Abstract
Aquaporins (AQPs) are integral membrane proteins, which play an important role in water homeostasis in the uterus. According to the literature, the expression of aquaporins in reproductive structures depends on the local hormonal milieu. The current study investigated the effect of selected PKA kinase inhibitor H89 and MAPK kinase inhibitor PD98059, on the expression of AQP1, 2, 5, and 7, and steroid hormones (E2), progesterone (P4), and arachidonic acid (AA) in the porcine endometrium on days 18–20 and 2–4 of the estrous cycle (the follicular phase where estrogen and follicle-stimulating hormone (FSH) are secreted increasingly in preparation for estrus and the luteal phase where the ovarian follicles begin the process of luteinization with the formation of the corpus luteum and progesterone secretion, respectively). The luminal epithelial cells were incubated in vitro in the presence of the aforementioned factors. The expression of mRNA was determined by the quantitative real-time PCR technique. In general, in Experiment 1, steroid hormones significantly increased expression of AQP1, 2, and 5 while arachidonic acid increased expression of AQP2 and AQP7. On the other hand, MAPK kinase inhibitor significantly decreased the expression of AQP1 and 5. In Experiment 2, E2, P4, or AA combined with kinase inhibitors differentially affected on AQPs expression. E2 in combination with PKA inhibitor significantly decreased expression of AQP1 but E2 or P4 combined with this inhibitor increased the expression of AQP5 and 7. On the contrary, E2 with PD98059 significantly increased AQP5 and AQP7 expression. Progesterone in combination with MAPK kinase inhibitor significantly downregulated the expression of AQP5 and upregulated AQP7. Arachidonic acid mixed with H89 or PD98059 caused a decrease in the expression of AQP5 and an increase of AQP7. The obtained results indicate that estradiol, progesterone, and arachidonic acid through PKA and MAPK signaling pathways regulate the expression of AQP1 and AQP5 in the porcine luminal epithelial cells in the periovulatory period.
1. Introduction
The endometrium undergoes diverse cell proliferation, growth, and apoptosis cycles, as a function of the estrous cycle and pregnancy. Sex hormones, mainly progesterone (P4) and estrogen (E2), are the key factors regulating these changes [1]. The uterine endometrium of swine is comprised of luminal epithelial, glandular epithelial, and stromal cells. These cells perceive and respond to their microenvironment, e.g., histotrophic, which is required for the growth and development of the conceptus and the receptivity of the uterus to implantation, forming the basis of endometrial homeostasis.
Aquaporins (AQPs) are considered to be important regulators of water homeostasis for normal uterus function, participating in water movement at an intraluminal, interstitial, and capillary level during the estrous cycle, implantation period, and parturition, creating the proper fluid microenvironment in the uterus [2,3,4]. Since their discovery in the uterus of mammals, they have been intensively studied, using molecular and pharmacological methods [5,6,7]. To date, eleven AQPs were found in the uterus (Table 1). The past few years have also seen a renewed interest in AQPs in different pathologies in the female reproductive system [8]. While it is known that some of the uterine AQP genes and proteins are regulated by E2 and/or P4 or other factors, [9,10,11] much remains to be learned about how different AQPs can be specifically regulated.

Table 1.
Expression of aquaporin isoforms in uterus tissues.
Several studies have been published on phosphorylation-dependent regulation of mammalian aquaporins [12,13,14,15]. A recent review of the literature on this subject has revealed phosphorylation as a ubiquitous mechanism in aquaporin regulation by both regulatory processes [16]. It has now been proposed that signaling pathways play a crucial role in the regulation of fluid homeostasis in the uterus. Researchers draw our attention to AMP-dependent protein (PKA) and mitogen-activated protein kinases (MAPK) [17,18,19]. PKA plays a role in the transcriptional control of genes, maintenance and control of several metabolic processes, and DNA replication [20,21]. N-[2-(p-bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide (H89) is frequently used to block signaling pathways in studies concerning cellular regulation [22]. In turn, the MAPK signal transduction pathway plays an essential role in the transduction of extracellular stimulating signals and induction of cellular responses, such as proliferation, transformation, differentiation, and apoptosis. The MAPK signal transduction pathway combines extracellular signal-regulated kinase (ERK) [23]. PD98059 prevents the phosphorylation and activation of MEK1 and MEK2 by upstream activators such as c-Raf, and then inhibits the ERK pathway [24].
In our recent study, we demonstrated the endometrial and myometrial expression of AQP1, 5, and 9 during different phases of the estrous cycle and pregnancy, which indicates that steroid hormones, arachidonic acid (AA), oxytocin (OT), forskolin (FSK), and cyclic adenosine monophosphate (cAMP) participate in controlling the distribution of AQP1 and 5 in the uterus [11,25,26,27]. Thus, following our previous results, the aim of this study was to (1) examine the gene expression of AQP1, 2, 5, and 7 in the porcine uterine epithelial cells during the periovulatory period; (2) determine the effect of E2, P4, and AA on the expression of studied AQPs in these cells; (3) find out whether the examined factors affect the PKA and MAPK signaling pathways during the periovulatory period.
2. Materials and Methods
2.1. Experimental Animals and Tissue Collection
All experiments were performed following the principles and procedures of the Animal Ethics Committee (number 32/2012), University of Warmia and Mazury in Olsztyn, Poland. Twenty crossbred gilts (Large White × Polish Landrace) of similar age, weight, and genetic background from one commercial herd were used. Gilts that exhibited two stages at the follicular phase (days 18–20; n = 10) and early luteal phase (days 2–4; n = 10) of the estrous cycle were chosen to collect uterus tissue for two in vitro experiments. The gilts were daily observed for estrus behavior in the presence of a boar. The day of onset of the second estrus was marked as day 0 of the estrous cycle. The phase of the estrous cycle was also confirmed based on the characteristic morphology of the ovaries [37]. After slaughter, the pig reproductive tracts were put into ice-cold PBS with an antibiotic mix and transported immediately to the laboratory.
2.2. Isolation of Uterine Luminal Epithelial Cells
The endometrial tissue was separated from the myometrium and digested using 0.2% dispase (Sigma-Aldrich, St. Louis, MO, USA) in Hank’s balanced salt solution (pH 7.4; Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 60 min with continuous stirring (37 °C). After this time, undigested tissue was removed by filtration through a 200 μm mesh filter. The collected supernatant was mixed with M199 medium (Sigma-Aldrich, St. Louis, MO, USA) with 5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) and centrifuged (15 °C, 1100 rpm, 10 min).
2.3. Cell Culture
From the supernatant, erythrocytes were removed by pipetting the precipitate with red blood cell lysis (Sigma-Aldrich, St. Louis, MO, USA) by 20 s and then mixed with M119 without phenol red with 5% BSA. The cell washes and centrifugation were repeated two times. Luminal epithelial (LE) cells released after this digestion were pelleted by centrifugation. The cell suspension was washed with Medium 199 without phenol red (Sigma-Aldrich, St. Louis, MO, USA) and counted. The next LE cells seeded onto the 12-wells plate (1 million cells in 1 mL medium) in M199 without phenol red medium supplemented with 2% BSA (Sigma-Aldrich, St. Louis, MO, USA), 10% dextran/charcoal-stripped FBS (Sigma-Aldrich, St. Louis, MO, USA), and antibiotics (Sigma-Aldrich, St. Louis, MO, USA). After 20 h preincubation, unattached cells were removed, and attached cells were supplemented with fresh medium. The LE cells were cultured approximately for 72 h when monolayers were estimated to be approximately 90% confluent. The culture medium was changed every two days. Depending on the type of experiment, LE cells were cultured separately in a twelve-well plate with factors E2, P4, AA, and kinases inhibitors H89 and PD98059 (Experiments 1, 2) at 37 °C in a humidified atmosphere of 95% air: 5% CO2. The doses of the agents were selected based on the following articles: estradiol 10−9 M, progesterone 10−6 M, arachidonic acid 10−5 M [27], H89—inhibitor of PKA [38,39], and PD98059—inhibitor of MAPK [23].
