Atherothrombosis in Acute Coronary Syndromes—From Mechanistic Insights to Targeted Therapies
Abstract
1. Introduction
2. Development and Progression of the Atherosclerotic Plaques
3. Plaque Destabilization and Disruption
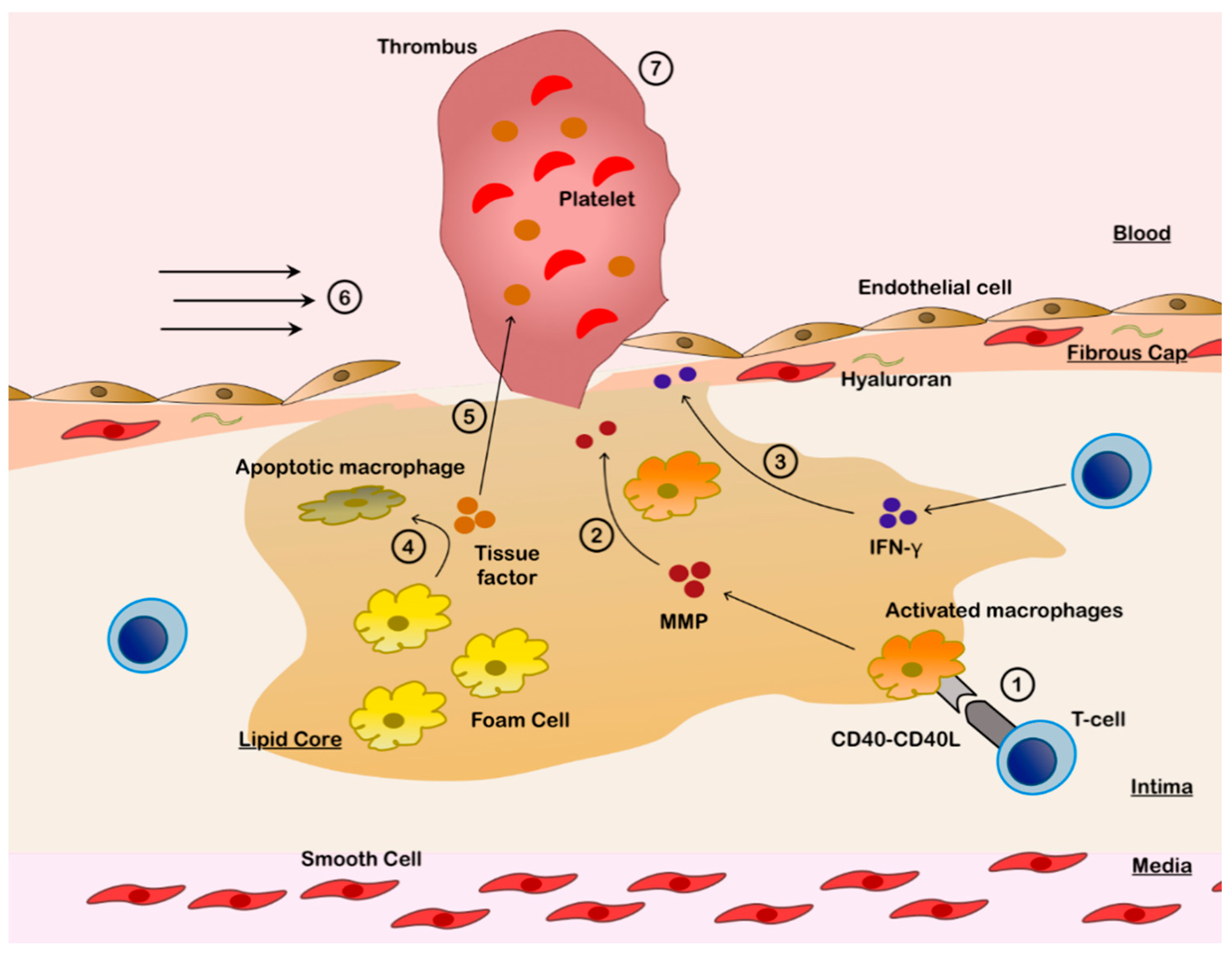
3.1. Plaque Rupture
3.2. Plaque Erosion
3.3. Calcified Nodules
4. Clinical and Angiographic Features of ACS According to the Culprit Lesion Subtypes
5. Culprit Plaque Evaluation Using Intravascular Imaging
5.1. Plaques with RFC on Intravascular Imaging
5.2. Plaques with IFC on Intravascular Imaging
5.3. Eruptive Calcified Nodules on Intravascular Imaging
6. OCT-Guided Treatment of ACS
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.H.; Smialek, J.; Virmani, R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 1997, 336, 1276–1282. [Google Scholar] [CrossRef]
- Farb, A.; Tang, A.L.; Burke, A.P.; Sessums, L.; Liang, Y.; Virmani, R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation 1995, 92, 1701–1709. [Google Scholar] [CrossRef]
- Virmani, R.; Frank, D.K.; Allen, P.B.; Farb, A.; Stephen, M.S. Lessons From Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994, 89, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Seimon, T.; Tabas, I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 2009, 50, S382–S387. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Chen, H.; Dan, J.; Cheng, J.; Guo, S.; Sun, X.; Wang, W.; Ai, Y.; Li, S.; et al. The Predominant Pathway of Apoptosis in THP-1 Macrophage-Derived Foam Cells Induced by 5-Aminolevulinic Acid-Mediated Sonodynamic Therapy is the Mitochondria-Caspase Pathway Despite the Participation of Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2014, 33, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Insull, W., Jr. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122, S3–S14. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Galliher-Beckley, A.J.; Lan, L.-Q.; Aono, S.; Wang, L.; Shi, J. Caspase-1 activation and mature interleukin-1β release are uncoupled events in monocytes. World J. Biol. Chem. 2013, 4, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feuerstein, G.Z.; Gu, J.-L.; Lysko, P.G.; Yue, T.-L. Interleukin-1β induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis 1995, 115, 89–98. [Google Scholar] [CrossRef]
- Darrieutort-Laffite, C.; Boutet, M.-A.; Chatelais, M.; Brion, R.; Blanchard, F.; Heymann, D.; Le Goff, B. IL-1β and TNFα Promote Monocyte Viability through the Induction of GM-CSF Expression by Rheumatoid Arthritis Synovial Fibroblasts. Mediat. Inflamm. 2014, 2014, 241840. [Google Scholar] [CrossRef]
- Hsu, L.C.; Enzler, T.; Seita, J.; Timmer, A.M.; Lee, C.Y.; Lai, T.Y.; Yu, G.Y.; Lai, L.C.; Temkin, V.; Sinzig, U.; et al. IL-1β-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKβ. Nat. Immunol. 2011, 12, 144–150. [Google Scholar] [CrossRef]
- Galis, Z.S.; Muszynski, M.; Sukhova, G.K.; Simon-Morrissey, E.; Unemori, E.N.; Lark, M.W.; Amento, E.; Libby, P. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ. Res. 1994, 75, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Sarén, P.; Welgus, H.G.; Kovanen, P.T. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J. Immunol. 1996, 157, 4159–4165. [Google Scholar] [PubMed]
