Sex Hormone Binding Globulin (SHBG) Mitigates ER Stress in Hepatocytes In Vitro and Ex Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Experimental Protocol
2.3. Staining of Intracellular Lipid
2.4. Visualization of ER
2.5. Immunofluorescence
2.6. Immunoblotting
2.7. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.8. Animal Qualification and Preparation of Liver Ex Vivo Specimens
2.9. ELISA Assays
2.10. Statistics
3. Results
3.1. The Effect of Lipid Overload on SHBG Levels
3.2. The Effects of SHBG on Lipid Metabolism
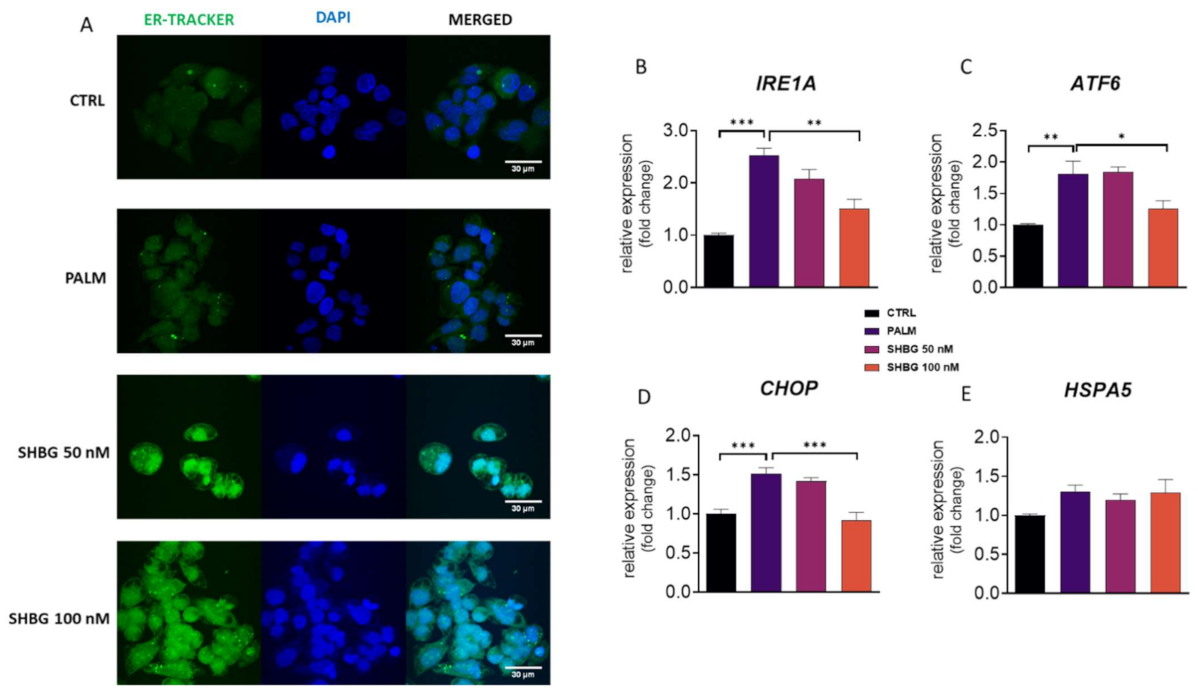
3.3. SHBG Protects against PA-Induced ER Stress
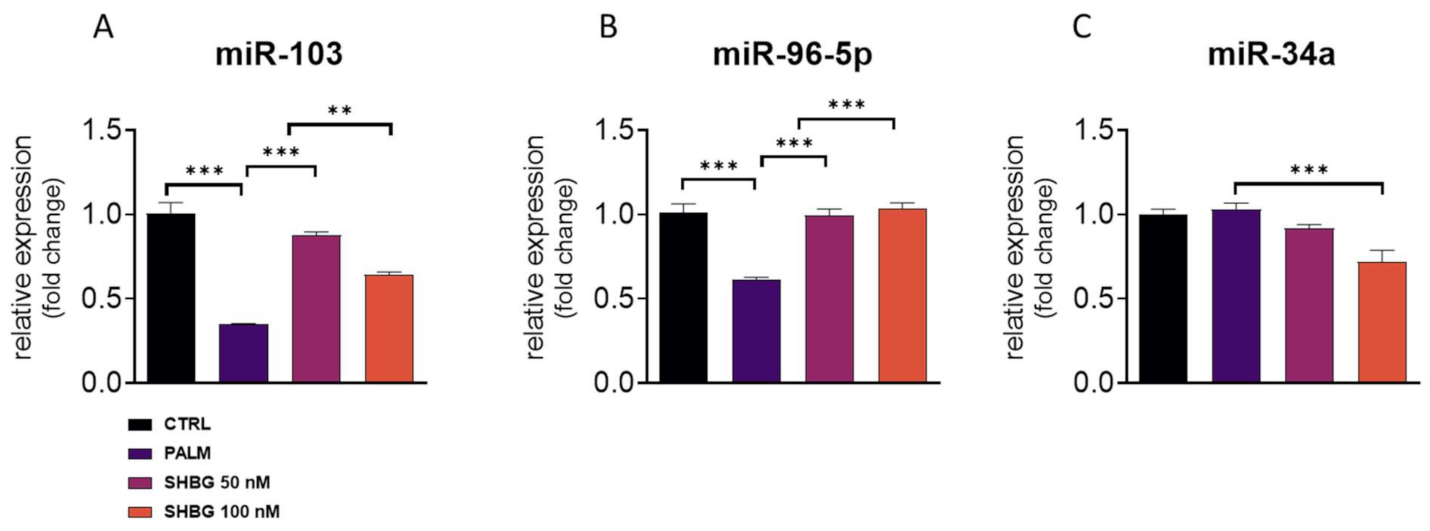
3.4. SHBG Pretreatment Affects miRNA Profile
3.5. Liver of MS Individuals Suffers from Lipotoxicicty, ER Stress, and Decreased SHBG Levels
3.6. The Effects of SHBG on Lipolytic Gene Expression Ex Vivo
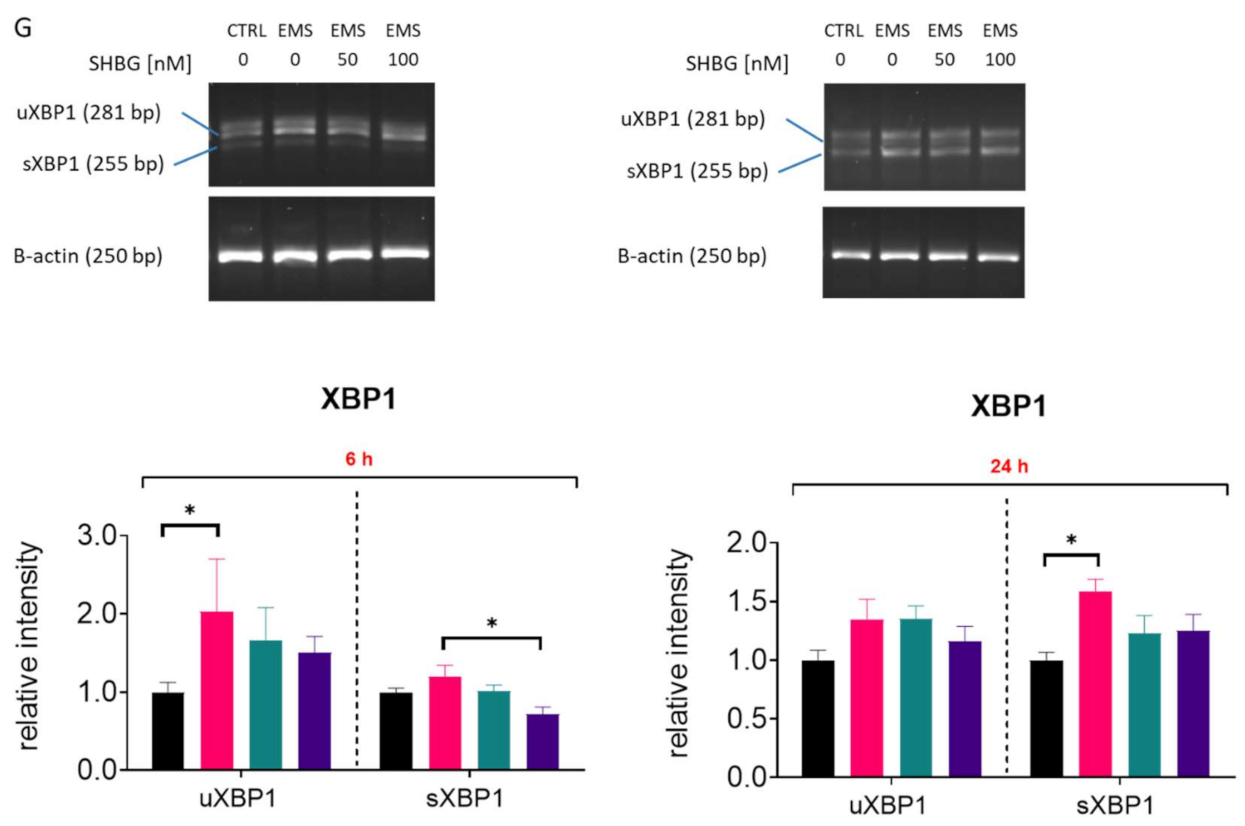
3.7. SHBG Reduces ER Stress in Liver Sections Ex Vivo
3.8. SHBG Modulates miRNA Expression in Liver Sections Ex Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Ethics Approval and Consent to Participate
Conflicts of Interest
References
- Ertelt, A.; Barton, A.-K.; Schmitz, R.R.; Gehlen, H. Metabolic syndrome: Is equine disease comparable to what we know in humans? Endocr. Connect. 2014, 3, R81–R93. [Google Scholar] [CrossRef] [Green Version]
- Mazidi, M.; Pennathur, S.; Afshinnia, F. Link of dietary patterns with metabolic syndrome: Analysis of the National Health and Nutrition Examination Survey. Nutr. Diabetes 2017, 7, e255. [Google Scholar] [CrossRef]
- Robin, C.A.; Ireland, J.L.; Wylie, C.E.; Collins, S.N.; Verheyen, K.L.P.; Newton, J.R. Prevalence of and risk factors for equine obesity in Great Britain based on owner-reported body condition scores. Equine Vet. J. 2015, 47, 196–201. [Google Scholar] [CrossRef]
- Stephenson, H.M.; Green, M.J.; Freeman, S.L.; Rivera, S.; Divers, S.J.; Knafo, S.E.; Martinez, P.; Cayot, L.J.; Tapia-Aguilera, W.; Flanagan, J. Prevalence of obesity in a population of horses in the UK. Vet. Rec. 2011, 168, 131. [Google Scholar] [CrossRef]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging. Oxidative Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Frank, N. Equine Metabolic Syndrome. J. Equine Vet. Sci. 2009, 29, 259–267. [Google Scholar] [CrossRef]
