Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Drug Administration
2.3. Microglia Depletion
2.4. Immunohistochemistry
2.5. Von Frey Test
2.6. Hargreaves Test
2.7. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers. 2017, 3, 17002. [Google Scholar] [CrossRef]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Bouhassira, D.; Lanteri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef]
- Jaggi, A.S.; Jain, V.; Singh, N. Animal models of neuropathic pain. Fundam. Clin. Pharmacol. 2011, 25, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Kierdorf, K.; Prinz, M. Microglia in steady state. J. Clin. Investig. 2017, 127, 3201–3209. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Kataoka, A.; Tozaki-Saitoh, H.; Koga, Y.; Tsuda, M.; Inoue, K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J. Neurochem. 2009, 108, 115–125. [Google Scholar] [CrossRef]
- Simpson, J.E.; Newcombe, J.; Cuzner, M.L.; Woodroofe, M.N. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J. Neuroimmunol. 1998, 84, 238–249. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Urban, D.J.; Roth, B.L. DREADDs (designer receptors exclusively activated by designer drugs): Chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Tohyama, S.; Martin, L.J. The Use of DREADDs to Deconstruct Behavior. Front. Genet. 2016, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Wang, X.; Strand, K.A.; Baratta, M.V.; Zhang, Y.; Galer, E.L.; Yin, H.; Maier, S.F.; Watkins, L.R. DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp. Neurol. 