Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models
Abstract
1. Introduction
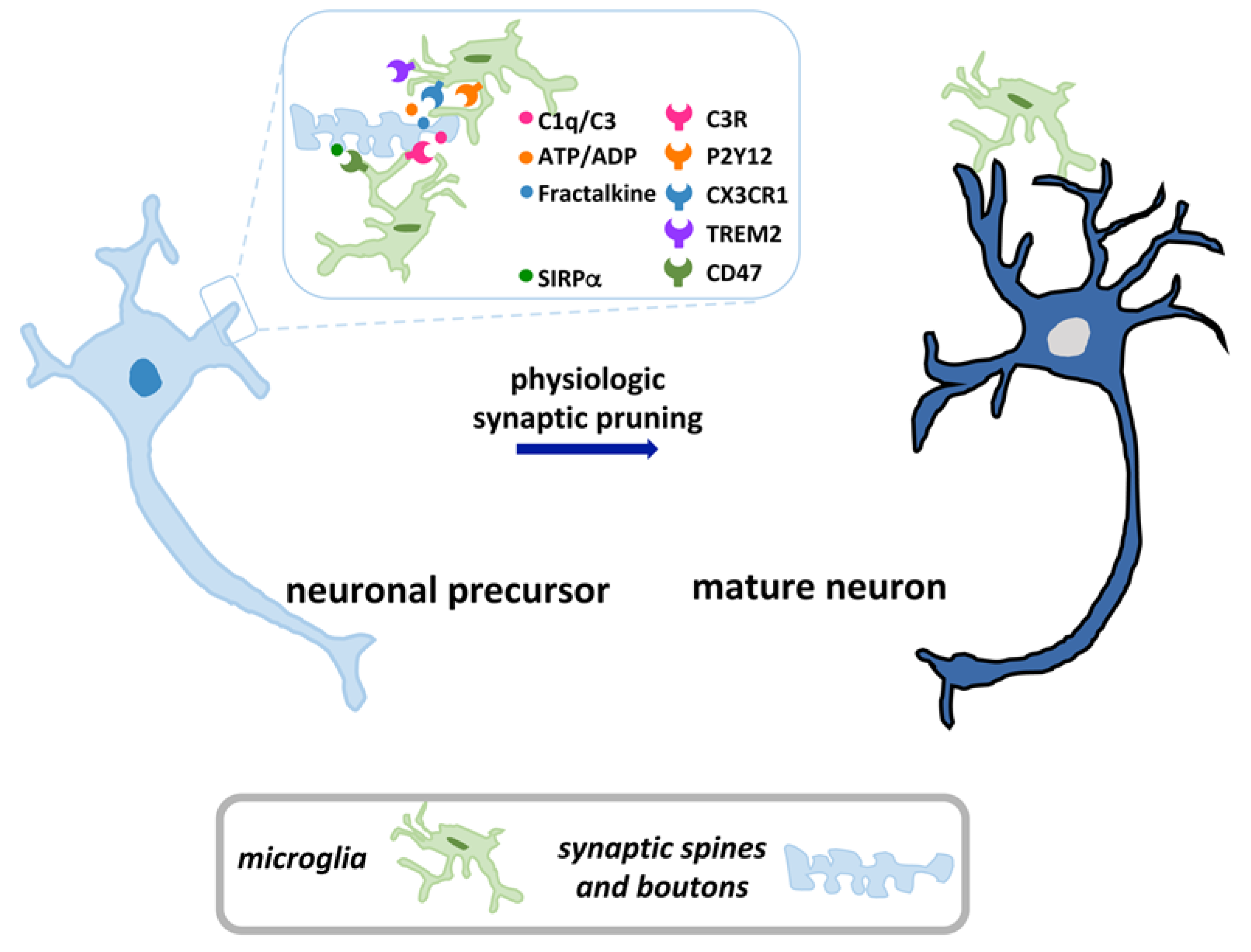
2. Overview of Microglia and Synaptic Pruning
3. Microglia and Synaptopathy in MS and Experimental Autoimmune Encephalomyelitis (EAE)
3.1. Schematic Timeline of MS Pathology
3.2. Overview of Synapse Loss in GM Damage Associated with MS and EAE
3.3. Role of Aberrant Microglial Pruning in Synaptic Elimination in MS and EAE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Filippo, M.; Portaccio, E.; Mancini, A.; Calabresi, P. Multiple Sclerosis and Cognition: Synaptic Failure and Network Dysfunction. Nat. Rev. Neurosci. 2018, 19, 599–609. [Google Scholar] [CrossRef]
- Schirmer, L.; Velmeshev, D.; Holmqvist, S.; Kaufmann, M.; Werneburg, S.; Jung, D.; Vistnes, S.; Stockley, J.H.; Young, A.; Steindel, M.; et al. Neuronal Vulnerability and Multilineage Diversity in Multiple Sclerosis. Nature 2019, 573, 75–82. [Google Scholar] [CrossRef]
- Zoupi, L.; Booker, S.A.; Eigel, D.; Werner, C.; Kind, P.C.; Spires-Jones, T.L.; Newland, B.; Williams, A.C. Selective Vulnerability of Inhibitory Networks in Multiple Sclerosis. Acta Neuropathol. 2021. [Google Scholar] [CrossRef]
- Jafari, M.; Schumacher, A.-M.; Snaidero, N.; Ullrich Gavilanes, E.M.; Neziraj, T.; Kocsis-Jutka, V.; Engels, D.; Jürgens, T.; Wagner, I.; Weidinger, J.D.F.; et al. Phagocyte-Mediated Synapse Removal in Cortical Neuroinflammation Is Promoted by Local Calcium Accumulation. Nat. Neurosci. 2021. [Google Scholar] [CrossRef]
- Mandolesi, G.; Gentile, A.; Musella, A.; Fresegna, D.; De Vito, F.; Bullitta, S.; Sepman, H.; Marfia, G.A.; Centonze, D. Synaptopathy Connects Inflammation and Neurodegeneration in Multiple Sclerosis. Nat. Rev. Neurol. 2015, 11, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.G.; Reynolds, R.; Martin, R. Exploring the Origins of Grey Matter Damage in Multiple Sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; Genova, H.M.; DeLuca, J. Cognitive Rehabilitation in Multiple Sclerosis: The Role of Plasticity. Front. Neurol. 2015, 6, 67. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Zivadinov, R. Risk Factors for and Management of Cognitive Dysfunction in Multiple Sclerosis. Nat. Rev. Neurol. 2011, 7, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Gillingwater, T.H.; Wishart, T.M. Mechanisms Underlying Synaptic Vulnerability and Degeneration in Neurodegenerative Disease: Mechanisms of Synapse Degeneration. Neuropathol. Appl. Neurobiol. 2013, 39, 320–334. [Google Scholar] [CrossRef]
- Casas, C.; Manzano, R.; Vaz, R.; Osta, R.; Brites, D. Synaptic Failure: Focus in an Integrative View of ALS. BPL 2016, 1, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, C.M.; Tzioras, M.; Paolicelli, R.C. Glial Contribution to Excitatory and Inhibitory Synapse Loss in Neurodegeneration. Front. Cell Neurosci. 2019, 13, 63. [Google Scholar] [CrossRef]
- Wishart, T.M.; Parson, S.H.; Gillingwater, T.H. Synaptic Vulnerability in Neurodegenerative Disease. J. Neuropathol. Exp. Neurol. 2006, 65, 733–739. [Google Scholar] [CrossRef]
- Harris, K.M.; Weinberg, R.J. Ultrastructure of Synapses in the Mammalian Brain. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Reshetniak, S.; Rizzoli, S.O. Interrogating Synaptic Architecture: Approaches for Labeling Organelles and Cytoskeleton Components. Front. Synaptic Neurosci. 2019, 11, 23. [Google Scholar] [CrossRef]
- Fedorovich, S.V.; Waseem, T.V.; Puchkova, L.V. Biogenetic and Morphofunctional Heterogeneity of Mitochondria: The Case of Synaptic Mitochondria. Rev. Neurosci. 2017, 28, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.V.; Verstreken, P. Mitochondria at the Synapse. Neuroscientist 2006, 12, 291–299. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Sideris, D.I.; Carroll, E.; Rotariu, S.; Salomonsson, S.; Tzioras, M.; McKenzie, C.-A.; Smith, C.; von Arnim, C.A.F.; Ludolph, A.C.; et al. Synapse Loss in the Prefrontal Cortex Is Associated with Cognitive Decline in Amyotrophic Lateral Sclerosis. Acta Neuropathol. 2018, 135, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.R.; Kim, S.H. Synapses in Neurodegenerative Diseases. BMB Rep. 2017, 50, 237–246. [Google Scholar] [CrossRef]