- Alicka, M.; Kornicka-Garbowska, K.; Roecken, M.; Marycz, K. Inhibition of the Low Molecular Weight Protein Tyrosine Phosphatase (LMPTP) as a Potential Therapeutic Strategy for Hepatic Progenitor Cells Lipotoxicity—Short Communication. Int. J. Mol. Sci. 2019, 20, 5873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alicka, M.; Marycz, K. The Effect of Chronic Inflammation and Oxidative and Endoplasmic Reticulum Stress in the Course of Metabolic Syndrome and Its Therapy. Stem Cells Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marycz, K.; Kornicka, K.; Szlapka-Kosarzewska, J.; Weiss, C. Excessive Endoplasmic Reticulum Stress Correlates with Impaired Mitochondrial Dynamics, Mitophagy and Apoptosis, in Liver and Adipose Tissue, but Not in Muscles in EMS Horses. Int. J. Mol. Sci. 2018, 19, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boppidi, H. Nonalcoholic Fatty Liver Disease: Hepatic Manifestation of Obesity and the Metabolic Syndrome. Postgrad. Med. 2008, 120, E01–E07. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Shu, L.; Song, G.; Ma, H. Resveratrol reduces liver endoplasmic reticulum stress and improves insulin sensitivity in vivo and in vitro. Drug Des. Dev. Ther. 2019, 13, 1473–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariyasu, D.; Yoshida, H.; Hasegawa, Y. Endoplasmic Reticulum (ER) Stress and Endocrine Disorders. Int. J. Mol. Sci. 2017, 18, 382. [Google Scholar] [CrossRef] [Green Version]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.H.; Wang, J.J.; Zhang, S.X. The Unfolded Protein Response and Diabetic Retinopathy. J. Diabetes Res. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Simó, R.; Sáez-López, C.; Barbosa-Desongles, A.; Hernández, C.; Selva, D.M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef]