2018, 304, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Saika, F.; Matsuzaki, S.; Kobayashi, D.; Ideguchi, Y.; Nakamura, T.Y.; Kishioka, S.; Kiguchi, N. Chemogenetic Regulation of CX3CR1-Expressing Microglia Using Gi-DREADD Exerts Sex-Dependent Anti-Allodynic Effects in Mouse Models of Neuropathic Pain. Front. Pharmacol. 2020, 11, 925. [Google Scholar] [CrossRef]
- Kiguchi, N.; Uta, D.; Ding, H.; Uchida, H.; Saika, F.; Matsuzaki, S.; Fukazawa, Y.; Abe, M.; Sakimura, K.; Ko, M.C.; et al. GRP receptor and AMPA receptor cooperatively regulate itch-responsive neurons in the spinal dorsal horn. Neuropharmacology 2020, 170, 108025. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.R.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef]
- Ma, D.; Zhao, Y.; Huang, L.; Xiao, Z.; Chen, B.; Shi, Y.; Shen, H.; Dai, J. A novel hydrogel-based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials 2020, 237, 119830. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; el Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Guan, Z.; Kuhn, J.A.; Wang, X.; Colquitt, B.; Solorzano, C.; Vaman, S.; Guan, A.K.; Evans-Reinsch, Z.; Braz, J.; Devor, M.; et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 2016, 19, 94–101. [Google Scholar] [CrossRef]
- Patel, S.; Player, M.R. Colony-stimulating factor-1 receptor inhibitors for the treatment of cancer and inflammatory disease. Curr. Top. Med. Chem. 2009, 9, 599–610. [Google Scholar] [CrossRef]
- Calvo, M.; Dawes, J.M.; Bennett, D.L. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012, 11, 629–642. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Saika, F.; Kishioka, S. CC-chemokine MIP-1alpha in the spinal cord contributes to nerve injury-induced neuropathic pain. Neurosci. Lett. 2010, 484, 17–21. [Google Scholar] [CrossRef]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef]
