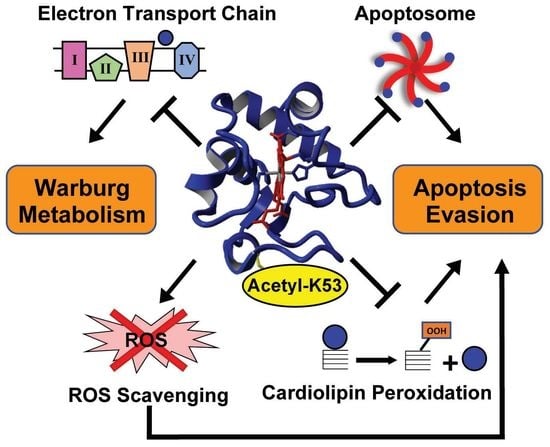
Lysine 53 Acetylation of Cytochrome c in Prostate Cancer: Warburg Metabolism and Evasion of Apoptosis
Abstract
Share and Cite
Bazylianska, V.; Kalpage, H.A.; Wan, J.; Vaishnav, A.; Mahapatra, G.; Turner, A.A.; Chowdhury, D.D.; Kim, K.; Morse, P.T.; Lee, I.; et al. Lysine 53 Acetylation of Cytochrome c in Prostate Cancer: Warburg Metabolism and Evasion of Apoptosis. Cells 2021, 10, 802. https://doi.org/10.3390/cells10040802
Bazylianska V, Kalpage HA, Wan J, Vaishnav A, Mahapatra G, Turner AA, Chowdhury DD, Kim K, Morse PT, Lee I, et al. Lysine 53 Acetylation of Cytochrome c in Prostate Cancer: Warburg Metabolism and Evasion of Apoptosis. Cells. 2021; 10(4):802. https://doi.org/10.3390/cells10040802
Chicago/Turabian StyleBazylianska, Viktoriia, Hasini A. Kalpage, Junmei Wan, Asmita Vaishnav, Gargi Mahapatra, Alice A. Turner, Dipanwita Dutta Chowdhury, Katherine Kim, Paul T. Morse, Icksoo Lee, and et al. 2021. "Lysine 53 Acetylation of Cytochrome c in Prostate Cancer: Warburg Metabolism and Evasion of Apoptosis" Cells 10, no. 4: 802. https://doi.org/10.3390/cells10040802
APA StyleBazylianska, V., Kalpage, H. A., Wan, J., Vaishnav, A., Mahapatra, G., Turner, A. A., Chowdhury, D. D., Kim, K., Morse, P. T., Lee, I., Brunzelle, J. S., Polin, L., Subedi, P., Heath, E. I., Podgorski, I., Marcus, K., Edwards, B. F. P., & Hüttemann, M. (2021). Lysine 53 Acetylation of Cytochrome c in Prostate Cancer: Warburg Metabolism and Evasion of Apoptosis. Cells, 10(4), 802. https://doi.org/10.3390/cells10040802