Cell Surface Profiling of Retinal Müller Glial Cells Reveals Association to Immune Pathways after LPS Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Stimulation of Cells and Biotinylation of Cell Surface Proteins
2.3. Preparation of Plasma Membrane Fraction and Protein Extraction from Affinity-Purified Plasma Membrane
2.4. Mass Spectrometric Analysis
2.5. Data Analysis
2.6. Data Processing
3. Results
3.1. Mass Spectrometric Analysis Reveals 507 Cell Surface Proteins in Human Müller Glia Cell Line MIO-M1 and 1425 in Primary RMG
3.2. Stimulation with LPS Results in Distinct Changes of RMG Surface Proteome
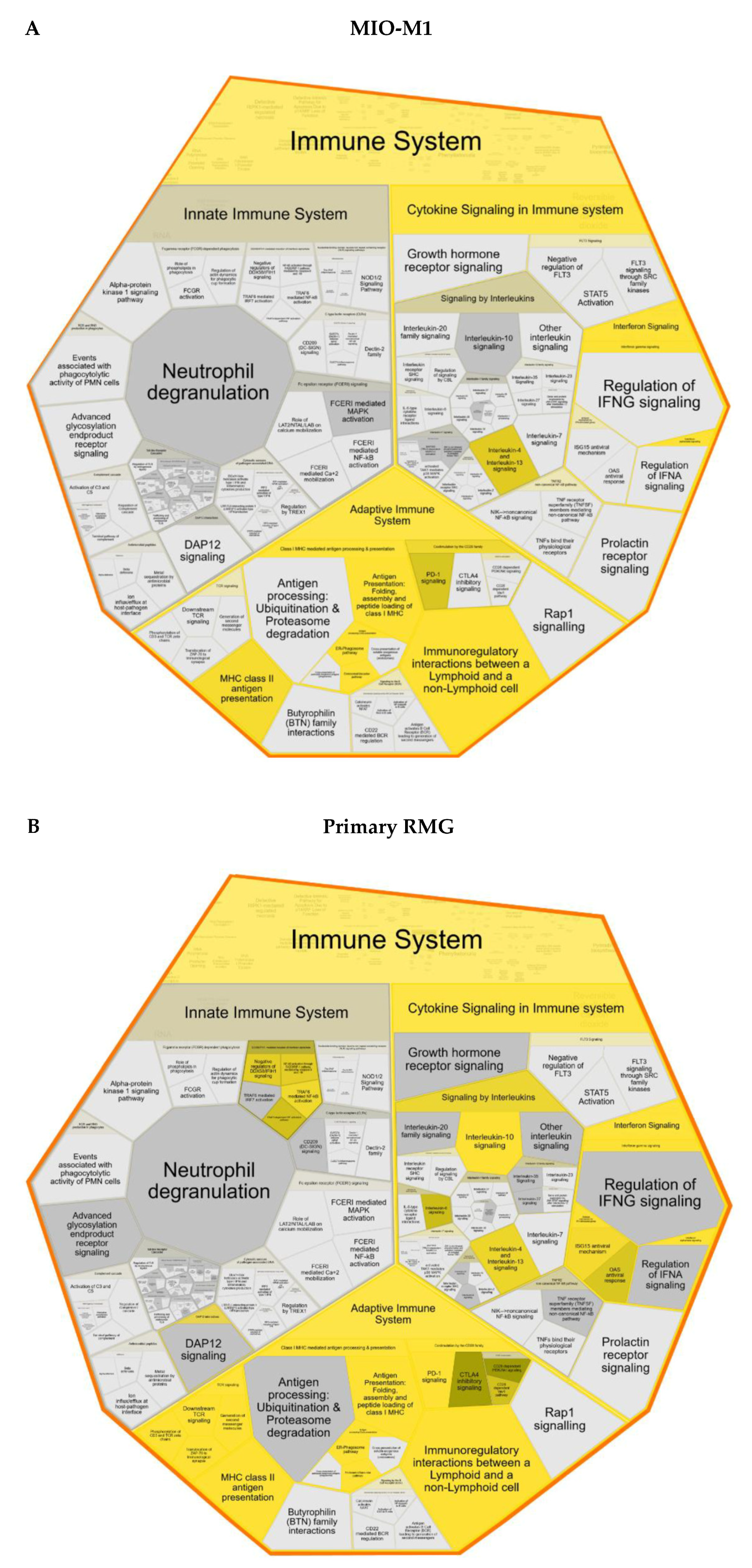
3.3. Proteins with Higher Abundance in Stimulated RMG Associated with Immune System Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Ghaseminejad, F.; Kaplan, L.; Pfaller, A.M.; Hauck, S.M.; Grosche, A. The role of Müller cell glucocorticoid signaling in diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Hoh, J.; Liu, J. Müller cells in pathological retinal angiogenesis. Transl. Res. 2019, 207, 96–106. [Google Scholar] [CrossRef]
- Devoldere, J.; Peynshaert, K.; De Smedt, S.C.; Remaut, K. Müller cells as a target for retinal therapy. Drug Discov. Today 2019, 24, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Müller, H. Zur histologie der netzhaut. Z. Wiss. Zool. 1851, 3, 234–237. [Google Scholar]
- Newman, E.; Reichenbach, A. The Müller cell: A functional element of the retina. Trends Neurosci. 1996, 19, 307–312. [Google Scholar] [CrossRef]
- Bringmann, A.; Reichenbach, A.; Wiedemann, P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004, 36, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tout, S.; Chan-Ling, T.; Holländer, H.; Stone, J. The role of Müller cells in the formation of the blood-retinal barrier. Neuroscience 1993, 55, 291–301. [Google Scholar] [CrossRef]
- Matsui, K.; Hosoi, N.; Tachibana, M. Active role of glutamate uptake in the synaptic transmission from retinal nonspiking neurons. J. Neurosci. 1999, 19, 6755–6766. [Google Scholar] [CrossRef]
- Poitry-Yamate, C.L.; Poitry, S.; Tsacopoulos, M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J. Neurosci. 1995, 15, 5179–5191. [Google Scholar] [CrossRef]
- Tsacopoulos, M.; Magistretti, P.J. Metabolic coupling between glia and neurons. J. Neurosci. 1996, 16, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular signaling and factors involved in Müller cell gliosis: Neuroprotective and detrimental effects. Prog. Retin Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef]
- Liberto, C.M.; Albrecht, P.J.; Herx, L.M.; Yong, V.W.; Levison, S.W. Pro-regenerative properties of cytokine-activated astrocytes. J. Neurochem. 2004, 89, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Roberge, F.G.; Caspi, R.R.; Nussenblatt, R.B. Glial retinal Müller cells produce IL-1 activity and have a dual effect on autoimmune T helper lymphocytes. Antigen presentation manifested after removal of suppressive activity. J. Immunol. 1988, 140, 2193–2196. [Google Scholar] [PubMed]
- Eastlake, K.; Banerjee, P.J.; Angbohang, A.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia 2016, 64, 495–506. [Google Scholar] [CrossRef]
