Inhibition of Cochlear HMGB1 Expression Attenuates Oxidative Stress and Inflammation in an Experimental Murine Model of Noise-Induced Hearing Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cochlear Cell Culture
2.2. Immunofluorescence Staining of 4-HNE
2.3. Quantitative PCR for Analysis of iNOS Gene (Nos2) Expression
2.4. Animals and Study Design
2.5. Noise Exposure
2.6. Tissue Preparation and Immunohistochemistry
2.7. Cochlear Cryosections and Immunostaining
2.8. Cochlear Surface Preparations and Outer Hair Cell Survival Rate
2.9. Western Blotting
2.10. Auditory Brainstem Response Recording
2.11. Statistical Analysis
3. Results
3.1. Recombinant HMGB1 Activated 4-HNE Production and Induced the Expression of iNOS Gene in Primary Cochlear Cells
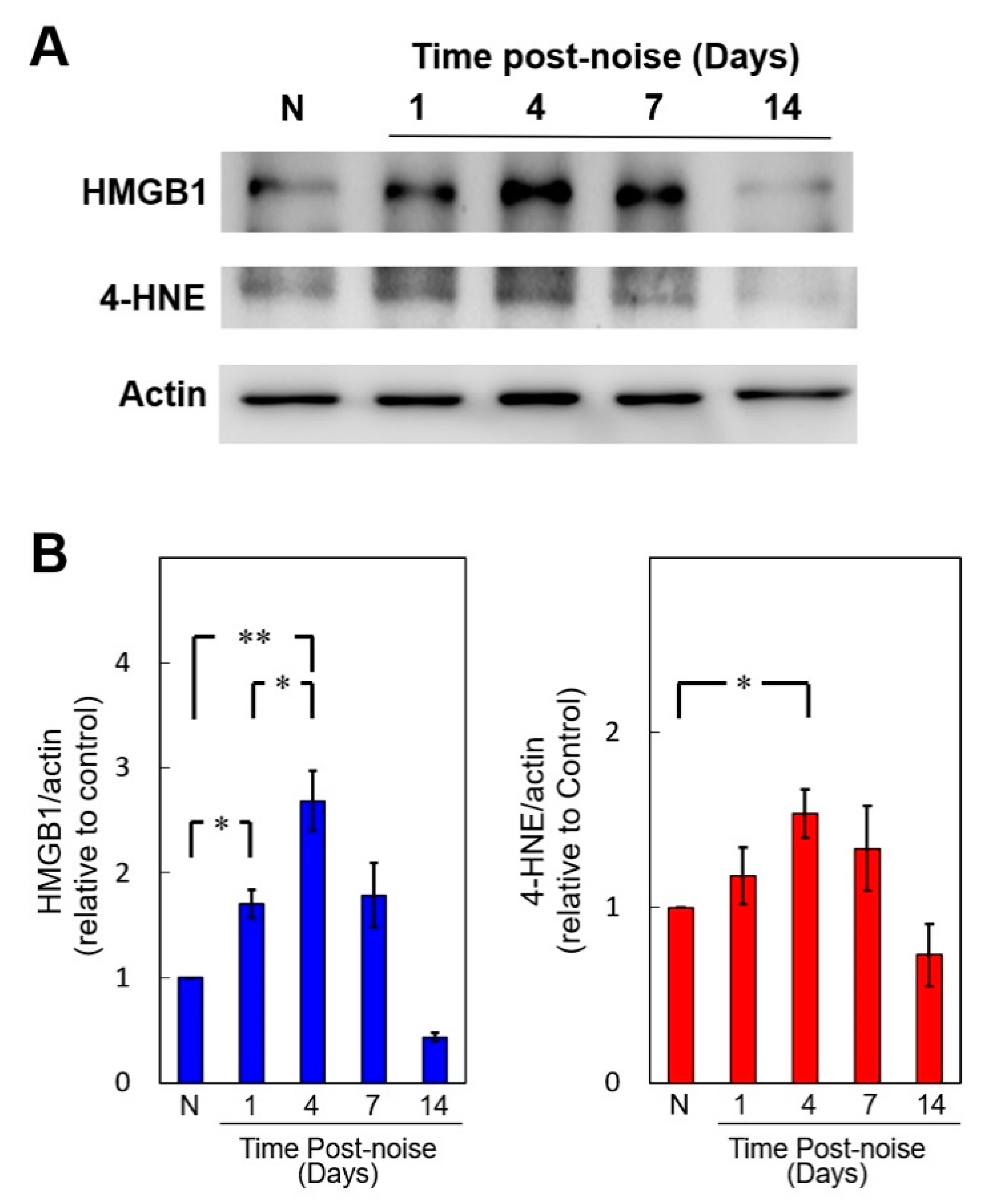
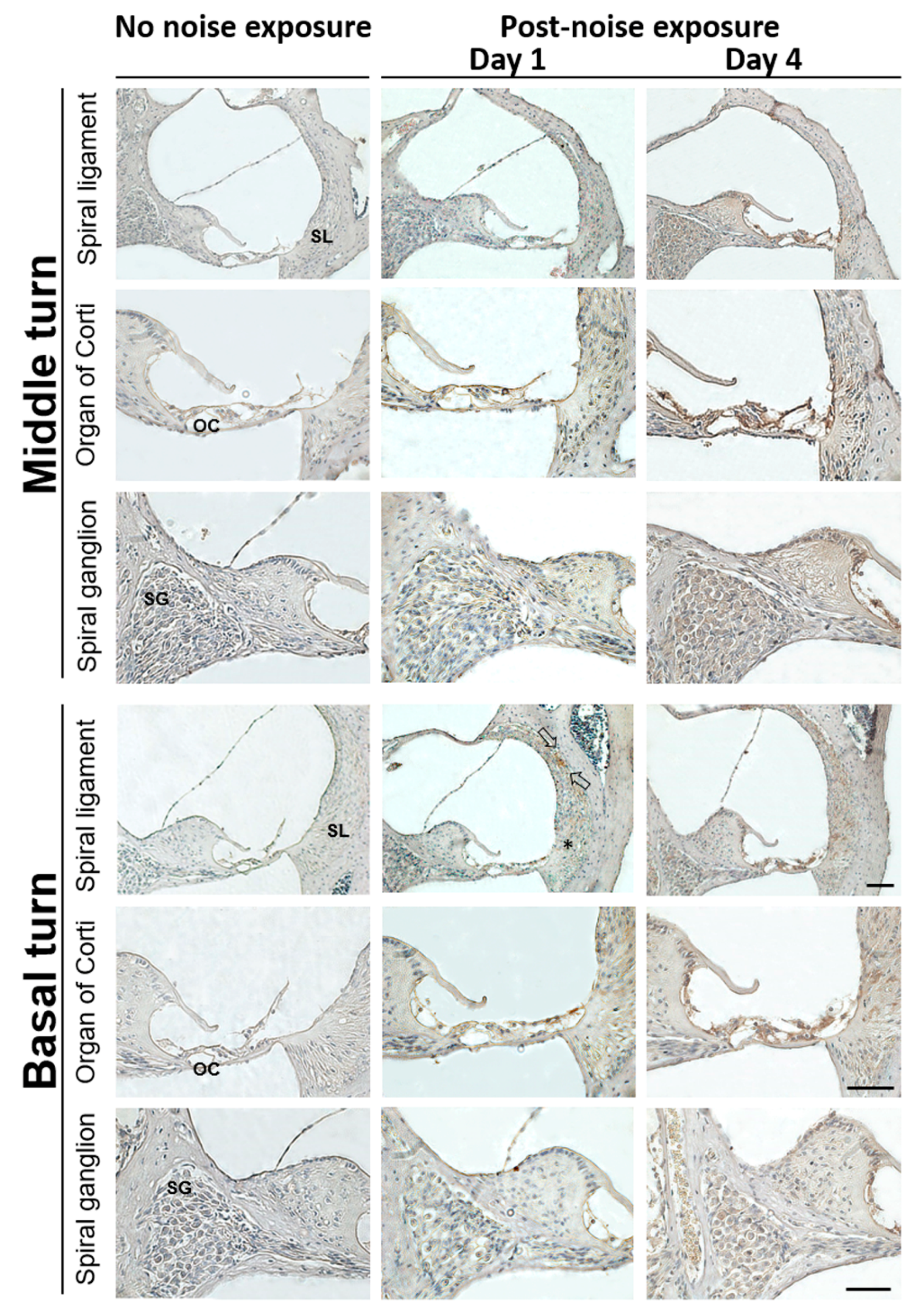
3.2. Noise Exposure Increased Cochlear HMGB1 Expression and Oxidative Stress
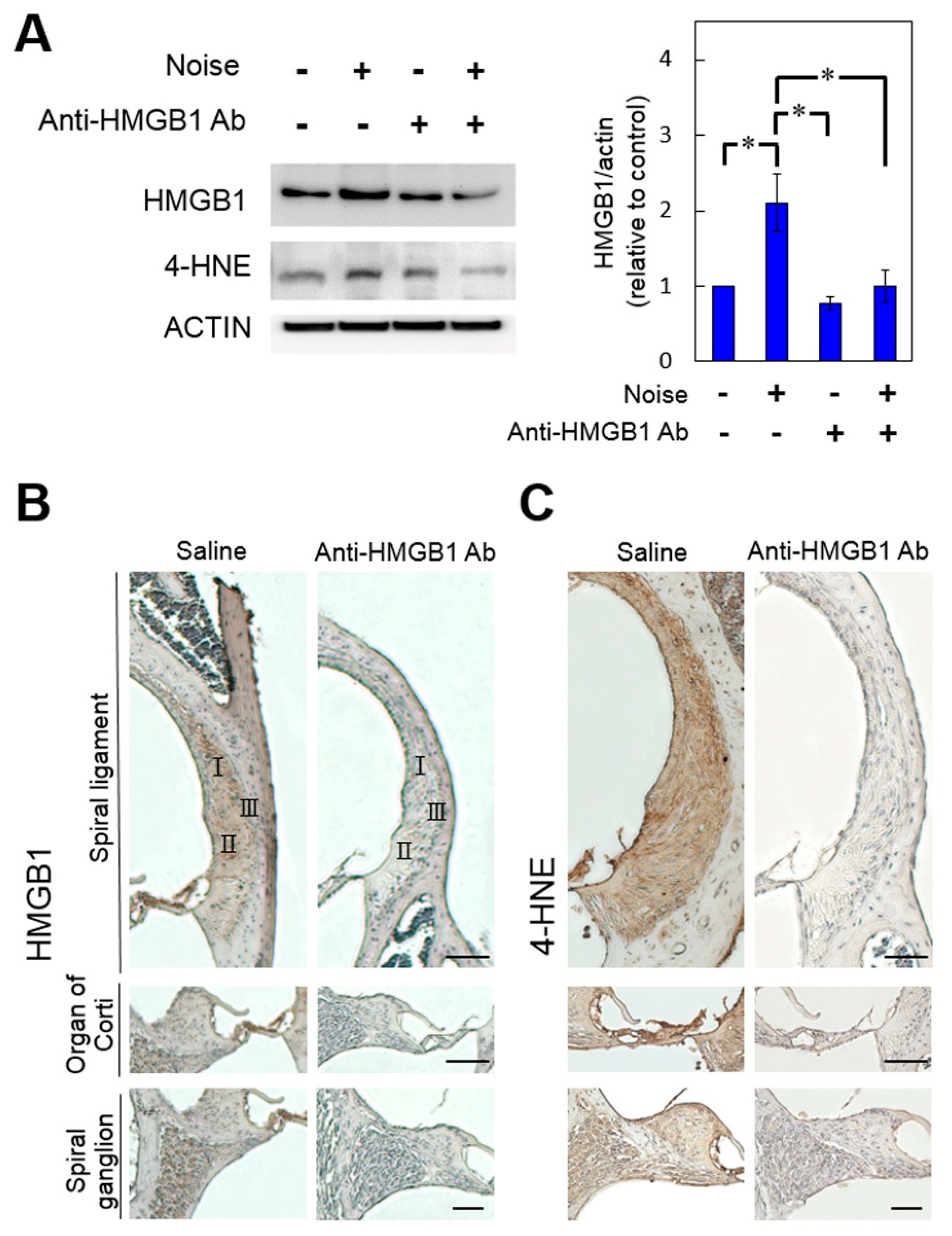
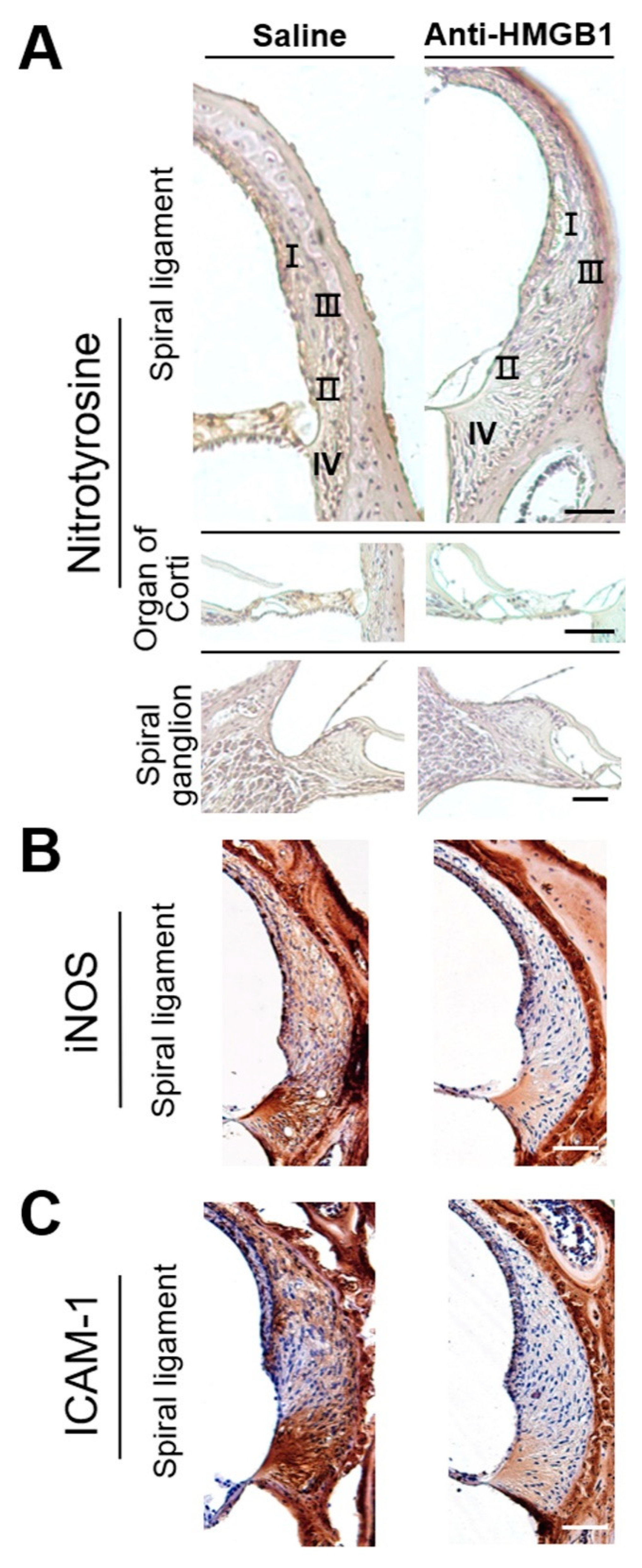
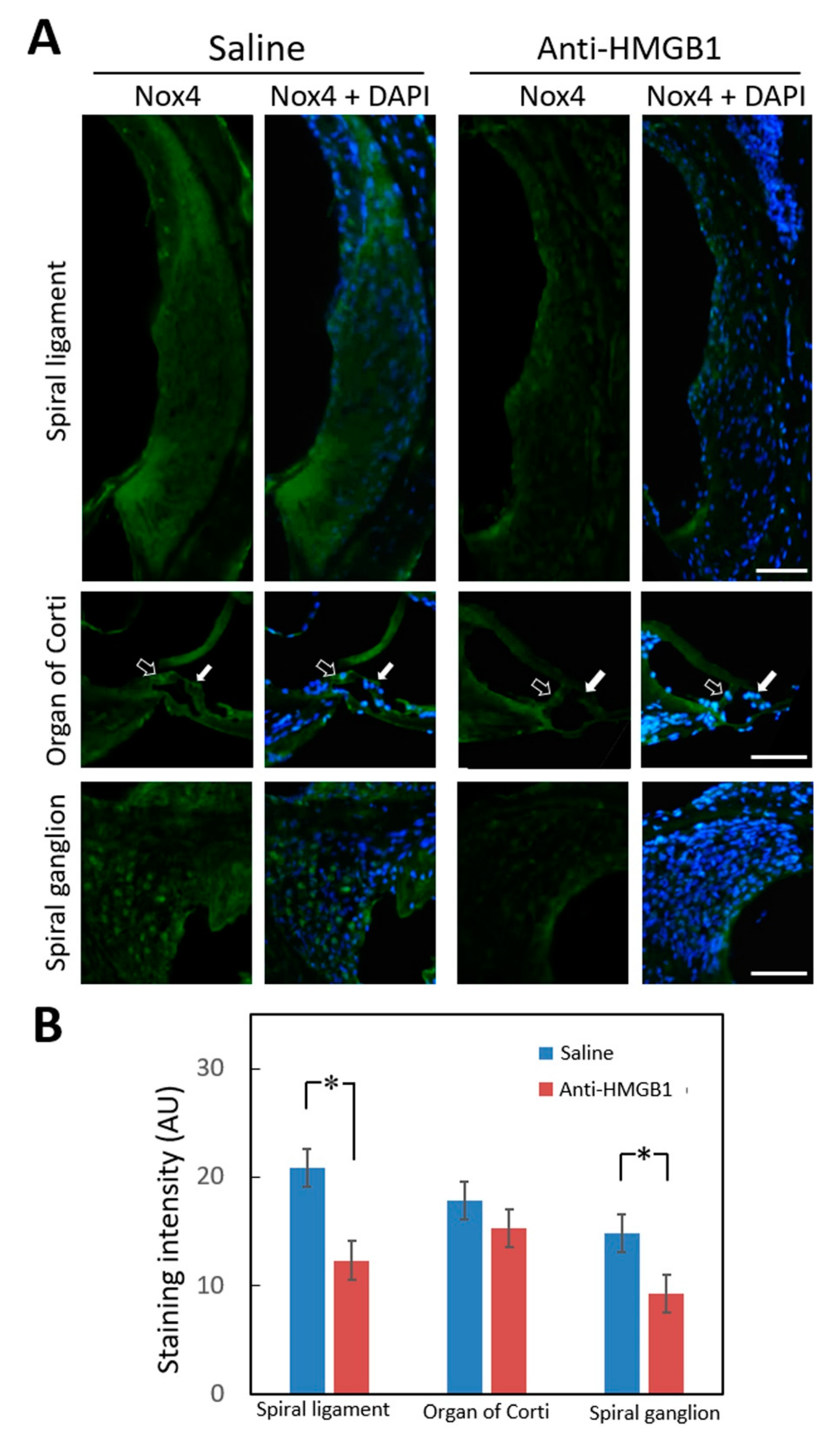
3.3. Pretreatment with Anti-HMGB1 Neutralizing Antibody Attenuated HMGB1 Expression, ROS Generation, and Subsequent Inflammation in Noise-Exposed Cochlea
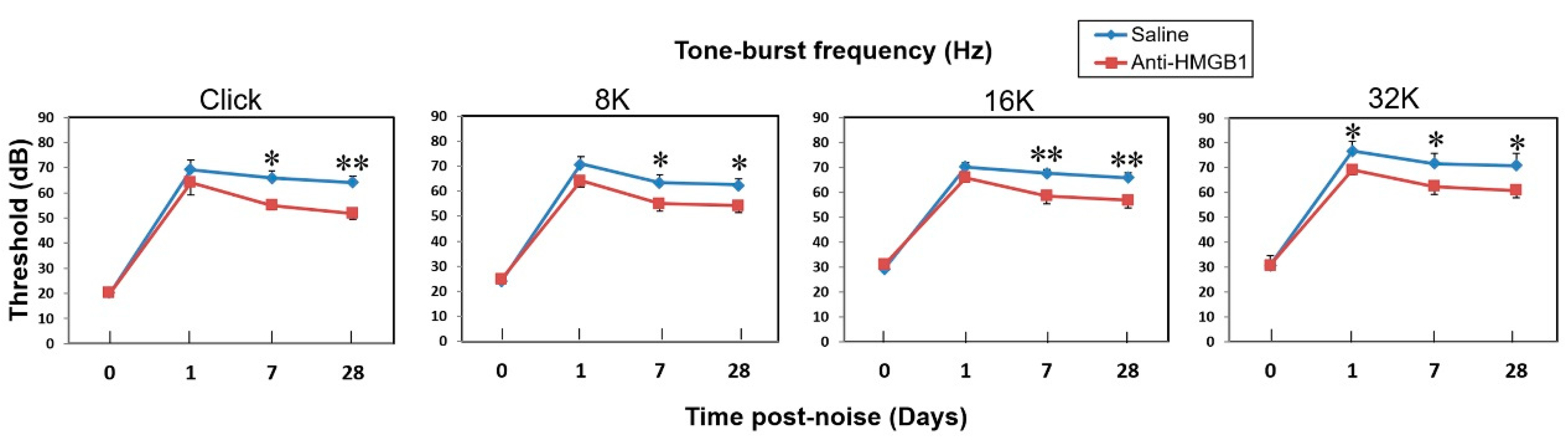
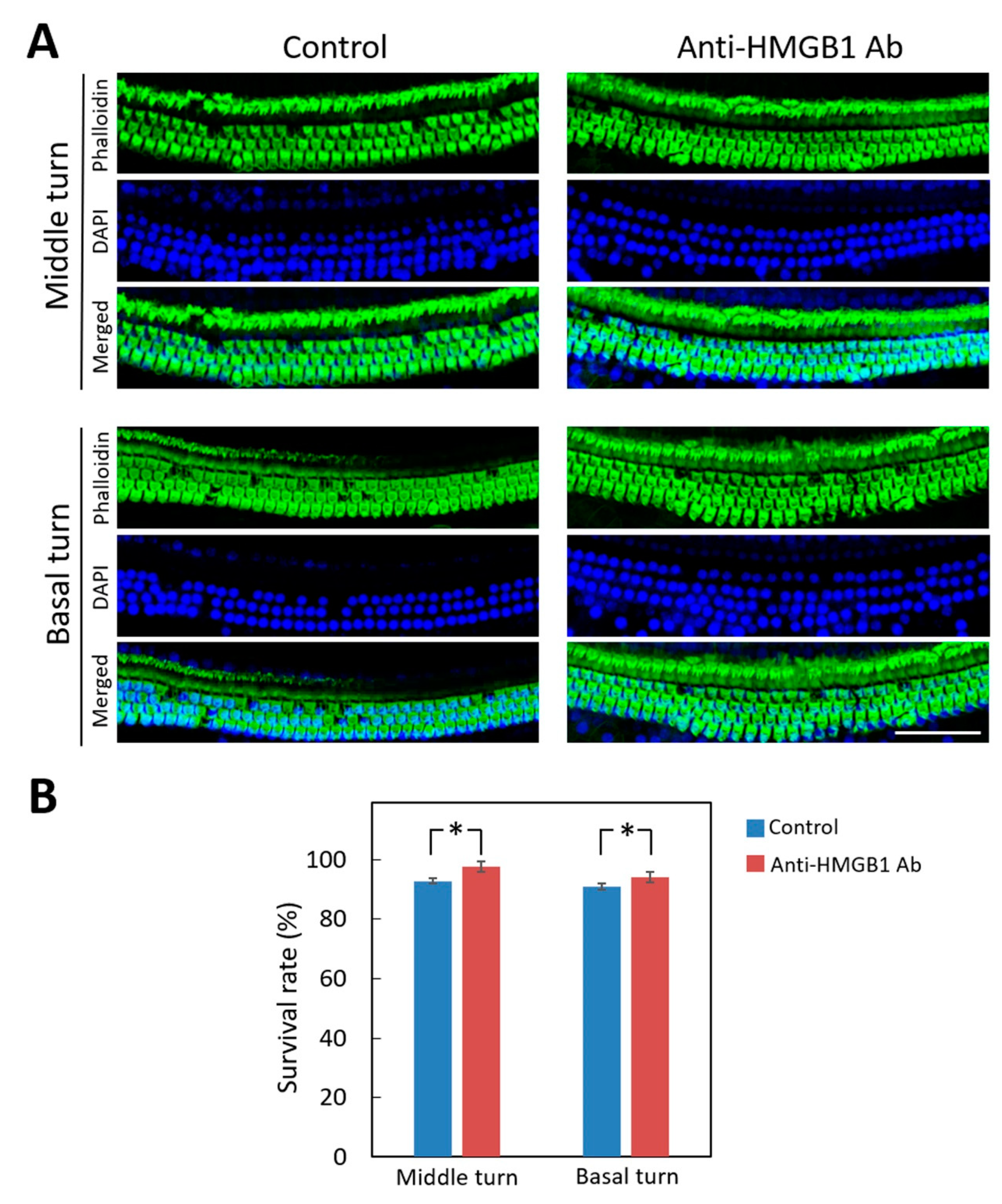
3.4. Suppression of HMGB1 Expression Ameliorated NIHL and Cochlear Hair Cell Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, D.I.; Nelson, R.Y.; Concha-Barrientos, M.; Fingerhut, M. The global burden of occupational noise—Induced hearing loss. Am. J. Ind. Med. 2005, 48, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Graydon, K.; Waterworth, C.; Miller, H.; Gunasekera, H. Global burden of hearing impairment and ear disease. J. Laryngol. Otol. 2019, 133, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise—Induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef]