- Hammond, G.L. Diverse Roles for Sex Hormone-Binding Globulin in Reproduction. Biol. Reprod. 2011, 85, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Pinós, T.; Barbosa-Desongles, A.; Hurtado, A.; Santamaria-Martínez, A.; De Torres, I.; Morote, J.; Reventós, J.; Munell, F. Identification, characterization and expression of novel Sex Hormone Binding Globulin alternative first exons in the human prostate. BMC Mol. Biol. 2009, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Hryb, D.J.; Nakhla, A.M.; Kahn, S.M.; George, J.S.; Levy, N.C.; Romas, N.A.; Rosner, W. Sex Hormone-binding Globulin in the Human Prostate Is Locally Synthesized and May Act as an Autocrine/Paracrine Effector. J. Biol. Chem. 2002, 277, 26618–26622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-M.; Bayliss, U.A.; Millhorn, D.E.; Petrusz, P.; Joseph, D.R. The Androgen-Binding Protein Gene Is Expressed in Male and Female Rat Brain*. Endocrinology 1990, 127, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Karpati, E.; Schneider, A.E.; Hetey, S.; Szilagyi, A.; Juhasz, K.; Laszlo, G.; Hupuczi, P.; Zavodszky, P.; Papp, Z.; et al. Sex hormone-binding globulin provides a novel entry pathway for estradiol and influences subsequent signaling in lymphocytes via membrane receptor. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forges, T.; Gerard, A.; Monnier-Barbarino, P.; Gérard, H. Immunolocalization of sex hormone-binding globulin (SHBG) in human ovarian follicles and corpus luteum. Histochem. Cell Biol. 2005, 124, 285–290. [Google Scholar] [CrossRef]
- Daka, B.; Rosen, T.; Jansson, P.A.; Råstam, L.; Larsson, C.A.; Lindblad, U. Inverse association between serum insulin and sex hormone-binding globulin in a population survey in Sweden. Endocr. Connect. 2013, 2, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Caglar, G.S.; Ozdemir, E.D.U.; Cengiz, S.D.; Demirtaş, S. Sex-hormone-binding globulin early in pregnancy for the prediction of severe gestational diabetes mellitus and related complications. J. Obstet Gynaecol. Res. 2012, 38, 1286–1293. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Manson, J.E.; Hunter, D.J.; Lee, C.C.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Sex Hormone–Binding Globulin and Risk of Type 2 Diabetes in Women and Men. N. Engl. J. Med. 2009, 361, 1152–1163. [Google Scholar] [CrossRef] [Green Version]