- Experiment 1:
LE cells were further incubated for 24 h with the control medium (M199 supplemented with 1% steroid-free FBS and antibiotics) and medium (M199 supplemented with 1% steroid-free FBS and antibiotics) with E2, P4, AA, H89 (1 µmol, 10 µmol), PD98059 (1 µmol, 10 µmol), and incubated for 24 h.
- Experiment 2:
LE cells were treated with control medium (M199 supplemented with 1% steroid-free FBS and antibiotics) and medium (M199 supplemented with 1% steroid-free FBS and antibiotics) with mixed factors: E2 + H89 (1 µmol, 10 µmol), P4 + H89 1 µmol, P4 + H89—10 µmol, AA + H89—1 µmol, AA + H89—10 µmol and E2 + PD98059—1 µmol, E2 + PD98059—10 µmol, P4 + PD98059 (1 µmol, 10 µmol), AA + PD98059 (1 µmol, 10 µmol) and incubated for 24 h.
All treatments were performed in triplicate in two separate experiments. After 24 h of culture, LE cells were washed with PBS, treated with TRI Reagent® (Sigma-Aldrich) for RNA extraction.
2.4. RNA Isolation
Total RNA was extracted, using the total RNA TRI Reagent® (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol, from luminal epithelium cells. Total RNA quality and quantity were determined with spectrophotometry (Infinite® 200 PRO NanoQuant, Tecan, Switzerland).
2.5. cDNA Synthesis and Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA samples were transcribed to cDNA using a TransScriba Kit (A&A Biotechnology, Gdansk, Poland). Real-time PCR was performed in triplicate for each sample using a AriaMx Real-Time PCR System (Agilent Technologies) and SYBR®Green PCR Master Mix (Life Technologies, Grand Island, NY, USA). Real-Time PCR reaction included 12.5 μL SYBR Green PCR master mix, 1 μM forward and reverse primers each, and reverse-transcribed cDNA (2 μL of diluted RT product) supplemented with water to a volume of 25 μL. The conditions of the thermal cycling for each gene were as follows: initial denaturation for 10 min at 95 °C, denaturation for 15 s at 95 °C, and primer annealing for 1 min at 60 °C. Specific primers for AQP1, AQP2, AQP5, and AQP7 (Table 2) were designed with the PrimerQuest Tool (Integrated DNA Technologies, Inc., Coralville, IA, USA) and