Mast Cell Mediated Regulation of Small Intestinal Chloride Malabsorption in SAMP1/YitFc Mouse Model of Spontaneous Chronic Ileitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Models and Drug Treatment
2.2. Cell Isolation
2.3. β-Hexosaminidase Activity Assay
2.4. BBM Vesicles (BBMV) Preparation
2.5. 36Cl− Uptake Studies to Determine Cl−/HCO3− Exchange in BBMV
2.6. 22Na Uptake Studies to Determine Na/H Exchange in BBMV
2.7. Cl−/HCO3− Exchange Kinetic Studies in Intact Villus Cells
2.8. Real Time qPCR Analysis
2.9. Western Blot Analysis
2.10. Histology and Immunofluorescence Study
2.11. Protein Assay
2.12. Statistical Analysis
3. Results
3.1. Effect of Ketotifen on Mast Cell Degranulation in Chronically Inflamed SAMP1 Mice
3.2. Effect of Ketotifen on Mast Cell β-Hexosaminidase in Intestinal Mucosa
3.3. Effect of Ketotifen on Cl−/HCO3− Exchange in the BBMV of Ileal Villus Cell
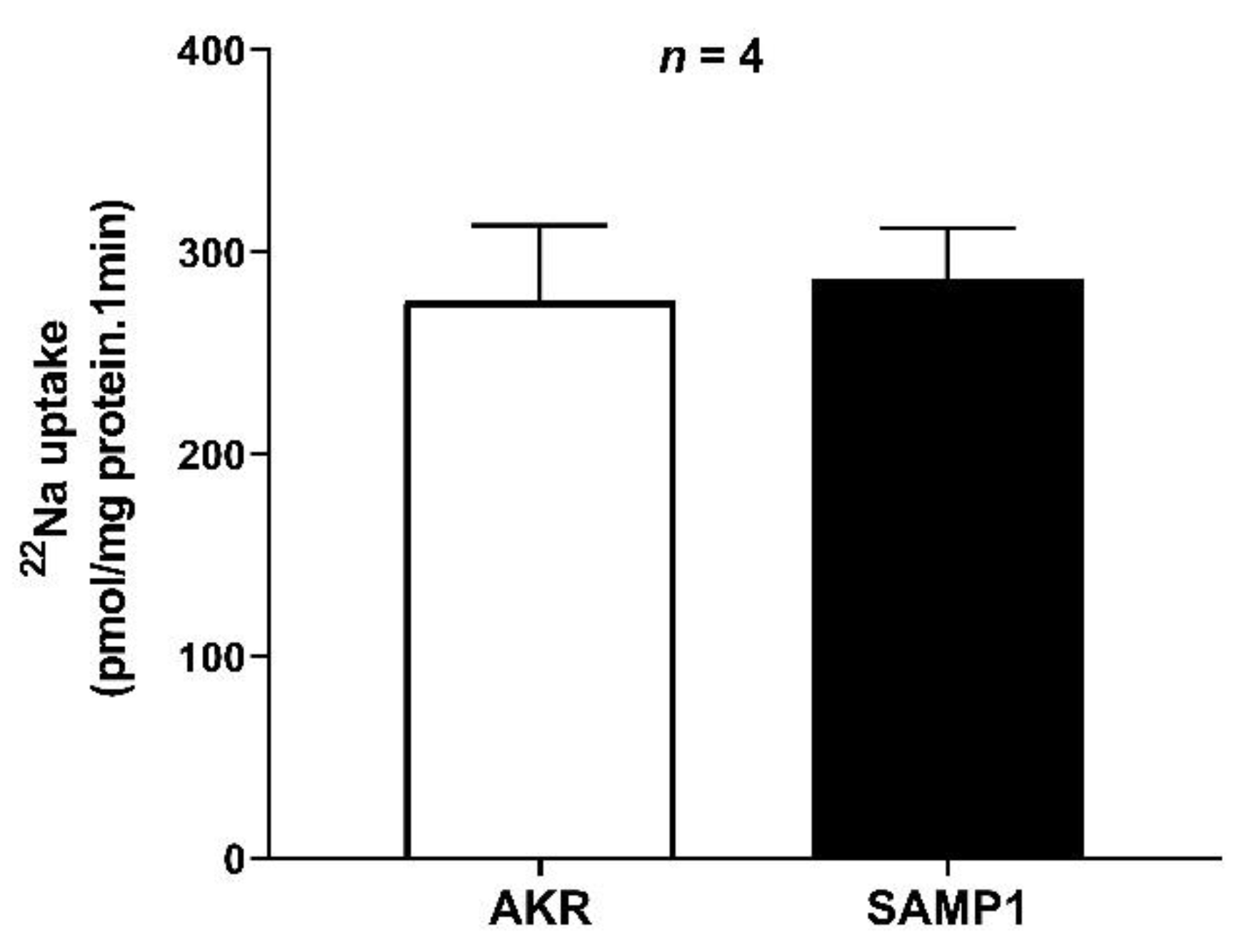
3.4. Na/H Exchanger (NHE3) Activity in the BBMV of Ileal Villus Cell
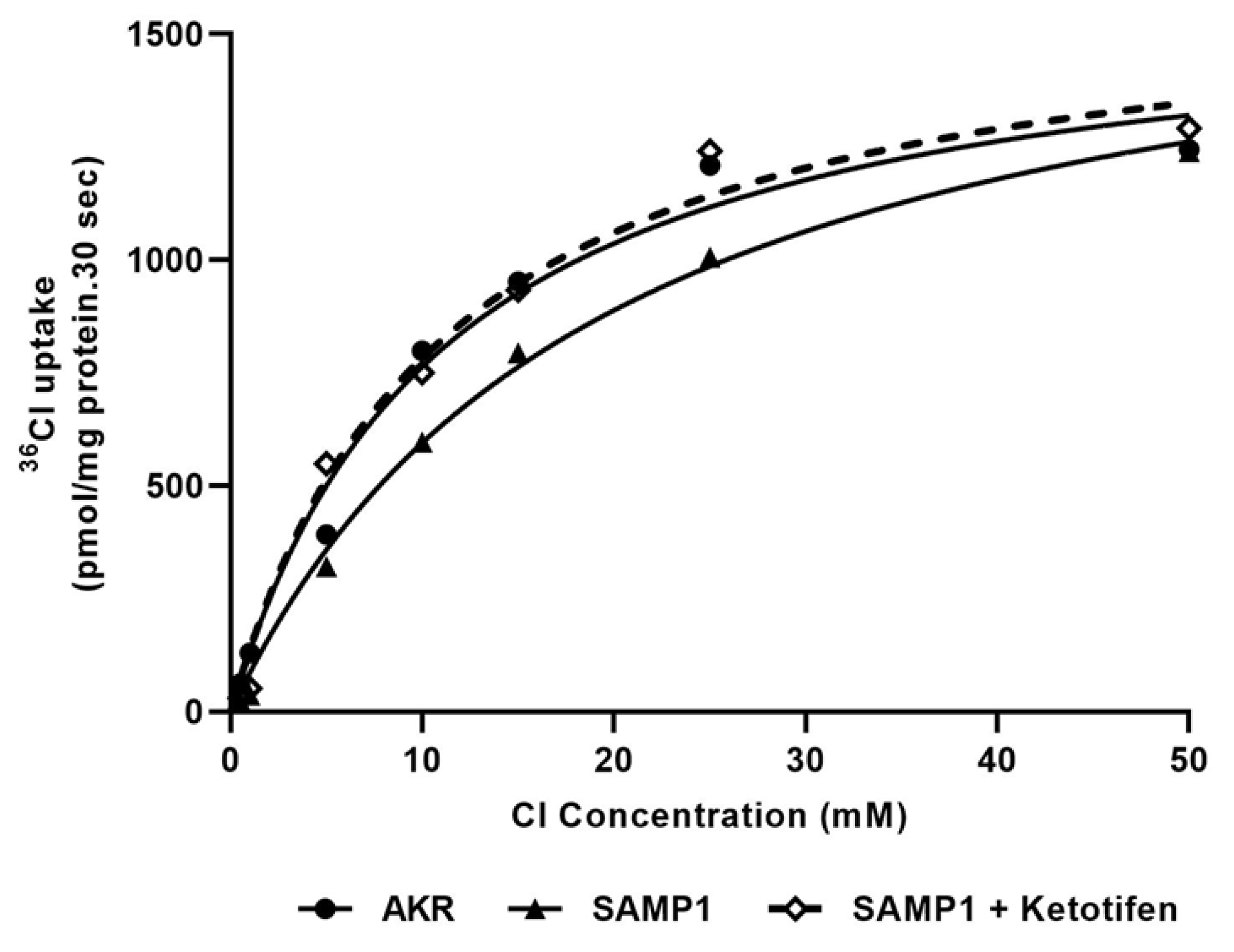
3.5. Kinetic Studies for Cl−/HCO3− Exchange
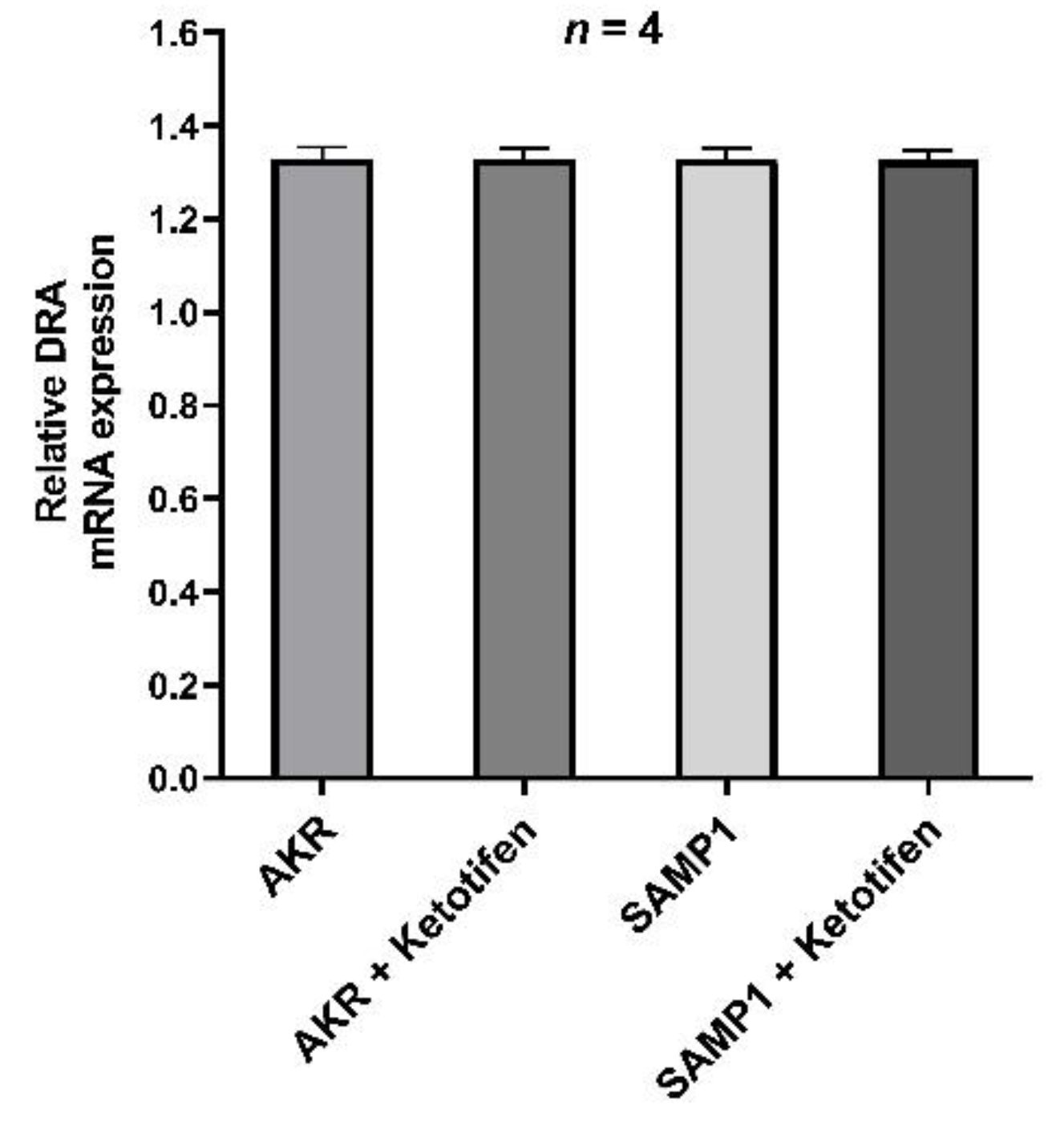
3.6. DRA mRNA Expression by RT-qPCR
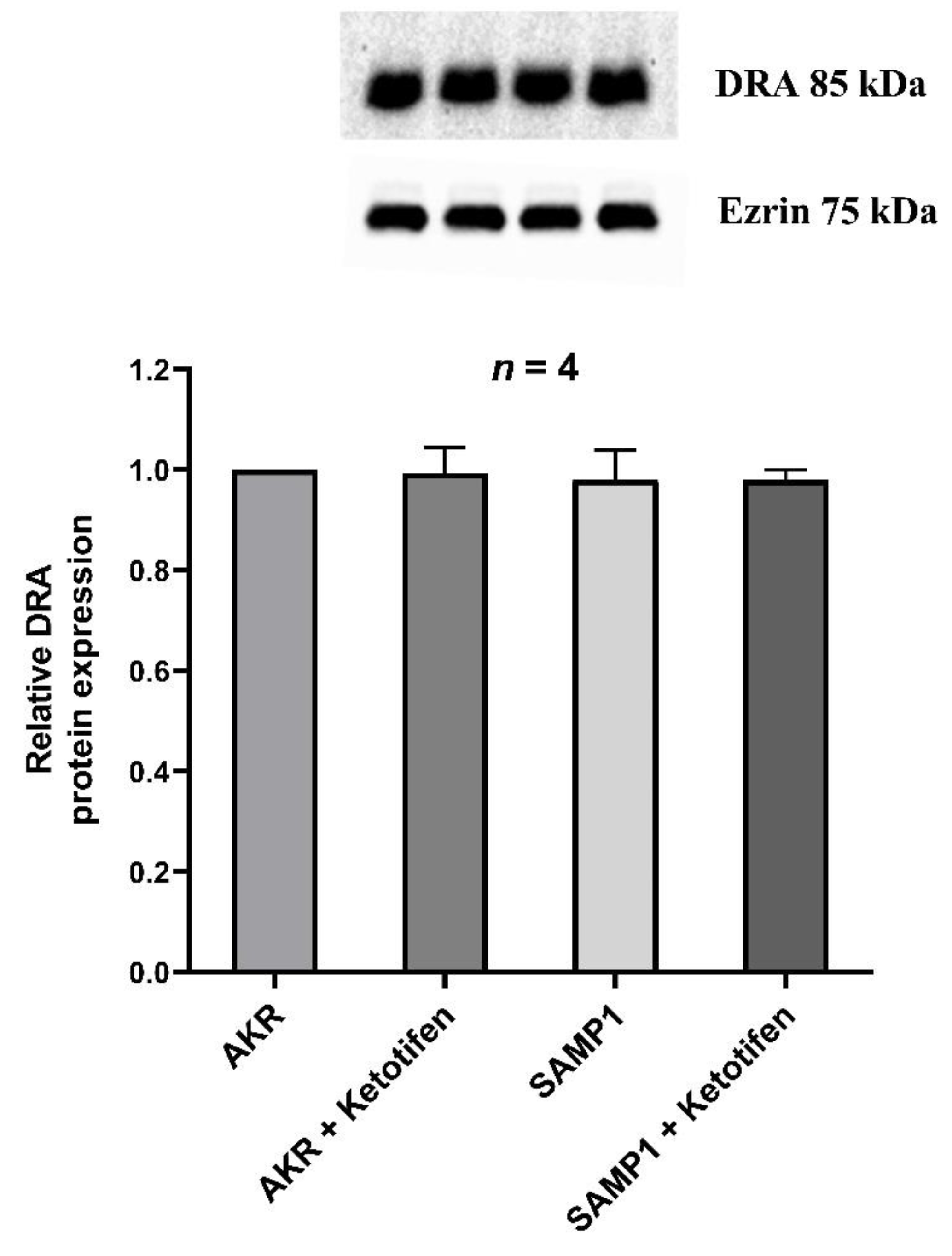
3.7. Western Blot Studies for DRA
3.8. Immunofluorescence Studies of DRA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parray, F.Q.; Wani, M.L.; Bijli, A.H.; Thakur, N.; Irshad, I.; Nayeem-ul-Hassan. Crohn′s disease: A surgeon′s perspective. Saudi J. Gastroenterol. 2011, 17, 6–15. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.E.; Cho, M.-L. Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD associated diarrhea. Tissue Barriers 2018, 6, e1463897. [Google Scholar] [CrossRef] [PubMed]
- Meerveld, B.G.-V.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. Snake Venoms 2017, 239, 1–16. [Google Scholar] [CrossRef]