- Teng, W.; Yin, W.; Zhao, L.; Ma, C.; Huang, J.; Ren, F. Resveratrol metabolites ameliorate insulin resistance in HepG2 hepatocytes by modulating IRS-1/AMPK. RSC Adv. 2018, 8, 36034–36042. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, L.; Li, J.; Cheng, Y.; Chen, J.; Shen, M.; Zhang, S.; Wei, H. Insulin resistance contributes to multidrug resistance in HepG2 cells via activation of the PERK signaling pathway and upregulation of Bcl-2 and P-gp. Oncol. Rep. 2016, 35, 3018–3024. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Wei, D.; Mo, C.; Zhang, J.; Wang, X.; Han, X.; Wang, Z.; Xiao, H. Saturated fatty acid palmitate-induced insulin resistance is accompanied with myotube loss and the impaired expression of health benefit myokine genes in C2C12 myotubes. Lipids Health Dis. 2013, 12, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Ren, H.; Wang, J.; Gao, Y.; Yang, F.; Huang, W. Metabolic syndrome and liver-related events: A systematic review and meta-analysis. BMC Endocr. Disord. 2019, 19, 40. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6485158/ (accessed on 29 March 2021). [CrossRef] [PubMed]
- Marycz, K.; Szłapka-Kosarzewska, J.; Geburek, F.; Kornicka-Garbowska, K. Systemic Administration of Rejuvenated Adipose-Derived Mesenchymal Stem Cells Improves Liver Metabolism in Equine Metabolic Syndrome (EMS)- New Approach in Veterinary Regenerative Medicine. Stem Cell Rev. Rep. 2019, 15, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Hong, T.; Ward, A.; Pi, J.; Liu, Z.; Liu, H.-Y.; Cao, W. Constitutive Role for IRE1α-XBP1 Signaling Pathway in the Insulin-Mediated Hepatic Lipogenic Program. Endocrinology 2011, 152, 2247–2255. [Google Scholar] [CrossRef] [Green Version]
- Tirosh, B.; Iwakoshi, N.N.; Glimcher, L.H.; Ploegh, H.L. Rapid Turnover of Unspliced Xbp-1 as a Factor That Modulates the Unfolded Protein Response. J. Biol. Chem. 2006, 281, 5852–5860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.; Scheuner, D.; Ron, D.; Pennathur, S.; Kaufman, R.J. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Investig. 2008, 118, 3378–3389. [Google Scholar] [CrossRef] [Green Version]