- Frye, M.D.; Ryan, A.F.; Kurabi, A. Inflammation associated with noise—Induced hearing loss. J. Acoust. Soc. Am. 2019, 146, 4020–4032. [Google Scholar] [CrossRef]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.-Y. Cellular mechanisms of noise—Induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef]
- Yamashita, D.; Jiang, H.-Y.; Schacht, J.; Miller, J.M. Delayed production of free radicals following noise exposure. Brain Res. 2004, 1019, 201–209. [Google Scholar] [CrossRef]
- Vande Walle, L.; Kanneganti, T.-D.; Lamkanfi, M. HMGB1 release by inflammasomes. Virulence 2011, 2, 162–165. [Google Scholar] [CrossRef]
- Gozlan, J.; Borde, C.; Marechal, V. Extracellular HMGB1: An ambiguous messenger during HIV-1 infection. Soluble Factors Mediat. Innate Immune Responses HIV Infect. 2010, 64–80. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Wang, H.; Czura, C.J.; Tracey, K.J. The cytokine activity of HMGB1. J. Leukoc. Biol. 2005, 78, 1–8. [Google Scholar] [CrossRef]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef]
- Kohno, T.; Anzai, T.; Kaneko, H.; Sugano, Y.; Shimizu, H.; Shimoda, M.; Miyasho, T.; Okamoto, M.; Yokota, H.; Yamada, S.; et al. High-mobility group box 1 protein blockade suppresses development of abdominal aortic aneurysm. J. Cardiol. 