Effects of TRPC6 Inactivation on Glomerulosclerosis and Renal Fibrosis in Aging Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunoblot Analysis and Enzyme-Linked Immunosorbent Assays
2.3. Histopathology and Immunohistochemistry
2.4. Statistical Analyses
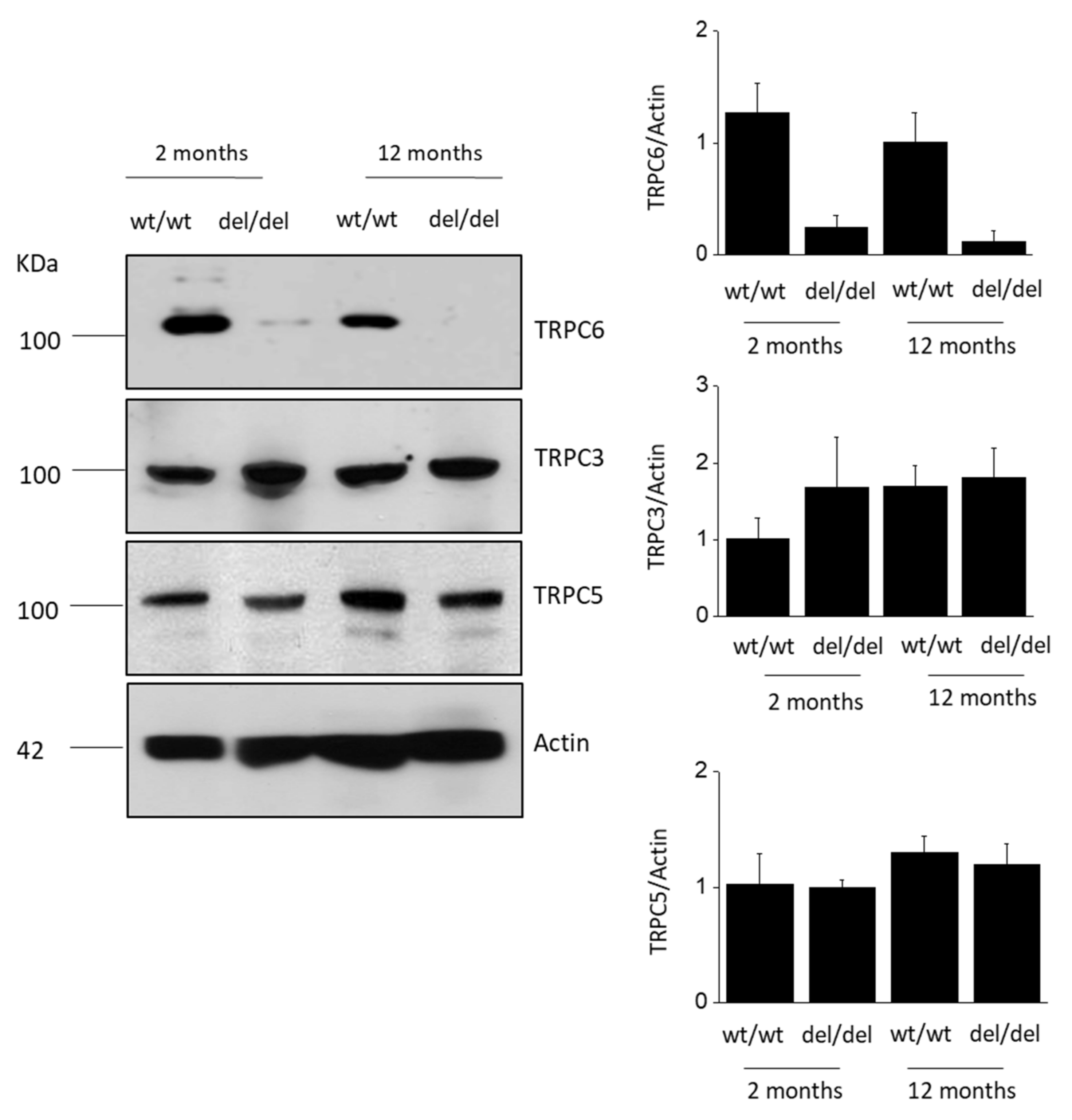
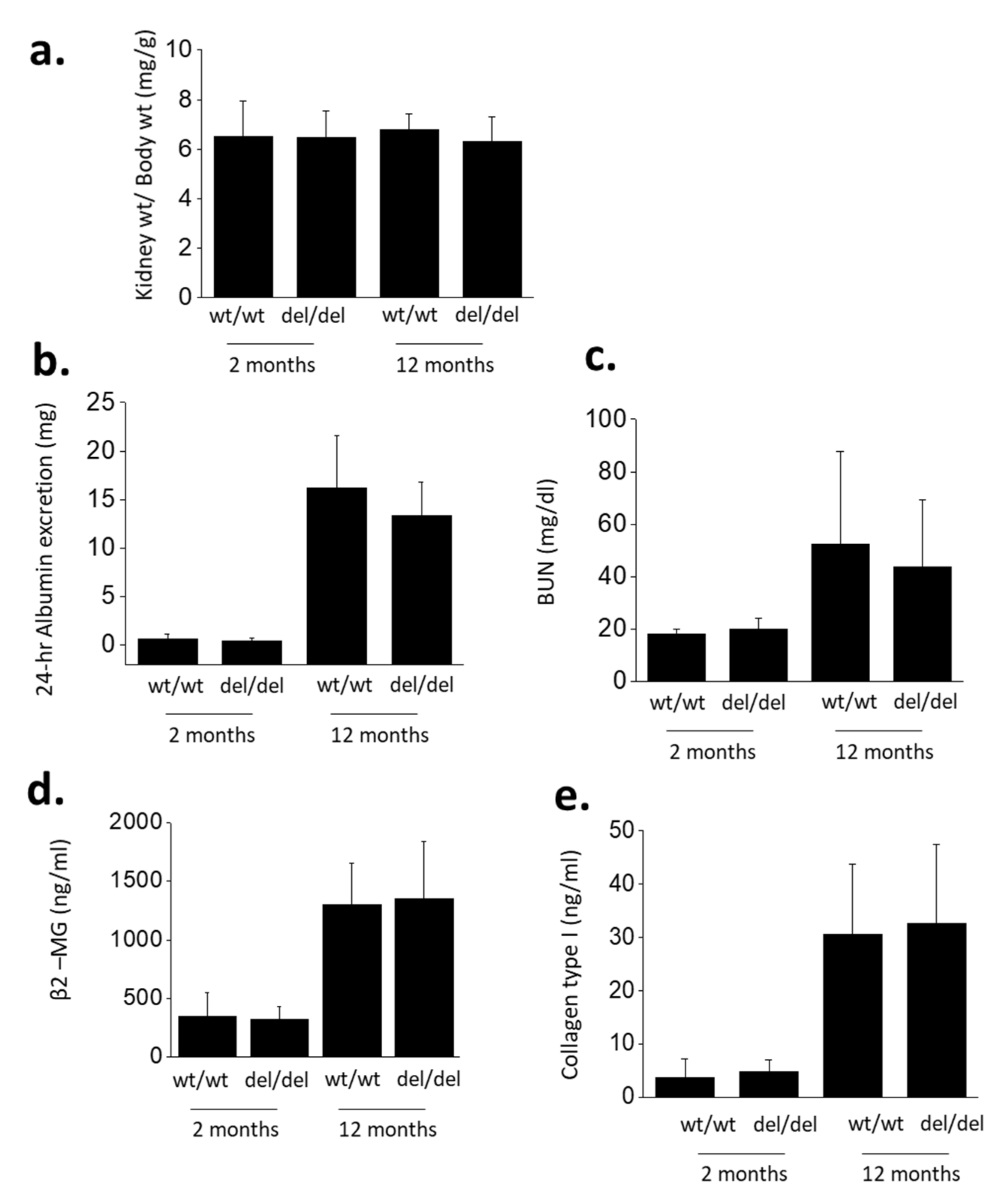
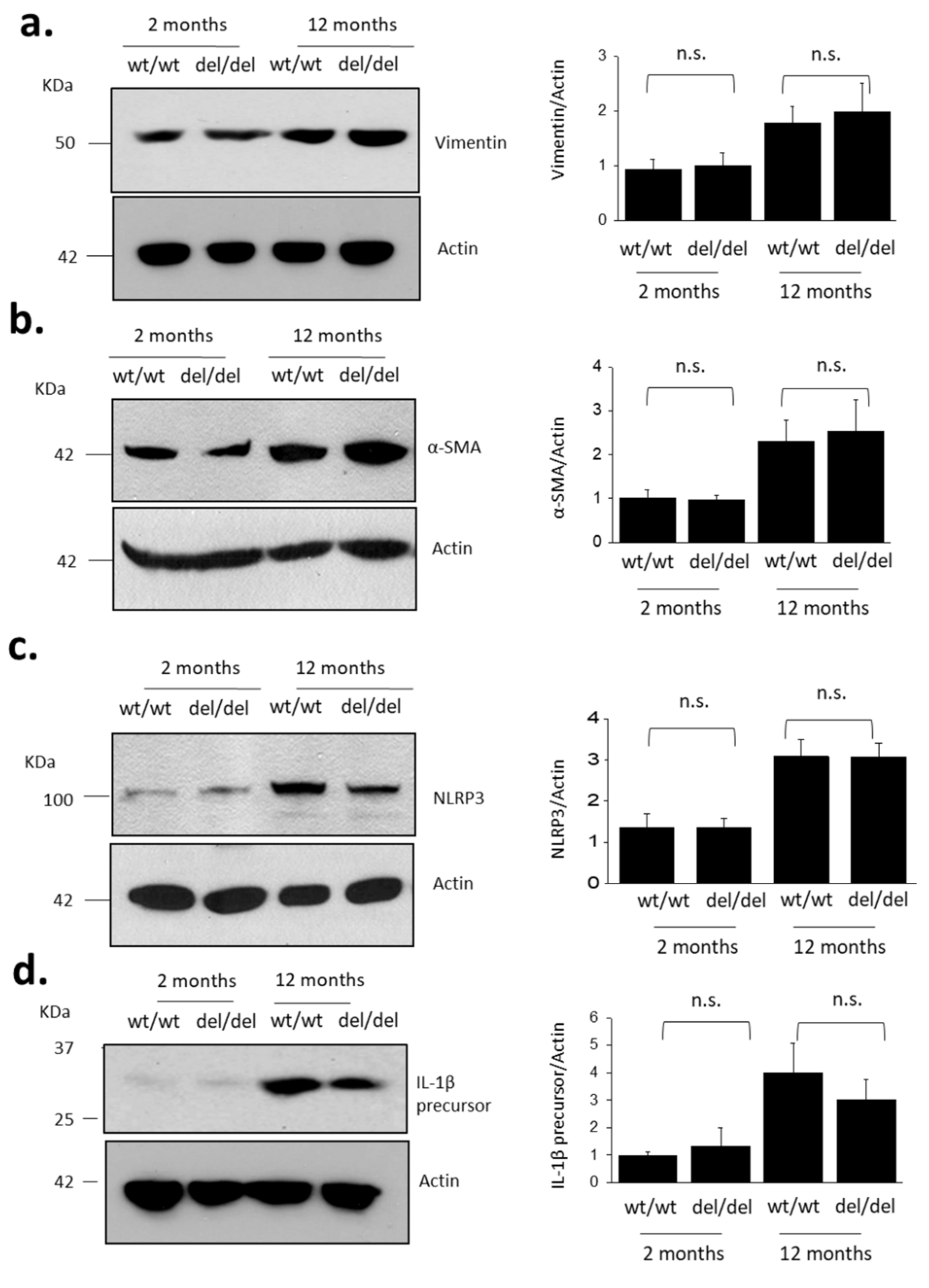
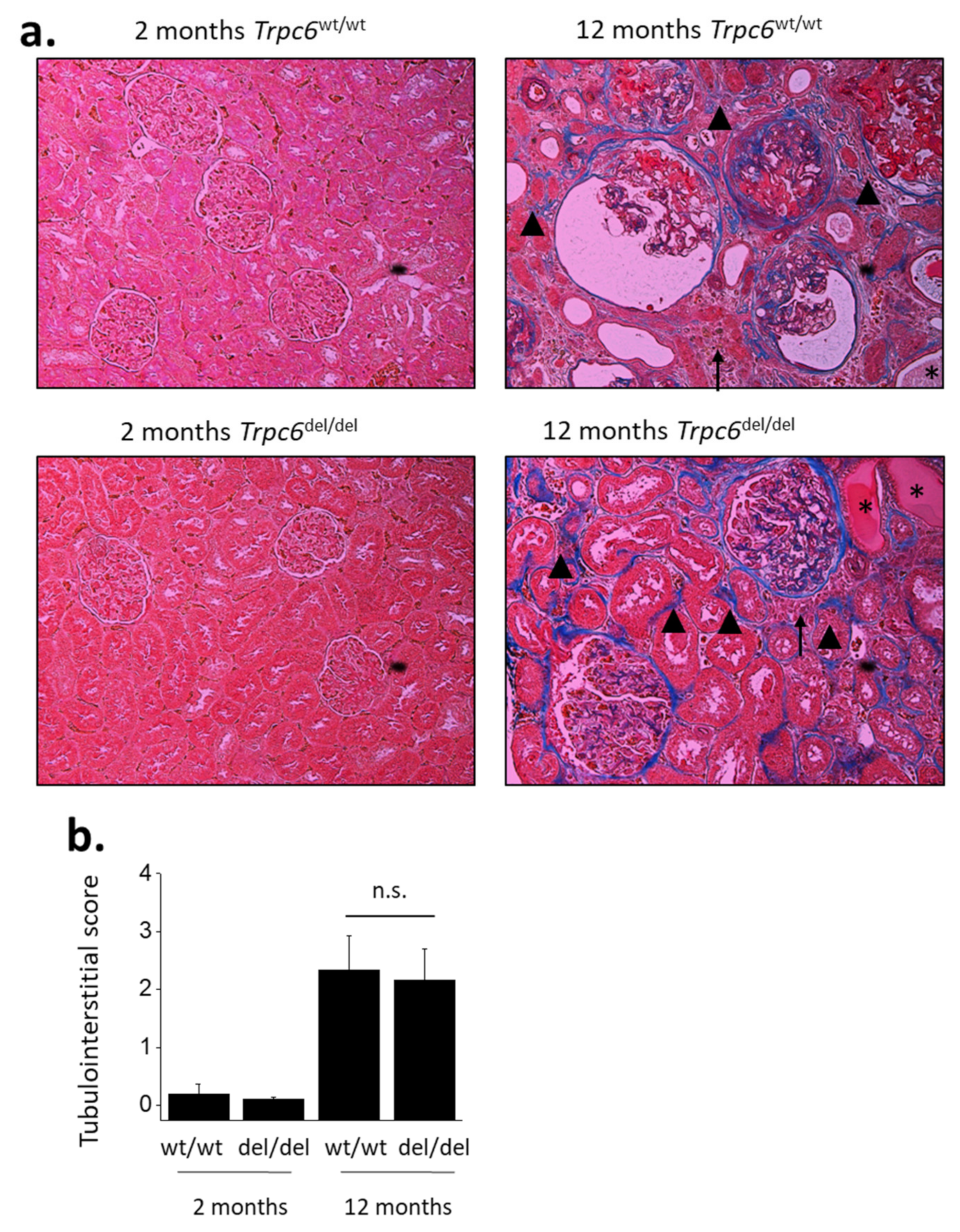
3. Results
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
References
- Davies, D.F.; Shock, N.W. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J. Clin. Investig. 1950, 29, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.; Pasternack, B.; Shah, H.; Gallo, G. Age-related incidence of sclerotic glomeruli in human kidneys. Am. J. Pathol. 1975, 80, 227–234. [Google Scholar] [PubMed]
- Jones, C.A.; Francis, M.E.; Eberhardt, M.S.; Chavers, B.; Coresh, J.; Engelgau, M.; Kusek, J.W.; Byrd-Holt, D.; Narayan, K.; Herman, W.H.; et al. Microalbuminuria in the US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2002, 39, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, N.K.; Adams, D.F.; Solomon, H.S.; Rashid, A.; Abrams, H.L.; Merrill, J.P. Senescence and the Renal Vasculature in Normal Man. Circ. Res. 1974, 34, 309–316. [Google Scholar] [CrossRef]