- Bennett, M.R.; Evan, G.I.; Schwartz, S.M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Investig. 1995, 95, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Antoniou, C.K.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Hear. Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Farb, A.; Burke, A.P.; Tang, A.L.; Liang, T.Y.; Mannan, P.; Smialek, J.; Virmani, R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996, 93, 1354–1363. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Schönbeck, U.; Libby, P. CD40 Signaling and Plaque Instability. Circ. Res. 2001, 89, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.G. The expression of CD40-CD40L and activities of matrix metalloproteinases in atherosclerotic rats. Mol. Cell Biochem. 2006, 282, 141–146. [Google Scholar] [CrossRef]
- Amento, E.P.; Ehsani, N.; Palmer, H.; Libby, P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arter. Thromb. 1991, 11, 1223–1230. [Google Scholar] [CrossRef]
- Cheng, G.C.; Loree, H.M.; Kamm, R.D.; Fishbein, M.C.; Lee, R.T. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation 1993, 87, 1179–1187. [Google Scholar] [CrossRef]
- Richardson, P.D.; Davies, M.J.; Born, G.V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 1989, 2, 941–944. [Google Scholar] [CrossRef]
- Araki, M.; Soeda, T.; Kim, H.O.; Thondapu, V.; Russo, M.; Kurihara, O.; Shinohara, H.; Minami, Y.; Higuma, T.; Lee, H.; et al. Spatial Distribution of Vulnerable Plaques: Comprehensive In Vivo Coronary Plaque Mapping. JACC Cardiovasc. Imaging 2020, 13, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.C.; Rittersma, S.Z.; de Winter, R.J.; Ladich, E.R.; Fowler, D.R.; Liang, Y.H.; Kutys, R.; Carter-Monroe, N.; Kolodgie, F.D.; van der Wal, A.C.; et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J. Am. Coll. Cardiol. 2010, 55, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lee, W.S.; Mintz, G.S.; Hong, Y.J.; Lee, S.Y.; Kim, K.S.; Hahn, J.Y.; Kumar, K.S.; Won, H.; Hyeon, S.H.; et al. Multimodality Intravascular Imaging Assessment of Plaque Erosion versus Plaque Rupture in Patients with Acute Coronary Syndrome. Korean Circ. J. 2016, 46, 499–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liuzzo, G.; Pedicino, D.; Vinci, R.; Crea, F. CD8 lymphocytes and plaque erosion: A new piece in the jigsaw. Eur. Heart J. 2020, 41, 3561–3563. [Google Scholar] [CrossRef] [PubMed]
- Leistner, D.M.; Krankel, N.; Meteva, D.; Abdelwahed, Y.S.; Seppelt, C.; Stahli, B.E.; Rai, H.; Skurk, C.; Lauten, A.; Mochmann, H.C.; et al. Differential immunological signature at the culprit site distinguishes acute coronary syndrome with intact from acute coronary syndrome with ruptured fibrous cap: Results from the prospective translational OPTICO-ACS study. Eur. Heart J. 2020, 41, 3549–3560. [Google Scholar] [CrossRef]
- di Mario, C.; Koskinas, K.C.; Räber, L. Clinical Benefit of IVUS Guidance for Coronary Stenting: The ULTIMATE Step Toward Definitive Evidence? J. Am. Coll. Cardiol. 2018, 72, 3138–3141. [Google Scholar] [CrossRef]
- Tricot, O.; Mallat, Z.; Heymes, C.; Belmin, J.; Lesèche, G.; Tedgui, A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation 2000, 101, 2450–2453. [Google Scholar] [CrossRef]
- Fracassi, F.; Crea, F.; Sugiyama, T.; Yamamoto, E.; Uemura, S.; Vergallo, R.; Porto, I.; Lee, H.; Fujimoto, J.; Fuster, V.; et al. Healed Culprit Plaques in Patients With Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2019, 73, 2253–2263. [Google Scholar] [CrossRef]
- Quillard, T.; Araújo, H.A.; Franck, G.; Shvartz, E.; Sukhova, G.; Libby, P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: Implications for superficial erosion. Eur. Heart J. 2015, 36, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Mullick, A.E.; Soldau, K.; Kiosses, W.B.; Bell, T.A., 3rd; Tobias, P.S.; Curtiss, L.K. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J. Exp. Med. 2008, 205, 373–383. [Google Scholar] [CrossRef]
