Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models
Abstract
:1. Introduction
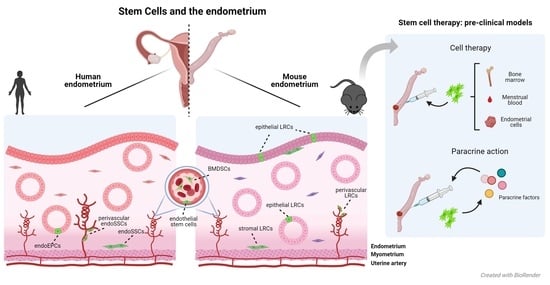
2. Endometrial Stem Cells and Specific Niches
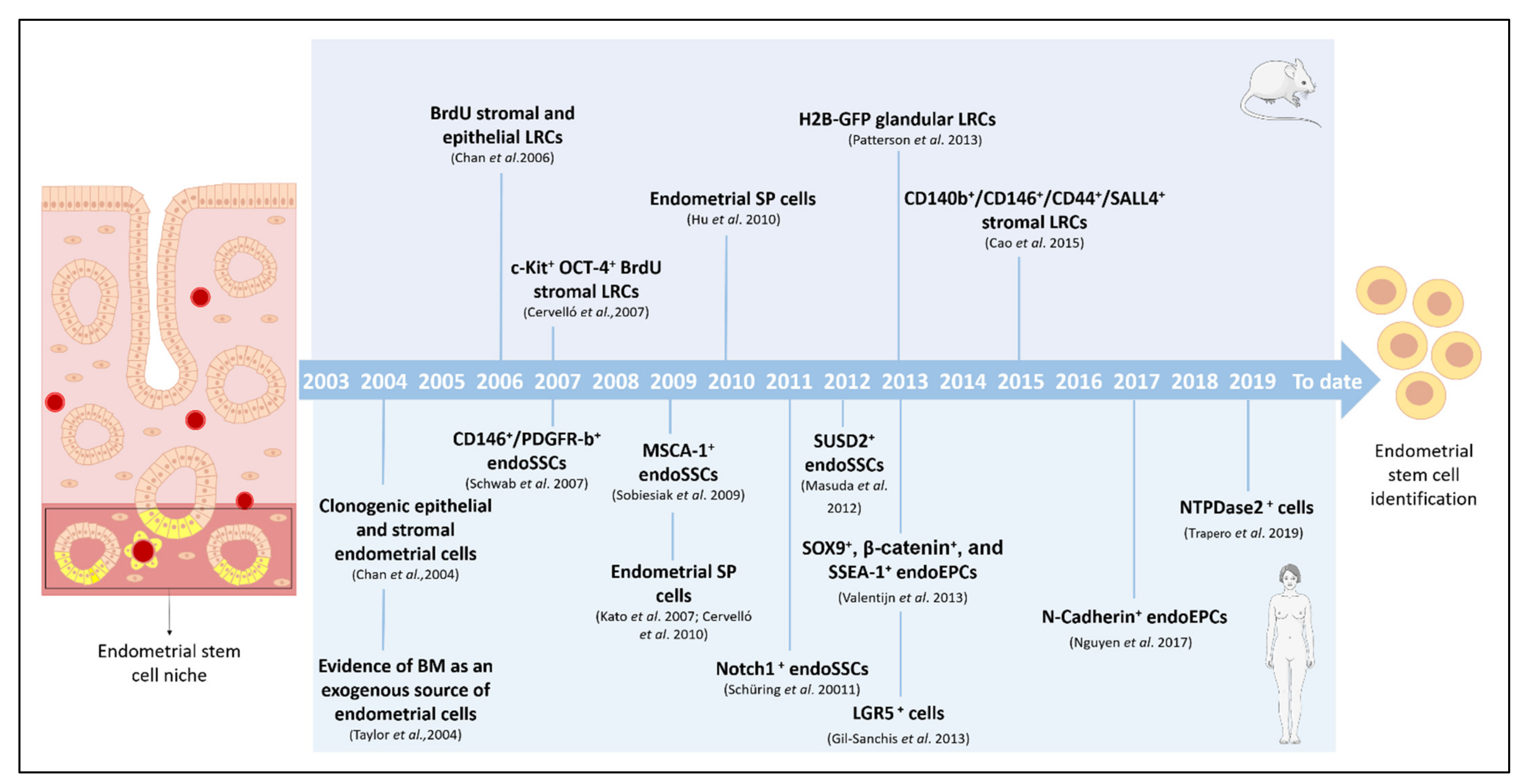
2.1. Identifying Endometrial Stem Cells in Murine Models
2.1.1. Label-Retention Methods in Murine Models
Bromodeoxyuridine Label-Retaining Cells
- (1)
- Stromal BrdU-LRCs
- (2)
- Epithelial BrdU-LRCs
Histone 2B-Green Fluorescent Protein-Label-Retaining Cells
2.1.2. Other Approaches to Identify Murine Endometrial Stem Cells
Side Population Cells
Progenitor Cell Markers in the Mouse Endometrium
2.2. Identifying Endometrial Stem Cells in Humans
2.2.1. Endogenous Endometrial Stem Cells
Endometrial Stromal Stem Cells
Endometrial Epithelial Progenitor Cells
Endothelial Stem Cells
2.2.2. Exogenous Endometrial Stem Cells
Contribution of Bone Marrow to the Endometrial Stem Cell Niche
2.3. Role of Stem Cells in Endometrial Pathologies
3. Stem Cell Therapy and the Endometrium: The Importance of Basic Research and Pre-Clinical Models
3.1. Paracrine Action of Stem Cells: Main Findings in the Endometrium
3.2. Stem Cell Therapy for Treating Endometrial Pathologies
Pre-Clinical Models of Endometrial Injury
4. Future Perspectives and Next Steps
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Józkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H. What is an adult stem cell? Science 2015, 350, 1319–1320. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Cervelló, I.; Santamaria, X.; Miyazaki, K.; Maruyama, T.; Simon, C. Cell Therapy and Tissue Engineering from and toward the Uterus. Semin. Reprod. Med. 2015, 33, 366–372. [Google Scholar] [CrossRef]
- Chacón-Martínez, C.A.; Koester, J.; Wickström, S.A. Signaling in the stem cell niche: Regulating cell fate, function and plasticity. Development 2018, 145, 165399. [Google Scholar] [CrossRef] [Green Version]