their specificities were confirmed by comparison of their sequences with the sequence of AQP1, AQP2, AQP5, and AQP7 deposited in a database and calculation of the statistical significance of the match was performed using the Basic Local Alignment Search Tool (BLAST). For the specificity control, non-template controls and dissociation curve analysis of the amplified products were used for each amplification. The specificity of the amplifications was further validated with electrophoresis of the putative amplicons in a 2% agarose gel. Levels of gene expression were calculated using the ΔΔ Ct method and normalized using the geometrical means of reference genes expression levels, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and 18S rRNA (Table 2).

Table 2.
Forward and reverse primers sequences, amplicons length, and GeneBank accession numbers of genes used during real-time PCR analysis.
2.6. Statistical Analysis
Data are presented as means ± SEM from five different observations. Differences between groups within each factor separately were analyzed by one-way ANOVA followed by Dunnet’s post hoc test. Statistical analyses were performed using Statistica Software (StatSoft, Hamburg, Germany). Values for p < 0.05 were considered statistically significant.
3. Results
3.1. The Effect of Estradiol, Progesterone, Arachidonic Acid, and Kinases Inhibitors (H89—PKA Inhibitor, PD98059—MAPK Inhibitor) on Aquaporin 1, 2, 5, and 7 mRNA Expressions in the Porcine Endometrial Luminal Epithelial Cells (Experiment 1)
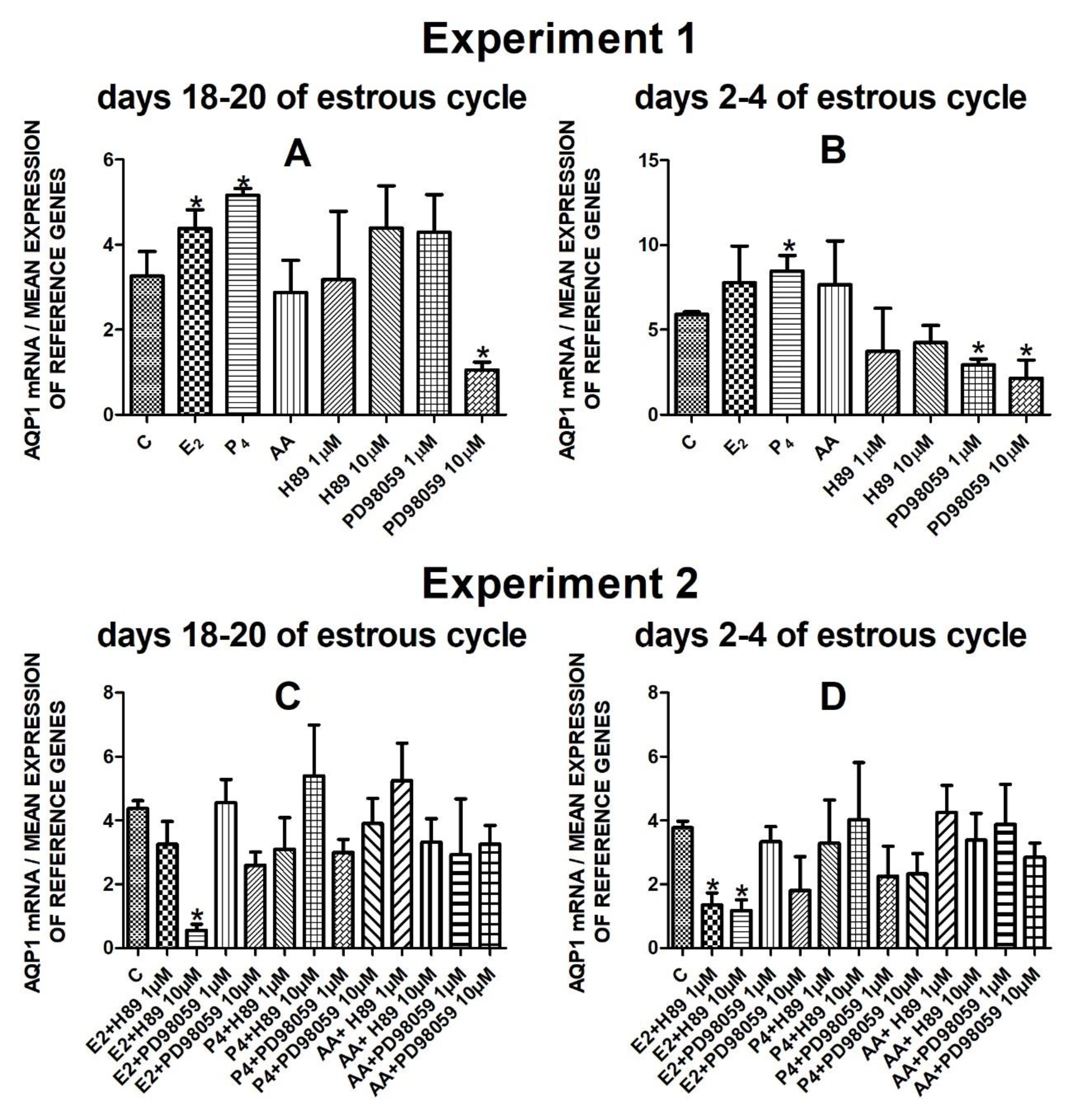
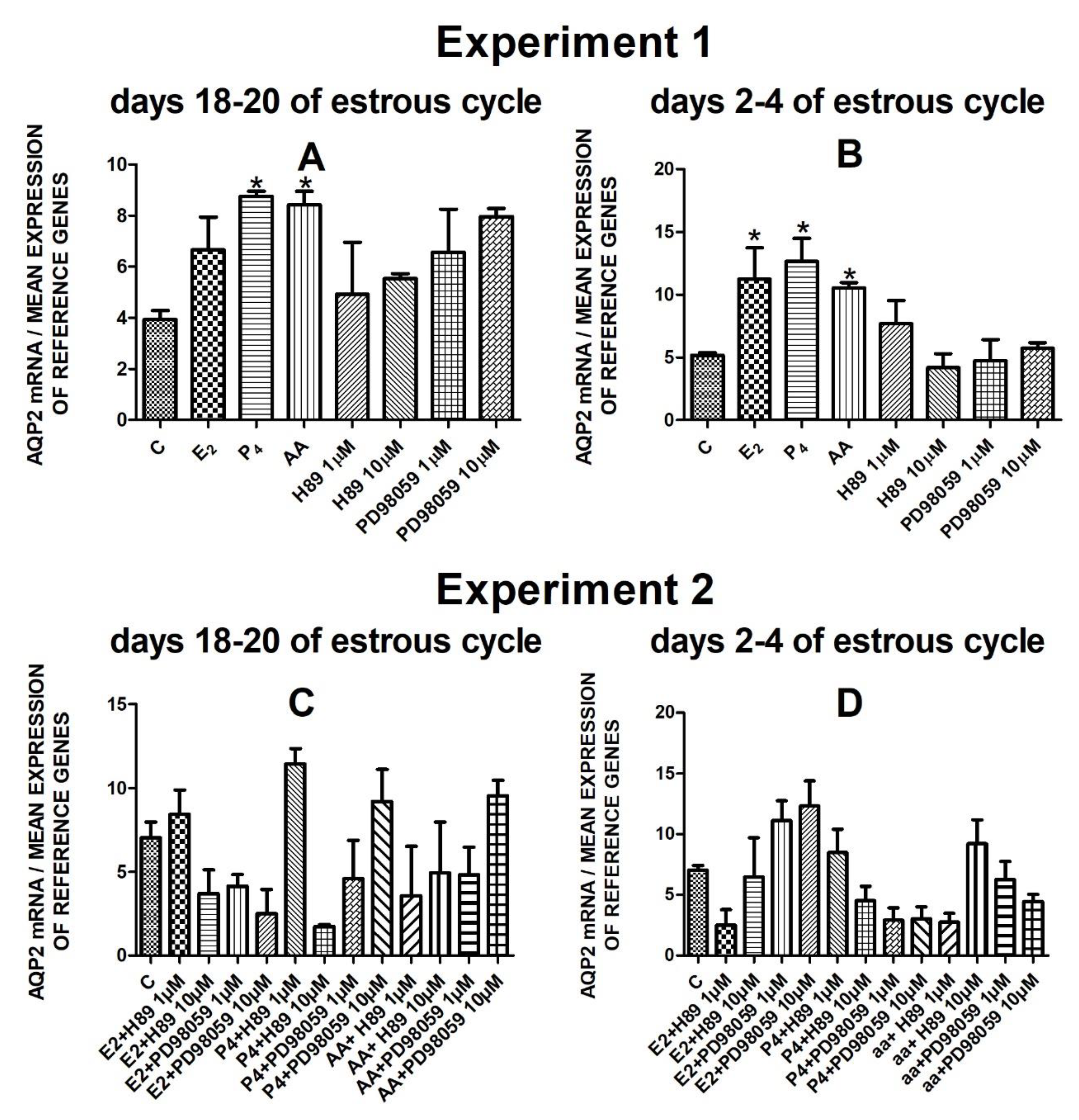
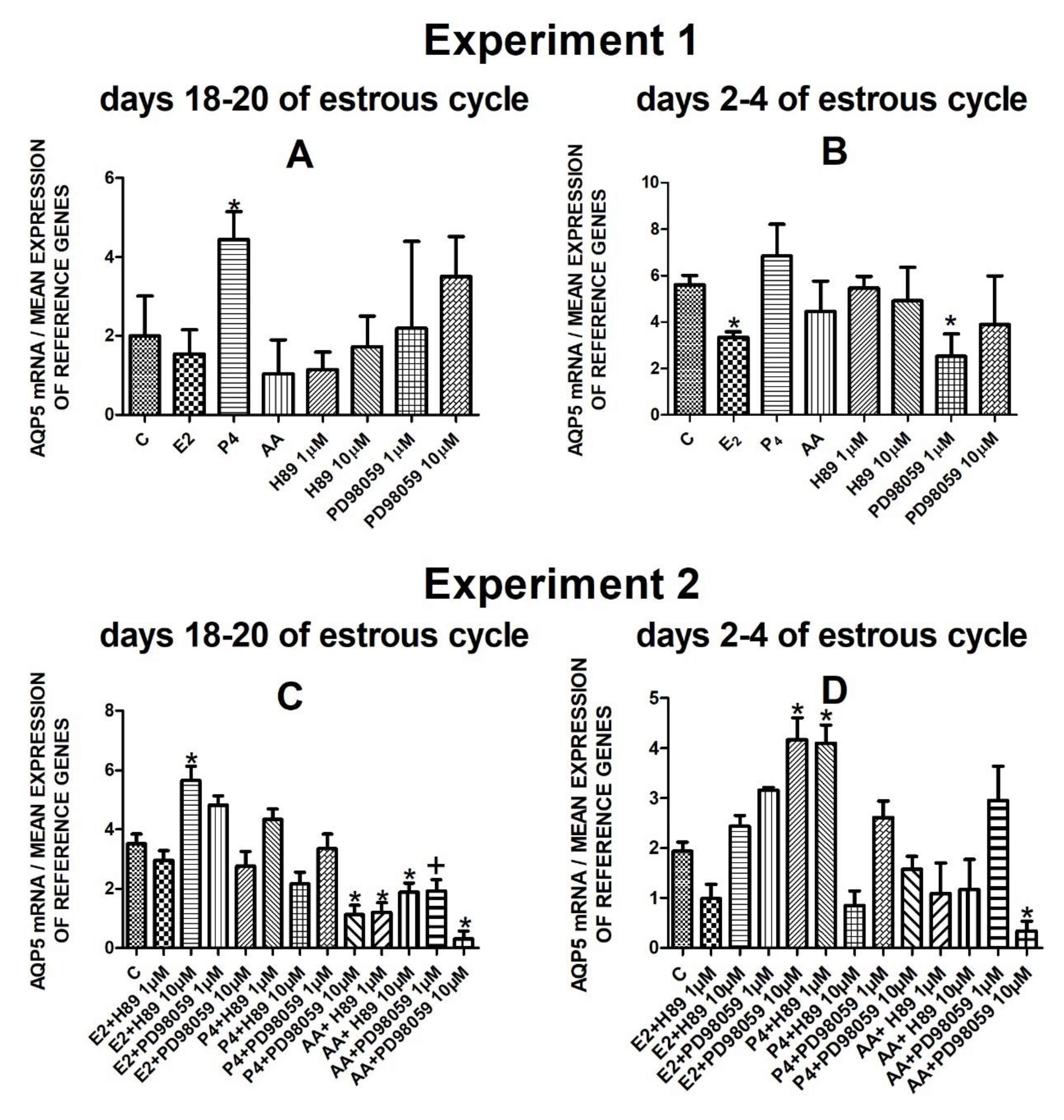
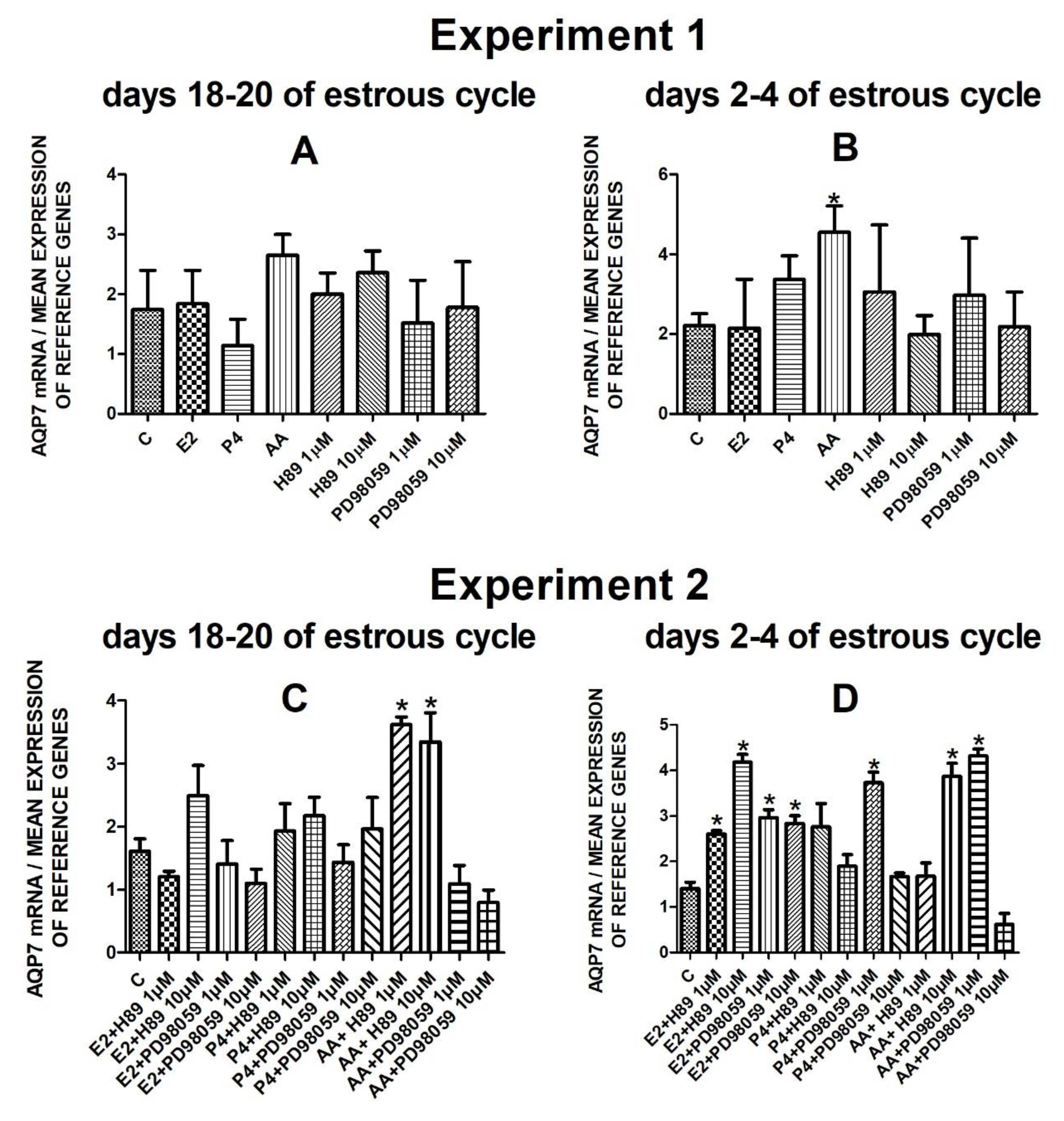
The data from Experiment 1 are presented in Figure 1A,B, Figure 2A,B, Figure 3A,B and Figure 4A,B. The summarized results of Experiment 1 are shown in Table 3. Estradiol and P4 significantly increased and 10 µM of PD98059 decreased AQP1 mRNA expression in the porcine uterine luminal epithelial cells on days 18–20 of the estrous cycle (Figure 1A) (p < 0.05). On the early luteal phase (days 2–4) of