- Wiggins, J. Podocytes and Glomerular Function with Aging. Semin. Nephrol. 2009, 29, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Rennke, H.G.; Zatz, R. Glomerular adaptations with normal aging and with long-term converting enzyme inhibition in rats. Am. J. Physiol. 1994, 267, F35–F43. [Google Scholar] [CrossRef] [PubMed]
- Bertani, T.; Zoja, C.; Abbate, M.; Rossini, M.; Remuzzi, G. Age-related nephropathy and proteinuria in rats with intact kidneys exposed to diets with different protein content. Lab. Investig. 1989, 6, 196–204. [Google Scholar]
- Ding, G.; Franki, N.; Kapasi, A.A.; Reddy, K.; Gibbons, N.; Singhal, P.C. Tubular Cell Senescence and Expression of TGF-β1 and p21WAF1/CIP1 in Tubulointerstitial Fibrosis of Aging Rats. Exp. Mol. Pathol. 2001, 70, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.P.; Conlon, P.J.; Lynn, K.L.; Farrington, M.K.; Creazzo, T.; Hawkins, A.F.; Daskalakis, N.; Kwan, S.Y.; Ebersviller, S.; Burchette, J.L.; et al. A Mutation in the TRPC6 Cation Channel Causes Familial Focal Segmental Glomerulosclerosis. Science 2005, 308, 1801–1804. [Google Scholar] [CrossRef]
- Möller, C.C.; Wei, C.; Altintas, M.M.; Li, J.; Greka, A.; Ohse, T.; Pippin, J.W.; Rastaldi, M.P.; Wawersik, S.; Schiavi, S.; et al. Induction of TRPC6 Channel in Acquired Forms of Proteinuric Kidney Disease. J. Am. Soc. Nephrol. 2007, 18, 29–36. [Google Scholar] [CrossRef]