- Komarova, Y.A.; Huang, F.; Geyer, M.; Daneshjou, N.; Garcia, A.; Idalino, L.; Kreutz, B.; Mehta, D.; Malik, A.B. VE-cadherin signaling induces EB3 phosphorylation to suppress microtubule growth and assemble adherens junctions. Mol. Cell 2012, 48, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Nakano, M.; Prati, F.; Niccoli, G.; Mallus, M.T.; Ramazzotti, V.; Montone, R.A.; Kolodgie, F.D.; Virmani, R.; Crea, F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: A clinicopathological study. Circulation 2010, 122, 2505–2513. [Google Scholar] [CrossRef]
- Gupta, A.K.; Joshi, M.B.; Philippova, M.; Erne, P.; Hasler, P.; Hahn, S.; Resink, T.J. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010, 584, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Travis, J.G.; Trang, T.V.; Laura, L.S.; Dhruva, J.D.; Safiah, H.C.M.; Jeffrey, I.W.; Patricia, C.L. Neutrophil Extracellular Traps Promote Thrombin Generation Through Platelet-Dependent and Platelet-Independent Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1977–1984. [Google Scholar] [CrossRef]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef]
- Pedicino, D.; Vinci, R.; Giglio, A.F.; Pisano, E.; Porto, I.; Vergallo, R.; Russo, G.; Ruggio, A.; D’Aiello, A.; Flego, D.; et al. Alterations of Hyaluronan Metabolism in Acute Coronary Syndrome: Implications for Plaque Erosion. J. Am. Coll. Cardiol. 2018, 72, 1490–1503. [Google Scholar] [CrossRef]
- Ali, Z.A.; Galougahi, K.K.; Maehara, A.; Shlofmitz, R.A.; Ben-Yehuda, O.; Mintz, G.S.; Stone, G.W. Intracoronary Optical Coherence Tomography 2018: Current Status and Future Directions. JACC Cardiovasc. Interv. 2017, 10, 2473–2487. [Google Scholar] [CrossRef]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef]
- Teder, P.; Vandivier, R.W.; Jiang, D.; Liang, J.; Cohn, L.; Puré, E.; Henson, P.M.; Noble, P.W. Resolution of lung inflammation by CD44. Science 2002, 296, 155–158. [Google Scholar] [CrossRef]
- Kong, X.; Chen, L.; Ye, P.; Wang, Z.; Zhang, J.; Ye, F.; Chen, S. The role of HYAL2 in LSS-induced glycocalyx impairment and the PKA-mediated decrease in eNOS-Ser-633 phosphorylation and nitric oxide production. Mol. Biol. Cell 2016, 27, 3972–3979. [Google Scholar] [CrossRef]
- Koshiishi, I.; Shizari, M.; Underhill, C.B. CD44 can mediate the adhesion of platelets to hyaluronan. Blood 1994, 84, 390–396. [Google Scholar] [CrossRef]
- Lesley, J.; Howes, N.; Perschl, A.; Hyman, R. Hyaluronan binding function of CD44 is transiently activated on T cells during an in vivo immune response. J. Exp. Med. 1994, 180, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Davis, H.R.; Arbustini, E.; Virmani, R. Sex differences in coronary artery disease: Pathological observations. Atherosclerosis 2015, 239, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Kolodgie, F.D.; Otsuka, F.; Finn, A.V.; Davis, H.R.; Joner, M.; Virmani, R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Mintz, G.S.; Matsumura, M.; Zhang, W.; Cao, Y.; Usui, E.; Kanaji, Y.; Murai, T.; Yonetsu, T.; Kakuta, T.; et al. Prevalence, Predictors, and Clinical Presentation of a Calcified Nodule as Assessed by Optical Coherence Tomography. JACC Cardiovasc. Imaging 2017, 10, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Dal Bello, B.; Morbini, P.; Burke, A.P.; Bocciarelli, M.; Specchia, G.; Virmani, R. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999, 82, 269. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Dai, J.; Xing, L.; Jia, H.; Zhu, Y.; Zhang, S.; Hu, S.; Lin, L.; Ma, L.; Liu, H.; Xu, M.; et al. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: A clinical, angiographical, and intravascular optical coherence tomography study. Eur. Heart J. 2018, 39, 2077–2085. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, Y.; Zhang, Y.; Dai, J.; Li, L.; Dauerman, H.; Soeda, T.; Wang, Z.; Lee, H.; Wang, C.; et al. Management and Outcome of Patients With Acute Coronary Syndrome Caused by Plaque Rupture Versus Plaque Erosion: An Intravascular Optical Coherence Tomography Study. J. Am. Heart Assoc. 2017, 6, e004730. [Google Scholar] [CrossRef]