- Natoli, R.; Fernando, N.; Madigan, M.; Chu-Tan, J.A.; Valter, K.; Provis, J.; Rutar, M. Microglia-derived IL-1β promotes chemokine expression by Müller cells and RPE in focal retinal degeneration. Mol. Neurodegener. 2017, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Chan, C.C.; Belfort, R., Jr.; Farah, M.; Burnier, M.P.; Nussenblatt, R.B.; Kuwabara, T.; Palestine, A.G. Histopathologic and immunohistopathologic features of subretinal fibrosis and uveitis syndrome. Am. J. Ophthalmol. 1987, 104, 15–23. [Google Scholar] [CrossRef]
- Hauck, S.M.; Schoeffmann, S.; Amann, B.; Stangassinger, M.; Gerhards, H.; Ueffing, M.; Deeg, C.A. Retinal Mueller glial cells trigger the hallmark inflammatory process in autoimmune uveitis. J. Proteome Res. 2007, 6, 2121–2131. [Google Scholar] [CrossRef]
- Eberhardt, C.; Amann, B.; Feuchtinger, A.; Hauck, S.M.; Deeg, C.A. Differential expression of inwardly rectifying K+ channels and aquaporins 4 and 5 in autoimmune uveitis indicates misbalance in Müller glial cell-dependent ion and water homeostasis. Glia 2011, 59, 697–707. [Google Scholar] [CrossRef]
- Deeg, C.A.; Amann, B.; Lutz, K.; Hirmer, S.; Lutterberg, K.; Kremmer, E.; Hauck, S.M. Aquaporin 11, a regulator of water efflux at retinal Muller glial cell surface decreases concomitant with immune-mediated gliosis. J. Neuroinflam. 2016, 13, 89. [Google Scholar] [CrossRef]
- Lorenz, L.; Amann, B.; Hirmer, S.; Degroote, R.L.; Hauck, S.M.; Deeg, C.A. NEU1 is more abundant in uveitic retina with concomitant desialylation of retinal cells. Glycobiology 2021. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef] [PubMed]
- Gaud, G.; Lesourne, R.; Love, P.E. Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 2018, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Yates, J.R. The application of mass spectrometry to membrane proteomics. Nat. Biotechnol. 2003, 21, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, B.; Matta, C.; Fellows, C.R.; Boocock, D.J.; Smith, J.R.; Liddell, S.; Lafeber, F.; van Spil, W.E.; Mobasheri, A. Alterations in the chondrocyte surfaceome in response to pro-inflammatory cytokines. BMC Mol. Cell Biol. 2020, 21, 47. [Google Scholar] [CrossRef]
- Uhl, P.B.; Szober, C.M.; Amann, B.; Alge-Priglinger, C.; Ueffing, M.; Hauck, S.M.; Deeg, C.A. In situ cell surface proteomics reveals differentially expressed membrane proteins in retinal pigment epithelial cells during autoimmune uveitis. J. Proteom. 2014, 109, 50–62. [Google Scholar] [CrossRef]
- Limb, G.A.; Salt, T.E.; Munro, P.M.; Moss, S.E.; Khaw, P.T. In vitro characterization of a spontaneously immortalized human Müller cell line (MIO-M1). Invest. Ophthalmol. Vis. Sci. 2002, 43, 864–869. [Google Scholar] [PubMed]
- Eberhardt, C.; Amann, B.; Stangassinger, M.; Hauck, S.M.; Deeg, C.A. Isolation, characterization and establishment of an equine retinal glial cell line: A prerequisite to investigate the physiological function of Muller cells in the retina. J. Anim. Physiol. Anim. Nutr. (Berl.) 2012, 96, 260–269. [Google Scholar] [CrossRef]
- Hauck, S.M.; Suppmann, S.; Ueffing, M. Proteomic profiling of primary retinal Müller glia cells reveals a shift in expression patterns upon adaptation to in vitro conditions. Glia 2003, 44, 251–263. [Google Scholar] [CrossRef]