- Sweitzer, S.; Martin, D.; DeLeo, J.A. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience 2001, 103, 529–539. [Google Scholar] [CrossRef]
- Reeve, A.J.; Patel, S.; Fox, A.; Walker, K.; Urban, L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur. J. Pain 2000, 4, 247–257. [Google Scholar] [CrossRef]
- Svensson, C.I.; Schafers, M.; Jones, T.L.; Powell, H.; Sorkin, L.S. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci. Lett. 2005, 379, 209–213. [Google Scholar] [CrossRef]
- Rojewska, E.; Zychowska, M.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front. Immunol. 2018, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, R.; Zecevic, N. Neuroprotective role of minocycline in co-cultures of human fetal neurons and microglia. Exp. Neurol. 2008, 211, 41–51. [Google Scholar] [CrossRef]
- Gonzalez, J.C.; Egea, J.; del Carmen-Godino, M.; Fernandez-Gomez, F.J.; Sanchez-Prieto, J.; Gandia, L.; Garcia, A.G.; Jordan, J.; Hernandez-Guijo, J.M. Neuroprotectant minocycline depresses glutamatergic neurotransmission and Ca(2+) signalling in hippocampal neurons. Eur. J. Neurosci. 2007, 26, 2481–2495. [Google Scholar] [CrossRef]
- Peng, H.Z.; Ma, L.X.; Lv, M.H.; Hu, T.; Liu, T. Minocycline enhances inhibitory transmission to substantia gelatinosa neurons of the rat spinal dorsal horn. Neuroscience 2016, 319, 183–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sweitzer, S.M.; Schubert, P.; DeLeo, J.A. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J. Pharmacol. Exp. Ther. 2001, 297, 1210–1217. [Google Scholar]