- Gurusamy, N.; Alsayari, A.; Rajasingh, S.; Rajasingh, J. Adult Stem Cells for Regenerative Therapy. Prog. Mol. Biol. Transl. Sci. 2018, 160, 1–22. [Google Scholar] [CrossRef]
- Amouzegar, A.; Dey, B.R.; Spitzer, T.R. Peripheral Blood or Bone Marrow Stem Cells? Practical Considerations in Hematopoietic Stem Cell Transplantation. Transfus. Med. Rev. 2019, 33, 43–50. [Google Scholar] [CrossRef]
- Ayyaz, A.; Kumar, S.; Chan, K.; Wrana, J.L.; Gregorieff, A.; Sangiorgi, B.; Ghoshal, B.; Gosio, J.; Ouladan, S.; Fink, M.; et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 2019, 569, 121–125. [Google Scholar] [CrossRef]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Mashinchian, O.; Pisconti, A.; Le Moal, E.; Bentzinger, C.F. The Muscle Stem Cell Niche in Health and Disease. In Current Topics in Developmental Biology, 1st ed.; David Sasson: Bombay, India, 2018; Volume 126, pp. 23–65. [Google Scholar]
- Kelava, I.; Lancaster, M.A. Stem Cell Models of Human Brain Development. Cell Stem Cell 2016, 18, 736–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervelló, I.; Mas, A.; Gil-Sanchis, C.; Simon, C. Somatic Stem Cells in the Human Endometrium. Semin. Reprod. Med. 2013, 31, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Simón, C.; Horcajadas, J.A.; García-Velasco, J.; Pellicer, A. El Endometrio Humano: Desde la Investigación a La Clínica, 1st ed.; Editorial Médica Panamericana: Buenos Aires, Argentina, 2009; pp. 2–42. [Google Scholar]
- Jabbour, H.N.; Kelly, R.W.; Fraser, H.M.; Critchley, H.O.D. Endocrine Regulation of Menstruation. Endocr. Rev. 2006, 27, 17–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef] [Green Version]
- Gargett, C.E.; Nguyen, H.P.T.; Ye, L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251. [Google Scholar] [CrossRef]
- Teixeira, J.; Rueda, B.R.; Pru, J.K. Uterine Stem cells. In StemBook, 1st ed.; The Stem Cell Research Community: Cambridge, MA, USA; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008; Available online: http://www.stembook.org (accessed on 2 January 2021).