- Sullivan, S.; Alex, P.; Dassopoulos, T.; Zachos, N.C.; Iacobuzio-Donahue, C.; Donowitz, M.; Brant, S.R.; Cuffari, C.; Harris, M.L.; Datta, L.W.; et al. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: Potential contributors to IBD-associated diarrhea. Inflamm. Bowel Dis. 2009, 15, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Arthur, S.; Sundaram, U. Mechanisms of Regulation of Transporters of Amino Acid Absorption in Inflammatory Bowel Diseases. Compr. Physiol. 2020, 10, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.; Sundaram, U. Inducible nitric oxide regulates intestinal glutamine assimilation during chronic intestinal inflammation. Nitric Oxide 2015, 44, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.; Manoharan, P.; Sundaram, S.; Rahman, M.M.; Palaniappan, B.; Sundaram, U. Unique Regulation of Enterocyte Brush Border Membrane Na-Glutamine and Na-Alanine Co-Transport by Peroxynitrite during Chronic Intestinal Inflammation. Int. J. Mol. Sci. 2019, 20, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaram, U.; West, A.B. Effect of chronic inflammation on electrolyte transport in rabbit ileal villus and crypt cells. Am. J. Physiol. Content 1997, 272, G732–G741. [Google Scholar] [CrossRef]
- Martínez-Augustin, O.; Romero-Calvo, I.; Suárez, M.D.; Zarzuelo, A.; De Medina, F.S. Molecular bases of impaired water and ion movements in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009, 15, 114–127. [Google Scholar] [CrossRef]
- Priyamvada, S.; Gomes, R.; Gill, R.K.; Saksena, S.; Alrefai, W.A.; Dudeja, P.K. Mechanisms Underlying Dysregulation of Electrolyte Absorption in Inflammatory Bowel Disease–Associated Diarrhea. Inflamm. Bowel Dis. 2015, 21, 2926–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, N.M.; Simpson, J.E.; Yen, P.; Gill, R.K.; Rigsby, E.V.; Brazill, J.M.; Dudeja, P.K.; Schweinfest, C.W.; Clarke, L.L. Down-regulated in Adenoma Cl/HCO3 Exchanger Couples With Na/H Exchanger 3 for NaCl Absorption in Murine Small Intestine. Gastroenterology 2008, 135, 1645–1653.e3. [Google Scholar] [CrossRef] [Green Version]
- Barkas, F.; Liberopoulos, E.; Kei, A.; Elisaf, M. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann. Gastroenterol. 2013, 26, 23–28. [Google Scholar]