- Gurlo, T.; Rivera, J.F.; Butler, A.E.; Cory, M.; Hoang, J.; Costes, S.; Butler, P.C. CHOP Contributes to, But Is Not the Only Mediator of, IAPP Induced β-Cell Apoptosis. Mol. Endocrinol. 2016, 30, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lopez, C.; Barbosa-Desongles, A.; Hernandez, C.; Dyer, R.A.; Innis, S.M.; Simó, R.; Selva, D.M. Sex Hormone-Binding Globulin Reduction in Metabolic Disorders May Play a Role in NAFLD Development. Endocrinology 2017, 158, 545–559. [Google Scholar] [PubMed]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nat. Cell Biol. 2011, 474, 649–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haflidadóttir, B.S.; Larne, O.; Martin, M.; Persson, M.; Edsjö, A.; Bjartell, A.; Ceder, Y. Upregulation of miR-96 Enhances Cellular Proliferation of Prostate Cancer Cells through FOXO1. PLoS ONE 2013, 8, e72400. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Liu, K.; Wang, Y.; Xu, Z.; Meng, J.; Gu, S. Upregulation of microRNA-96 and its oncogenic functions by targeting CDKN1A in bladder cancer. Cancer Cell Int. 2015, 15, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Liang, H.; Uzair-ur-Rehman; Wang, Y.; Zhang, W.; Zhou, Y.; Chen, S.; Yu, M.; Cui, S.; Liu, M.; et al. miR-96 promotes cell proliferation, migration and invasion by targeting PTPN9 in breast cancer. Scientific Reports. Nat. Publ. Group 2016, 6, 37421. [Google Scholar]
- Böhlig, L.; Friedrich, M.; Engeland, K. p53 activates the PANK1/ miRNA-107 gene leading to downregulation of CDK6 and p130 cell cycle proteins. Nucleic Acids Res. 2011, 39, 440–453. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ma, G.; Zhu, H.; Lv, C.; Chu, H.; Tong, N.; Wu, D.; Qiang, F.; Gong, W.; Zhao, Q.; et al. miR-107 regulates tumor progression by targeting NF1 in gastric cancer. Sci. Rep. 2016, 6, 36531. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Yu, F.; Zhong, X.-M.; Lu, G.-X.; Cong, X.-L.; Xue, S.-B.; Xie, W.-T.; Hou, L.-K.; Pang, L.-J.; Wu, W.; et al. miR-30 Family Reduction Maintains Self-Renewal and Promotes Tumorigenesis in NSCLC-Initiating Cells by Targeting Oncogene TM4SF1. Mol. Ther. 2018, 26, 2751–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, R.M.; Theoret, C.L.; Van De Walle, G.R. The Horse as a Model for the Study of Cutaneous Wound Healing. Adv. Wound Care 2019. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef]