- Kim, E.Y.; Roshanravan, H.; Dryer, S.E. Changes in podocyte TRPC channels evoked by plasma and sera from patients with recurrent FSGS and by putative glomerular permeability factors. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2342–2354. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Khayyat, N.H.; Dryer, S.E. Mechanisms underlying modulation of podocyte TRPC6 channels by suPAR: Role of NADPH oxidases and Src family tyrosine kinases. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Shotorbani, P.Y.; Dryer, S.E. Trpc6 inactivation confers protection in a model of severe nephrosis in rats. J. Mol. Med. 2018, 96, 631–644. [Google Scholar] [CrossRef]
- Wu, Y.L.; Xie, J.; An, S.W.; Oliver, N.; Barrezueta, N.X.; Lin, M.H.; Birnbaumer, L.; Huang, C.L. Inhibition of TRPC6 channels ameliorates renal fibrosis and contributes to renal protection by soluble klotho. Kidney Int. 2017, 91, 830–841. [Google Scholar] [CrossRef]
- Riazanski, V.; Gabdoulkhakova, A.G.; Boynton, L.S.; Eguchi, R.R.; Deriy, L.V.; Hogarth, D.K.; Loaëc, N.; Oumata, N.; Galons, H.; Brown, M.E.; et al. TRPC6 channel translocation into phagosomal membrane augments phagosomal function. Proc. Natl. Acad. Sci. USA 2015, 112, E6486–E6495. [Google Scholar] [CrossRef]
- Lindemann, O.; Umlauf, D.; Frank, S.; Schimmelpfennig, S.; Bertrand, J.; Pap, T.; Hanley, P.J.; Fabian, A.; Dietrich, A.; Schwab, A. TRPC6 Regulates CXCR2-Mediated Chemotaxis of Murine Neutrophils. J. Immunol. 2013, 190, 5496–5505. [Google Scholar] [CrossRef]