- Binder, H.J. Mechanisms of Diarrhea in Inflammatory Bowel Diseases. Ann. N. Y. Acad. Sci. 2009, 1165, 285–293. [Google Scholar] [CrossRef]
- Manoharan, P.; Coon, S.; Baseler, W.; Sundaram, S.; Kekuda, R.; Sundaram, U. Prostaglandins, not the leukotrienes, regulate Cl−/HCO3− exchange (DRA, SLC26A3) in villus cells in the chronically inflamed rabbit ileum. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, S.C. Physiological and pathophysiological functions of intestinal mast cells. Semin. Immunopathol. 2009, 31, 185–205. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [Green Version]
- De Winter, B.Y.; Wijngaard, R.M.V.D.; De Jonge, W.J. Intestinal mast cells in gut inflammation and motility disturbances. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 66–73. [Google Scholar] [CrossRef] [Green Version]
- He, S.-H. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 309–318. [Google Scholar] [CrossRef]
- Crowe, S.E.; Luthra, G.K.; Perdue, M.H. Mast cell mediated ion transport in intestine from patients with and without inflammatory bowel disease. Gut 1997, 41, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Perdue, M.H.; Masson, S.; Wershil, B.K.; Galli, S.J. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J. Clin. Investig. 1991, 87, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizarro, T.T.; Pastorelli, L.; Bamias, G.; Garg, R.R.; Reuter, B.K.; Mercado, J.R.; Chieppa, M.; Arseneau, K.O.; Ley, K.; Cominelli, F. SAMP1/YitFc mouse strain: A spontaneous model of Crohnʼs disease-like ileitis. Inflamm. Bowel Dis. 2011, 17, 2566–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, S.; Palaniappan, B.; Mani, K.; Sundaram, U. 1020—Inducible Nitric Oxide Mediates the Inhibitiion of Coupled Nacl Abosorption in a Mouse Model of Spontaneous Ileitis. Gastroenterology 2018, 154, S-193. [Google Scholar] [CrossRef]