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef]
- Deane, J.A.; Gualano, R.C.; Gargett, C.E. Regenerating endometrium from stem/progenitor cells: Is it abnormal in endometriosis, Asherman’s syndrome and infertility? Curr. Opin. Obstet. Gynecol. 2013, 25, 193–200. [Google Scholar] [CrossRef]
- Dreisler, E.; Kjer, J.J. Asherman’s syndrome: Current perspectives on diagnosis and management. Int. J. Women Health 2019, 11, 191–198. [Google Scholar] [CrossRef]
- Lebovitz, O.; Orvieto, R. Treating patients with “thin” endometrium-an ongoing challenge. Gynecol. Endocrinol. 2014, 30, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, J.; Lara, E.; Pacha, P.; Rojas, D.; Veraguas, D.; Saravia, F.; Rodríguez-Alvarez, L.; Castro, F.O. The Endometrium of Cycling Cows Contains Populations of Putative Mesenchymal Progenitor Cells. Reprod. Domest. Anim. 2014, 49, 550–559. [Google Scholar] [CrossRef]
- Miernik, K.; Karasinski, J. Porcine uterus contains a population of mesenchymal stem cells. Reproduction 2012, 143, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letouzey, V.; Tan, K.S.; Deane, J.A.; Ulrich, D.; Gurung, S.; Ong, Y.R.; Gargett, C.E. Isolation and Characterisation of Mesenchymal Stem/Stromal Cells in the Ovine Endometrium. PLoS ONE 2015, 10, e0127531. [Google Scholar] [CrossRef]
- Cabezas, J.; Rojas, D.; Navarrete, F.; Ortiz, R.; Rivera, G.; Saravia, F.; Rodriguez-Alvarez, L.; Castro, F. Equine mesenchymal stem cells derived from endometrial or adipose tissue share significant biological properties, but have distinctive pattern of surface markers and migration. Theriogenology 2018, 106, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Padykula, H.A.; Coles, L.G.; Okulicz, W.C.; Rapaport, S.I.; McCracken, J.A.; King, N.W., Jr.; Longcope, C.; Kaiserman-Abramof, I.R. The Basalis of the Primate Endometrium: A Bifunctional Germinal Compartment. Biol. Reprod. 1989, 40, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar] [PubMed]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraro, F.; Lo Celso, C.; Scadden, D. Adult stem cells and their niches. Adv. Exp. Med. Biol. 2010, 695, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Prianishnikov, V.A. A functional model of the structure of the epithelium of normal, hyperplastic, and malignant human endometrium: A review. Gynecol. Oncol. 1978, 6, 420–428. [Google Scholar] [CrossRef]
- Cousins, F.L.; Dorien, F.O.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38. [Google Scholar] [CrossRef]
- Padykula, H.A. Regeneration in the Primate Uterus: The Role of Stem Cells. Ann. N. Y. Acad. Sci. 1991, 622, 47–56. [Google Scholar] [CrossRef]
- Santamaria, X.; Mas, A.; Cervelló, I.; Taylor, H.; Simon, C. Uterine stem cells: From basic research to advanced cell therapies. Hum. Reprod. Update 2018, 24, 673–693. [Google Scholar] [CrossRef]
- Chan, R.W.S.; Gargett, C.E. Identification of Label-Retaining Cells in Mouse Endometrium. Stem Cells 2006, 24, 1529–1538. [Google Scholar] [CrossRef]
- Cervelló, I.; Martínez-Conejero, J.A.; Horcajadas, J.A.; Pellicer, A.; Simón, C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum. Reprod. 2007, 22, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Chan, R.W.S.; Yeung, W.S.B. Label-Retaining Stromal Cells in Mouse Endometrium Awaken for Expansion and Repair After Parturition. Stem Cells Dev. 2015, 24, 768–780. [Google Scholar] [CrossRef] [Green Version]
- Chan, R.W.S.; Kaitu’U-Lino, T.; Gargett, C.E. Role of Label-Retaining Cells in Estrogen-Induced Endometrial Regeneration. Reprod. Sci. 2012, 19, 102–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaitu’U-Lino, T.J.; Ye, L.; Gargett, C.E. Reepithelialization of the Uterine Surface Arises from Endometrial Glands: Evidence from a Functional Mouse Model of Breakdown and Repair. Endocrinology 2010, 151, 3386–3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-C.; Orvis, G.D.; Wang, Y.; Behringer, R.R. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS ONE 2012, 7, e44285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaitu’U-Lino, T.J.; Ye, L.; Salamonsen, L.A.; Girling, J.E.; Gargett, C.E.; Kaitu’U-Lino, T. Identification of Label-Retaining Perivascular Cells in a Mouse Model of Endometrial Decidualization, Breakdown, and Repair. Biol. Reprod. 2012, 86, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deane, J.A.; Ong, Y.R.; Cain, J.E.; Jayasekara, W.S.N.; Tiwari, A.; Carlone, D.L.; Watkins, D.N.; Breault, D.T.; Gargett, C.E. The mouse endometrium contains epithelial, endothelial and leucocyte populations expressing the stem cell marker telomerase reverse transcriptase. Mol. Hum. Reprod. 