2012, 59, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Matsuda, T.; Hashimoto, S.; Amaya, F.; Kitamura, Y.; Tanaka, M.; Kobayashi, A.; Maruyama, I.; Yamada, S.; Hasegawa, N.; et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. j. Respir. Crit. Care Med. 2004, 170, 1310–1316. [Google Scholar] [CrossRef]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, J.; Wang, P.; Corpuz, T.M.; Panchapakesan, U.; Wyburn, K.R.; Chadban, S.J. HMGB1 contributes to kidney ischemia reperfusion injury. J. Am. Soc. Nephrol. 2010, 21, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, W.; Frenz, D. Cisplatin ototoxicity to the rat inner ear: A role for HMG1 and iNOS. Neurotoxicology 2006, 27, 22–30. [Google Scholar] [CrossRef]
- Daffu, G.; Del Pozo, C.H.; O’Shea, K.M.; Ananthakrishnan, R.; Ramasamy, R.; Schmidt, A.M. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci. 2013, 14, 19891–19910. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hattori, K.; Hamanaka, J.; Murase, T.; Egashira, Y.; Mishiro, K.; Ishiguro, M.; Tsuruma, K.; Hirose, Y.; Tanaka, H.; et al. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci. Rep. 2012, 2, 896. [Google Scholar] [CrossRef]
- Tsung, A.; Klune, J.R.; Zhang, X.; Jeyabalan, G.; Cao, Z.; Peng, X.; Stolz, D.B.; Geller, D.A.; Rosengart, M.R.; Billiar, T.R.; et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4—Dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 2007, 204, 2913–2923. [Google Scholar] [CrossRef]
- Shih, C.P.; Chen, H.C.; Lin, Y.C.; Chen, H.K.; Wang, H.; Kuo, C.Y.; Lin, Y.Y.; Wang, C.H. Middle-ear dexamethasone delivery via ultrasound microbubbles attenuates noise—Induced hearing loss. Laryngoscope 2019, 129, 1907–1914. [Google Scholar] [CrossRef]