- Gwak, Y.S.; Crown, E.D.; Unabia, G.C.; Hulsebosch, C.E. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain 2008, 138, 410–422. [Google Scholar] [CrossRef]
- Binning, W.; Hogan-Cann, A.E.; Yae-Sakae, D.; Maksoud, M.; Ostapchenko, V.; Al-Onaizi, M.; Matovic, S.; Lu, W.Y.; Prado, M.A.M.; Inoue, W.; et al. Chronic hM3Dq signaling in microglia ameliorates neuroinflammation in male mice. Brain Behav. Immun. 2020, 88, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Richards, J.; Mancl, M.; Hanash, S.; Beretta, L.; Pitha, P.M. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J. Biol. Chem. 2004, 279, 45194–45207. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, A.; Barnes, B.J.; Amrute, S.; Yeow, W.S.; Megjugorac, N.; Dai, J.; Feng, D.; Chung, E.; Pitha, P.M.; Fitzgerald-Bocarsly, P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 2003, 74, 1125–1138. [Google Scholar] [CrossRef]
- Takaoka, A.; Yanai, H.; Kondo, S.; Duncan, G.; Negishi, H.; Mizutani, T.; Kano, S.; Honda, K.; Ohba, Y.; Mak, T.W.; et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 2005, 434, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Iwamoto, S.; Yoshinaga, R.; Tozaki-Saitoh, H.; Nishiyama, A.; Mak, T.W.; Tamura, T.; Tsuda, M.; Inoue, K. Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat. Commun. 2014, 5, 3771. [Google Scholar] [CrossRef]