- Vargas, L.M.; Cerpa, W.; Muñoz, F.J.; Zanlungo, S.; Alvarez, A.R. Amyloid-β Oligomers Synaptotoxicity: The Emerging Role of EphA4/c-Abl Signaling in Alzheimer’s Disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1148–1159. [Google Scholar] [CrossRef]
- Gandhi, P.N.; Chen, S.G.; Wilson-Delfosse, A.L. Leucine-Rich Repeat Kinase 2 (LRRK2): A Key Player in the Pathogenesis of Parkinson’s Disease. J. Neurosci. Res. 2009, 87, 1283–1295. [Google Scholar] [CrossRef]
- Chung, W.-S.; Welsh, C.A.; Barres, B.A.; Stevens, B. Do Glia Drive Synaptic and Cognitive Impairment in Disease? Nat. Neurosci. 2015, 18, 1539–1545. [Google Scholar] [CrossRef]
- Neniskyte, U.; Gross, C.T. Errant Gardeners: Glial-Cell-Dependent Synaptic Pruning and Neurodevelopmental Disorders. Nat. Rev. Neurosci. 2017, 18, 658–670. [Google Scholar] [CrossRef]
- Chung, W.-S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Stevens, B. The “Quad-Partite” Synapse: Microglia-Synapse Interactions in the Developing and Mature CNS. Glia 2013, 61, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Wake, H.; Moorhouse, A.J.; Nabekura, J. Microglia and Synapse Interactions: Fine Tuning Neural Circuits and Candidate Molecules. Front. Cell Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Dissing-Olesen, L.; Stevens, B. New Insights on the Role of Microglia in Synaptic Pruning in Health and Disease. Curr. Opin. Neurobiol. 2016, 36, 128–134. [Google Scholar] [CrossRef]
- Cardozo, P.L.; de Lima, I.B.Q.; Maciel, E.M.A.; Silva, N.C.; Dobransky, T.; Ribeiro, F.M. Synaptic Elimination in Neurological Disorders. CN 2019, 17, 1071–1095. [Google Scholar] [CrossRef] [PubMed]
- Sancho, L.; Contreras, M.; Allen, N.J. Glia as Sculptors of Synaptic Plasticity. Neurosci. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Podleśny-Drabiniok, A.; Marcora, E.; Goate, A.M. Microglial Phagocytosis: A Disease-Associated Process Emerging from Alzheimer’s Disease Genetics. Trends Neurosci. 2020, 43, 965–979. [Google Scholar] [CrossRef]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New Roles for the Synaptic Stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef]
- Barik, A.; Li, L.; Sathyamurthy, A.; Xiong, W.-C.; Mei, L. Schwann Cells in Neuromuscular Junction Formation and Maintenance. J. Neurosci. 2016, 36, 9770–9781. [Google Scholar] [CrossRef]
- Wilton, D.K.; Dissing-Olesen, L.; Stevens, B. Neuron-Glia Signaling in Synapse Elimination. Annu. Rev. Neurosci. 2019, 42, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.L.; Nelson, C.A. Brain Development and the Role of Experience in the Early Years. Zero Three 2009, 30, 9–13. [Google Scholar] [PubMed]
- Hammad, A.; Westacott, L.; Zaben, M. The Role of the Complement System in Traumatic Brain Injury: A Review. J. Neuroinflammation 2018, 15, 24. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Thion, M.S.; Garel, S. Microglia under the Spotlight: Activity and Complement-Dependent Engulfment of Synapses. Trends Neurosci. 2018, 41, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Sipe, G.O.; Lowery, R.L.; Tremblay, M.-È.; Kelly, E.A.; Lamantia, C.E.; Majewska, A.K. Microglial P2Y12 Is Necessary for Synaptic Plasticity in Mouse Visual Cortex. Nat. Commun. 2016, 7, 10905. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Filipello, F.; Morini, R.; Corradini, I.; Zerbi, V.; Canzi, A.; Michalski, B.; Erreni, M.; Markicevic, M.; Starvaggi-Cucuzza, C.; Otero, K.; et al. The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 2018, 48, 979–991.e8. [Google Scholar] [CrossRef]
- Weinhard, L.; di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia Remodel Synapses by Presynaptic Trogocytosis and Spine Head Filopodia Induction. Nat. Commun. 2018, 9, 1228. [Google Scholar] [CrossRef]
- Elward, K.; Gasque, P. “Eat Me” and “Don’t Eat Me” Signals Govern the Innate Immune Response and Tissue Repair in the CNS: Emphasis on the Critical Role of the Complement System. Mol. Immunol. 2003, 40, 85–94. [Google Scholar] [CrossRef]