the estrous cycle, P4 significantly increased and PD98059 (1 µM and 10 µM) decreased expression of AQP1 mRNA in these cells (Figure 1B) (p < 0.05). The mRNA expression of AQP2 in the porcine uterine luminal epithelial cells on days 18–20 and days 2–4 of the estrous cycle was significantly upregulated by P4, AA (Figure 2A, p < 0.05) and E2, P4 as well as AA (Figure 2B, p < 0.05), respectively. Progesterone significantly increased the expression of AQP5 mRNA in the porcine luminal epithelial cells during the follicular phase of the estrous cycle (Figure 3A, p < 0.05). Following treatment with E2 and PD98059 (1 µM), AQP5 mRNA expression was significantly downregulated in the cells on the early luteal phase of the estrous cycle (Figure 3B, p < 0.05). Treatment of these cells with AA caused a significant decrease in the expression of AQP7 mRNA on days 2–4 of the estrous cycle (Figure 4B, p < 0.05).
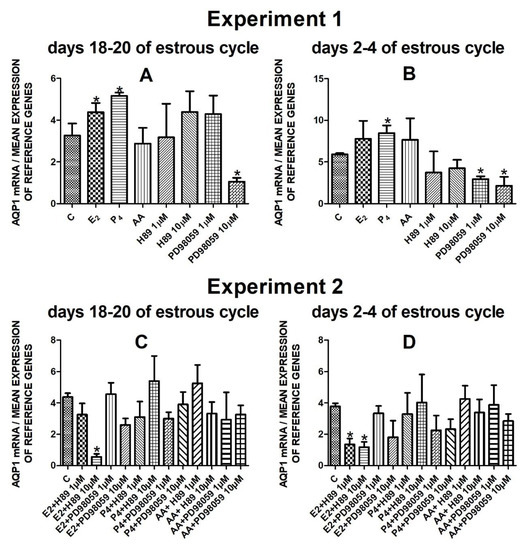
Figure 1.
The influence of estradiol (10 nM), progesterone (10−6 M), arachidonic acid (10−5 M), and kinase inhibitors H89 (1 µM, 10 µM) and PD98059 (1 µM, 10 µM) and mix of these factors on Aquaporin 1 mRNA expression (Experiment 1: (A,B), Experiment 2: (C,D)) in the porcine luminal epithelial cells from days 18–20 and 2–4 of the estrous cycle. The gene expression was determined by quantitative real-time PCR. Results are reported as the means ± S.E.M. (n = 5). Bars with different superscripts differ (p < 0.05) and are marked by (*).
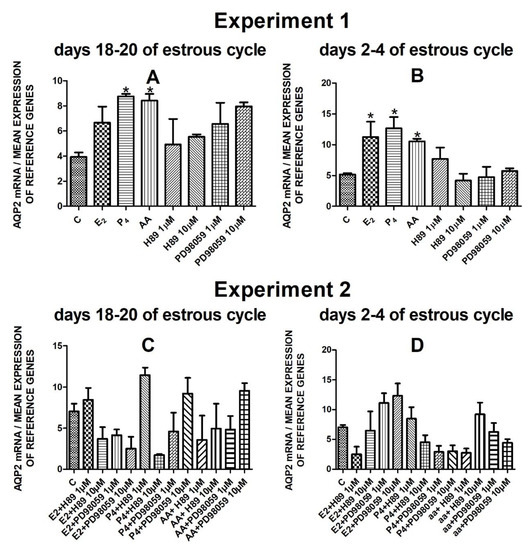
Figure 2.
The influence of estradiol (10 nM), progesterone (10−6 M), arachidonic acid (10−5 M), and kinase inhibitors H89 (1 µM, 10 µM) and PD98059 (1 µM, 10 µM) and mix of these factors on Aquaporin 2 mRNA expression (Experiment 1: (A,B), Experiment 2: (C,D)) in the porcine luminal epithelial cells from days 18–20 and 2–4 of the estrous cycle. The gene expression was determined by quantitative real-time PCR. Results are reported as the means ± S.E.M. (n = 5). Bars with different superscripts differ (p < 0.05) and are marked by (*).
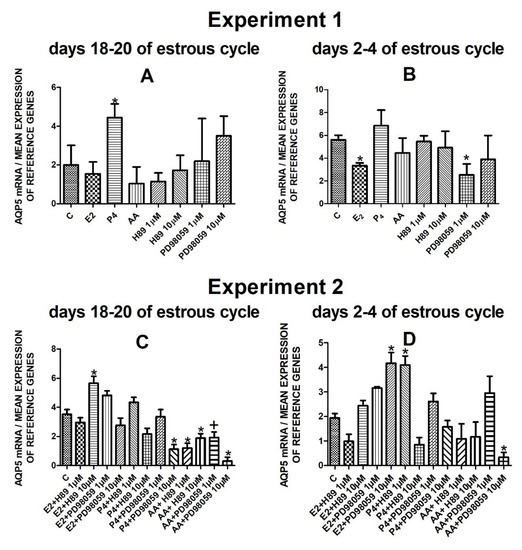
Figure 3.