- Theuerle, J.; Yudi, M.B.; Farouque, O.; Andrianopoulos, N.; Scott, P.; Ajani, A.E.; Brennan, A.; Duffy, S.J.; Reid, C.M.; Clark, D.J. Utility of the ACC/AHA lesion classification as a predictor of procedural, 30-day and 12-month outcomes in the contemporary percutaneous coronary intervention era. Catheter. Cardiovasc. Interv. 2018, 92, E227–E234. [Google Scholar] [CrossRef]
- Burzotta, F.; Leone, A.M.; Aurigemma, C.; Zambrano, A.; Zimbardo, G.; Arioti, M.; Vergallo, R.; De Maria, G.L.; Cerracchio, E.; Romagnoli, E.; et al. Fractional Flow Reserve or Optical Coherence Tomography to Guide Management of Angiographically Intermediate Coronary Stenosis: A Single-Center Trial. JACC Cardiovasc. Interv. 2020, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.A.; Winters, S.L.; Arora, R.R.; Haft, J.I.; Goldstein, J.; Rentrop, K.P.; Gorlin, R.; Fuster, V. Coronary angiographic morphology in myocardial infarction: A link between the pathogenesis of unstable angina and myocardial infarction. J. American Coll. Cardiol. 1985, 6, 1233–1238. [Google Scholar] [CrossRef]
- Zir, L.M.; Miller, S.W.; Dinsmore, R.E.; Gilbert, J.P.; Harthorne, J.W. Interobserver variability in coronary angiography. Circulation 1976, 53, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Kotani, J.; Mintz, G.S.; Castagna, M.T.; Pinnow, E.; Berzingi, C.O.; Bui, A.B.; Pichard, A.D.; Satler, L.F.; Suddath, W.O.; Waksman, R.; et al. Intravascular ultrasound analysis of infarct-related and non-infarct-related arteries in patients who presented with an acute myocardial infarction. Circulation 2003, 107, 2889–2893. [Google Scholar] [CrossRef]
- Madder, R.D.; Goldstein, J.A.; Madden, S.P.; Puri, R.; Wolski, K.; Hendricks, M.; Sum, S.T.; Kini, A.; Sharma, S.; Rizik, D.; et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2013, 6, 838–846. [Google Scholar] [CrossRef]
- Kubo, T.; Ino, Y.; Tanimoto, T.; Kitabata, H.; Tanaka, A.; Akasaka, T. Optical coherence tomography imaging in acute coronary syndromes. Cardiol. Res. Pract. 2011, 2011, 312978. [Google Scholar] [CrossRef]
- Kume, T.; Akasaka, T.; Kawamoto, T.; Watanabe, N.; Toyota, E.; Neishi, Y.; Sukmawan, R.; Sadahira, Y.; Yoshida, K. Assessment of coronary arterial plaque by optical coherence tomography. Am. J. Cardiol. 2006, 97, 1172–1175. [Google Scholar] [CrossRef]
- Yabushita, H.; Bouma, B.E.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Kang, D.H.; Halpern, E.F.; et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002, 106, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Guagliumi, G.; Capodanno, D.; Saia, F.; Musumeci, G.; Tarantini, G.; Garbo, R.; Tumminello, G.; Sirbu, V.; Coccato, M.; Fineschi, M.; et al. Mechanisms of atherothrombosis and vascular response to primary percutaneous coronary intervention in women versus men with acute myocardial infarction: Results of the OCTAVIA study. JACC Cardiovasc. Interv. 2014, 7, 958–968. [Google Scholar] [CrossRef]