- Alves, K.A.; Alves, B.G.; Gastal, G.D.A.; De Tarso, S.G.S.; Gastal, M.O.; Figueiredo, J.R.; Gambarini, M.L.; Gastal, E.L. The Mare Model to Study the Effects of Ovarian Dynamics on Preantral Follicle Features. PLoS ONE 2016, 11, e0149693. [Google Scholar] [CrossRef] [PubMed]
- Saéz-López, C.; Rivera-Giménez, M.; Hernández, C.; Simó, R.; Selva, D.M. SHBG-C57BL/ksJ-db/db: A New Mouse Model to Study SHBG Expression and Regulation During Obesity Development. Endocrinology 2015, 156, 4571–4581. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Protein Abbreviation | Protein Full Name | Manufacturer/Cat No. | Dilution |
|---|---|---|---|
| eIF2α (p-S51) | Eukaryotic translation initiation factor 2 alpha (phospho-S51) | Novus Biologicals/NBP2-67353 | 1:1000 |
| IRE1α (p-S724) | Inositol-requiring enzyme 1 alpha (phospho-S724) | Biorbyt/orb184380 | 1:1000 |
| ATF6 | Activating transcription factor 6 | Novus Biologicals/NBP1-40256 | 1:1000 |
| SHBG | Sex hormone binding globulin | Biorbyt/orb11366 | 1:1000 |
| CHOP | DNA damage-inducible transcript 3 | Aviva/arp31591 | 1:1000 |
| Β-actin | Beta-actin | Sigma Aldrich/a5441 | 1:5000 |
| Gene | Full Gene Name | Primer Sequence 5′→3′ | Accession No. |
|---|---|---|---|
| Hu FASN | Fatty acid synthase | F: CCCAAGCAGGCACACACG R: GGCCTCCGAGGTCTCAG | NM_004104.5 |
| Hu ACLY | ATP citrate lyase | F: TGTAACAGAGCCAGGAACCC R: CTGTACCCCAGTGGCTGTTT | NM_001096.3 |
| Eq ACLY | ATP citrate lyase | F: CCACTTCAGAGCCCAGACAA R: AACTAGGCCCAGCTTTCCAC | XM_005597396.3 |
| Hu PPARG | Peroxisome proliferator-activated receptor gamma | F: AGTCCTCACAGCTGTTTGCCAAGC R: GAGCGGGTGAAGACTCATGTCTGTC | XM_011533844.1 |
| Hu IRE1A | Inositol-requiring enzyme 1 alpha | F: CGGCCTCGGGATTTTTGGA R: AGAAAGGCAGGCTCTTCCAC | NM_001433.5 |
| Hu ATF6 | Activating transcription factor 6 | F: ACCTCCTTGTCAGCCCCTAA R: CACTCCCTGAGTTCCTGCTG | NM_007348.4 |
| Eq ATF6 | Activating transcription factor 6 | F: CAGGGTGCACTAGAACAGGG R: AATGTGTCTCCCCTTCTGCG | XM_023640315.1 |