2016, 22, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sacchetti, A.; Van Dijk, M.R.; Van Der Zee, M.; Van Der Horst, P.H.; Joosten, R.; Burger, C.W.; Grootegoed, J.A.; Blok, L.J.; Fodde, R. Identification of Quiescent, Stem-Like Cells in the Distal Female Reproductive Tract. PLoS ONE 2012, 7, e40691. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L.; Pru, J.K. Long-term label retaining cells localize to distinct regions within the female reproductive epithelium. Cell Cycle 2013, 12, 2888–2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervelló, I.; Gil-Sanchis, C.; Mas, A.; Delgado-Rosas, F.; Martínez-Conejero, J.A.; Galán, A.; Martínez-Romero, A.; Martínez, S.; Navarro, I.; Ferro, J.; et al. Human Endometrial Side Population Cells Exhibit Genotypic, Phenotypic and Functional Features of Somatic Stem Cells. PLoS ONE 2010, 5, e10964. [Google Scholar] [CrossRef] [Green Version]
- Cervelló, I.; Mas, A.; Gil-Sanchis, C.; Peris, L.; Faus, A.; Saunders, P.T.K.; Critchley, H.O.D.; Simón, C. Reconstruction of Endometrium from Human Endometrial Side Population Cell Lines. PLoS ONE 2011, 6, e21221. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Maruyama, T.; Masuda, H.; Yamasaki, A.; Uchida, S.; Oda, H.; Uchida, H.; Yoshimura, Y. Stem Cell-Like Differentiation Potentials of Endometrial Side Population Cells as Revealed by a Newly Developed In Vivo Endometrial Stem Cell Assay. PLoS ONE 2012, 7, e50749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, S.; Yoshimoto, M.; Takahashi, K.; Noda, Y.; Nakahata, T.; Heike, T. Side population cells contribute to the genesis of human endometrium. Fertil. Steril. 2008, 90, 1528–1537. [Google Scholar] [CrossRef]
- Masuda, H.; Matsuzaki, Y.; Hiratsu, E.; Ono, M.; Nagashima, T.; Kajitani, T.; Arase, T.; Oda, H.; Uchida, H.; Asada, H.; et al. Stem Cell-Like Properties of the Endometrial Side Population: Implication in Endometrial Regeneration. PLoS ONE 2010, 5, e10387. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yoshimoto, M.; Kato, K.; Adachi, S.; Yamayoshi, A.; Arima, T.; Asanoma, K.; Kyo, S.; Nakahata, T.; Wake, N. Characterization of side-population cells in human normal endometrium. Hum. Reprod. 2007, 22, 1214–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervelló, I.; Gil-Sanchis, C.; Mas, A.; Faus, A.; Sanz, J.; Moscardó, F.; Higueras, G.; Sanz, M.A.; Pellicer, A.; Simón, C. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS ONE 2012, 7, e30260. [Google Scholar] [CrossRef]
- Hu, F.-F.; Xu, J.; Cui, Y.-G.; Qian, X.-Q.; Mao, Y.-D.; Liao, L.-M.; Liu, J.-Y. Isolation and Characterization of Side Population Cells in the Postpartum Murine Endometrium. Reprod. Sci. 2010, 17, 629–642. [Google Scholar] [CrossRef]
- Janzen, D.M.; Cheng, D.; Schafenacker, A.M.; Paik, D.Y.; Goldstein, A.S.; Witte, O.N.; Jaroszewicz, A.; Pellegrini, M.; Memarzadeh, S. Estrogen and progesterone together expand murine endometrial epithelial progenitor cells. Stem Cells 2013, 31, 808–822. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Anwar, S.S.; Bühring, H.-J.; Rao, J.R.; Gargett, C.E. A Novel Marker of Human Endometrial Mesenchymal Stem-Like Cells. Cell Transplant. 2012, 21, 2201–2214. [Google Scholar] [CrossRef]
- Schwab, K.E.; Gargett, C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007, 22, 2903–2911. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, D.; Tan, K.S.; Deane, J.; Schwab, K.; Cheong, A.; Rosamilia, A.; Gargett, C.E. Mesenchymal stem/stromal cells in post-menopausal endometrium. Hum. Reprod. 2014, 29, 1895–1905. [Google Scholar] [CrossRef] [Green Version]
- Spitzer, T.L.; Rojas, A.; Giudice, L.C.; Zelenko, Z.; Aghajanova, L.; Erikson, D.W.; Barragan, F.; Meyer, M.; Tamaresis, J.S.; Hamilton, A.E.; et al. Perivascular Human Endometrial Mesenchymal Stem Cells Express Pathways Relevant to Self-Renewal, Lineage Specification, and Functional Phenotype. Biol. Reprod. 2012, 86, 58. [Google Scholar] [CrossRef]
- Sivasubramaniyan, K.; Harichandan, A.; Schumann, S.; Sobiesiak, M.; Lengerke, C.; Maurer, A.; Kalbacher, H.; Bühring, H.-J. Prospective Isolation of Mesenchymal Stem Cells from Human Bone Marrow Using Novel Antibodies Directed Against Sushi Domain Containing 2. Stem Cells Dev. 2013, 22, 1944–1954. [Google Scholar] [CrossRef]