- Abeyama, K.; Stern, D.M.; Ito, Y.; Kawahara, K.-I.; Yoshimoto, Y.; Tanaka, M.; Uchimura, T.; Ida, N.; Yamazaki, Y.; Yamada, S.; et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Investig. 2005, 115, 1267–1274. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Nuttall, A.L. Altered expression of inducible nitric oxide synthase (iNOS) in the cochlea. Hear. Res. 2003, 177, 43–52. [Google Scholar] [CrossRef]
- Xiong, M.; Lai, H.; He, Q.; Wang, J. Astragaloside IV attenuates impulse noise-induced trauma in guinea pig. Acta Oto-Laryngol. 2011, 131, 809–816. [Google Scholar] [CrossRef]
- Nagashima, R.; Yamaguchi, T.; Tanaka, H.; Ogita, K. Mechanism underlying the protective effect of tempol and Nω-nitro-L-arginine methyl ester on acoustic injury: Possible involvement of c-Jun N-terminal kinase pathway and connexin26 in the cochlear spiral ligament. J. Pharmacol. Sci. 2010, 114, 50. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, S.V.; Sato, K.; Pham, L.; Billings, P.; Keithley, E.M. Immune cell recruitment following acoustic trauma. Hear. Res. 2006, 222, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Morioka, S.; Sakaguchi, H.; Yamaguchi, T.; Ninoyu, Y.; Mohri, H.; Nakamura, T.; Hisa, Y.; Ogita, K.; Saito, N.; Ueyama, T.; et al. Hearing vulnerability after noise exposure in a mouse model of reactive oxygen species overproduction. J. Neurochem. 2018, 146, 459–473. [Google Scholar] [CrossRef]
- Wu, H.; Li, R.; Pei, L.G.; Wei, Z.H.; Kang, L.N.; Wang, L.; Xie, J.; Xu, B. Emerging role of high mobility group box-1 in thrombosis-related diseases. Cell. Physiol. Biochem. 2018, 47, 1319–1337. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Jung, H.Y.; Park, E.Y.; Kim, J.; Lee, W.J.; Bae, Y.S. Cutting edge: Direct interaction of TLR4 with NAD (P) H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-κB. J. Immunol. 