- Arthur, S.; Palaniappan, B.; Sundaram, U. Su1830—Inducible Nitric Oxide Mediated Phosphorylation Regulates the Altered Activity of Downregulated in Adenoma (DRA) in a Mouse Model of Spontaneous Ileitis. Gastroenterology 2019, 156, 627. [Google Scholar] [CrossRef]
- Sundaram, U.; Knickelbein, R.G.; Dobbins, J.W. Mechanism of intestinal secretion. Effect of serotonin on rabbit ileal crypt and villus cells. J. Clin. Investig. 1991, 87, 743–746. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, U.; Knickelbein, R.G.; Dobbins, J.W. pH regulation in ileum: Na(+)-H+ and Cl(-)-HCO3- exchange in isolated crypt and villus cells. Am. J. Physiol. Liver Physiol. 1991, 260, G440–G449. [Google Scholar] [CrossRef] [PubMed]
- Coon, S.; Sundaram, U. Unique regulation of anion/HCO3- exchangers by constitutive nitric oxide in rabbit small intestine. Am. J. Physiol. Liver Physiol. 2003, 285, G1084–G1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manokas, T.; Fromkes, J.J.; Sundaram, U. Effect of chronic inflammation on ileal short-chain fatty acid/bicarbonate exchange. Am. J. Physiol. Liver Physiol. 2000, 278, G585–G590. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Chin, B.C. Inflammatory Mediators in Gastrointestinal Defense and Injury. Exp. Biol. Med. 1997, 214, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef]
- Andoh, A.; Deguchi, Y.; Inatomi, O.; Yagi, Y.; Bamba, S.; Tsujikawa, T.; Fujiyama, Y. Immunohistochemical study of chymase-positive mast cells in inflammatory bowel disease. Oncol. Rep. 2006, 16, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Raithel, H.T.S.M. Effect of Substance P on Histamine Secretion from Gut Mucosa in Inflammatory Bowel Disease. Scand. J. Gastroenterol. 1999, 34, 496–503. [Google Scholar] [CrossRef]
- Raithel, S.W.M. Release of Mast Cell Tryptase from Human Colorectal Mucosa in Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2001, 36, 174–179. [Google Scholar] [CrossRef]
- Kurashima, Y.; Amiya, T.; Nochi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 2012, 3, 1034. [Google Scholar] [CrossRef]