- Xinxin, Z.; Fei, Y.; Guijun, Y.; Yali, H.; Haixiang, S.; Lijun, D. Human endometrial perivascular stem cells exhibit a limited potential to regenerate endometrium after xenotransplantation. Hum. Reprod. 2020, 36, 145–159. [Google Scholar]
- López-Pérez, N.; Gil-Sanchis, C.; Ferrero, H.; Faus, A.; Díaz, A.; Pellicer, A.; Cervelló, I.; Simón, C. Human endometrial reconstitution from somatic stem cells: The importance of niche-like cells. Reprod. Sci. 2019, 26, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Cervelló, I.; Gil-Sanchis, C.; Santamaría, X.; Faus, A.; Vallvé-Juanico, J.; Díaz-Gimeno, P.; Genolet, O.; Pellicer, A.; Simón, C. Leucine-rich repeat-containing G-protein-coupled receptor 5-positive cells in the endometrial stem cell niche. Fertil. Steril. 2017, 107, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Sobiesiak, M.; Sivasubramaniyan, K.; Hermann, C.; Tan, C.; Örgel, M.; Treml, S.; Cerabona, F.; De Zwart, P.; Ochs, U.; Müller, C.A.; et al. The Mesenchymal Stem Cell Antigen MSCA-1 is Identical to Tissue Non-specific Alkaline Phosphatase. Stem Cells Dev. 2010, 19, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Schüring, A.N.; Schulte, N.; Kelsch, R.; Röpke, A.; Kiesel, L.; Götte, M. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil. Steril. 2011, 95, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, A.J.; Palial, K.; Al-Lamee, H.; Tempest, N.; Drury, J.; Von Zglinicki, T.; Saretzki, G.; Murray, P.; Gargett, C.E.; Hapangama, D.K. SSEA-1 isolates human endometrial basal glandular epithelial cells: Phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum. Reprod. 2013, 28, 2695–2708. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.P.T.; Xiao, L.; Deane, J.A.; Tan, K.-S.; Cousins, F.L.; Masuda, H.; Sprung, C.N.; Rosamilia, A.; Gargett, C.E. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum. Reprod. 2017, 32, 2254–2268. [Google Scholar] [CrossRef] [Green Version]
- Tal, R.; Shaikh, S.; Pallavi, P.; Tal, A.; López-Giráldez, F.; Lyu, F.; Fang, Y.-Y.; Chinchanikar, S.; Liu, Y.; Kliman, H.J.; et al. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol. 2019, 17, e3000421. [Google Scholar] [CrossRef]
- Ong, Y.R.; Cousins, F.L.; Yang, X.; Al Mushafi, A.A.A.; Breault, D.T.; Gargett, C.E.; Deane, J.A. Bone Marrow Stem Cells Do Not Contribute to Endometrial Cell Lineages in Chimeric Mouse Models. Stem Cells 2018, 36, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersoy, G.S.; Zolbin, M.M.; Cosar, E.; Moridi, I.; Mamillapalli, R.; Taylor, H.S. CXCL12 Promotes Stem Cell Recruitment and Uterine Repair after Injury in Asherman’s Syndrome. Mol. Ther. Methods Clin. Dev. 2017, 4, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.; Schwab, K.E.; Gargett, C.E. Clonogenicity of Human Endometrial Epithelial and Stromal Cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S. Endometrial Cells Derived From Donor Stem Cells in Bone Marrow Transplant Recipients. JAMA 2004, 292, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Sutermaster, B.A.; Darling, E.M. Considerations for high-yield, high-throughput cell enrichment: Fluorescence versus magnetic sorting. Sci. Rep. 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Maleki, M.; Ghanbarvand, F.; Behvarz, M.R.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Fernández, R.; De La Mata, C.; Requena, F.; Martín, F.; Fernandez-Rubio, P.; Llorca, T.; Ruiz-Magaña, M.J.; Ruiz-Ruiz, C.; Olivares, E.G. Human predecidual stromal cells are mesenchymal stromal/stem cells and have a therapeutic effect in an immune-based mouse model of recurrent spontaneous abortion. Stem Cell Res. Ther. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, S.A.; Friel, A.M.; Kumar, B.; Zhang, L.; Rueda, B.R.; Gargett, C.E. Cell, tumor, and stem cell biology evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009, 69, 8241–8248. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, B.D. Cutting to the chase: Taking the pulse of label-retaining cells in kidney. Am. J. Physiol. Ren. Physiol. 2015, 308, F29–F30. [Google Scholar] [CrossRef] [Green Version]
- Ivanovs, A.; Rybtsov, S.; Anderson, R.A.; Turner, M.L.; Medvinsky, A. Identification of the Niche and Phenotype of the First Human Hematopoietic Stem Cells. Stem Cell Rep. 2014, 2, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Jackson, L.; Dey, S.K.; Daikoku, T. In Pursuit of Leucine-Rich Repeat-Containing G Protein-Coupled Receptor-5 Regulation and Function in the Uterus. Endocrinology 2009, 150, 5065–5073. [Google Scholar] [CrossRef] [Green Version]