- Hauck, S.M.; Hofmaier, F.; Dietter, J.; Swadzba, M.E.; Blindert, M.; Amann, B.; Behler, J.; Kremmer, E.; Ueffing, M.; Deeg, C.A. Label-free LC-MSMS analysis of vitreous from autoimmune uveitis reveals a significant decrease in secreted Wnt signalling inhibitors DKK3 and SFRP2. J. Proteom. 2012, 75, 4545–4554. [Google Scholar] [CrossRef]
- Hauck, S.M.; Dietter, J.; Kramer, R.L.; Hofmaier, F.; Zipplies, J.K.; Amann, B.; Feuchtinger, A.; Deeg, C.A.; Ueffing, M. Deciphering Membrane-Associated Molecular Processes in Target Tissue of Autoimmune Uveitis by Label-Free Quantitative Mass Spectrometry. Mol. Cell Proteom. 2010, 9, 2292–2305. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, A.; Wang, J.; Markand, S.; Perry, R.L.; Tawfik, A.; Zorrilla, E.; Ganapathy, V.; Smith, S.B. Sigma receptor 1 activation attenuates release of inflammatory cytokines MIP1γ, MIP2, MIP3α, and IL12 (p40/p70) by retinal Müller glial cells. J. Neurochem. 2015, 132, 546–558. [Google Scholar] [CrossRef]
- Iwami, K.-I.; Matsuguchi, T.; Masuda, A.; Kikuchi, T.; Musikacharoen, T.; Yoshikai, Y. Cutting Edge: Naturally Occurring Soluble Form of Mouse Toll-Like Receptor 4 Inhibits Lipopolysaccharide Signaling. J. Immunol. 2000, 165, 6682–6686. [Google Scholar] [CrossRef]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Pacholewska, A.; Marti, E.; Leeb, T.; Jagannathan, V.; Gerber, V. LPS-induced modules of co-expressed genes in equine peripheral blood mononuclear cells. BMC Genom. 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Chew, W.L.; Tabebordbar, M.; Cheng, J.K.W.; Mali, P.; Wu, E.Y.; Ng, A.H.M.; Zhu, K.; Wagers, A.J.; Church, G.M. A multifunctional AAV–CRISPR–Cas9 and its host response. Nat. Methods 2016, 13, 868–874. [Google Scholar] [CrossRef]
- Perng, Y.-C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Malakhova, O.; Malakhov, M.; Hetherington, C.; Zhang, D.E. Lipopolysaccharide activates the expression of ISG15-specific protease UBP43 via interferon regulatory factor 3. J. Biol. Chem. 2002, 277, 14703–14711. [Google Scholar] [CrossRef]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmueller, U.; Mann, M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol. Cell Proteom. 2009, 8, 443–450. [Google Scholar] [CrossRef]
- Hollborn, M.; Ulbricht, E.; Rillich, K.; Dukic-Stefanovic, S.; Wurm, A.; Wagner, L.; Reichenbach, A.; Wiedemann, P.; Limb, G.A.; Bringmann, A.; et al. The human Müller cell line MIO-M1 expresses opsins. Mol. Vis. 2011, 17, 2738–2750. [Google Scholar] [PubMed]
- Lawrence, J.M.; Singhal, S.; Bhatia, B.; Keegan, D.J.; Reh, T.A.; Luthert, P.J.; Khaw, P.T.; Limb, G.A. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells 2007, 25, 2033–2043. [Google Scholar] [CrossRef]
- Pereiro, X.; Ruzafa, N.; Acera, A.; Urcola, A.; Vecino, E. Optimization of a Method to Isolate and Culture Adult Porcine, Rats and Mice Müller Glia in Order to Study Retinal Diseases. Front. Cell Neurosci 2020, 14, 7. [Google Scholar] [CrossRef]