- Lehrman, E.K.; Wilton, D.K.; Litvina, E.Y.; Welsh, C.A.; Chang, S.T.; Frouin, A.; Walker, A.J.; Heller, M.D.; Umemori, H.; Chen, C.; et al. CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development. Neuron 2018, 100, 120–134.e6. [Google Scholar] [CrossRef]
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased Synapse Elimination by Microglia in Schizophrenia Patient-Derived Models of Synaptic Pruning. Nat. Neurosci. 2019, 22, 374–385. [Google Scholar] [CrossRef]
- Gupta, S.; Ellis, S.E.; Ashar, F.N.; Moes, A.; Bader, J.S.; Zhan, J.; West, A.B.; Arking, D.E. Transcriptome Analysis Reveals Dysregulation of Innate Immune Response Genes and Neuronal Activity-Dependent Genes in Autism. Nat. Commun. 2014, 5, 5748. [Google Scholar] [CrossRef]
- McQuade, A.; Kang, Y.J.; Hasselmann, J.; Jairaman, A.; Sotelo, A.; Coburn, M.; Shabestari, S.K.; Chadarevian, J.P.; Fote, G.; Tu, C.H.; et al. Gene Expression and Functional Deficits Underlie TREM2-Knockout Microglia Responses in Human Models of Alzheimer’s Disease. Nat. Commun. 2020, 11, 5370. [Google Scholar] [CrossRef]
- Sakai, J. Core Concept: How Synaptic Pruning Shapes Neural Wiring during Development and, Possibly, in Disease. Proc. Natl. Acad. Sci. USA 2020, 117, 16096–16099. [Google Scholar] [CrossRef]
- Schartz, N.D.; Tenner, A.J. The Good, the Bad, and the Opportunities of the Complement System in Neurodegenerative Disease. J. Neuroinflammation 2020, 17, 354. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s Disease. J. Cell. Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Zhou, J.; Botto, M.; Tenner, A.J. Absence of C1q Leads to Less Neuropathology in Transgenic Mouse Models of Alzheimer’s Disease. J. Neurosci. 2004, 24, 6457–6465. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and Microglia Mediate Early Synapse Loss in Alzheimer Mouse Models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Paolicelli, R.C. Microglia-Mediated Synapse Loss in Alzheimer’s Disease. J. Neurosci. 2018, 38, 2911–2919. [Google Scholar] [CrossRef]
- Wu, G.F.; Alvarez, E. The Immunopathophysiology of Multiple Sclerosis. Neurol. Clin. 2011, 29, 257–278. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Lucchinetti, C.F.; Noseworthy, J.H. Multiple Sclerosis: Current Pathophysiological Concepts. Lab. Investig. 2001, 81, 263–281. [Google Scholar] [CrossRef]
- Van der Valk, P.; Amor, S. Preactive Lesions in Multiple Sclerosis. Curr. Opin. Neurol. 2009, 22, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Correale, J. The Role of Microglial Activation in Disease Progression. Mult. Scler. 2014, 20, 1288–1295. [Google Scholar] [CrossRef]
- Brambilla, R. The Contribution of Astrocytes to the Neuroinflammatory Response in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Acta Neuropathol. 2019, 137, 757–783. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental Autoimmune Encephalomyelitis (EAE) as a Model for Multiple Sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Geurts, J.J.; Barkhof, F. Grey Matter Pathology in Multiple Sclerosis. Lancet Neurol. 2008, 7, 841–851. [Google Scholar] [CrossRef]
- Musella, A.; Mandolesi, G.; Mori, F.; Gentile, A.; Centonze, D. Linking Synaptopathy and Gray Matter Damage in Multiple Sclerosis. Mult. Scler. 2016, 22, 146–149. [Google Scholar] [CrossRef]
- Centonze, D.; Muzio, L.; Rossi, S.; Cavasinni, F.; De Chiara, V.; Bergami, A.; Musella, A.; D’Amelio, M.; Cavallucci, V.; Martorana, A.; et al. Inflammation Triggers Synaptic Alteration and Degeneration in Experimental Autoimmune Encephalomyelitis. J. Neurosci. 2009, 29, 3442–3452. [Google Scholar] [CrossRef]
- Centonze, D.; Muzio, L.; Rossi, S.; Furlan, R.; Bernardi, G.; Martino, G. The Link between Inflammation, Synaptic Transmission and Neurodegeneration in Multiple Sclerosis. Cell. Death Differ. 2010, 17, 1083–1091. [Google Scholar] [CrossRef]
- Jürgens, T.; Jafari, M.; Kreutzfeldt, M.; Bahn, E.; Brück, W.; Kerschensteiner, M.; Merkler, D. Reconstruction of Single Cortical Projection Neurons Reveals Primary Spine Loss in Multiple Sclerosis. Brain 2016, 139, 39–46. [Google Scholar] [CrossRef]