- Vivinus-Nébot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G. Mast cells and inflammatory bowel disease. Curr. Opin. Pharmacol. 2015, 25, 45–49. [Google Scholar] [CrossRef]
- Wang, L.; Stanisz, A.M.; Wershil, B.K.; Galli, S.J.; Perdue, M.H. Substance P induces ion secretion in mouse small intestine through effects on enteric nerves and mast cells. Am. J. Physiol. Liver Physiol. 1995, 269, G85–G92. [Google Scholar] [CrossRef]
- Barrett, K.E. Immune-related intestinal chloride secretion. III. Acute and chronic effects of mast cell mediators on chloride secretion by a human colonic epithelial cell line. J. Immunol. 1991, 147, 959–964. [Google Scholar] [PubMed]
- Russell, D.A. Mast cells in the regulation of intestinal electrolyte transport. Am. J. Physiol. Liver Physiol. 1986, 251, G253–G262. [Google Scholar] [CrossRef]
- Perdue, M.H.; Marshall, J.; Masson, S. Ion transport abnormalities in inflamed rat jejunum. Gastroenterology 1990, 98, 561–567. [Google Scholar] [CrossRef]
- Fukuishi, N.; Murakami, S.; Ohno, A.; Yamanaka, N.; Matsui, N.; Fukutsuji, K.; Yamada, S.; Itoh, K.; Akagi, M. Does β-Hexosaminidase Function Only as a Degranulation Indicator in Mast Cells? The Primary Role of β-Hexosaminidase in Mast Cell Granules. J. Immunol. 2014, 193, 1886–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Arthur, S.; Talukder, J.; Palaniappan, B.; Coon, S.; Sundaram, U. Mast cell regulation of Na-glutamine co-transporters B0AT1 in villus and SN2 in crypt cells during chronic intestinal inflammation. BMC Gastroenterol. 2015, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.B.; McGrath, J.; Baird, A.W.; Campion, D.P. Effect of Mast Cell Degranulation on Chicken Ileal Ion Transport In Vitro. Poult. Sci. 2007, 86, 843–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Kumar, A.; Raheja, G.; Anbazhagan, A.N.; Priyamvada, S.; Saksena, S.; Jhandier, M.N.; Gill, R.K.; Alrefai, W.A.; Borthakur, A.; et al. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am. J. Physiol. Liver Physiol. 