- Deeg, C.A.; Eberhardt, C.; Hofmaier, F.; Amann, B.; Hauck, S.M. Osteopontin and fibronectin levels are decreased in vitreous of autoimmune uveitis and retinal expression of both proteins indicates ECM re-modeling. PLoS ONE 2011, 6, e27674. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schnitzer, J. Astrocytes in the guinea pig, horse, and monkey retina: Their occurrence coincides with the presence of blood vessels. Glia 1988, 1, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Ehrenhofer, M.C.A.; Deeg, C.A.; Reese, S.; Liebich, H.-G.; Stangassinger, M.; Kaspers, B. Normal structure and age-related changes of the equine retina. Vet. Ophthalmol. 2002, 5, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Wurm, A.; Pannicke, T.; Iandiev, I.; Wiedemann, P.; Bringmann, A. Müller cells as players in retinal degeneration and edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 627–636. [Google Scholar] [CrossRef]
- Blees, A.; Januliene, D.; Hofmann, T.; Koller, N.; Schmidt, C.; Trowitzsch, S.; Moeller, A.; Tampé, R. Structure of the human MHC-I peptide-loading complex. Nature 2017, 551, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.C.; Meyer, C.; Bhatia, U.; Yshii, L.; Kleffner, I.; Bauer, J.; Tröscher, A.R.; Schulte-Mecklenbeck, A.; Herich, S.; Schneider-Hohendorf, T.; et al. CD8+ T cell-mediated endotheliopathy is a targetable mechanism of neuro-inflammation in Susac syndrome. Nat. Commun. 2019, 10, 5779. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.S.; Li, E.; Scharnagl, L.; Poupardin, R.; Altendorfer, B.; Mrowetz, H.; Hutter-Paier, B.; Weiger, T.M.; Heneka, M.T.; Attems, J.; et al. CD8(+) T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav. Immun. 2020, 89, 67–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, W.; Liu, J.; Yuan, X.; Mao, W.; Yin, J.; Peng, B.; Liu, W.; Han, S.; He, X. Blockade of Kv1.3 potassium channel inhibits CD8(+) T cell-mediated neuroinflammation via PD-1/Blimp-1 signaling. FASEB J. 2020, 34, 15492–15503. [Google Scholar] [CrossRef] [PubMed]
- Guerder, S.; Flavell, R.A. T-cell activation. Two for T. Curr. Biol. 1995, 5, 866–868. [Google Scholar] [CrossRef]
- Lipski, D.A.; Dewispelaere, R.; Foucart, V.; Caspers, L.E.; Defrance, M.; Bruyns, C.; Willermain, F. MHC class II expression and potential antigen-presenting cells in the retina during experimental autoimmune uveitis. J. Neuroinflam. 2017, 14, 136. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, G.S.; Ishimoto, S.; Pararajasegaram, G.; Rao, N.A. Expression of major histocompatibility complex molecules in rodent retina. Immunohistochemical study. Invest. Ophthalmol. Vis. Sci. 1997, 38, 1848–1857. [Google Scholar] [PubMed]
- Romeike, A.; Brügmann, M.; Drommer, W. Immunohistochemical studies in equine recurrent uveitis (ERU). Vet. Pathol. 1998, 35, 515–526. [Google Scholar] [CrossRef]
- Aicher, A.; Hayden-Ledbetter, M.; Brady, W.A.; Pezzutto, A.; Richter, G.; Magaletti, D.; Buckwalter, S.; Ledbetter, J.A.; Clark, E.A. Characterization of human inducible costimulator ligand expression and function. J. Immunol. 2000, 164, 4689–4696. [Google Scholar] [CrossRef]
- Usui, Y.; Akiba, H.; Takeuchi, M.; Kezuka, T.; Takeuchi, A.; Hattori, T.; Okunuki, Y.; Yamazaki, T.; Yagita, H.; Usui, M.; et al. The role of the ICOS/B7RP-1 T cell costimulatory pathway in murine experimental autoimmune uveoretinitis. Eur. J. Immunol. 2006, 36, 3071–3081. [Google Scholar] [CrossRef]