- Potter, L.E.; Paylor, J.W.; Suh, J.S.; Tenorio, G.; Caliaperumal, J.; Colbourne, F.; Baker, G.; Winship, I.; Kerr, B.J. Altered Excitatory-Inhibitory Balance within Somatosensory Cortex Is Associated with Enhanced Plasticity and Pain Sensitivity in a Mouse Model of Multiple Sclerosis. J. Neuroinflammation 2016, 13, 142. [Google Scholar] [CrossRef]
- Dutta, R.; Chang, A.; Doud, M.K.; Kidd, G.J.; Ribaudo, M.V.; Young, E.A.; Fox, R.J.; Staugaitis, S.M.; Trapp, B.D. Demyelination Causes Synaptic Alterations in Hippocampi from Multiple Sclerosis Patients. Ann. Neurol. 2011, 69, 445–454. [Google Scholar] [CrossRef]
- Michailidou, I.; Willems, J.G.P.; Kooi, E.-J.; van Eden, C.; Gold, S.M.; Geurts, J.J.G.; Baas, F.; Huitinga, I.; Ramaglia, V. Complement C1q-C3-Associated Synaptic Changes in Multiple Sclerosis Hippocampus. Ann. Neurol. 2015, 77, 1007–1026. [Google Scholar] [CrossRef]
- Friese, M.A. Widespread Synaptic Loss in Multiple Sclerosis. Brain 2016, 139, 2–4. [Google Scholar] [CrossRef][Green Version]
- Ziehn, M.O.; Avedisian, A.A.; Tiwari-Woodruff, S.; Voskuhl, R.R. Hippocampal CA1 Atrophy and Synaptic Loss during Experimental Autoimmune Encephalomyelitis, EAE. Lab. Investig. 2010, 90, 774–786. [Google Scholar] [CrossRef]
- Ziehn, M.O.; Avedisian, A.A.; Dervin, S.M.; O’Dell, T.J.; Voskuhl, R.R. Estriol Preserves Synaptic Transmission in the Hippocampus during Autoimmune Demyelinating Disease. Lab. Investig. 2012, 92, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Ziehn, M.O.; Avedisian, A.A.; Dervin, S.M.; Umeda, E.A.; O’Dell, T.J.; Voskuhl, R.R. Therapeutic Testosterone Administration Preserves Excitatory Synaptic Transmission in the Hippocampus during Autoimmune Demyelinating Disease. J. Neurosci. 2012, 32, 12312–12324. [Google Scholar] [CrossRef] [PubMed]
- Chanaday, N.L.; Vilcaes, A.A.; de Paul, A.L.; Torres, A.I.; Degano, A.L.; Roth, G.A. Glutamate Release Machinery Is Altered in the Frontal Cortex of Rats with Experimental Autoimmune Encephalomyelitis. Mol. Neurobiol. 2015, 51, 1353–1367. [Google Scholar] [CrossRef]
- Marchese, E.; Valentini, M.; Di Sante, G.; Cesari, E.; Adinolfi, A.; Corvino, V.; Ria, F.; Sette, C.; Geloso, M.C. Alternative Splicing of Neurexins 1–3 Is Modulated by Neuroinflammation in the Prefrontal Cortex of a Murine Model of Multiple Sclerosis. Exp. Neurol. 2021, 335, 113497. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neuroligins and Neurexins Link Synaptic Function to Cognitive Disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef]
- Südhof, T.C. Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell 2017, 171, 745–769. [Google Scholar] [CrossRef]
- Zanni, G.; Bertini, E.S. X-Linked Disorders with Cerebellar Dysgenesis. Orphanet J. Rare Dis. 2011, 6, 24. [Google Scholar] [CrossRef]
- Ehrmann, I.; Gazzara, M.R.; Pagliarini, V.; Dalgliesh, C.; Kheirollahi-Chadegani, M.; Xu, Y.; Cesari, E.; Danilenko, M.; Maclennan, M.; Lowdon, K.; et al. A SLM2 Feedback Pathway Controls Cortical Network Activity and Mouse Behavior. Cell. Rep. 2016, 17, 3269–3280. [Google Scholar] [CrossRef]
- Traunmüller, L.; Gomez, A.M.; Nguyen, T.-M.; Scheiffele, P. Control of Neuronal Synapse Specification by a Highly Dedicated Alternative Splicing Program. Science 2016, 352, 982–986. [Google Scholar] [CrossRef]
- Murphy, K.L.; Fischer, R.; Swanson, K.A.; Bhatt, I.J.; Oakley, L.; Smeyne, R.; Bracchi-Ricard, V.; Bethea, J.R. Synaptic Alterations and Immune Response Are Sexually Dimorphic in a Non-Pertussis Toxin Model of Experimental Autoimmune Encephalomyelitis. Exp. Neurol. 2020, 323, 113061. [Google Scholar] [CrossRef]
- Sarchielli, P.; Greco, L.; Floridi, A.; Floridi, A.; Gallai, V. Excitatory Amino Acids and Multiple Sclerosis: Evidence from Cerebrospinal Fluid. Arch. Neurol. 2003, 60, 1082–1088. [Google Scholar] [CrossRef]
- Mandolesi, G.; Gentile, A.; Musella, A.; Centonze, D. Il-1β Dependent Cerebellar Synaptopathy in a Mouse Mode of Multiple Sclerosis. Cerebellum 2015, 14, 19–22. [Google Scholar] [CrossRef]
- Di Bari, M.; Di Pinto, G.; Reale, M.; Mengod, G.; Tata, A.M. Cholinergic System and Neuroinflammation: Implication in Multiple Sclerosis. CNSAMC 2017, 17. [Google Scholar] [CrossRef]