2014, 307, G623–G631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anbazhagan, A.N.; Thaqi, M.; Priyamvada, S.; Jayawardena, D.; Kumar, A.; Gujral, T.; Chatterjee, I.; Mugarza, E.; Saksena, S.; Onyuksel, H.; et al. GLP-1 nanomedicine alleviates gut inflammation. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 659–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayawardena, D.; Anbazhagan, A.N.; Guzman, G.; Dudeja, P.K.; Onyuksel, H. Vasoactive Intestinal Peptide Nanomedicine for the Management of Inflammatory Bowel Disease. Mol. Pharm. 2017, 14, 3698–3708. [Google Scholar] [CrossRef]
- Jayawardena, D.; Tyagi, S.; Nazmi, A.; Olivares-Villagómez, D.; Dudeja, P.K. Ion Transport Basis of Diarrhea in a Mouse Model of Adoptive T Cell Transfer Colitis. Dig. Dis. Sci. 2020, 65, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Höglund, P.; Sormaala, M.; Haila, S.; Socha, J.; Rajaram, U.; Scheurlen, W.; Sinaasappel, M.; De Jonge, H.; Holmberg, C.; Yoshikawa, H.; et al. Identification of seven novel mutations including the first two genomic rearrangements in SLC26A3 mutated in congenital chloride diarrhea. Hum. Mutat. 2001, 18, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.M.; Khan, H.Y.; El-Shabrawi, M.H.; Sherief, L.M. Congenital chloride losing diarrhea. Medicine 2019, 98, e15928. [Google Scholar] [CrossRef]
- Mäkelä, S.; Kere, J.; Holmberg, C.; Höglund, P. SLC26A3 mutations in congenital chloride diarrhea. Hum. Mutat. 2002, 20, 425–438. [Google Scholar] [CrossRef]
- Wedenoja, S.; Pekansaari, E.; Höglund, P.; Mäkelä, S.; Holmberg, C.; Kere, J. Update on SLC26A3 mutations in congenital chloride diarrhea. Hum. Mutat. 2011, 32, 715–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, F.; Cardillo, G.; Liguori, R.; Scorza, M.; Comegna, M.; Elce, A.; Giordano, S.; Lucaccioni, L.; Lugli, L.; Cardile, S.; et al. Twelve Novel Mutations in the SLC26A3 Gene in 17 Sporadic Cases of Congenital Chloride Diarrhea. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Schweinfest, C.W.; Spyropoulos, D.D.; Henderson, K.W.; Kim, J.