- Shibagaki, N.; Hanada, K.; Yamaguchi, S.; Yamashita, H.; Shimada, S.; Hamada, H. Functional analysis of CD82 in the early phase of T cell activation: Roles in cell adhesion and signal transduction. Eur. J. Immunol. 1998, 28, 1125–1133. [Google Scholar] [CrossRef]
- Shibagaki, N.; Hanada, K.; Yamashita, H.; Shimada, S.; Hamada, H. Overexpression of CD82 on human T cells enhances LFA-1/ICAM-1-mediated cell-cell adhesion: Functional association between CD82 and LFA-1 in T cell activation. Eur. J. Immunol. 1999, 29, 4081–4091. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Karnell, J.L.; Rieder, S.A.; Ettinger, R.; Kolbeck, R. Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Adv. Drug Deliv. Rev. 2019, 141, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Kelsall, B.L.; Stüber, E.; Neurath, M.; Strober, W. Interleukin-12 Production by Dendritic Cells. Ann. N. Y. Acad. Sci. 1996, 795, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Portillo, J.A.; Okenka, G.; Kern, T.S.; Subauste, C.S. Identification of primary retinal cells and ex vivo detection of proinflammatory molecules using flow cytometry. Mol. Vis. 2009, 15, 1383–1389. [Google Scholar]
- Portillo, J.A.; Greene, J.A.; Okenka, G.; Miao, Y.; Sheibani, N.; Kern, T.S.; Subauste, C.S. CD40 promotes the development of early diabetic retinopathy in mice. Diabetologia 2014, 57, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Nerrière-Daguin, V.; Vanhove, B.; Naveilhan, P.; Neunlist, M.; Nicot, A.; Boudin, H. Targeting the CD80/CD86 costimulatory pathway with CTLA4-Ig directs microglia toward a repair phenotype and promotes axonal outgrowth. Glia 2015, 63, 2298–2312. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Sham, C.W.; Chan, A.M.; Kwong, J.M.K.; Caprioli, J.; Nusinowitz, S.; Chen, B.; Lee, J.G.; Gandhi, N.M.; Francisco, L.M.; Sharpe, A.H.; et al. Neuronal Programmed Cell Death-1 Ligand Expression Regulates Retinal Ganglion Cell Number in Neonatal and Adult Mice. J. Neuroophthalmol. 2012, 32, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, H.; Chen, P.W.; Alizadeh, H.; He, Y.; Hogan, R.N.; Niederkorn, J.Y. PD-L1 Expression on Human Ocular Cells and Its Possible Role in Regulating Immune-Mediated Ocular Inflammation. Invest. Ophthalmol. Vis. Sci. 2009, 50, 273–280. [Google Scholar] [CrossRef]
- Drescher, K.M.; Whittum-Hudson, J.A. Modulation of immune-associated surface markers and cytokine production by murine retinal glial cells. J. Neuroimmunol. 1996, 64, 71–81. [Google Scholar] [CrossRef]
- Gu, R.; Ding, X.; Tang, W.; Lei, B.; Jiang, C.; Xu, G. A Synthesized Glucocorticoid- Induced Leucine Zipper Peptide Inhibits Retinal Müller Cell Gliosis. Front. Pharm. 2018, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. VASC Biol. 2020, 2, H1–h18. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.S.; Schewitz-Bowers, L.P.; Wei, L.; Lee, R.W.J.; Smith, J.R. Intercellular Adhesion Molecule 1 Mediates Migration of Th1 and Th17 Cells Across Human Retinal Vascular Endothelium. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6917–6925. [Google Scholar] [CrossRef]
- Whitcup, S.M.; Chan, C.-C.; Li, Q.; Nussenblatt, R.B. Expression of Cell Adhesion Molecules in Posterior Uveitis. Arch. Ophthalmol. 1992, 110, 662–666. [Google Scholar] [CrossRef]