- Chandran, S.; Watkins, J.; Abdul-Aziz, A.; Shafat, M.; Calvert, P.A.; Bowles, K.M.; Flather, M.D.; Rushworth, S.A.; Ryding, A.D. Inflammatory Differences in Plaque Erosion and Rupture in Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e005868. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Montone, R.A.; Di Vito, L.; Gramegna, M.; Refaat, H.; Scalone, G.; Leone, A.M.; Trani, C.; Burzotta, F.; Porto, I.; et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur. Heart J. 2015, 36, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Kajander, O.A.; Pinilla-Echeverri, N.; Jolly, S.S.; Bhindi, R.; Huhtala, H.; Niemela, K.; Fung, A.; Vijayaraghavan, R.; Alexopoulos, D.; Sheth, T. Culprit plaque morphology in STEMI-an optical coherence tomography study: Insights from the TOTAL-OCT substudy. EuroIntervention 2016, 12, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 1166–1176. [Google Scholar] [CrossRef]
- Kubo, T.; Imanishi, T.; Takarada, S.; Kuroi, A.; Ueno, S.; Yamano, T.; Tanimoto, T.; Matsuo, Y.; Masho, T.; Kitabata, H.; et al. Assessment of culprit lesion morphology in acute myocardial infarction: Ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 2007, 50, 933–939. [Google Scholar] [CrossRef]
- Khalifa, A.K.M.; Kubo, T.; Ino, Y.; Terada, K.; Emori, H.; Higashioka, D.; Katayama, Y.; Takahata, M.; Shimamura, K.; Shiono, Y.; et al. Optical Coherence Tomography Comparison of Percutaneous Coronary Intervention Among Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. Circ. J. 2020, 84, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Ino, Y.; Kubo, T.; Matsuo, Y.; Yamaguchi, T.; Shiono, Y.; Shimamura, K.; Katayama, Y.; Nakamura, T.; Aoki, H.; Taruya, A.; et al. Optical Coherence Tomography Predictors for Edge Restenosis After Everolimus-Eluting Stent Implantation. Circ. Cardiovasc. Interv. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, E.; Weisz, G.; Tanaka, A.; Kashiwagi, M.; Shimizu, T.; Wang, L.; Souza, C.; Bouma, B.E.; Suter, M.J.; Shishkov, M.; et al. Multi-laboratory inter-institute reproducibility study of IVOCT and IVUS assessments using published consensus document definitions. Eur. Heart J. Cardiovasc. Imaging 2015, 17, 756–764. [Google Scholar] [CrossRef]
- Fang, C.; Dai, J.; Zhang, S.; Wang, Y.; Wang, J.; Li, L.; Wang, Y.; Yu, H.; Wei, G.; Zhang, X.; et al. Culprit lesion morphology in young patients with ST-segment elevated myocardial infarction: A clinical, angiographic and optical coherence tomography study. Atherosclerosis 2019, 289, 94–100. [Google Scholar] [CrossRef]
- Shibuya, J.; Kobayashi, N.; Asai, K.; Tsurumi, M.; Shibata, Y.; Uchiyama, S.; Okazaki, H.; Goda, H.; Tani, K.; Shirakabe, A.; et al. Comparison of Coronary Culprit Lesion Morphology Determined by Optical Coherence Tomography and Relation to Outcomes in Patients Diagnosed with Acute Coronary Syndrome During Winter -vs- Other Seasons. Am. J. Cardiol. 2019, 124, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Takano, M.; Tsurumi, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Okazaki, H.; Shirakabe, A.; Seino, Y.; Hata, N.; et al. Features and Outcomes of Patients with Calcified Nodules at Culprit Lesions of Acute Coronary Syndrome: An Optical Coherence Tomography Study. Cardiology 2018, 139, 90–100. [Google Scholar] [CrossRef]
- Sun, R.; Sun, L.; Fu, Y.; Liu, H.; Xu, M.; Ren, X.; Yu, H.; Dong, H.; Liu, Y.; Zhu, Y.; et al. Culprit plaque characteristics in women vs men with a first ST-segment elevation myocardial infarction: In vivo optical coherence tomography insights. Clin. Cardiol. 2017, 40, 1285–1290. [Google Scholar] [CrossRef]
- Yonetsu, T.; Hoshino, M.; Lee, T.; Kanaji, Y.; Yamaguchi, M.; Hada, M.; Sumino, Y.; Ohya, H.; Kanno, Y.; Hirano, H.; et al. Plaque morphology assessed by optical coherence tomography in the culprit lesions of the first episode of acute myocardial infarction in patients with low low-density lipoprotein cholesterol level. J. Cardiol. 2020, 75, 485–493. [Google Scholar] [CrossRef]