2004, 173, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Chen, L.; Li, J.; Zhang, H.; Guo, X. Glycine suppresses AGE/RAGE signaling pathway and subsequent oxidative stress by restoring Glo1 function in the aorta of diabetic rats and in HUVECs. Oxidative Med. Cell. Longev. 2019, 2019, 4628962. [Google Scholar] [CrossRef]
- Qin, J.; Peng, Z.; Yuan, Q.; Li, Q.; Peng, Y.; Wen, R.; Hu, Z.; Liu, J.; Xia, X.; Deng, H.; et al. AKF-PD alleviates diabetic nephropathy via blocking the RAGE/AGEs/NOX and PKC/NOX pathways. Sci. Rep. 2019, 9, 4407. [Google Scholar] [CrossRef] [PubMed]
- Ladrech, S.; Mathieu, M.; Puel, J.L.; Lenoir, M. Supporting cells regulate the remodelling of aminoglycoside—Injured organ of C orti, through the release of high mobility group box 1. Eur. J. Neurosci. 2013, 38, 2962–2972. [Google Scholar]
- Chen, L.; Han, M.; Lu, Y.; Chen, D.; Sun, X.; Yang, S.; Sun, W.; Yu, N.; Zhai, S. Molecular mechanisms underlying the protective effects of hydrogen-saturated saline on noise-induced hearing loss. Acta Oto-Laryngol. 2017, 137, 1063–1068. [Google Scholar] [CrossRef]
- Amin, A.R.; Islam, A.B. Genomic analysis and differential expression of HMG and S100A family in human arthritis: Upregulated expression of chemokines, IL-8 and nitric oxide by HMGB1. DNA cell Biol. 2014, 33, 550–565. [Google Scholar] [CrossRef]
- Oh, G.S.; Kim, H.J.; Choi, J.H.; Shen, A.; Kim, C.H.; Kim, S.J.; Shin, S.R.; Hong, S.H.; Kim, Y.; Park, C.; et al. Activation of lipopolysaccharide-TLR4 signaling accelerates the ototoxic potential of cisplatin in mice. J. Immunol. 