- Dewispelaere, R.; Lipski, D.; Foucart, V.; Bruyns, C.; Frère, A.; Caspers, L.; Willermain, F. ICAM-1 and VCAM-1 are differentially expressed on blood-retinal barrier cells during experimental autoimmune uveitis. Exp. Eye Res. 2015, 137, 94–102. [Google Scholar] [CrossRef]
- Richardson, P.R.; Boulton, M.E.; Duvall-Young, J.; McLeod, D. Immunocytochemical study of retinal diode laser photocoagulation in the rat. Br. J. Ophthalmol. 1996, 80, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Fraemohs, L.; Dejana, E. The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. Immunol. 2007, 7, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Martin-Blondel, G.; Pignolet, B.; Tietz, S.; Yshii, L.; Gebauer, C.; Perinat, T.; Van Weddingen, I.; Blatti, C.; Engelhardt, B.; Liblau, R. Migration of encephalitogenic CD8 T cells into the central nervous system is dependent on the α4β1-integrin. Eur. J. Immunol. 2015, 45, 3302–3312. [Google Scholar] [CrossRef] [PubMed]
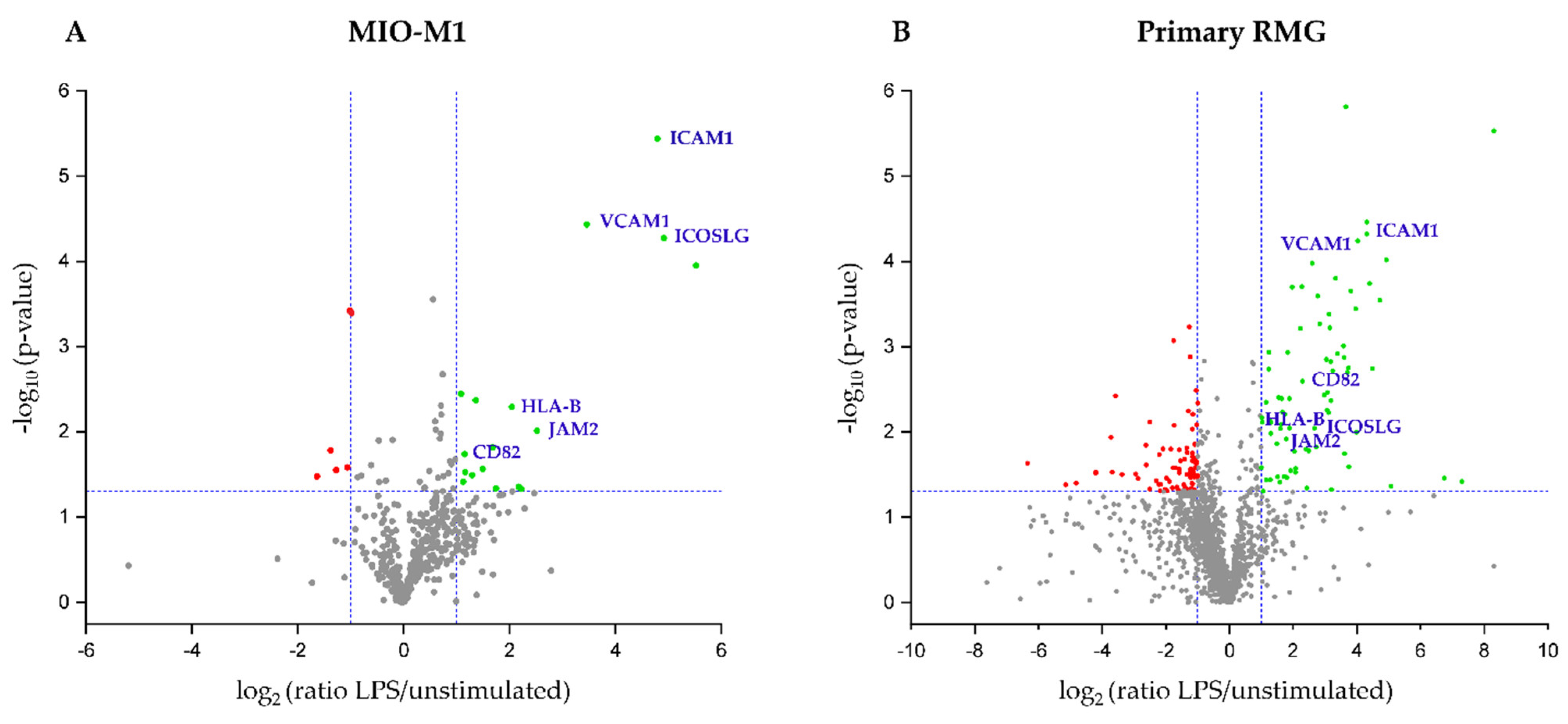

| Protein ID | Description | Gene Name | Peptides Used for Quantification | p-Value | Ratio LPS/Unstim |
|---|---|---|---|---|---|
| (1) MIO-M1 | |||||
| ENSP00000006053 | C-X3-C motif chemokine ligand 1 | CX3CL1 | 4 | 0 | 46 |
| ENSP00000339477 | Inducible T cell costimulator ligand | ICOSLG | 9 | 0 | 30.1 |
| ENSP00000264832 | Intercellular adhesion molecule 1 | ICAM1 | 29 | 0 | 27.7 |
| ENSP00000294728 | Vascular cell adhesion molecule 1 | VCAM1 | 47 | 0 | 11 |
| ENSP00000318416 | Junctional adhesion molecule 2 | JAM2 | 1 | 0.01 | 5.7 |
| ENSP00000166534 | Prolyl 4-hydroxylase subunit alpha 2 | P4HA2 | 2 | 0.047 | 4.7 |
| ENSP00000412429 | Transgelin 2 | TAGLN2 | 2 | 0.045 | 4.5 |
| ENSP00000399168 | Major histocompatibility complex, class I, B | HLA-B | 2 | 0.005 | 4.1 |
| ENSP00000304592 | Fatty acid synthase | FASN | 1 | 0.047 | 3.4 |
| ENSP00000336799 | Tubulin alpha 1b | TUBA1B | 2 | 0.015 | 3.2 |
| ENSP00000341289 | Tubulin beta 4B class IVb | TUBB4B | 2 | 0.028 | 2.8 |
| ENSP00000240095 | Solute carrier family 39 member 14 | SLC39A14 | 13 | 0.004 | 2.6 |
| ENSP00000330054 | Eukaryotic translation elongation factor 1 alpha 1 | EEF1A1 | 3 | 0.033 | 2.4 |
| ENSP00000307046 | Syndecan 2 | SDC2 | 3 | 0.03 | 2.2 |
| ENSP00000227155 | CD82 molecule | CD82 | 8 | 0.018 | 2.2 |
| ENSP00000245185 | Metallothionein 2A | MT2A | 1 | 0.039 | 2.2 |
| ENSP00000319782 | Podocalyxin like | PODXL | 10 | 0.004 | 2.1 |
| ENSP00000380855 | Programmed cell death 1 ligand 2 | PDCD1LG2 | 2 | 0.021 | 2 |
| (2) Primary RMG | |||||
| ENSECAP00000013197 | TAP binding protein | TAPBP | 1 | 0 | infinity |
| ENSECAP00000010634 | SLAM family member 7 | SLAMF7 | 1 | 0.039 | 158.6 |
| ENSECAP00000009810 | 5,-nucleotidase, cytosolic IIIA | NT5C3A | 1 | 0.035 | 107.7 |
| ENSECAP00000009600 | Transmembrane protein 33 | TMEM33 | 1 | 0.044 | 33.8 |
| ENSECAP00000018879 | Neuregulin 1 | NRG1 | 2 | 0 | 30.5 |
| ENSECAP00000014003 | CD274 molecule | CD274 | 2 | 0 | 26.4 |
| ENSECAP00000019916 | Betacellulin | BTC | 1 | 0.002 | 22.4 |
| ENSECAP00000000924 | ISG15 ubiquitin like modifier | ISG15 | 7 | 0 | 21.1 |
| ENSECAP00000011996 | Intercellular adhesion molecule 1 | ICAM1 | 25 | 0 | 19.9 |
| ENSECAP00000009944 | CD86 molecule | CD86 | 7 | 0 | 19.9 |