-H.; Chapman, J.M.; Barone, S.; Worrell, R.T.; Wang, Z.; Soleimani, M. slc26a3 (dra)-deficient Mice Display Chloride-losing Diarrhea, Enhanced Colonic Proliferation, and Distinct Up-regulation of Ion Transporters in the Colon. J. Biol. Chem. 2006, 281, 37962–37971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alper, S.L.; Sharma, A.K. The SLC26 gene family of anion transporters and channels. Mol. Asp. Med. 2013, 34, 494–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coldwell, J.R.; Phillis, B.D.; Sutherland, K.; Howarth, G.S.; Blackshaw, L.A. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J. Physiol. 2007, 579, 203–213. [Google Scholar] [CrossRef]
- Menozzi, A.; Pozzoli, C.; Poli, E.; Lazzaretti, M.; Grandi, D.; Coruzzi, G. Long-term study of TNBS-induced colitis in rats: Focus on mast cells. Inflamm. Res. 2006, 55, 416–422. [Google Scholar] [CrossRef]
- Xu, X.; Weksler-Zangen, S.; Pikarsky, A.; Pappo, O.; Wengrower, D.; Bischoff, S.C.; Pines, M.; Rivkind, A.; Goldin, E.; Levi-Schaffer, F. Mast cells involvement in the inflammation and fibrosis development of the TNBS-induced rat model of colitis. Scand. J. Gastroenterol. 2002, 37, 330–337. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Kasai, H.; Takahashi, H.; Sugiyama, H.; Hase, N.; Kaneko, H.; Hamamura, I.; Aoki, Y.; Ota, M.; Kobayashi, T.; et al. The role of mast cells in the development of 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis in rats. Scand. J. Gastroenterol. 2002, 37, 555–560. [Google Scholar] [CrossRef]
- Chichlowski, M.; Westwood, G.S.; Abraham, S.N.; Hale, L.P. Role of Mast Cells in Inflammatory Bowel Disease and Inflammation-Associated Colorectal Neoplasia in IL-10-Deficient Mice. PLoS ONE 2010, 5, e12220. [Google Scholar] [CrossRef]
- Barbara, G.; Stanghellini, V.; de Giorgio, R.; Corinaldesi, R. Functional gastrointestinal disorders and mast cells: Implications for therapy. Neurogastroenterol. Motil. 2005, 18, 6–17. [Google Scholar] [CrossRef]