- Lazo-Gomez, R.; Velázquez, G.; de Lourdes Llamosa-García Velázquez, G.; Mireles-Jacobo, D.; Sotomayor-Sobrino, M.A. Mechanisms of Neurobehavioral Abnormalities in Multiple Sclerosis: Contributions from Neural and Immune Components. Clin. Neurophysiol. Pract. 2019, 4, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ellwardt, E.; Pramanik, G.; Luchtman, D.; Novkovic, T.; Jubal, E.R.; Vogt, J.; Arnoux, I.; Vogelaar, C.F.; Mandal, S.; Schmalz, M.; et al. Maladaptive Cortical Hyperactivity upon Recovery from Experimental Autoimmune Encephalomyelitis. Nat. Neurosci. 2018, 21, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Prinz, M.; van Loo, G. Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef]
- Guerrero, B.L.; Sicotte, N.L. Microglia in Multiple Sclerosis: Friend or Foe? Front. Immunol. 2020, 11, 374. [Google Scholar] [CrossRef]
- Giunti, D.; Parodi, B.; Cordano, C.; Uccelli, A.; Kerlero de Rosbo, N. Can We Switch Microglia’s Phenotype to Foster Neuroprotection? Focus on Multiple Sclerosis. Immunology 2014, 141, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Laroni, A.; Weiner, H.L. Role of the Innate Immune System in the Pathogenesis of Multiple Sclerosis. J. Neuroimmunol. 2010, 221, 7–14. [Google Scholar] [CrossRef]
- Zrzavy, T.; Hametner, S.; Wimmer, I.; Butovsky, O.; Weiner, H.L.; Lassmann, H. Loss of ‘Homeostatic’ Microglia and Patterns of Their Activation in Active Multiple Sclerosis. Brain 2017, 140, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Metz, I.; Amor, S.; van der Valk, P.; Stadelmann, C.; Brück, W. Microglial Nodules in Early Multiple Sclerosis White Matter Are Associated with Degenerating Axons. Acta Neuropathol. 2013, 125, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, M.; Ulas, T.; Mizee, M.R.; Hsiao, C.-C.; Miedema, S.S.M.; Adelia; Schuurman, K.G.; Helder, B.; Tas, S.W.; Schultze, J.L.; et al. Transcriptional Profiling of Human Microglia Reveals Grey–White Matter Heterogeneity and Multiple Sclerosis-Associated Changes. Nat. Commun. 2019, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Hagens, M.H.J.; Golla, S.V.; Wijburg, M.T.; Yaqub, M.; Heijtel, D.; Steenwijk, M.D.; Schober, P.; Brevé, J.J.P.; Schuit, R.C.; Reekie, T.A.; et al. In Vivo Assessment of Neuroinflammation in Progressive Multiple Sclerosis: A Proof of Concept Study with [18F]DPA714 PET. J. Neuroinflammation 2018, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, M.; Merola, A.; Piacentino, C.; Votta, B.; Capello, E.; Mancardi, G.L.; Mutani, R.; Giordana, M.T.; Cavalla, P. Altered Glutamate Reuptake in Relapsing-Remitting and Secondary Progressive Multiple Sclerosis Cortex: Correlation with Microglia Infiltration, Demyelination, and Neuronal and Synaptic Damage. J. Neuropathol. Exp. Neurol. 2007, 66, 732–739. [Google Scholar] [CrossRef]
- Mrdjen, D.; Pavlovic, A.; Hartmann, F.J.; Schreiner, B.; Utz, S.G.; Leung, B.P.; Lelios, I.; Heppner, F.L.; Kipnis, J.; Merkler, D.; et al. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 2018, 48, 380–395.e6. [Google Scholar] [CrossRef]
- Plemel, J.R.; Stratton, J.A.; Michaels, N.J.; Rawji, K.S.; Zhang, E.; Sinha, S.; Baaklini, C.S.; Dong, Y.; Ho, M.; Thorburn, K.; et al. Microglia Response Following Acute Demyelination Is Heterogeneous and Limits Infiltrating Macrophage Dispersion. Sci. Adv. 2020, 6, eaay6324. [Google Scholar] [CrossRef]
- Heppner, F.L.; Greter, M.; Marino, D.; Falsig, J.; Raivich, G.; Hövelmeyer, N.; Waisman, A.; Rülicke, T.; Prinz, M.; Priller, J.; et al. Experimental Autoimmune Encephalomyelitis Repressed by Microglial Paralysis. Nat. Med. 2005, 11, 146–152. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Jiang, Y.; Pi, R.; Liu, Y.; Ma, L. The Prospects of Minocycline in Multiple Sclerosis. J. Neuroimmunol. 2011, 235, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Saitoh, S.; Miyajima, H.; Itokazu, T.; Yamashita, T. Microglia Suppress the Secondary Progression of Autoimmune Encephalomyelitis. Glia 2019, 67, 23640. [Google Scholar] [CrossRef]
- Rasmussen, S.; Wang, Y.; Kivisäkk, P.; Bronson, R.T.; Meyer, M.; Imitola, J.; Khoury, S.J. Persistent Activation of Microglia Is Associated with Neuronal Dysfunction of Callosal Projecting Pathways and Multiple Sclerosis-like Lesions in Relapsing--Remitting Experimental Autoimmune Encephalomyelitis. Brain 2007, 130, 2816–2829. [Google Scholar] [CrossRef]