| ENSECAP00000014290 | Vascular cell adhesion molecule 1 | VCAM1 | 51 | 0 | 16.3 |
| ENSECAP00000004905 | Doublesex- and mab-3-related transcription factor C2 | DMRTC2 | 1 | 0.01 | 15.9 |
| ENSECAP00000017760 | Neuromedin U receptor 2 | NMUR2 | 2 | 0 | 15.7 |
| ENSECAP00000011171 | 2,-5,-oligoadenylate synthetase 1 | OAS1 | 2 | 0 | 14 |
| ENSECAP00000000811 | Beta-2-microglobulin | B2M | 1 | 0.026 | 13.4 |
| ENSECAP00000010646 | MX dynamin like GTPase 2 | MX1 | 8 | 0.002 | 13.4 |
| ENSECAP00000021590 | MHC class I heavy chain | HLA-A | 3 | 0.002 | 13.1 |
| ENSECAP00000018982 | Guanylate binding protein 5 | GBP5 | 10 | 0 | 12.6 |
| ENSECAP00000009324 | Serum amyloid A1 | SAA1 | 3 | 0.018 | 12.2 |
| ENSECAP00000020078 | MHC class I heavy chain | HLA-A | 11 | 0.001 | 12.2 |
| ENSECAP00000006405 | Interferon induced with helicase C domain 1 | IFIH1 | 22 | 0.001 | 12 |
| ENSECAP00000020119 | Very low-density lipoprotein receptor | VLDLR | 1 | 0.001 | 10.5 |
| ENSECAP00000003048 | Interferon-induced protein with tetratricopeptide repeats 1 | IFIT1 | 6 | 0 | 10 |
| ENSECAP00000009264 | MHC class I heavy chain | HLA-A | 7 | 0.002 | 9.5 |
| ENSECAP00000008591 | ATP binding cassette subfamily A member 1 | ABCA1 | 1 | 0.048 | 9.2 |
| ENSECAP00000015028 | Carbonic anhydrase 12 | CA12 | 3 | 0.004 | 9.1 |
| ENSECAP00000016719 | Pleckstrin | PLEK | 3 | 0.002 | 9 |
| ENSECAP00000019669 | DExD/H-box helicase 58 | DDX58 | 18 | 0.001 | 8.9 |
| ENSECAP00000002356 | MHC class I heavy chain | HLA-A | 1 | 0 | 8.7 |
| ENSECAP00000006671 | Semaphorin 4A | SEMA4A | 2 | 0.006 | 8.7 |
| ENSECAP00000022282 | Intercellular adhesion molecule 3 | ICAM3 | 3 | 0.003 | 8.5 |
| ENSECAP00000017347 | Versican | VCAN | 4 | 0.006 | 8.4 |
| ENSECAP00000014907 | CD40 molecule | CD40 | 8 | 0.001 | 8.2 |
| ENSECAP00000000620 | Galectin 3 binding protein | LGALS3BP | 2 | 0.004 | 7.9 |
| ENSECAP00000007529 | DnaJ heat shock protein family (Hsp40) member C3 | DNAJC3 | 1 | 0.001 | 7.2 |
| ENSECAP00000012605 | HIRA interacting protein 3 | HIRIP3 | 1 | 0 | 6.8 |
| ENSECAP00000010541 | MHC class II DR alpha chain | HLA-DRA | 2 | 0.015 | 6.6 |
| ENSECAP00000006847 | Inducible T cell costimulator ligand | ICOSLG | 4 | 0.009 | 6.4 |
| ENSECAP00000008012 | Interferon induced protein with tetratricopeptide repeats 3 | IFIT3 | 3 | 0 | 6.1 |
| ENSECAP00000017943 | Syndecan 4 | SDC4 | 4 | 0.017 | 5.6 |
| ENSECAP00000017562 | CXADR like membrane protein | CLMP | 3 | 0.046 | 5.4 |
| ENSECAP00000003412 | Major prion protein | PRNP | 2 | 0.016 | 5.3 |
| ENSECAP00000017893 | CD82 molecule | CD82 | 7 | 0.003 | 4.9 |
| ENSECAP00000015940 | C-X-C motif chemokine ligand 16 | CXCL16 | 1 | 0 | 4.8 |
| ENSECAP00000019909 | MHC class II DR-beta chain | HLA-DRB1 | 1 | 0.001 | 4.7 |
| ENSECAP00000018161 | MHC class I heavy chain | HLA-C | 2 | 0.03 | 4.2 |
| ENSECAP00000006146 | Praja ring finger ubiquitin ligase 2 | PJA2 | 2 | 0.027 | 4.2 |
| ENSECAP00000010650 | DnaJ heat shock protein family (Hsp40) member C8 | DNAJC8 | 1 | 0.017 | 4.1 |
| ENSECAP00000001275 | Carcinoembryonic antigen-related cell adhesion molecule 1 | CEACAM21 | 9 | 0 | 3.9 |
| ENSECAP00000015833 | Lumican | LUM | 1 | 0.029 | 3.8 |
| ENSECAP00000018257 | CD38 molecule | CD38 | 2 | 0.009 | 3.7 |
| ENSECAP00000017093 | Versican | VCAN | 5 | 0.004 | 3.7 |