- Trapero, C.; Vidal, A.; Rodríguez-Martínez, A.; Sévigny, J.; Ponce, J.; Coroleu, B.; Matias-Guiu, X.; Martín-Satué, M. The ectonucleoside triphosphate diphosphohydrolase-2 (NTPDase2) in human endometrium: A novel marker of basal stroma and mesenchymal stem cells. Purinergic Signal. 2019, 15, 225–236. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Ménard, C.; Dulong, J.; Roulois, D.; Hébraud, B.; Verdière, L.; Pangault, C.; Sibut, V.; Bezier, I.; Bescher, N.; Monvoisin, C.; et al. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells 2020, 38, 146–159. [Google Scholar] [CrossRef] [Green Version]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tempest, N.; MacLean, A.; Hapangama, D.K. Endometrial Stem Cell Markers: Current Concepts and Unresolved Questions. Int. J. Mol. Sci. 2018, 19, 3240. [Google Scholar] [CrossRef] [Green Version]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. A re-appraisal of the morphological changes within the endometrium during menstruation: A hysteroscopic, histological and scanning electron microscopic study. Hum. Reprod. 2009, 24, 1393–1401. [Google Scholar] [CrossRef]
- Hayashi, R.; Yamato, M.; Sugiyama, H.; Sumide, T.; Yang, J.; Okano, T.; Tano, Y.; Nishida, K. N-Cadherin Is Expressed by Putative Stem/Progenitor Cells and Melanocytes in the Human Limbal Epithelial Stem Cell Niche. Stem Cells 2007, 25, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.; Kato, N.; Yoshida, S.; Ogawa, Y.; Shimazaki, J.; Tsubota, K.; Shimmura, S. Aquaporin 1-positive stromal niche-like cells directly interact with N-cadherin-positive clusters in the basal limbal epithelium. Stem Cell Res. 2013, 10, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, H.; Polak, L.; Fuchs, E. Lhx2 Maintains Stem Cell Character in Hair Follicles. Science 2006, 312, 1946–1949. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Niu, C.; Harris, S.E.; Wiedemann, L.M.; Mishina, Y.; Li, L.; Ye, L.; Huang, H.; He, X.; Tong, W.-G.; et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucia, M.; Reca, R.; Jala, V.R.; Dawn, B.; Ratajczak, J.; Ratajczak, M.Z. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia 2005, 19, 1118–1127. [Google Scholar] [CrossRef]
- Mints, M.; Jansson, M.; Sadeghi, B.; Westgren, M.; Uzunel, M.; Hassan, M.; Palmblad, J. Endometrial Endothelial Cells are Derived from Donor Stem Cells in a Bone Marrow Transplant Recipient. Obstet. Gynecol. Surv. 2008, 63, 437–438. [Google Scholar] [CrossRef]
- Campo, H.; Murphy, A.; Yildiz, S.; Woodruff, T.; Cervelló, I.; Kim, J.J. Microphysiological Modeling of the Human Endometrium. Tissue Eng. Part A 2020, 26, 759–768. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, 191. [Google Scholar] [CrossRef]
- Giannone, G.; Attademo, L.; Scotto, G.; Genta, S.; Ghisoni, E.; Tuninetti, V.; Aglietta, M.; Pignata, S.; Valabrega, G. Endometrial Cancer Stem Cells: Role, Characterization and Therapeutic Implications. Cancers 2019, 11, 1820. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Ju, D.-D.; Yang, G.-D.; Zhu, L.-Y.; Yang, X.-M.; Li, J.; Song, W.-W.; Wang, J.-H.; Zhang, C.-C.; Zhang, Z.-G.; et al. Targeting cancer stem cell signature gene SMOC-2 Overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma. EBioMedicine 2019, 40, 276–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasson, I.E.; Taylor, H.S. Stem Cells and the Pathogenesis of Endometriosis. Ann. N Y Acad. Sci. 2008, 1127, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Brosens, J.J.; Puttemans, P.; Benagiano, G.; Brosens, I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 2014, 20, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Gargett, C.E.; Gurung, S. Endometrial Mesenchymal Stem/Stromal Cells, Their Fibroblast Progeny in Endometriosis, and More. Biol. Reprod. 2016, 94, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.G.; Chiantera, V.; Frangini, S.; Younes, S.; Köhler, C.; Taube, E.T.; Plendl, J.; Mechsner, S. Ultramicro-trauma in the endometrial-myometrial junctional zone and pale cell migration in adenomyosis. Fertil. Steril. 2015, 104, 1475–1483.e3. [Google Scholar] [CrossRef] [Green Version]
- Terrovitis, J.V.; Smith, R.R.; Marbán, E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ. Res. 2010, 106, 479–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Bahr, L.; Batsis, I.; Moll, G.; Hägg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Le Blanc, K. Analysis of Tissues Following Mesenchymal Stromal Cell Therapy in Humans Indicates Limited Long-Term Engraftment and No Ectopic Tissue Formation. Stem Cells 2012, 30, 1575–1578. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef]