The influence of estradiol (10 nM), progesterone (10−6 M), arachidonic acid (10−5 M), and kinase inhibitors H89 (1 µM, 10 µM) and PD98059 (1 µM, 10 µM) and a mix of these factors on Aquaporin 5 mRNA expression (Experiment 1: (A,B), Experiment 2: (C,D)) in the porcine luminal epithelial cells from days 18–20 and 2–4 of the estrous cycle. The gene expression was determined by quantitative real-time PCR. Results are reported as the means ± S.E.M. (n = 5). Bars with different superscripts differ (p < 0.05) and are marked by (*), tendency (p < 0.1) and are marked by plus (+).
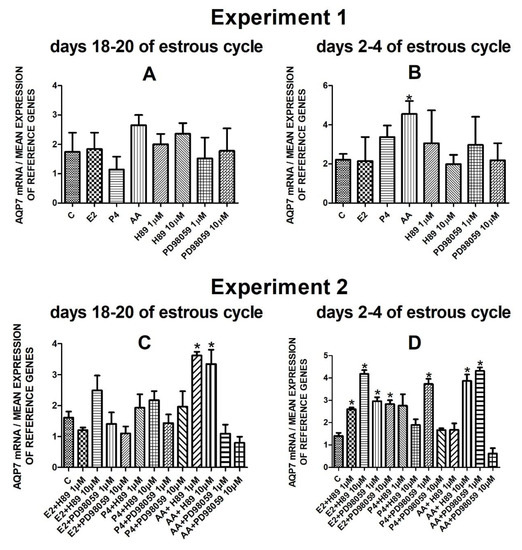
Figure 4.
The influence of estradiol (10 nM), progesterone (10−6 M), arachidonic acid (10−5 M), and kinase inhibitors H89 (1 µM, 10 µM) and PD98059 (1 µM, 10 µM) and mix of these factors on Aquaporin 7 mRNA expression (Experiment 1: (A,B), Experiment 2: (C,D)) in the porcine luminal epithelial cells from days 18–20 and 2–4 of the estrous cycle. The gene expression was determined by quantitative real-time PCR. Results are reported as the means ± S.E.M. (n = 5). Bars with different superscripts differ (p < 0.05) and are marked by (*).

Table 3.
A summary of the results from Experiment 1.
3.2. The Effect of Estradiol, Progesterone, and Arachidonic Acid Combined with Kinases Inhibitors (H89—PKA Inhibitor, PD98059—MAPK Inhibitor) on Aquaporin 1, 2, 5, and 7 mRNA Expressions in the Porcine Endometrial Luminal Epithelial Cells (Experiment 2)
Data of Experiment 2 are presented in Figure 1C,D, Figure 2C,D, Figure 3C,D and Figure 4C,D. The summarized results of Experiment 2 are shown in Table 4.

Table 4.
A summary of the results from Experiment 2.
Treatment of the porcine endometrial luminal epithelial cells with E2 combined with PKA inhibitor (10 µM) significantly decreased expression of AQP1 mRNA during the follicular phase of the estrous cycle (Figure 1C, p < 0.05). On the early luteal phase of the estrous cycle, estradiol in combination with two doses of PKA inhibitor (1 µM or 10 µM) significantly downregulated AQP1 mRNA expression in these cells (Figure 1D, p < 0.05).
The AQP2 mRNA expression did not significantly change after treatment with studied factors (Figure 2C,D).
On days 18–20 of the estrous cycle, E2 with the addition of H89 inhibitor to the cells had a significant stimulatory effect on AQP5 mRNA expression. After treatment with progesterone and MAPK kinase inhibitor on dose 10 µM and arachidonic acid with two doses (1 µM or 10 µM) of PKA inhibitor or MAPK inhibitor AQP5 expression significantly decreased in these cells in the follicular phase of the estrous cycle (Figure 3C, p < 0.05). In the early luteal phase of the estrous cycle, AQP5 was significantly upregulated by E2 combined with PD98059 (10 µM) and P4 in combination with H89 (1 µM) and downregulated by the combination of arachidonic acid with MAPK inhibitor in dose 10 µM (Figure 3D, p < 0.05).
Following treatment of the cells with arachidonic acid mixed with two doses (1 µM, 10 µM) of PKA inhibitor expression of AQP7 mRNA significantly increased on days 18–20 of the estrous cycle. On days 2–4 of the estrous cycle, treatment with estradiol and two doses of H89 or PD98059, progesterone with PD98059 (1 µM) as well as arachidonic acid with H89 (10 µM) or PD98059 (1 µM) upregulated the expression of AQP7 mRNA in the porcine endometrial luminal epithelial cells (Figure 4D, p < 0.05).
4. Discussion
Our experiments confirmed the AQP1 and AQP5 gene expression in the porcine uterine luminal epithelial cells [11,25,26,27], a novel finding was that AQP2 and AQP7 are also expressed in these cells. In addition, in this study, we investigated the effects of the estradiol, progesterone, arachidonic acid, H89 (selective PKA inhibitor), and PD98059 (MAPK inhibitor) on the AQP1, AQP2, AQP5, and AQP7 gene expression in the uterine epithelial cells.
The transport of water across the secretory epithelia involves two distinct pathways, i.e., the paracellular and transcellular pathways. In the transcellular pathway, aquaporins are mainly responsible for a large amount of transport of water, which is driven by the osmotic gradient [6]. It has been already shown that several factors, including hormones, regulate uterine transepithelial water transport via modulating the expression level of AQPs [6,25,26,27,40]. As shown in our results, treatment with steroid hormones (E2 and P4) resulted in a predominantly stimulatory effect on AQP1 and AQP2 mRNA expression. Moreover, luminal epithelial cells from the follicular phase treated with P4 upregulated AQP5 mRNA expression. Conversely, E2 treatment significantly downregulated AQP5 mRNA expression. In the present study, there was no significant change of AQP7 mRNA expression in the epithelial cells after P4 and E2 treatment. Chinigarzadeh et al. [40] reported that AQP2 might participate in estradiol-induced uterine fluid accumulation in the rat. While in humans, the levels of endometrial AQP2 positively correlate with plasma estrogen levels [31]. Others have shown the regulatory effects of E2 on AQP2 in the human endometrium [41]. Contrary to the research of Chinigarzadeh et al. [40], in our study, progesterone did not induce the expression of AQP7 at the mRNA level. AQP7, which also transports urea and glycerol was found to be involved in decidualization [36]. Recently, the expression of AQP7 in the uterus was reported to be influenced by testosterone [42]. Thus, our results provide further evidence of hormonal regulation of the water channels in the porcine uterine epithelial cells. Notably, the fluid produced in the uterus provides a physiological medium for its normal function and early embryonic development [25,26]. Very recent transcriptomes throughout swine estrous cycle studies revealed that ovarian steroids and cytokines regulate endometrial gene expression during the estrous cycle [43]. Molecular studies have already elucidated the physiological functions of aquaporins and their contribution to the mechanism responsible for balancing water concentration within the uterus [2,6,40,44]. Furthermore, the quality and quantity of the uterine fluid are modified in correlation with fluctuations of estrogen and progesterone during the estrous and menstrual cycle [11,25,26,27].
In this study, apart from hormones, we have shown that arachidonic acid (AA) could enhance AQP1 and 7 mRNA expression in the porcine uterus. Arachidonic acid is important for the biosynthesis of prostaglandins, which play an essential role in the regulation of reproductive processes [45,46]. Several articles report a significant effect of prostaglandins on water homeostasis. The COX-2-derived prostaglandins can regulate the expression of AQP2 and AQP3 in the collecting duct and additionally the role of prostaglandins in AQPs translocation, which can stimulate water permeability [47]. Selective decrease in urinary AQP2 and increase in PGE2 excretion are associated with post obstructive polyuria in human congenital hydronephrosis [48]. The results obtained in this study revealed that AA differentially regulated the expression of AQP genes in the luminal epithelial cells and that this regulation was dependent on the phase of the estrous cycle. In the follicular phase, AA led to the upregulation of AQP2 mRNA, but, in the early luteal phase, AA upregulated both aquaporins AQP2 and AQP7. We can presume that prostaglandins may exert regulatory effects on AQP2 and 7. However, given that the present findings are based on gene expression, the results should be treated with caution. In consistence with these findings, our previous study also revealed the participation of AA, steroid hormones (E2 and P4), OT, FSK, and cAMP in the regulation of AQP1 and AQP5 expression at mRNA and protein level in the endometrium and myometrium of cyclic gilts during the mid-luteal phase and luteolysis [11,27]. The above results support the notion that steroid hormones, as well as other factors, including prostaglandins, cAMP, oxytocin, and arachidonic acid are important for the regulation of uterine AQPs, and may affect endometrial cellular functions.
