP Activators Reduces Glioblastoma Growth and Invasion as Assessed by Two-Photon Microscopy. Cells, 10(3), 556. https://doi.org/10.3390/cells10030556