- Kubo, T.; Shinke, T.; Okamura, T.; Hibi, K.; Nakazawa, G.; Morino, Y.; Shite, J.; Fusazaki, T.; Otake, H.; Kozuma, K.; et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): One-year angiographic and clinical results. Eur. Heart J. 2017, 38, 3139–3147. [Google Scholar] [CrossRef]
- Yamamoto, E.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; Takano, M.; Niccoli, G.; Higuma, T.; et al. Clinical and Laboratory Predictors for Plaque Erosion in Patients With Acute Coronary Syndromes. J. Am. Heart Assoc. 2019, 8, e012322. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Tanaka, A.; Ozaki, Y.; Taruya, A.; Fukuda, S.; Taguchi, H.; Iwaguro, T.; Ueno, S.; Okumoto, Y.; Akasaka, T. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 263–270. [Google Scholar] [CrossRef]
- Wang, C.; Hu, S.; Feng, N.; Li, J.; Wang, Y.; Dong, H.; Sun, R.; Yu, H.; Li, L.; Qin, Y.; et al. Classification of Culprit Ruptured Plaque Morphologies in Patients With STEMI: An OCT Study. JACC Cardiovasc. Imaging 2019, 12, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, G.; Gatto, L.; Romagnoli, E.; Di Vito, L.; Fabbiocchi, F.; Marco, V.; Versaci, F.; Di Giorgio, A.; Taglieri, N.; La Manna, A.; et al. Assessment of Mechanisms of Acute Coronary Syndromes and Composition of Culprit Plaques in Patients With and Without Diabetes. JACC Cardiovasc. Imaging 2019, 12, 1111–1112. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Jeong, M.H.; Choi, Y.H.; Ko, J.S.; Lee, M.G.; Kang, W.Y.; Lee, S.E.; Kim, S.H.; Park, K.H.; Sim, D.S.; et al. Plaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patients. JACC Cardiovasc. Imaging 2009, 2, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Yonetsu, T.; Kurihara, O.; Nakajima, A.; Lee, H.; Soeda, T.; Minami, Y.; Higuma, T.; Kimura, S.; Takano, M.; et al. Circadian variations in pathogenesis of ST-segment elevation myocardial infarction: An optical coherence tomography study. J. Thromb. Thrombolysis 2020, 51, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kusama, I.; Hibi, K.; Kosuge, M.; Nozawa, N.; Ozaki, H.; Yano, H.; Sumita, S.; Tsukahara, K.; Okuda, J.; Ebina, T.; et al. Impact of plaque rupture on infarct size in ST-segment elevation anterior acute myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 1230–1237. [Google Scholar] [CrossRef]
- Johnson, T.W.; Raber, L.; di Mario, C.; Bourantas, C.; Jia, H.; Mattesini, A.; Gonzalo, N.; de la Torre Hernandez, J.M.; Prati, F.; Koskinas, K.; et al. Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2019, 40, 2566–2584. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Farooq, V.; Zhang, Y.; Muramatsu, T.; Gogas, B.D.; Thuesen, L.; McClean, D.; Chevalier, B.; Windecker, S.; Koolen, J.; et al. Circumferential distribution of the neointima at six-month and two-year follow-up after a bioresorbable vascular scaffold implantation: A substudy of the ABSORB Cohort B Clinical Trial. EuroIntervention 2015, 10, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Al-Hussaini, A.; Joseph, S.; van Soest, G.; Wood, A.; Macaya, F.; Gonzalo, N.; Cade, J.; Caixeta, A.; Hlinomaz, O.; et al. Spontaneous Coronary Artery Dissection: Pathophysiological Insights From Optical Coherence Tomography. JACC Cardiovasc. Imaging 2019, 12, 2475–2488. [Google Scholar] [CrossRef]
- Ali, Z.A.; Galougahi, K.K.; Finn, A.V. Covering our tracks-optical coherence tomography to assess vascular healing. EuroIntervention 2018, 14, e1247–e1251. [Google Scholar] [CrossRef]