2011, 186, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Bhatt, K.H.; Sodhi, A. High mobility group box 1 protein synergizes with lipopolysaccharide and peptidoglycan for nitric oxide production in mouse peritoneal macrophages in vitro. Mol. Immunol. 2013, 54, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Vlajkovic, S.M.; Lin, S.C.-Y.; Wong, A.C.Y.; Wackrow, B.; Thorne, P.R. Noise-induced changes in expression levels of NADPH oxidases in the cochlea. Hear. Res. 2013, 304, 145–152. [Google Scholar] [CrossRef]
- Bielefeld, E.C. Reduction in impulse noise-induced permanent threshold shift with intracochlear application of an NADPH oxidase inhibitor. J. Am. Acad. Audiol. 2013, 24, 461–473. [Google Scholar] [CrossRef]
- Rousset, F.; Carnesecchi, S.; Senn, P.; Krause, K.-H. Nox3-targeted therapies for inner ear pathologies. Curr. Pharm. Des. 2015, 21, 5977–5987. [Google Scholar] [CrossRef]
- Shi, L.; Chen, H.; Yu, X.; Wu, X. Advanced glycation end products delay corneal epithelial wound healing through reactive oxygen species generation. Mol. Cell. Biochem. 2013, 383, 253–259. [Google Scholar] [CrossRef]
- Hanusek, C.; Setz, C.; Radojevic, V.; Brand, Y.; Levano, S.; Bodmer, D. Expression of advanced glycation end—product receptors in the cochlea. Laryngoscope 2010, 120, 1227–1232. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting inflammation driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Zeh, H.J., III; Lotze, M.T. High-mobility group box 1, oxidative stress, and disease. Antioxid. redox Signal. 2011, 14, 1315–1335. [Google Scholar] [CrossRef]