| Hu HSPA5 | Heat shock protein family A member | F: TGACCAGAATCGCCTGACAC R: TGTCAGCATCTTGGTGGCTT | NM_005347.5 |
| Hu CHOP | DNA damage-inducible transcript 3 | F: TAAAGATGAGCGGGTGGCAG R: GGATAATGGGGAGTGGCTGG | NM_001195053.1 |
| Eq CHOP | DNA damage-inducible transcript 3 | F: AGCCAAAATCAGAGCCGGAA R: GGGGTCAAGAGTGGTGAAGG | XM_001488999.4 |
| Hu XBP1 | X-box binding protein 1 | F: CGCGGATCCGAATGAAGTGAGGCCAGTG R: GGGGCTTGGTATATATGTGG | XM_014742035.2 |
| Eq XBP1 | X-box binding protein 1 | F: TTACGCGAGAAAACTCATGGCC R: GGGTCCAAGTTGAACAGAATGC | XM_014742035.2 |
| Hu SHBG | Sex hormone binding globulin | F: GCTGATTATGGAGAGCAGAGG R: GGTCATGACAGCGATAGGCT | NM_001146281.3 |
| Eq SREBP1C | Sterol regulatory element-binding transcription factor 1 | F: TCAGCGAGGCGGCTTTGGAGCAG R: CATGTCTTCGATGTCGGTCAG | XM_008542859.1 |
| Eq BIP | binding immunoglobulin protein | F: CTGTAGCGTATGGTGCTGCT R: CATGACACCTCCCACGGTTT | XM_023628864.1 |
| Hu GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | F: GTCAGTGGTGGACCTGACCT R: CACCACCCTGTTGCTGTAGC | NM_001289746.1 |
| Eq GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | F: GATGCCCCAATGTTTGTGA R: GATGCCCCAATGTTTGTGA | NM 001163856.1 |
| miRNAs | Primer Sequence 5′→3′ |
|---|---|
| miR-103 | AGCAGCATTGTACAGGGCTATGA |
| miR-93-5p | CAAAGTGCTGTTCGTGCAGGTAG |
| miR-34a | TGGCAGTGTCTTAGCTGGTTGT |
| miR-30c | TGTAAACATCCTACACTCTCAGC |
| miR-107 | AGCAGCATTGTACAGGGCTATCA |
| miR-34a | AGGCAGTGTAGTTAGCTGATTGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornicka-Garbowska, K.; Bourebaba, L.; Röcken, M.; Marycz, K. Sex Hormone Binding Globulin (SHBG) Mitigates ER Stress in Hepatocytes In Vitro and Ex Vivo. Cells 2021, 10, 755. https://doi.org/10.3390/cells10040755
Kornicka-Garbowska K, Bourebaba L, Röcken M, Marycz K. Sex Hormone Binding Globulin (SHBG) Mitigates ER Stress in Hepatocytes In Vitro and Ex Vivo. Cells. 2021; 10(4):755. https://doi.org/10.3390/cells10040755
Chicago/Turabian StyleKornicka-Garbowska, Katarzyna, Lynda Bourebaba, Michael Röcken, and Krzysztof Marycz. 2021. "Sex Hormone Binding Globulin (SHBG) Mitigates ER Stress in Hepatocytes In Vitro and Ex Vivo" Cells 10, no. 4: 755. https://doi.org/10.3390/cells10040755