- Hayashi, T.; Kiyoshima, T.; Matsuura, M.; Ueno, M.; Kobayashi, N.; Yabushita, H.; Kurooka, A.; Taniguchi, M.; Miyataka, M.; Kimura, A.; et al. Plaque erosion in the culprit lesion is prone to develop a smaller myocardial infarction size compared with plaque rupture. Am. Heart J. 2005, 149, 284–290. [Google Scholar] [CrossRef]
- Alfonso, F.; Rivero, F. Superficial Calcific Sheets: A Novel Substrate for Acute Coronary Syndromes? JACC Cardiovasc. Interv. 2019, 12, 541–544. [Google Scholar] [CrossRef]
- Sabate, M.; Windecker, S.; Iniguez, A.; Okkels-Jensen, L.; Cequier, A.; Brugaletta, S.; Hofma, S.H.; Raber, L.; Christiansen, E.H.; Suttorp, M.; et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: Results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur. Heart J. 2016, 37, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Limbruno, U.; Versaci, F.; Fabbiocchi, F.; Di Giorgio, A.; Marco, V.; et al. Clinical Impact of Suboptimal Stenting and Residual Intrastent Plaque/Thrombus Protrusion in Patients With Acute Coronary Syndrome: The CLI-OPCI ACS Substudy (Centro per la Lotta Contro L’Infarto-Optimization of Percutaneous Coronary Intervention in Acute Coronary Syndrome). Circ. Cardiovasc. Interv. 2016, 9, e003726. [Google Scholar] [CrossRef]
- Sheth, T.N.; Kajander, O.A.; Lavi, S.; Bhindi, R.; Cantor, W.J.; Cheema, A.N.; Stankovic, G.; Niemela, K.; Natarajan, M.K.; Shestakovska, O.; et al. Optical Coherence Tomography-Guided Percutaneous Coronary Intervention in ST-Segment-Elevation Myocardial Infarction: A Prospective Propensity-Matched Cohort of the Thrombectomy Versus Percutaneous Coronary Intervention Alone Trial. Circ. Cardiovasc. Interv. 2016, 9, e003414. [Google Scholar] [CrossRef]
- Meneveau, N.; Ecarnot, F.; Souteyrand, G.; Motreff, P.; Caussin, C.; Van Belle, E.; Ohlmann, P.; Morel, O.; Grentzinger, A.; Angioi, M.; et al. Does optical coherence tomography optimize results of stenting? Rationale and study design. Am. Heart J. 2014, 168, 175–181.e2. [Google Scholar] [CrossRef]
- Kim, N.; Lee, J.H.; Jang, S.Y.; Bae, M.H.; Yang, D.H.; Park, H.S.; Cho, Y.; Jeong, M.H.; Park, J.S.; Kim, H.S.; et al. Intravascular modality-guided versus angiography-guided percutaneous coronary intervention in acute myocardial infarction. Catheter. Cardiovasc. Interv. 2020, 95, 696–703. [Google Scholar] [CrossRef]
- Ali, Z.; Landmesser, U.; Galougahi, K.K.; Maehara, A.; Matsumura, M.; Shlofmitz, R.A.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; et al. OPtical Coherence Tomography Guided Coronary Stent IMplantation Compared to Angiography: A Multicenter Randomized TriaL in PCI-Design and Rationale of ILUMIEN IV: OPTIMAL PCI. EuroIntervention 2021, 16, 1092–1099. [Google Scholar] [CrossRef]
- Hu, S.; Wang, C.; Zhe, C.; Zhu, Y.; Yonetsu, T.; Jia, H.; Hou, J.; Zhang, S.; Jang, I.K.; Yu, B. Plaque erosion delays vascular healing after drug eluting stent implantation in patients with acute coronary syndrome: An In Vivo Optical Coherence Tomography Study. Catheter. Cardiovasc. Interv. 2017, 89, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Yamamoto, E.; Sugiyama, T.; Jia, H.; Ma, L.; Hu, S.; Wang, C.; Zhu, Y.; Li, L.; Xu, M.; et al. EROSION Study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion): A 1-Year Follow-Up Report. Circ. Cardiovasc. Interv. 2017, 10, e005860. [Google Scholar] [CrossRef]