The above studies allow us to more efficiently investigate the effect of AA and other factors/inhibitors in the endometrial cells in vitro on AQPs expression. In cultures of luminal epithelium, we found that, in the follicular phase of the estrous cycle, AA combined with H89 decreased expression of AQP5, but increased expression of AQP7. A different situation was observed on days 2–4 of the estrous cycle when progesterone in combination with the H89 upregulated expression of AQP5, while E2 combined with two doses of H89 (1 µM and 10 µM) and AA with H89 (10 µM) increased expression of AQP7 mRNA. The PKA signaling pathway is responsible for all the cellular responses induced by the cAMP second messenger system and plays an essential role in the integration of the signaling pathway networks in cells [49,50]. cAMP-dependent protein kinase via the transcription factor CREB plays a role in the regulation of the cell cycle, cell proliferation, and differentiation, as well as controlling of several metabolic reproductive processes like progesterone-induced oocyte maturation [51,52,53]. Lochner and Moolman showed that high-affinity N-[2-(p-bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide has been used greatly for the evaluation of the role of PKA in various cell types, e.g., epithelial, smooth muscle, embryonic, and neural cells [54,55]. H89 is involved in the regulation of PKA, which is required for estrogen binding and signaling to PI3K and it also plays role in placental steroidogenesis [56,57]. Yang et al. suggest that H89 also inhibited injury stimulated by AA in the podocyte [58].
We found that the expression of AQP1 and AQP5 at the mRNA level significantly decreased when endometrial luminal epithelial cells were treated with PD98059. In addition, following progesterone combined with PD98059 treatment, PD98059 attenuated the effect of P4 on the expression of AQP1 and AQP5 mRNA (days 18–20 of the estrous cycle). We have demonstrated by the use of a specific and potent MAPK inhibitor that the regulation of AQP1 and 5 expression at the mRNA level primarily occurs by the MAPK pathway. Incubation of uterine cells with PD98059, prevented the activation of ERK and blocked both AQPs expression at the mRNA level. This supports previous findings in the literature that MAPK signaling is implicated in the regulation of AQP1 and 5 [59,60]. Furthermore, it has also been shown that the p38 MAPK-dependent pathway is possibly the primary mechanism in controlling the altered expression of a number of major AQPs including AQP4 and AQP9 [61], as well as AQPs 3, 5, and 8 [62]. In contrast, when endometrial luminal epithelial cells from the early luteal phase of the estrous cycle were treated with E2 combined with PD98059, the expression of AQP5 mRNA was upregulated. As shown in our results, treatment with E2 combined with PD98059, and P4 with PD98059 significantly increased the expression of AQP7 mRNA in luminal epithelial cells derived from 2–4 days of the estrous cycle. These results demonstrate that examined AQPs isoforms are differentially regulated and can respond independently to environmental changes.
MAPKs are a family of serine-threonine kinases that integrate signals from a diverse range of stimuli and elicit an appropriate physiological response, including cellular differentiation, proliferation, inflammatory responses, and apoptosis in mammalian cells [63]. It was demonstrated that two isoforms of MAPKs, ERK1 (p44) and ERK2 (p42), express widely in mammalian oocytes and play a pivotal role in meiosis [64]. Similarly, MAP kinase is also involved in oocytes maturation, it encodes serine/threonine protein kinase, which can phosphorylate and activate MEK1. The pre-treatment of cells with the MEK1/MEK2 inhibitors, to which we include PD98059, resulted in a direct reduction in ERK1/ERK2 MAPK phosphorylation. MEK1/2 are dual-specificity kinases that phosphorylate and activate ERK, the classical MAP kinase [65]. The critical role of MAPK/ERK signaling on the expression of essential genes involved in the regulation of gonadotropic hormones was found. It has been proposed that the MAPK pathways are tightly regulated and cross-communicated with other signaling pathways [66].
The regulatory mechanisms underlying AQP gene and protein expression are complex and could be influenced by various physiological, pathological, or regulatory stimuli, including hormones, cytokines, and/or stress-activated signals.
5. Conclusions
Therefore, we can conclude that AQP1 and AQP5 gene expression in the porcine uterine luminal epithelial cells are regulated by estradiol, progesterone, and arachidonic acid through PKA and MAPK signaling pathways in the periovulatory period. The presented data may contribute to the existing knowledge of the mechanism linking signaling pathways and factors, which may affect uterine water homeostasis in pigs. Additionally, these data might be used as a basic reference for further studies in this research area.
Author Contributions
D.T. and M.T.S. conceived and designed the experiments; D.T. performed the tissue cultures, participated in data and statistical analysis, prepared the figures, prepared the manuscript; M.T.S. analyzed the data, interpreted the results of the experiments, supervised the manuscript preparation, A.S. interpreted the results of the experiments, participated writing the manuscript; M.T. analyzed the data; and E.L. contributed to the reagents. All authors have read and agreed to the published version of the manuscript.
Funding
A.S. is a recipient of the Statutory Fund of the School of Medicine, Collegium Medicum (61.610.001-300), University of Warmia and Mazury in Olsztyn. MTS is a recipient of the Grants 2013/09/B/NZ9/03129 and 2016/21/B/NZ9/03535 from the National Science Center (NSC).
Institutional Review Board Statement
All experiments were performed in accordance with the principles and procedures of the Animal Ethics Committee (number 32/2012), University of Warmia and Mazury in Olsztyn, Poland.