- Trapp, B.D.; Wujek, J.R.; Criste, G.A.; Jalabi, W.; Yin, X.; Kidd, G.J.; Stohlman, S.; Ransohoff, R. Evidence for Synaptic Stripping by Cortical Microglia. Glia 2007, 55, 360–368. [Google Scholar] [CrossRef]
- Donzis, E.J.; Tronson, N.C. Modulation of Learning and Memory by Cytokines: Signaling Mechanisms and Long Term Consequences. Neurobiol. Learn. Mem. 2014, 115, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Fresegna, D.; Bullitta, S.; Musella, A.; Rizzo, F.R.; De Vito, F.; Guadalupi, L.; Caioli, S.; Balletta, S.; Sanna, K.; Dolcetti, E.; et al. Re-Examining the Role of TNF in MS Pathogenesis and Therapy. Cells 2020, 9, 2290. [Google Scholar] [CrossRef] [PubMed]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9. [Google Scholar] [CrossRef]
- Dorostkar, M.M.; Zou, C.; Blazquez-Llorca, L.; Herms, J. Analyzing Dendritic Spine Pathology in Alzheimer’s Disease: Problems and Opportunities. Acta Neuropathol. 2015, 130, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.J.; Mu, E.W.H.; Lavidis, N.A.; Noakes, P.G.; Bellingham, M.C. Motor Areas Show Altered Dendritic Structure in an Amyotrophic Lateral Sclerosis Mouse Model. Front. Neurosci. 2017, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Verghese, P.B.; Chakraborty, C.; Joung, J.; Hyman, B.T.; Ulrich, J.D.; Holtzman, D.M.; Barres, B.A. Novel Allele-Dependent Role for APOE in Controlling the Rate of Synapse Pruning by Astrocytes. Proc. Natl. Acad. Sci. USA 2016, 113, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Chen, M.; Cai, K.; Song, Y.; Yu, D.; Zhang, H.; Xu, G. Microglial Trem2 Induces Synaptic Impairment at Early Stage and Prevents Amyloidosis at Late Stage in APP/PS1 Mice. FASEB J. 2019, 33, 10425–10442. [Google Scholar] [CrossRef]
- Piccio, L.; Buonsanti, C.; Cella, M.; Tassi, I.; Schmidt, R.E.; Fenoglio, C.; Rinker, J.; Naismith, R.T.; Panina-Bordignon, P.; Passini, N.; et al. Identification of Soluble TREM-2 in the Cerebrospinal Fluid and Its Association with Multiple Sclerosis and CNS Inflammation. Brain 2008, 131, 3081–3091. [Google Scholar] [CrossRef]
- Öhrfelt, A.; Axelsson, M.; Malmeström, C.; Novakova, L.; Heslegrave, A.; Blennow, K.; Lycke, J.; Zetterberg, H. Soluble TREM-2 in Cerebrospinal Fluid from Patients with Multiple Sclerosis Treated with Natalizumab or Mitoxantrone. Mult. Scler. 2016, 22, 1587–1595. [Google Scholar] [CrossRef]
- Zetterberg, H. Fluid Biomarkers for Microglial Activation and Axonal Injury in Multiple Sclerosis. Acta Neurol. Scand. 2017, 136, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.A.; Nawrocki, A.; Molnar, V.; Elkjaer, M.L.; Thygesen, E.K.; Palkovits, M.; Acs, P.; Sejbaek, T.; Nielsen, H.H.; Hegedus, Z.; et al. Orthologous Proteins of Experimental De- and Remyelination Are Differentially Regulated in the CSF Proteome of Multiple Sclerosis Subtypes. PLoS ONE 2018, 13, e0202530. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 Activation on Microglia Promotes Myelin Debris Clearance and Remyelination in a Model of Multiple Sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Piccio, L.; Buonsanti, C.; Mariani, M.; Cella, M.; Gilfillan, S.; Cross, A.H.; Colonna, M.; Panina-Bordignon, P. Blockade of TREM-2 Exacerbates Experimental Autoimmune Encephalomyelitis. Eur. J. Immunol. 2007, 37, 1290–1301. [Google Scholar] [CrossRef]
- Cantoni, C.; Bollman, B.; Licastro, D.; Xie, M.; Mikesell, R.; Schmidt, R.; Yuede, C.M.; Galimberti, D.; Olivecrona, G.; Klein, R.S.; et al. TREM2 Regulates Microglial Cell Activation in Response to Demyelination in Vivo. Acta Neuropathol. 2015, 129, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Poliani, P.L.; Wang, Y.; Fontana, E.; Robinette, M.L.; Yamanishi, Y.; Gilfillan, S.; Colonna, M. TREM2 Sustains Microglial Expansion during Aging and Response to Demyelination. J. Clin. Investig. 2015, 125, 2161–2170. [Google Scholar] [CrossRef]