- Le, K.; Daliv, E.C.; Wu, S.; Qian, F.; Ali, A.I.; Yu, D.; Guo, Y. SIRT1-regulated HMGB1 release is partially involved in TLR4 signal transduction: A possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain injury. Int. Immunopharmacol. 2019, 75, 105779. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, J.; Xu, K.; Zhang, W. Resveratrol protects against asthma—Induced airway inflammation and remodeling by inhibiting the HMGB1/TLR4/NF-κB pathway. Exp. Ther. Med. 2019, 18, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E.; et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Juhn, S.K.; Rybak, L.P.; Fowlks, W.L. Transport characteristics of the blood—Perilymph barrier. Am. J. Otolaryngol. 1982, 3, 392–396. [Google Scholar] [CrossRef]
- Mogi, G.; Lim, D.J.; Watanabe, N. Immunologic study on the inner ear. Immunoglobulins in perilymph. Arch. Otolaryngol. 1982, 108, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Mogi, G.; Kawauchi, H.; Suzuki, M.; Sato, N. Inner ear immunology. Am. J. Otolaryngol. 1985, 6, 142–147. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Skundric, D.S.; Segal, M.B.; Lipovac, M.N.; Mackic, J.B.; Davson, H. A saturable mechanism for transport of immunoglobulin G across the blood-brain barrier of the guinea pig. Exp. Neurol. 1990, 107, 263–270. [Google Scholar] [CrossRef]
- Hei, Y.; Chen, R.; Yi, X.; Long, Q.; Gao, D.; Liu, W. HMGB1 neutralization attenuates hippocampal neuronal death and cognitive impairment in rats with chronic cerebral hypoperfusion via suppressing inflammatory responses and oxidative stress. Neuroscience 2018, 383, 150–159. [Google Scholar] [CrossRef]