- Molina, E.R.; Smith, B.T.; Shah, S.R.; Shin, H.; Mikos, A.G. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J. Control. Release 2015, 219, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Cervelló, I.; Gil-Sanchis, C.; Santamaría, X.; Cabanillas, S.; Díaz, A.; Faus, A.; Pellicer, A.; Simón, C. Human CD133+ bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil. Steril. 2015, 104, 1552–1560.e3. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ju, B.; Pan, C.; Gu, Y.; Zhang, Y.; Sun, L.; Zhang, B.; Zhang, Y. Application of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Intrauterine Adhesions in Rats. Cell. Physiol. Biochem. 2016, 39, 1553–1560. [Google Scholar] [CrossRef]
- De Miguel–Gómez, L.; Ferrero, H.; López-Martínez, S.; Campo, H.; López-Pérez, N.; Faus, A.; Hervás, D.; Santamaría, X.; Pellicer, A.; Cervelló, I. Stem cell paracrine actions in tissue regeneration and potential therapeutic effect in human endometrium: A retrospective study. BJOG Int. J. Obstet. Gynecol. 2019, 127, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ding, L.; Wang, L.; Cao, Y.; Zhu, H.; Lu, J.; Li, X.; Song, T.; Hu, Y.; Dai, J. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res. Ther. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, Y.; Guan, C.-Y.; Tian, S.; Lv, X.-D.; Li, J.-H.; Ma, X.; Xia, H.-F. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res. Ther. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.; Duan, H.; Xu, Q.; Tang, Y.-Q.; Li, J.-J.; Sun, F.-Q.; Wang, S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy 2017, 19, 603–616. [Google Scholar] [CrossRef]
- Ouyang, X.; You, S.; Zhang, Y.; Zhang, C.; Zhang, G.; Shao, X.; He, F.; Hu, L. Transplantation of Human Amnion Epithelial Cells Improves Endometrial Regeneration in Rat Model of Intrauterine Adhesions. Stem Cells Dev. 2020, 29, 1346–1362. [Google Scholar] [CrossRef]
- Kilic, S.Ş.; Yuksel, B.; Pinarli, F.A.; Albayrak, A.; Boztok, B.; Delibasi, T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J. Assist. Reprod. Genet. 2014, 31, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Song, K.; Zhang, J.; Zhang, Y.; Tan, B. Effects of menstrual blood-derived stem cells on endometrial injury repair. Mol. Med. Rep. 2019, 19, 813–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, Y.; Park, K.; Jin, Y.; Suk, M.; Ching, H.; Rosenwaks, Z.; Ku, S. Acta Biomaterialia Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater. 2019, 89, 139–151. [Google Scholar]
- Domnina, A.; Novikova, P.; Obidina, J.; Fridlyanskaya, I.; Alekseenko, L.; Kozhukharova, I.; Lyublinskaya, O.; Zenin, V.; Nikolsky, N. Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium. Stem Cell Res. Ther. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champlin, R. Selection of Autologous or Allogeneic Transplantation. In Cancer Medicine; Bast, R.C., Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Hollan, J.F., Frei, E., Eds.; BC Decker: Hamilton, Canada, 2003. [Google Scholar]
- Queckbörner, S.; Davies, L.C.; Von Grothusen, C.; Santamaria, X.; Simon, C.; Gemzell-Danielsson, K. Cellular therapies for the endometrium: An update. Acta Obstet. Gynecol. Scand. 2019, 98, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, M.D.; Alstrup, A.K.O.; Duvald, C.S.; Mikkelsen, E.F.R.; Vendelbo, M.H.; Ovesen, P.G.; Pedersen, M. Animal Models of Fetal Medicine and Obstetrics. In Experimental Animal Models of Human Diseases—An Effective Therapeutic Strategy, 1st ed.; Bartholomew, I., Ed.; IntechOpen: London, UK, 2018; pp. 343–374. [Google Scholar]
- Jing, Z.; Qiong, Z.; Yonggang, W.; Yanping, L. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil. Steril. 2014, 101, 587–594.e3. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Huang, Z.; Lin, H.; Tian, Y.; Li, P.; Lin, S. Bone marrow mesenchymal stem cells (BMSCs) restore functional endometrium in the rat model for severe sherman syndrome. Reprod. Sci. 2019, 26, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, S.; Feng, R.; Huang, J.; Liu, L.; Liu, F.; Chen, Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res. Ther. 2017, 8, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alawadhi, F.; Du, H.; Cakmak, H.; Taylor, H.S. Bone Marrow-Derived Stem Cell (BMDSC) Transplantation Improves Fertility in a Murine Model of Asherman’s Syndrome. PLoS ONE 2014, 9, e96662. [Google Scholar] [CrossRef]