Data Availability Statement
All data are included in the paper. There are no databases associated with this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Bazer, F.W. Progesterone and Placental Hormone Actions on the Uterus: Insights from Domestic Animals. Biol. Reprod. 2004, 71, 2–10. [Google Scholar] [CrossRef]
- Ducza, E.; Csányi, A.; Gáspár, R. Aquaporins during Pregnancy: Their Function and Significance. Int. J. Mol. Sci. 2017, 18, 2593. [Google Scholar] [CrossRef]
- Kordowitzki, P.; Kranc, W.; Bryl, R.; Kempisty, B.; Skowronska, A.; Skowronski, M.T. The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals. Cells 2020, 9, 2570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tan, Y.-J.; Qu, F.; Sheng, J.-Z.; Huang, H.-F. Functions of water channels in male and female reproductive systems. Mol. Asp. Med. 2012, 33, 676–690. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Koide, S.S. The water channel gene in human uterus. Biochem. Mol. Boil. Int. 1994, 32, 371–377. [Google Scholar]
- Huang, H.-F.; He, R.-H.; Sun, C.-C.; Zhang, Y.; Meng, Q.-X.; Ma, Y.-Y. Function of aquaporins in female and male reproductive systems. Hum. Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jiang, Z.; Bazer, F.W.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Aquaporins in the female reproductive system of mammals. Front. Biosci. 2015, 20, 838–871. [Google Scholar] [CrossRef]
- Khan, S.; Ricciardelli, C.; Yool, A. Targeting Aquaporins in Novel Therapies for Male and Female Breast and Reproductive Cancers. Cells 2021, 10, 215. [Google Scholar] [CrossRef]
- Jablonski, E.M.; McConnell, N.A.; Hughes, F.M., Jr.; Huet-Hudson, Y.M. Estrogen Regulation of Aquaporins in the Mouse Uterus: Potential Roles in Uterine Water Movement. Biol. Reprod. 2003, 69, 1481–1487. [Google Scholar] [CrossRef]
- Zhu, X. Expression of AQP3 protein in hAECs is regulated by Camp-PKA-CREB signalling pathway. Front. Biosci. 2015, 20, 1047–1055. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skowrońska, A.; Młotkowska, P.; Wojciechowicz, B.; Okrasa, S.; Nielsen, S.; Skowronski, M.T. Progesterone, estradiol, arachidonic acid, oxytocin, forskolin and cAMP influence on aquaporin 1 and 5 expression in porcine uterine explants during the mid-luteal phase of the estrous cycle and luteolysis: An in vitro study. Reprod. Biol. Endocrinol. 2015, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Day, R.E.; Taylor, L.H.J.; Salman, M.M.; Bill, R.M.; Conner, M.T.; Conner, A.C. Identification and Molecular Mechanisms of the Rapid Tonicity-induced Relocalization of the Aquaporin 4 Channel. J. Biol. Chem. 2015, 290, 16873–16881. [Google Scholar] [CrossRef]
- Roche, J.V.; Survery, S.; Kreida, S.; Nesverova, V.; Ampah-Korsah, H.; Gourdon, M.; Deen, P.M.T.; Törnroth-Horsefield, S. Phosphorylation of human aquaporin 2 (AQP2) allosterically controls its interaction with the lysosomal trafficking protein LIP. J. Biol. Chem. 2017, 292, 14636–14648. [Google Scholar] [CrossRef]
- Yang, F.; Kawedia, J.D.; Menon, A.G. Cyclic AMP Regulates Aquaporin 5 Expression at Both Transcriptional and Post-transcriptional Levels through a Protein Kinase A Pathway. J. Biol. Chem. 2003, 278, 32173–32180. [Google Scholar] [CrossRef]
- Woo, J.; Chae, Y.K.; Jang, S.J.; Kim, M.S.; Baek, J.H.; Park, J.C.; Trink, B.; Ratovitski, E.; Lee, T.; Park, B.; et al. Membrane trafficking of AQP5 and cAMP dependent phosphorylation in bronchial epithelium. Biochem. Biophys. Res. Commun. 2008, 366, 321–327. [Google Scholar] [CrossRef]
- Nesverova, V.; Törnroth-Horsefield, S. Phosphorylation-Dependent Regulation of Mammalian Aquaporins. Cells 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, O.-H.; Lee, S.; Lee, J.; Park, H.; Park, M.; Chang, E.M.; Park, K.H.; Choi, Y. STK3/4 Expression Is Regulated in Uterine Endometrial Cells during the Estrous Cycle. Cells 2019, 8, 1643. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.; Sun, W.L.; Zhou, L.; Kreiter, C.; Jenab, S.; Quiñones-Jenab, V. PKA-mediated responses in females’ estrous cycle affect cocaine-induced responses in dopamine-mediated intracellular cascades. Neuroscience 2009, 161, 865–876. [Google Scholar] [CrossRef]
- Kim, S.T.; Moley, K.H. Regulation of Facilitative Glucose Transporters and AKT/MAPK/PRKAA Signaling via Estradiol and Progesterone in the Mouse Uterine Epithelium. Biol. Reprod. 2009, 81, 188–198. [Google Scholar] [CrossRef]
- Søberg, K.; Moen, L.V.; Skålhegg, B.S.; Laerdahl, J.K. Evolution of the cAMP-dependent protein kinase (PKA) catalytic subunit isoforms. PLoS ONE 2017, 12, e0181091. [Google Scholar] [CrossRef]
- Taylor, S.S.; Zhang, P.; Steichen, J.M.; Keshwani, M.M.; Kornev, A.P. PKA: Lessons learned after twenty years. Biochim. Biophys. Acta BBA Proteins Proteom. 2013, 1834, 1271–1278. [Google Scholar] [CrossRef]
- Engh, R.A.; Girod, A.; Kinzel, V.; Huber, R.; Bossemeyer, D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and HStructural implications for selectivity. J. Biol. Chem. 1996, 271. [Google Scholar] [CrossRef]
- Wang, K.; Hou, Y.; Gu, C.; Zhao, D.; Duan, Y.; Ran, Z.; Li, Q.; Li, X. Inhibitory effect of the mitogen activated protein kinase specific inhibitor PD98059 on Mtb-Ag-activated γδΤ cells. Int. J. Clin. Exp. Pathol. 2017, 10, 9644–9648. [Google Scholar] [PubMed]
- Ouyang, L.; Chen, Y.; Wang, X.Y.; Lu, R.F.; Zhang, S.Y.; Tian, M.; Xie, T.; Liu, B.; He, G. Polygonatum odoratum lectin induces apoptosis and autophagy via targeting EGFR-mediated Ras-Raf-MEK-ERK pathway in human MCF-7 breast cancer cells. Phytomedicine 2014, 21, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Okrasa, S.; Nielsen, S.; Skowronski, M.T. Modulatory effects of steroid hormones, oxytocin, arachidonic acid, forskolin and cyclic AMP on the expression of aquaporin 1 and aquaporin 5 in the porcine uterus during placentation. J. Physiol. Pharmacol. 2016, 67, 311–319. [Google Scholar]
- Skowronska, A.; Mlotkowska, P.; Majewski, M.; Nielsen, S.; Skowronski, M.T. Expression of aquaporin 1 and 5 and their regulation by ovarian hormones, arachidonic acid, forskolin and cAMP during implantation in pigs. Physiol. Res. 2016, 65, 637–650. [Google Scholar] [CrossRef]
- Skowronska, A.; Mlotkowska, P.; Nielsen, S.R.K.; Skowronski, M.T. Difference in expression between AQP1 and AQP5 in porcine endometrium and myometrium in response to steroid hormones, oxytocin, arachidonic acid, forskolin and cAMP during the mid-luteal phase of the estrous cycle and luteolysis. Reprod. Biol. Endocrinol. 2015, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Kwon, T.-H.; Nielsen, S. Immunolocalization of Aquaporin 1, 5, and 9 in the Female Pig Reproductive System. J. Histochem. Cytochem. 2008, 57, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T. Distribution and quantitative changes in amounts of aquaporin 1, 5 and 9 in the pig uterus during the estrous cycle and early pregnancy. Reprod. Biol. Endocrinol. 2010, 8, 109. [Google Scholar] [CrossRef]
- Wojtanowicz-Markiewicz, K.; Kulus, M.; Knap, S.; Kocherova, I.; Jankowski, M.; Stefańska, K.; Jeseta, M.; Piotrowska-Kempisty, H.; Bukowska, D.; Zabel, M.; et al. Expression of Selected Connexin and Aquaporin Genes and Real-Time Proliferation of Porcine Endometrial Luminal Epithelial Cells in Primary Culture Model. BioMed Res. Int. 2020, 1–15. [Google Scholar] [CrossRef]
- Ferré-Dolcet, L.; Yeste, M.; Vendrell, M.; Rigau, T.; Rodríguez-Gil, J.E.; Del Alamo, M.M.R. Uterine and placental specific localization of AQP2 and AQP8 is related with changes of serum progesterone levels in pregnant queens. Theriogenology 2020, 142, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Gao, J.; Brown, N.; Reese, J. Aquaporin Water Channel Genes Are Differentially Expressed and Regulated by Ovarian Steroids during the Periimplantation Period in the Mouse. Endocrinology 2003, 144, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.A.; Murphy, C.R. Aquaporins are upregulated in glandular epithelium at the time of implantation in the rat. J. Mol. Histol. 2007, 38, 87–95. [Google Scholar] [CrossRef] [PubMed]