- Sheridan, G.K.; Murphy, K.J. Neuron–Glia Crosstalk in Health and Disease: Fractalkine and CX 3 CR1 Take Centre Stage. Open Biol. 2013, 3, 130181. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Pino, P.A.; Mizutani, M.; Cardona, S.M.; Charo, I.F.; Ransohoff, R.M.; Forsthuber, T.G.; Cardona, A.E. Regulation of Adaptive Immunity by the Fractalkine Receptor during Autoimmune Inflammation. J. Immunol. 2013, 191, 1063–1072. [Google Scholar] [CrossRef]
- Arli, B.; Irkec, C.; Menevse, S.; Yilmaz, A.; Alp, E. Fractalkine Gene Receptor Polymorphism in Patients with Multiple Sclerosis. Int. J. Neurosci. 2013, 123, 31–37. [Google Scholar] [CrossRef]
- Cardona, S.M.; Kim, S.V.; Church, K.A.; Torres, V.O.; Cleary, I.A.; Mendiola, A.S.; Saville, S.P.; Watowich, S.S.; Parker-Thornburg, J.; Soto-Ospina, A.; et al. Role of the Fractalkine Receptor in CNS Autoimmune Inflammation: New Approach Utilizing a Mouse Model Expressing the Human CX3CR1I249/M280 Variant. Front. Cell Neurosci. 2018, 12, 365. [Google Scholar] [CrossRef]
- Linnartz-Gerlach, B.; Bodea, L.; Klaus, C.; Ginolhac, A.; Halder, R.; Sinkkonen, L.; Walter, J.; Colonna, M.; Neumann, H. TREM2 Triggers Microglial Density and Age-related Neuronal Loss. Glia 2019, 67, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Tenner, A.J.; Stevens, B.; Woodruff, T.M. New Tricks for an Ancient System: Physiological and Pathological Roles of Complement in the CNS. Mol. Immunol. 2018, 102, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.; Hakobyan, S.; Robertson, N.P.; Morgan, B.P. Complement in Multiple Sclerosis: Its Role in Disease and Potential as a Biomarker. Clin. Exp. Immunol. 2009, 155, 128–139. [Google Scholar] [CrossRef]
- Pinto, M.V.; Fernandes, A. Microglial Phagocytosis—Rational but Challenging Therapeutic Target in Multiple Sclerosis. IJMS 2020, 21, 5960. [Google Scholar] [CrossRef] [PubMed]
- Rus, H.; Cudrici, C.; Niculescu, F.; Shin, M.L. Complement Activation in Autoimmune Demyelination: Dual Role in Neuroinflammation and Neuroprotection. J. Neuroimmunol. 2006, 180, 9–16. [Google Scholar] [CrossRef]
- Barnum, S.R.; Szalai, A.J. Complement and Demyelinating Disease: No MAC Needed? Brain Res. Rev. 2006, 52, 58–68. [Google Scholar] [CrossRef]
- Becquart, P.; Vilariño-Güell, C.; Quandt, J.A. Enhanced Expression of Complement and Microglial-Specific Genes Prior to Clinical Progression in the MOG-Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Brain Res. Bull. 2020, 165, 63–69. [Google Scholar] [CrossRef]
- Aeinehband, S.; Lindblom, R.P.F.; Al Nimer, F.; Vijayaraghavan, S.; Sandholm, K.; Khademi, M.; Olsson, T.; Nilsson, B.; Ekdahl, K.N.; Darreh-Shori, T.; et al. Complement Component C3 and Butyrylcholinesterase Activity Are Associated with Neurodegeneration and Clinical Disability in Multiple Sclerosis. PLoS ONE 2015, 10, e0122048. [Google Scholar] [CrossRef]
- Bhargava, P.; Nogueras-Ortiz, C.; Kim, S.; Delgado-Peraza, F.; Calabresi, P.A.; Kapogiannis, D. Synaptic and Complement Markers in Extracellular Vesicles in Multiple Sclerosis. Mult. Scler. 2020, 1352458520924590. [Google Scholar] [CrossRef]
- Roostaei, T.; Sadaghiani, S.; Mashhadi, R.; Falahatian, M.; Mohamadi, E.; Javadian, N.; Nazeri, A.; Doosti, R.; Naser Moghadasi, A.; Owji, M.; et al. Convergent Effects of a Functional C3 Variant on Brain Atrophy, Demyelination, and Cognitive Impairment in Multiple Sclerosis. Mult. Scler. 2019, 25, 532–540. [Google Scholar] [CrossRef]
- Lindblom, R.P.F.; Berg, A.; Ström, M.; Aeinehband, S.; Dominguez, C.A.; Al Nimer, F.; Abdelmagid, N.; Heinig, M.; Zelano, J.; Harnesk, K.; et al. Complement Receptor 2 Is up Regulated in the Spinal Cord Following Nerve Root Injury and Modulates the Spinal Cord Response. J. Neuroinflammation 2015, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, R.P.F.; Aeinehband, S.; Ström, M.; Al Nimer, F.; Sandholm, K.; Khademi, M.; Nilsson, B.; Piehl, F.; Ekdahl, K.N. Complement Receptor 2 Is Increased in Cerebrospinal Fluid of Multiple Sclerosis Patients and Regulates C3 Function. Clin. Immunol. 2016, 166–167, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Prineas, J.W.; Kwon, E.E.; Cho, E.S.; Sharer, L.R.; Barnett, M.H.; Oleszak, E.L.; Hoffman, B.; Morgan, B.P. Immunopathology of Secondary-Progressive Multiple Sclerosis. Ann. Neurol. 2001, 50, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.H.; Parratt, J.D.E.; Cho, E.-S.; Prineas, J.W. Immunoglobulins and Complement in Postmortem Multiple Sclerosis Tissue. Ann. Neurol. 2009, 65, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.; Loveless, S.; Howell, O.W.; Hakobyan, S.; Dancey, B.; Harris, C.L.; Robertson, N.P.; Neal, J.W.; Morgan, B.P. Complement Activation in Multiple Sclerosis Plaques: An Immunohistochemical Analysis. Acta Neuropathol. Commun. 2014, 2, 53. [Google Scholar] [CrossRef]