- Yi, K.W.; Mamillapalli, R.; Sahin, C.; Song, J.; Tal, R.; Taylor, H.S. Bone marrow-derived cells or C-X-C motif chemokine 12 (CXCL12) treatment improve thin endometrium in a mouse model. Biol. Reprod. 2019, 100, 61–70. [Google Scholar] [CrossRef]
- Wolff, E.F.; Mutlu, L.; Massasa, E.E.; Elsworth, J.D.; Eugene Redmond, D.; Taylor, H.S. Endometrial stem cell transplantation in MPTP-exposed primates: An alternative cell source for treatment of Parkinson’s disease. J. Cell. Mol. Med. 2015, 19, 249–256. [Google Scholar] [CrossRef]
- Peron, J.P.S.; Jazedje, T.; Brandão, W.N.; Perin, P.M.; Maluf, M.; Evangelista, L.P.; Halpern, S.; Nisenbaum, M.G.; Czeresnia, C.E.; Zatz, M.; et al. Human Endometrial-Derived Mesenchymal Stem Cells Suppress Inflammation in the Central Nervous System of EAE Mice. Stem Cell Rev. Rep. 2012, 8, 940–952. [Google Scholar] [CrossRef]
- Zhang, S.; Li, P.; Yuan, Z.; Tan, J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res. Ther. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Ding, L.; Sun, H.; Su, J.; Lin, N.; Péault, B.; Song, T.; Yang, J.; Dai, J.; Hu, Y. Biomaterials transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials 2014, 35, 4888–4900. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S. Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of Asherman ’s syndrome. Adv. Healthc. Mater. 2019, 1900411, 1–10. [Google Scholar]
- Zhao, S.; Qi, W.; Zheng, J.; Tian, Y.; Qi, X.; Kong, D.; Zhang, J. Exosomes derived from adipose mesenchymal stem cells restore functional endometrium in a raa model of intrauterine adhesions. Reprod. Sci. 2020, 27, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yuan, Q.; Qu, Y.; Zhou, Y.; Bei, J. Endometrial Mesenchymal Stem Cells Isolated from Menstrual Blood by Adherence. Stem Cells Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ren, Y.; Yang, F.; He, Y.; Liang, S.; Guan, L.; Cheng, F.; Liu, Y.; Lin, J. High-yield isolation of menstrual blood-derived endometrial stem cells by direct red blood cell lysis treatment. Biol. Open 2019, 8, bio038885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Identification/Isolation Method | Main Characteristic | Application in Murine Endometrial ASCs | Application in Human Endometrial ASCs | References | |
|---|---|---|---|---|---|
| Label-retention methods | BrdU | DNA analog Pulse-chase assays | YES | NO | [34,35,36,37,38,39,40,41] |
| H2B-GFP | Transgenic system Allows detection of viable cells | YES | NO | [42,43] | |
| Side population | ABC-transporter-positive cell identification | YES | YES | [44,45,46,47,48,49,50,51] | |
| Stem cell marker identification | Flow cytometry | Allows identification and isolation; Compatible with multiple-marker profiles | YES | YES | [41,44,45,47,48,49,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] |
| MACS | Magnetic labeling-based cell isolation method; Preferable for single marker procedures | NO | YES | [49,53,55,57,61,63,64] | |
| Clonogenicity assays | Evaluates the ability of a single cell to produce a colony | YES | YES | [41,44,45,47,48,49,51,52,53,56,57,61,62,64,68,69,70,71,72,73,74,75] | |
| Long-term culture | Stem cells exhibit long-term proliferative potential | NO | YES | [45,53,62,75] | |
| Multi-lineage differentiation | MSCs can differentiate into adipocytes, osteoblasts, myocytes, and chondrocytes in vivo and in vitro | YES | YES | [44,45,46,52,53,54,55,56,57,58,62,65,67,74] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Miguel-Gómez, L.; López-Martínez, S.; Francés-Herrero, E.; Rodríguez-Eguren, A.; Pellicer, A.; Cervelló, I. Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells 2021, 10, 595. https://doi.org/10.3390/cells10030595
de Miguel-Gómez L, López-Martínez S, Francés-Herrero E, Rodríguez-Eguren A, Pellicer A, Cervelló I. Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells. 2021; 10(3):595. https://doi.org/10.3390/cells10030595
Chicago/Turabian Stylede Miguel-Gómez, Lucía, Sara López-Martínez, Emilio Francés-Herrero, Adolfo Rodríguez-Eguren, Antonio Pellicer, and Irene Cervelló. 2021. "Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models" Cells 10, no. 3: 595. https://doi.org/10.3390/cells10030595
APA Stylede Miguel-Gómez, L., López-Martínez, S., Francés-Herrero, E., Rodríguez-Eguren, A., Pellicer, A., & Cervelló, I. (2021). Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells, 10(3), 595. https://doi.org/10.3390/cells10030595