- Al Mamun, A.; Chauhan, A.; Yu, H.; Xu, Y.; Sharmeen, R.; Liu, F. Interferon regulatory factor 4/5 signaling impacts on microglial activation after ischemic stroke in mice. Eur. J. Neurosci. 2018, 47, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Pagano, J.S.; Barber, G.N. IRF7: Activation, regulation, modification and function. Genes Immun. 2011, 12, 399–414. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef]
- Chen, G.; Luo, X.; Qadri, M.Y.; Berta, T.; Ji, R.R. Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neurosci. Bull. 2018, 34, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Berta, T.; Qadri, Y.J.; Chen, G.; Ji, R.R. Microglial Signaling in Chronic Pain with a Special Focus on Caspase 6, p38 MAP Kinase, and Sex Dependence. J. Dent. Res. 2016, 95, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e2776. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Muere, A.; Einstein, G. Ovarian hormones and chronic pain: A comprehensive review. Pain 2014, 155, 2448–2460. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Ju, B.G.; Yune, T.Y. Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation. Biochim. Biophys. Acta 2018, 1864, 2472–2480. [Google Scholar] [CrossRef]
- Li, L.; Fan, X.; Warner, M.; Xu, X.J.; Gustafsson, J.A.; Wiesenfeld-Hallin, Z. Ablation of estrogen receptor alpha or beta eliminates sex differences in mechanical pain threshold in normal and inflamed mice. Pain 2009, 143, 37–40. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Hamel, K.A.; Morvan, M.G.; Yu, S.; Leff, J.; Guan, Z.; Braz, J.M.; Basbaum, A.I. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat. Commun. 2020, 11, 264. [Google Scholar] [CrossRef]
- Yi, M.H.; Liu, Y.U.; Liu, K.; Chen, T.; Bosco, D.B.; Zheng, J.; Xie, M.; Zhou, L.; Qu, W.; Wu, L.J. Chemogenetic manipulation of microglia inhibits neuroinflammation and neuropathic pain in mice. Brain Behav. Immun. 2020. [Google Scholar] [CrossRef]