- Kim, E.Y.; Shotorbani, P.Y.; Dryer, S.E. TRPC6 inactivation does not affect loss of renal function in nephrotoxic serum glomerulonephritis in rats, but reduces severity of glomerular lesions. Biochem. Biophys. Rep. 2019, 17, 139–150. [Google Scholar] [CrossRef]
- Wiggins, J.E.; Goyal, M.; Sanden, S.K.; Wharram, B.L.; Shedden, K.A.; Misek, D.E.; Kuick, R.D.; Wiggins, R.C. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J. Am. Soc. Nephrol. 2005, 16, 2953–2966. [Google Scholar] [CrossRef]
- Dickson, L.E.; Wagner, M.C.; Sandoval, R.M.; Molitoris, B.A. The Proximal Tubule and Albuminuria: Really! J. Am. Soc. Nephrol. 2014, 25, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A. Progression in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2005, 12, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G.; Johnson, R.J. Mechanisms of Progressive Renal Disease in Glomerulonephritis. Am. J. Kidney Dis. 1994, 23, 193–198. [Google Scholar] [CrossRef]
- Latz, E.; Duewell, P. NLRP3 inflammasome activation in inflammaging. Semin. Immunol. 2018, 40, 61–73. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Niemir, Z.I.; Stein, H.; Dworacki, G.; Mundel, P.; Koehl, N.; Koch, B.; Autschbach, F.; Andrassy, K.; Ritz, E.; Waldherr, R.; et al. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritides. Kidney Int. 1997, 52, 393–403. [Google Scholar] [CrossRef]