| ENSECAP00000002944 | Interferon induced protein with tetratricopeptide repeats 5 | IFIT5 | 7 | 0.001 | 3.6 |
| ENSECAP00000016198 | Folate receptor beta | FOLR2 | 1 | 0.034 | 3.5 |
| ENSECAP00000012733 | Junctional adhesion molecule 2 | JAM2 | 2 | 0.012 | 3.4 |
| ENSECAP00000011113 | Guanylate-binding protein 6 | GBP6 | 9 | 0.006 | 3.3 |
| ENSECAP00000007853 | Signal transducer and activator of transcription 1 | STAT1 | 14 | 0.034 | 3.3 |
| ENSECAP00000014392 | Endothelin converting enzyme 1 | ECE1 | 17 | 0.006 | 3.2 |
| ENSECAP00000002864 | Vasorin | VASN | 16 | 0.004 | 3.1 |
| ENSECAP00000009001 | Integrin subunit alpha 4 | ITGA4 | 9 | 0.008 | 3.1 |
| ENSECAP00000015309 | Colony stimulating factor 1 | CSF1 | 5 | 0.009 | 3 |
| ENSECAP00000002058 | Sphingosine-1-phosphate receptor 3 | S1PR3 | 2 | 0.039 | 3 |
| ENSECAP00000001010 | Hydroxycarboxylic acid receptor 2 | HCAR2 | 2 | 0.004 | 2.9 |
| ENSECAP00000019459 | Mitochondria localized glutamic acid rich protein | MGARP | 1 | 0.034 | 2.9 |
| ENSECAP00000001529 | CD80 molecule | CD80 | 1 | 0.014 | 2.8 |
| ENSECAP00000017208 | Solute carrier family 1 member 3 | SLC1A3 | 12 | 0.008 | 2.5 |
| ENSECAP00000012239 | Solute carrier family 20 member 1 | SLC20A1 | 2 | 0.01 | 2.5 |
| ENSECAP00000019326 | Tissue factor pathway inhibitor | TFPI | 2 | 0.037 | 2.4 |
| ENSECAP00000019780 | MHC class I heavy chain | HLA-B | 7 | 0.007 | 2.4 |
| ENSECAP00000005675 | Plasminogen activator, urokinase | PLAU | 5 | 0.001 | 2.4 |
| ENSECAP00000000171 | Semaphorin 7A | SEMA7A | 1 | 0.002 | 2.4 |
| ENSECAP00000011356 | CD53 molecule | CD53 | 2 | 0.037 | 2.2 |
| ENSECAP00000012737 | LDL receptor related protein associated protein 1 | LRPAP1 | 3 | 0.005 | 2.2 |
| ENSECAP00000008846 | Epidermal growth factor receptor | EGFR | 22 | 0.05 | 2.1 |
| ENSECAP00000002079 | CD68 molecule | CD68 | 2 | 0.008 | 2 |
| ENSECAP00000006770 | Tetraspanin 6 | TSPAN6 | 2 | 0.007 | 2 |
| ENSECAP00000016635 | CD47 molecule | CD47 | 5 | 0.026 | 2 |
| ENSECAP00000019515 | Mannose-6-phosphate receptor, cation dependent | M6PR | 2 | 0.015 | 2 |
| ENSECAP00000004902 | Slit guidance ligand 3 | SLIT3 | 4 | 0.007 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenz, L.; Hirmer, S.; Schmalen, A.; Hauck, S.M.; Deeg, C.A. Cell Surface Profiling of Retinal Müller Glial Cells Reveals Association to Immune Pathways after LPS Stimulation. Cells 2021, 10, 711. https://doi.org/10.3390/cells10030711
Lorenz L, Hirmer S, Schmalen A, Hauck SM, Deeg CA. Cell Surface Profiling of Retinal Müller Glial Cells Reveals Association to Immune Pathways after LPS Stimulation. Cells. 2021; 10(3):711. https://doi.org/10.3390/cells10030711
Chicago/Turabian StyleLorenz, Lea, Sieglinde Hirmer, Adrian Schmalen, Stefanie M. Hauck, and Cornelia A. Deeg. 2021. "Cell Surface Profiling of Retinal Müller Glial Cells Reveals Association to Immune Pathways after LPS Stimulation" Cells 10, no. 3: 711. https://doi.org/10.3390/cells10030711
APA StyleLorenz, L., Hirmer, S., Schmalen, A., Hauck, S. M., & Deeg, C. A. (2021). Cell Surface Profiling of Retinal Müller Glial Cells Reveals Association to Immune Pathways after LPS Stimulation. Cells, 10(3), 711. https://doi.org/10.3390/cells10030711