- He, R.-H.; Sheng, J.-Z.; Luo, Q.; Jin, F.; Wang, B.; Qian, Y.-L.; Zhou, C.-Y.; Sheng, X.; Huang, H.-F. Aquaporin-2 expression in human endometrium correlates with serum ovarian steroid hormones. Life Sci. 2006, 79, 423–429. [Google Scholar] [CrossRef]
- Cui, D.; Sui, L.; Zhu, C.; Ma, Y.; Zhang, M.; Guo, Z.; Xie, Y.; Kong, Y. Expression of AQP 3 in the mice endometrium during estrous cycle and early pregnancy. Int. J. Clin. Exp. Pathol. 2016, 9, 8037–8046. [Google Scholar]
- Peng, H.; Zhang, Y.; Lei, L.; Chen, Q.; Yue, J.; Tan, Y.; Duan, E. Aquaporin 7 expression in postimplantation mouse uteri: A potential role for glycerol transport in uterine decidualization. Fertil. Steril. 2011, 95, 1514.e3–1517.e3. [Google Scholar] [CrossRef] [PubMed]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Veter. Res. 1968, 29, 1953–1957. [Google Scholar]
- Valenti, G.; Procino, G.; Carmosino, M.; Frigeri, A.; Mannucci, R.; Nicoletti, I.; Svelto, M. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J. Cell Sci. 2000, 113, 1985–1992. [Google Scholar]
- Yuan, W.; Bers, D.M. Protein kinase inhibitor H-89 reverses forskolin stimulation of cardiac L-type calcium current. Am. J. Physiol. Physiol. 1995, 268, C651–C659. [Google Scholar] [CrossRef]
- Chinigarzadeh, A.; Muniandy, S.; Salleh, N. Estradiol, progesterone and genistein differentially regulate levels of aquaporin (AQP)-1, 2, 5 and 7 expression in the uteri of ovariectomized, sex-steroid deficient rats. Steroids 2016, 115, 47–55. [Google Scholar] [CrossRef]
- He, R.; Han, W.; Hu, Y.; Chen, X.; Hu, X.; Zhu, Y. AQP2 is regulated by estradiol in human endometrium and is associated with spheroid attachment in vitro. Mol. Med. Rep. 2019, 20, 1306–1312. [Google Scholar] [CrossRef]
- Salleh, N.; Mokhtar, H.M.; Kassim, N.M.; Giribabu, N. Testosterone Induces Increase in Aquaporin (AQP)-1, 5, and 7 Expressions in the Uteri of Ovariectomized Rats. J. Membr. Biol. 2015, 248, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Park, J.E.; Yoo, I.; Han, J.; Kim, N.; Lim, W.J.; Cho, E.S.; Choi, B.; Choi, S.; Kim, T.H.; et al. Integrated transcriptomes throughout swine oestrous cycle reveal dynamic changes in reproductive tissues interacting networks. Sci. Rep. 2018, 8, 5436. [Google Scholar] [CrossRef] [PubMed]
- Zannetti, A.; Benga, G.; Brunetti, A.; Napolitano, F.; Avallone, L.; Pelagalli, A. Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells. Cells 2020, 9, 2678. [Google Scholar] [CrossRef]
- Norman, S.; Poyser, N. Effects of inhibitors of arachidonic acid turnover on the production of prostaglandins by the guinea-pig uterus. J. Reprod. Fertil. 2000, 118, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hertelendy, F.; Zakár, T. Prostaglandins and the myometrium and cervix. Prostaglandins, Leukot. Essent. Fat. Acids 2004, 70, 207–222. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; Zheng, F.; Guan, Y.; Zhang, X. Prostaglandin E2 in the Regulation of Water Transport in Renal Collecting Ducts. Int. J. Mol. Sci. 2017, 18, 2539. [Google Scholar] [CrossRef] [PubMed]
- Murer, L.; Addabbo, F.; Carmosino, M.; Procino, G.; Tamma, G.; Montini, G.; Rigamonti, W.; Zucchetta, P.; Della Vella, M.; Venturini, A.; et al. Selective Decrease in Urinary Aquaporin 2 and Increase in Prostaglandin E2 Excretion Is Associated with Postobstructive Polyuria in Human Congenital Hydronephrosis. J. Am. Soc. Nephrol. 2004, 15, 2705–2712. [Google Scholar] [CrossRef]
- Yarwood, S.J. Special Issue on “New Advances in Cyclic AMP Signalling”—An Editorial Overview. Cells 2020, 9, 2274. [Google Scholar] [CrossRef]
- Lochner, A.; Moolman, J.A. The many faces of H89: A review. Cardiovasc. Drug Rev. 2006, 24, 261–274. [Google Scholar] [CrossRef]
- Rosenberg, D.; Groussin, L.; Jullian, E.; Perlemoine, K.; Bertagna, X.; Bertherat, J. Role of the PKA-Regulated Transcription Factor CREB in Development and Tumorigenesis of Endocrine Tissues. Ann. N. Y. Acad. Sci. 2002, 968, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Eyers, P.A.; Liu, J.; Hayashi, N.R.; Lewellyn, A.L.; Gautier, J.; Maller, J.L. Regulation of the G2/M Transition in Xenopus Oocytes by the cAMP-dependent Protein Kinase. J. Biol. Chem. 2005, 280, 24339–24346. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Conti, M. New Pathways from PKA to the Cdc2/cyclin B Complex in Oocytes: Wee1B as a Potential PKA Substrate. Cell Cycle 2005, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Burvall, K.; Palmberg, L.; Larsson, K. Expression of TNFalpha and its receptors R1 and R2 in human alveolar epithelial cells exposed to organic dust and the effects of 8-bromo-cAMP and protein kinase A modulation. Inflamm. Res. 2005, 54, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, H.; Livnah, N.; Geiger, T.; Klein, S.; Ohne, O.; Cohen, I.; Benhar, M.; Gellerman, G.; Levitzki, A. Toward a PKB Inhibitor: Modification of a Selective PKA Inhibitor by Rational Design. Biochemistry 2002, 41, 10304–10314. [Google Scholar] [CrossRef]
- Cosentino, C.; Di Domenico, M.; Porcellini, A.; Cuozzo, C.; De Gregorio, G.; Santillo, M.R.; Agnese, S.; Di Stasio, R.; Feliciello, A.; Migliaccio, A.; et al. p85 regulatory subunit of PI3K mediates cAMP–PKA and estrogens biological effects on growth and survival. Oncogene 2006, 26, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Concha, C.; Flores-Herrera, O.; Olvera-Sanchez, S.; Espinosa-Garcia, M.T.; Martinez, F. Progesterone synthesis by human placental mitochondria is sensitive to PKA inhibition by H. Int. J. Biochem. Cell Biol. 2011, 43, 1402–1411. [Google Scholar] [CrossRef]
- Yang, L.; Pan, Y.; Wu, Y.; Lin, S.; Dai, B.; Chen, H.; Wan, J. Excessive arachidonic acid induced actin bunching remodeling and podocyte injury via a PKA-c-Abl dependent pathway. Exp. Cell Res. 2020, 388, 111808. [Google Scholar] [CrossRef]
- Umenishi, F.; Schrier, R.W. Hypertonicity-induced Aquaporin-1 (AQP1) Expression Is Mediated by the Activation of MAPK Pathways and Hypertonicity-responsive Element in the AQP1 Gene. J. Biol. Chem. 2003, 278, 15765–15770. [Google Scholar] [CrossRef]
- Ren, Y.; Lu, H.; Reinach, P.S.; Zheng, Q.; Li, J.; Tan, Q.; Zhu, H.; Chen, W. Hyperosmolarity-induced AQP5 upregulation promotes inflammation and cell death via JNK1/2 Activation in human corneal epithelial cells. Sci. Rep. 2017, 7, 4727. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamamoto, N.; Sobue, K.; Inagaki, M.; Ito, H.; Arima, H.; Morishima, T.; Takeuchi, A.; Tsuda, T.; Katsuya, H.; et al. Effect of mild hypothermia on the expression of aquaporin family in cultured rat astrocytes under hypoxic condition. Neurosci. Res. 2003, 47, 437–444. [Google Scholar] [CrossRef]
- Yang, M.; Gao, F.; Liu, H.; Yu, W.H.; Zhuo, F.; Qiu, G.P.; Ran, J.H.; Sun, S.Q. Hyperosmotic induction of aquaporin expression in rat astrocytes through a different MAPK pathway. J. Cell. Biochem. 2012, 114, 111–119. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Y.; Breitbart, H.; Schatten, H. Role of the MAPK cascade in mammalian germ cells. Reprod. Fertil. Dev. 1999, 11, 443–450. [Google Scholar] [CrossRef]
- Gotoh, Y.; Masuyama, N.; Dell, K.; Shirakabe, K.; Nishida, E. Initiation of Xenopus Oocyte Maturation by Activation of the Mitogen-activated Protein Kinase Cascade. J. Biol. Chem. 1995, 270, 25898–25904. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Weiskirchen, R. Usage of Mitogen-Activated Protein Kinase Small Molecule Inhibitors: More Than Just Inhibition! Front. Pharmacol. 2018, 9, 98. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).