- Sasaki, Y.; Tanaka, M.; Kudo, H. Differentiation between ulcerative colitis and Crohn’s disease by a quantitative immunohistochemical evaluation of T lymphocytes, neutrophils, histiocytes and mast cells. Pathol. Int. 2002, 52, 277–285. [Google Scholar] [CrossRef]
- Eliakim, R.; Karmeli, F.; Okon, E.; Rachmilewitz, D. Ketotifen effectively prevents mucosal damage in experimental colitis. Gut 1992, 33, 1498–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, P.; McGarity, B.; Walls, A.F.; Church, M.K.; Millward-Sadler, G.H.; Robertson, D.A.F. Corticosteroid treatment reduces mast cell numbers in inflammatory bowel disease. Dig. Dis. Sci. 1990, 35, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.C.; Moore, W.C.; Lichtenstein, L.M. Modulation of mediator release from human intestinal mast cells by sulfasalazine and 5-aminosalicylic acid. Dig. Dis. Sci. 1991, 36, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.K.; Irvine, E.J. Ketotifen Treatment of Active Colitis in Patients with 5-Aminosalicylate Intolerance. Can. J. Gastroenterol. 1998, 12, 273–275. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.L.; Roifman, C.M.; Griffiths, A.M.; Sherman, P. Ketotifen therapy for acute ulcerative colitis in children: A pilot study. Dig. Dis. Sci. 1998, 43, 609–615. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Afroz, S.; Arthur, S.; Sundaram, U. Mast Cell Mediated Regulation of Small Intestinal Chloride Malabsorption in SAMP1/YitFc Mouse Model of Spontaneous Chronic Ileitis. Cells 2021, 10, 697. https://doi.org/10.3390/cells10030697
Rahman MM, Afroz S, Arthur S, Sundaram U. Mast Cell Mediated Regulation of Small Intestinal Chloride Malabsorption in SAMP1/YitFc Mouse Model of Spontaneous Chronic Ileitis. Cells. 2021; 10(3):697. https://doi.org/10.3390/cells10030697
Chicago/Turabian StyleRahman, M Motiur, Sheuli Afroz, Subha Arthur, and Uma Sundaram. 2021. "Mast Cell Mediated Regulation of Small Intestinal Chloride Malabsorption in SAMP1/YitFc Mouse Model of Spontaneous Chronic Ileitis" Cells 10, no. 3: 697. https://doi.org/10.3390/cells10030697