- Brink, B.P.; Veerhuis, R.; Breij, E.C.W.; van der Valk, P.; Dijkstra, C.D.; Bö, L. The Pathology of Multiple Sclerosis Is Location-Dependent: No Significant Complement Activation Is Detected in Purely Cortical Lesions. J. Neuropathol. Exp. Neurol. 2005, 64, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.M.; Neal, J.W.; Loveless, S.; Michailidou, I.; Ramaglia, V.; Rees, M.I.; Reynolds, R.; Robertson, N.P.; Morgan, B.P.; Howell, O.W. Complement Is Activated in Progressive Multiple Sclerosis Cortical Grey Matter Lesions. J. Neuroinflammation 2016, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, S.; Jung, J.; Kunjamma, R.B.; Ha, S.-K.; Luciano, N.J.; Willis, C.M.; Gao, G.; Biscola, N.P.; Havton, L.A.; Crocker, S.J.; et al. Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity 2020, 52, 167–182.e7. [Google Scholar] [CrossRef]
- Xin, W.; Chan, J.R. That Wasn’t a Complement—Too Much C3 in Demyelinating Disease. Immunity 2020, 52, 11–13. [Google Scholar] [CrossRef]
- Alawieh, A.; Langley, E.F.; Weber, S.; Adkins, D.; Tomlinson, S. Identifying the Role of Complement in Triggering Neuroinflammation after Traumatic Brain Injury. J. Neurosci. 2018, 38, 2519–2532. [Google Scholar] [CrossRef]
- Hammond, J.W.; Bellizzi, M.J.; Ware, C.; Qiu, W.Q.; Saminathan, P.; Li, H.; Luo, S.; Ma, S.A.; Li, Y.; Gelbard, H.A. Complement-Dependent Synapse Loss and Microgliosis in a Mouse Model of Multiple Sclerosis. Brain Behav. Immun. 2020, 87, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, I.; Jongejan, A.; Vreijling, J.P.; Georgakopoulou, T.; de Wissel, M.B.; Wolterman, R.A.; Ruizendaal, P.; Klar-Mohamad, N.; Grootemaat, A.E.; Picavet, D.I.; et al. Systemic Inhibition of the Membrane Attack Complex Impedes Neuroinflammation in Chronic Relapsing Experimental Autoimmune Encephalomyelitis. Acta Neuropathol. Commun. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Gitik, M.; Liraz-Zaltsman, S.; Oldenborg, P.-A.; Reichert, F.; Rotshenker, S. Myelin Down-Regulates Myelin Phagocytosis by Microglia and Macrophages through Interactions between CD47 on Myelin and SIRPα (Signal Regulatory Protein-α) on Phagocytes. J. Neuroinflammation 2011, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Koning, N.; Bö, L.; Hoek, R.M.; Huitinga, I. Downregulation of Macrophage Inhibitory Molecules in Multiple Sclerosis Lesions. Ann. Neurol. 2007, 62, 504–514. [Google Scholar] [CrossRef]
- Junker, A.; Krumbholz, M.; Eisele, S.; Mohan, H.; Augstein, F.; Bittner, R.; Lassmann, H.; Wekerle, H.; Hohlfeld, R.; Meinl, E. MicroRNA Profiling of Multiple Sclerosis Lesions Identifies Modulators of the Regulatory Protein CD47. Brain 2009, 132, 3342–3352. [Google Scholar] [CrossRef]
- Han, M.H.; Lundgren, D.H.; Jaiswal, S.; Chao, M.; Graham, K.L.; Garris, C.S.; Axtell, R.C.; Ho, P.P.; Lock, C.B.; Woodard, J.I.; et al. Janus-like Opposing Roles of CD47 in Autoimmune Brain Inflammation in Humans and Mice. J. Exp. Med. 2012, 209, 1325–1334. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geloso, M.C.; D’Ambrosi, N. Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models. Cells 2021, 10, 686. https://doi.org/10.3390/cells10030686
Geloso MC, D’Ambrosi N. Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models. Cells. 2021; 10(3):686. https://doi.org/10.3390/cells10030686
Chicago/Turabian StyleGeloso, Maria Concetta, and Nadia D’Ambrosi. 2021. "Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models" Cells 10, no. 3: 686. https://doi.org/10.3390/cells10030686
APA StyleGeloso, M. C., & D’Ambrosi, N. (2021). Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models. Cells, 10(3), 686. https://doi.org/10.3390/cells10030686