- Nishibori, M.; Mori, S.; Takahashi, H.K. Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J. Pharmacol. Sci. 2019, 140, 94–101. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, W.; Li, S.; Chen, X.; Chang, Y.; Yi, X.; Kang, P.; Yang, Y.; Chen, J.; Liu, L.; et al. Oxidative stress-induced hmgb1 release from melanocytes: A paracrine mechanism underlying the cutaneous inflammation in vitiligo. J. Investig. Dermatol. 2019, 139, 2174–2184.e4. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, C.-P.; Kuo, C.-Y.; Lin, Y.-Y.; Lin, Y.-C.; Chen, H.-K.; Wang, H.; Chen, H.-C.; Wang, C.-H. Inhibition of Cochlear HMGB1 Expression Attenuates Oxidative Stress and Inflammation in an Experimental Murine Model of Noise-Induced Hearing Loss. Cells 2021, 10, 810. https://doi.org/10.3390/cells10040810
Shih C-P, Kuo C-Y, Lin Y-Y, Lin Y-C, Chen H-K, Wang H, Chen H-C, Wang C-H. Inhibition of Cochlear HMGB1 Expression Attenuates Oxidative Stress and Inflammation in an Experimental Murine Model of Noise-Induced Hearing Loss. Cells. 2021; 10(4):810. https://doi.org/10.3390/cells10040810
Chicago/Turabian StyleShih, Cheng-Ping, Chao-Yin Kuo, Yuan-Yung Lin, Yi-Chun Lin, Hang-Kang Chen, Hao Wang, Hsin-Chien Chen, and Chih-Hung Wang. 2021. "Inhibition of Cochlear HMGB1 Expression Attenuates Oxidative Stress and Inflammation in an Experimental Murine Model of Noise-Induced Hearing Loss" Cells 10, no. 4: 810. https://doi.org/10.3390/cells10040810
APA StyleShih, C.-P., Kuo, C.-Y., Lin, Y.-Y., Lin, Y.-C., Chen, H.-K., Wang, H., Chen, H.-C., & Wang, C.-H. (2021). Inhibition of Cochlear HMGB1 Expression Attenuates Oxidative Stress and Inflammation in an Experimental Murine Model of Noise-Induced Hearing Loss. Cells, 10(4), 810. https://doi.org/10.3390/cells10040810