- He, L.; Qin, Y.; Xu, Y.; Hu, S.; Wang, Y.; Zeng, M.; Feng, X.; Liu, Q.; Syed, I.; Demuyakor, A.; et al. Predictors of Non-Stenting Strategy for Acute Coronary Syndrome Caused by Plaque Erosion: 4-Year Outcomes of the EROSION Study. EuroIntervention 2020. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Vaidya, K.; Tucker, B.; Kurup, R.; Khandkar, C.; Pandzic, E.; Barraclough, J.; Machet, J.; Misra, A.; Kavurma, M.; Martinez, G.; et al. Colchicine Inhibits Neutrophil Extracellular Trap Formation in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2021, 10, e018993. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, K.; Arnott, C.; Martínez, G.J.; Ng, B.; McCormack, S.; Sullivan, D.R.; Celermajer, D.S.; Patel, S. Colchicine Therapy and Plaque Stabilization in Patients With Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc. Imaging 2018, 11, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Galougahi, K.K.; Patel, S.; Shlofmitz, R.A.; Maehara, A.; Kereiakes, D.J.; Hill, J.M.; Stone, G.W.; Ali, Z.A. Calcific Plaque Modification by Acoustic Shockwaves: Intravascular Lithotripsy in Coronary Interventions. Circ. Cardiovasc. Interv. 2021, 14, e009354. [Google Scholar] [CrossRef]
- Patel, S.; Madhavan, M.V.; Spina, R.; Figtree, G.A.; Galougahi, K.K. Deferred Intravascular Lithotripsy-Facilitated Stenting in ACS–Novel Approach to Improve PCI Outcomes in Severe Calcification? J. Am. Coll. Cardiol. Case Rep. 2020, 2, 1700–1701. [Google Scholar]



| Study | Cohort | Lesions | RFC-ACS | IFC-ACS | Calcified Nodule | Other Causes |
|---|---|---|---|---|---|---|
| Leistner et al. [33] | ACS | 170 | 98 | 32 | 3 | 37 |
| Guagliumi et al. [70] | STEMI | 128 | 63 | 32 | - | 33 |
| Dai et al. [58] | STEMI | 822 | 564 | 209 | 5 | 44 |
| Yamamoto et al. [85] | ACS | 1241 | 607 | 477 | 157 | - |
| Jia et al. [57] | ACS | 126 | 55 | 39 | 10 | 22 |
| Chandran et al. [71] | STEMI | 40 | 23 | 15 | - | 2 |
| Niccoli et al. [72] | ACS | 139 | 82 | 57 | - | - |
| Yonetsu et al. [83] | ACS | 318 | 141 | 131 | - | 46 |
| Kajander et al. [73] | STEMI | 70 | 34 | 31 | 5 | - |
| Kwon et al. [33] | ACS | 133 | 90 | 43 | - | - |
| Higuma et al. [74] | STEMI | 112 | 72 | 30 | 9 | 1 |
| Kubo et al. [75] | STEMI | 30 | 22 | 7 | - | 1 |
| Khalifa et al. [76] | ACS | 288 | 172 | 82 | 34 | - |
| Nishiguchi et al. [86] | ACS | 326 | 160 | 153 | - | 13 |
| Wang et al. [87] | STEMI | 80 | 37 | 25 | 2 | 16 |
| Fang et al. [79] | STEMI | 1442 | 972 | 348 | 23 | 99 |
| Shibuya et al. [80] | ACS | 483 | 237 | 218 | 28 | - |
| Kobayashi et al. [81] | ACS | 362 | 163 | 149 | 21 | 29 |
| Sun et al. [82] | STEMI | 211 | 123 | 82 | 6 | 0 |
| Hu et al. [59] | ACS | 141 | 79 | 62 | - | - |
| Total, n (%) | 6662 (100%) | 3794 (56.9%) | 2222 (33.3%) | 303 (4.5%) | 343 (5.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandkar, C.; Madhavan, M.V.; Weaver, J.C.; Celermajer, D.S.; Karimi Galougahi, K. Atherothrombosis in Acute Coronary Syndromes—From Mechanistic Insights to Targeted Therapies. Cells 2021, 10, 865. https://doi.org/10.3390/cells10040865
Khandkar C, Madhavan MV, Weaver JC, Celermajer DS, Karimi Galougahi K. Atherothrombosis in Acute Coronary Syndromes—From Mechanistic Insights to Targeted Therapies. Cells. 2021; 10(4):865. https://doi.org/10.3390/cells10040865
Chicago/Turabian StyleKhandkar, Chinmay, Mahesh V. Madhavan, James C. Weaver, David S. Celermajer, and Keyvan Karimi Galougahi. 2021. "Atherothrombosis in Acute Coronary Syndromes—From Mechanistic Insights to Targeted Therapies" Cells 10, no. 4: 865. https://doi.org/10.3390/cells10040865
APA StyleKhandkar, C., Madhavan, M. V., Weaver, J. C., Celermajer, D. S., & Karimi Galougahi, K. (2021). Atherothrombosis in Acute Coronary Syndromes—From Mechanistic Insights to Targeted Therapies. Cells, 10(4), 865. https://doi.org/10.3390/cells10040865