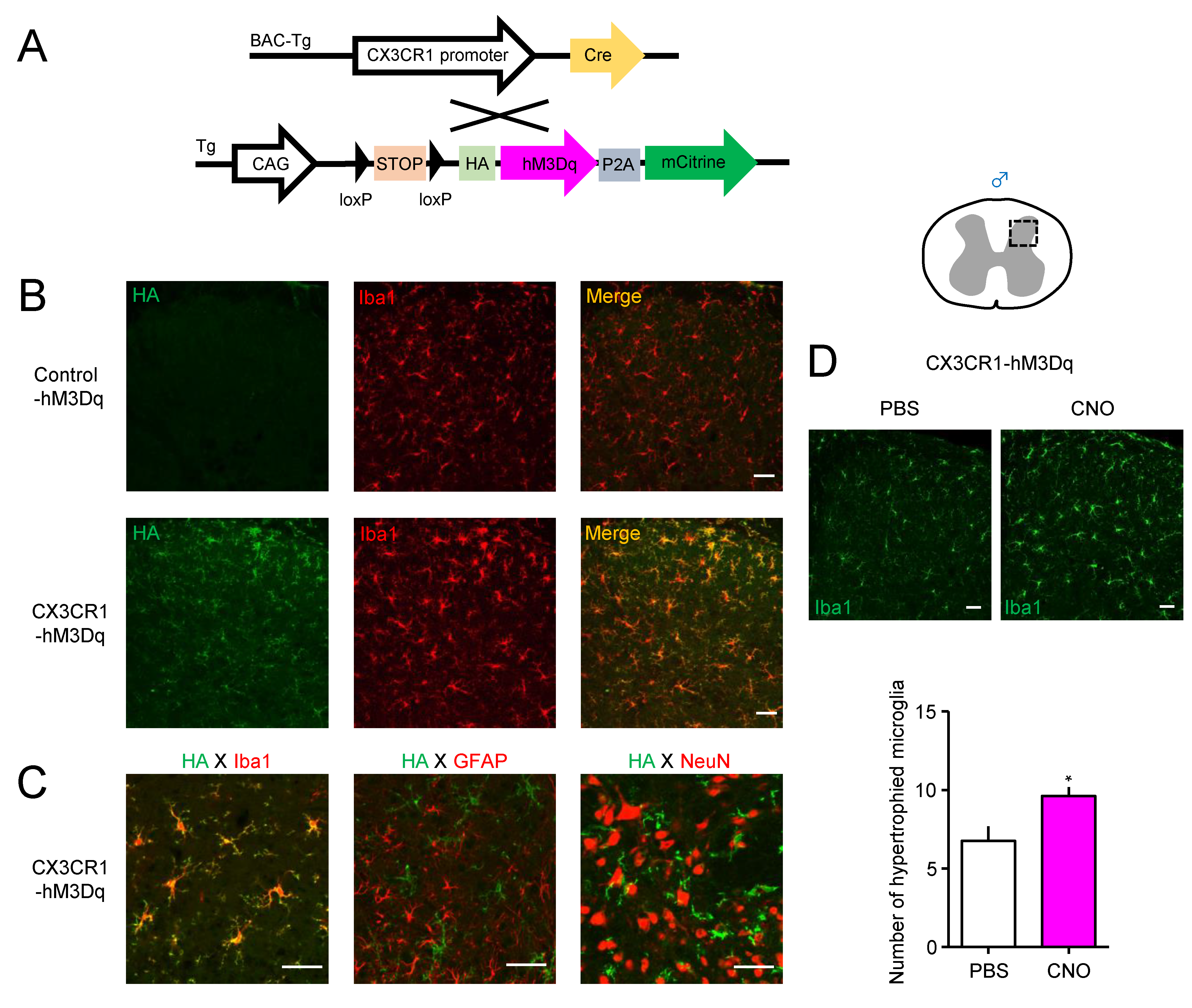

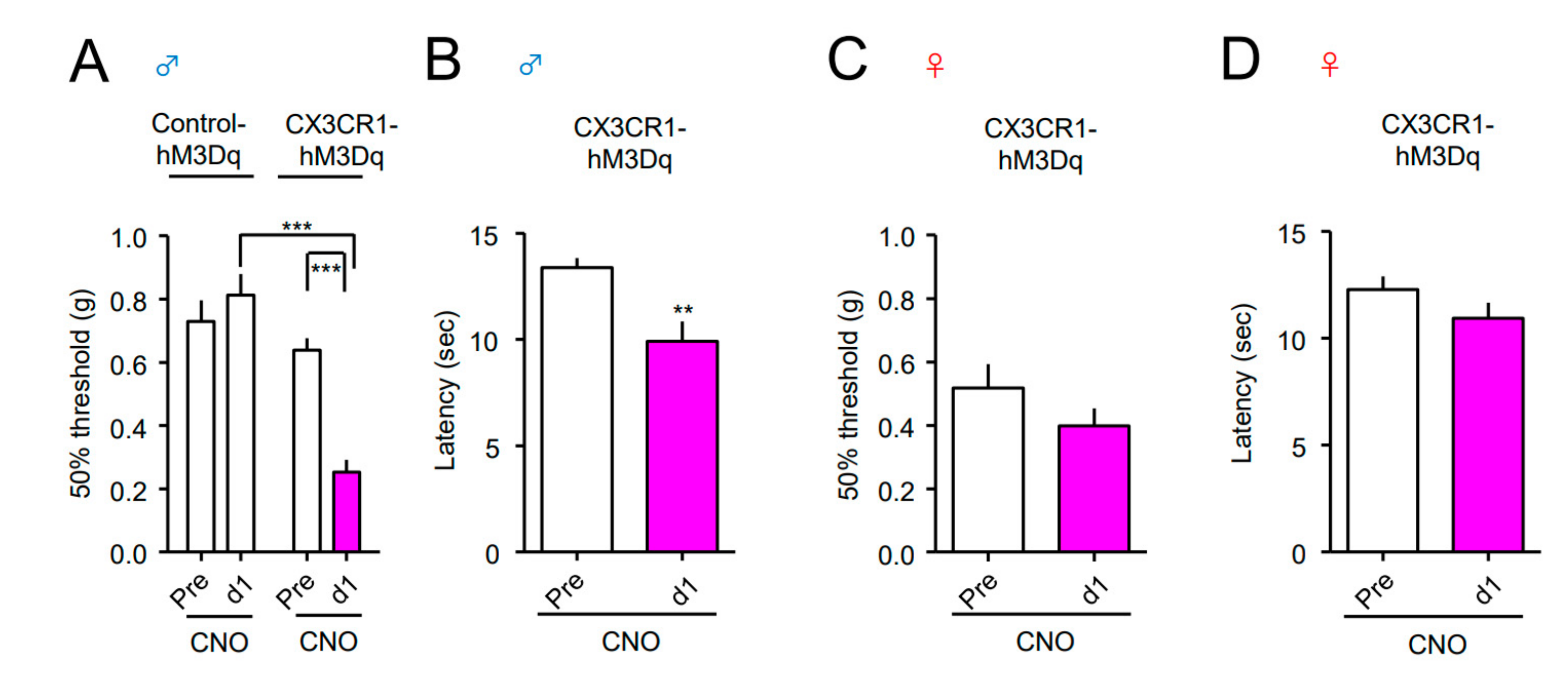
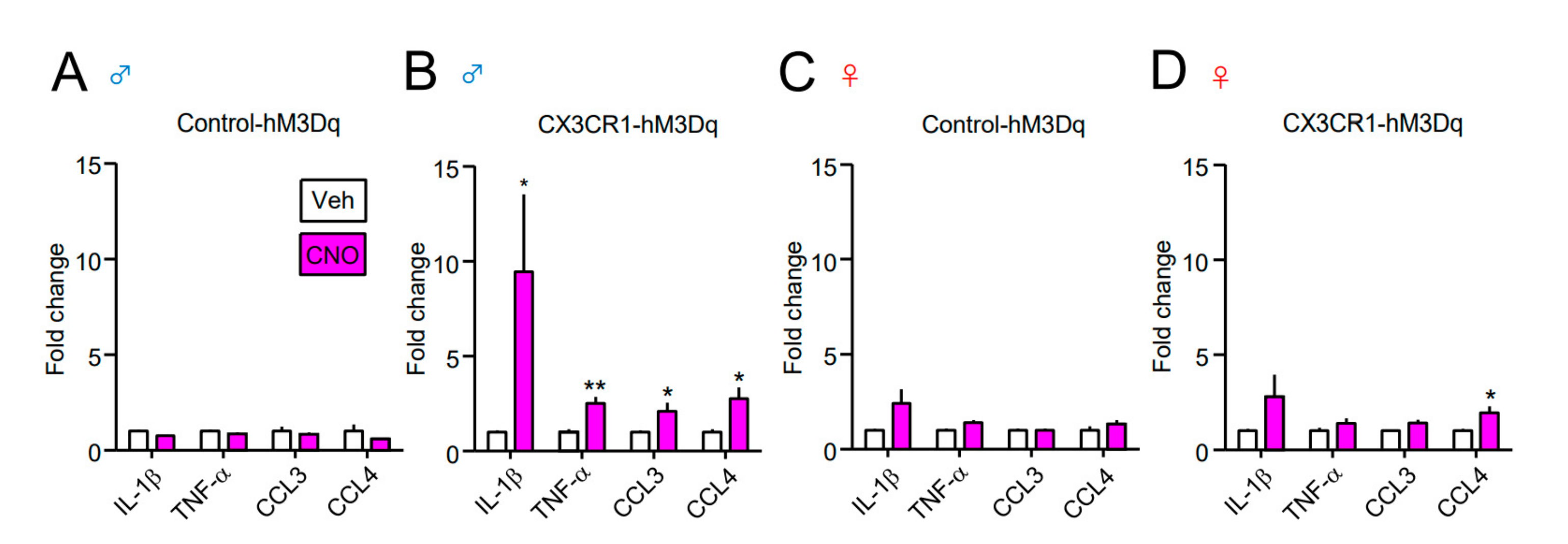
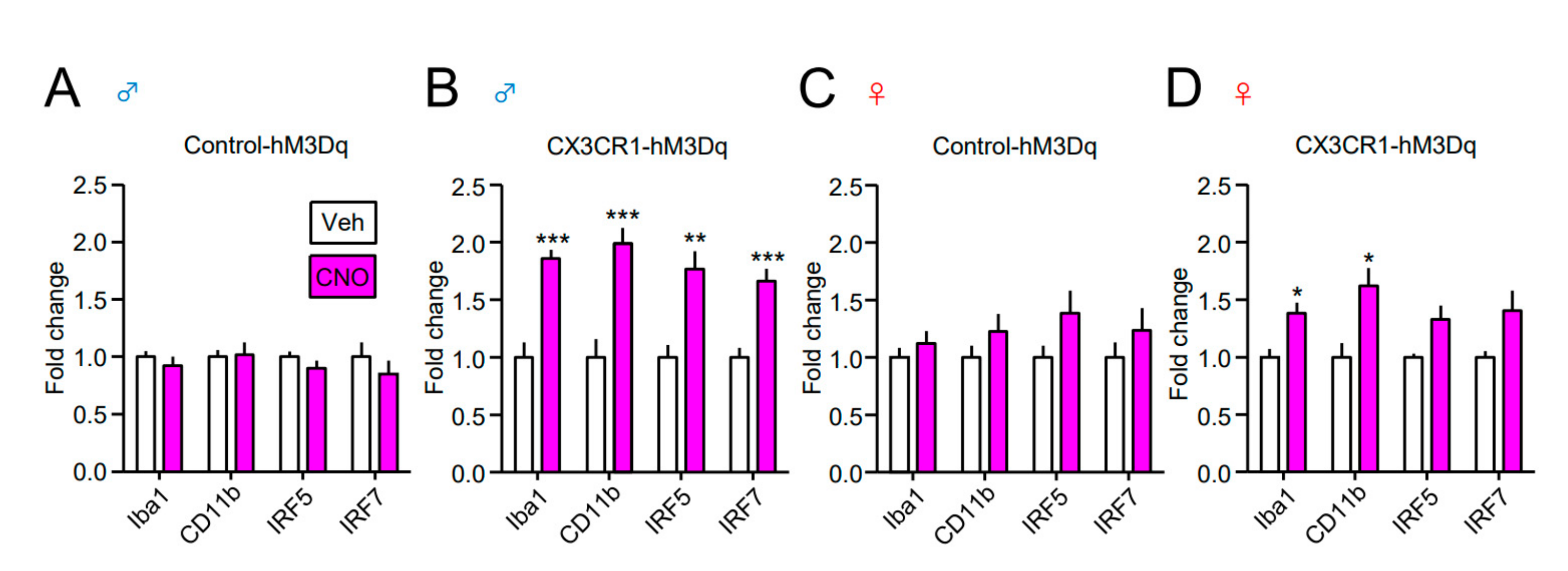

| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| GAPDH | GGGTGTGAACCACGAGAAAT | ACTGTGGTCATGAGCCCTTC |
| IL-1β | AAAGCTCTCCACCTCAATGG | AGGCCACAGGTATTTTGTCG |
| TNF-α | CCCCAAAGGGATGAGAAGTT | TGGGCTACAGGCTTGTCACT |
| CCL3 | CTGCCCTTGCTGTTCTTCTC | GTGGAATCTTCCGGCTGTAG |
| CCL4 | ATGAACTCTGCGTGTCTGC | GCCGGGAGGTGTAAGAGAAA |
| Iba1 | ATGAGCCAAAGCAGGGATTT | TTGGGATCATCGAGGAATTG |
| CD11b | GTTTCTACTGTCCCCCAGCA | GTTGGAGCCGAACAAATAGC |
| IRF5 | ACACTGAAGGGGTGATGAG | CGAGGGCCATCATAGAACAG |
| IRF7 | GTGTGTCCCCAGGATCATTT | CTGCAGAACCTGAAGCAAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saika, F.; Matsuzaki, S.; Kishioka, S.; Kiguchi, N. Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice. Cells 2021, 10, 874. https://doi.org/10.3390/cells10040874
Saika F, Matsuzaki S, Kishioka S, Kiguchi N. Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice. Cells. 2021; 10(4):874. https://doi.org/10.3390/cells10040874
Chicago/Turabian StyleSaika, Fumihiro, Shinsuke Matsuzaki, Shiroh Kishioka, and Norikazu Kiguchi. 2021. "Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice" Cells 10, no. 4: 874. https://doi.org/10.3390/cells10040874
APA StyleSaika, F., Matsuzaki, S., Kishioka, S., & Kiguchi, N. (2021). Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice. Cells, 10(4), 874. https://doi.org/10.3390/cells10040874