- Kim, J.-H.; Xie, J.; Hwang, K.-H.; Wu, Y.-L.; Oliver, N.; Eom, M.; Park, K.-S.; Barrezueta, N.; Kong, I.-D.; Fracasso, R.P.; et al. Klotho May Ameliorate Proteinuria by Targeting TRPC6 Channels in Podocytes. J. Am. Soc. Nephrol. 2017, 28, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh Khayyat, N.; Kim, E.Y.; Dryer, S.E. TRPC6 inactivation does not protect against diabetic kidney disease in streptozotocin (STZ)-treated Sprague-Dawley rats. FASEB BioAdv. 2019, 1, 773–782. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, M.; Kalhorn, T.F.; Ho, H.T.B.; Wang, J. Podocyte-specific expression of organic cation transporter PMAT: Implication in puromycin aminonucleoside nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2009, 296, F1307–F1313. [Google Scholar] [CrossRef]
- Kim, Y.H.; Goyal, M.; Kurnit, D.; Wharram, B.; Wiggins, J.; Holzman, L.; Kershaw, D.; Wiggins, R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001, 60, 957–968. [Google Scholar] [CrossRef] [PubMed]
- LeHir, M.; Kriz, W. New insights into structural patterns encountered in glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 2007, 16, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Kim, E.Y.; Hagmann, H.; Benzing, T.; Dryer, S.E. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am. J. Physiol. Cell Physiol. 2013, 305, C276–C289. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Li, A.; Westhorpe, C.L.; Lo, C.Y.; Abeynaike, L.D.; Snelgrove, S.L.; Hall, P.; Ooi, J.D.; Sobey, C.G.; Kitching, A.R.; et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat. Med. 2013, 19, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, N.; Gao, Y.; Kohan, D.E. Characterization of flow-regulated cortical collecting duct endothelin-1 production. Physiol. Rep. 2017, 5, e13126. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, C.; Zhang, D.; Xin, Y.; Li, J.; Zhang, Y.; Chu, X. Increased TRPC6 expression is associated with tubular epithelial cell proliferation and inflammation in diabetic nephropathy. Mol. Immunol. 2018, 94, 75–81. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.Y.; Dryer, S.E. Effects of TRPC6 Inactivation on Glomerulosclerosis and Renal Fibrosis in Aging Rats. Cells 2021, 10, 856. https://doi.org/10.3390/cells10040856
Kim EY, Dryer SE. Effects of TRPC6 Inactivation on Glomerulosclerosis and Renal Fibrosis in Aging Rats. Cells. 2021; 10(4):856. https://doi.org/10.3390/cells10040856
Chicago/Turabian StyleKim, Eun Young, and Stuart E. Dryer. 2021. "Effects of TRPC6 Inactivation on Glomerulosclerosis and Renal Fibrosis in Aging Rats" Cells 10, no. 4: 856. https://doi.org/10.3390/cells10040856
APA StyleKim, E. Y., & Dryer, S. E. (2021). Effects of TRPC6 Inactivation on Glomerulosclerosis and Renal Fibrosis in Aging Rats. Cells, 10(4), 856. https://doi.org/10.3390/cells10040856




