Abstract
Under physiological conditions, cAMP signaling plays a key role in the regulation of cardiac function. Activation of this intracellular signaling pathway mirrors cardiomyocyte adaptation to various extracellular stimuli. Extracellular ligand binding to seven-transmembrane receptors (also known as GPCRs) with G proteins and adenylyl cyclases (ACs) modulate the intracellular cAMP content. Subsequently, this second messenger triggers activation of specific intracellular downstream effectors that ensure a proper cellular response. Therefore, it is essential for the cell to keep the cAMP signaling highly regulated in space and time. The temporal regulation depends on the activity of ACs and phosphodiesterases. By scaffolding key components of the cAMP signaling machinery, A-kinase anchoring proteins (AKAPs) coordinate both the spatial and temporal regulation. Myocardial infarction is one of the major causes of death in industrialized countries and is characterized by a prolonged cardiac ischemia. This leads to irreversible cardiomyocyte death and impairs cardiac function. Regardless of its causes, a chronic activation of cardiac cAMP signaling is established to compensate this loss. While this adaptation is primarily beneficial for contractile function, it turns out, in the long run, to be deleterious. This review compiles current knowledge about cardiac cAMP compartmentalization under physiological conditions and post-myocardial infarction when it appears to be profoundly impaired.
1. Introduction
The beating heart ensures an essential function by supplying an oxygen- and nutrient-rich blood flow to the organs. Efficient blood flow distribution in both the pulmonary and systemic circulations allows the exchange of oxygen and carbon dioxide. Pump function is maintained by the automatic cardiac excitation that spontaneously triggers rhythmic and periodic contractions. This dynamic process is modulated in response to various physiological and pathophysiological stimuli such as hormones, neurotransmitters, cardiac injuries and stresses that adapt/maladapt heart function [1,2]. Cardiac injuries and stresses are defined as events leading to cardiac diseases (e.g., medication, pollution, xenohormones, hypertension and myocardial infarction (MI)). Under physiological conditions, the sympathetic nervous system releases catecholamines (e.g., epinephrine and norepinephrine), which bind to α- or β-adreno receptors (α- or β-AR) and adapt the cardiac function by increasing the heart rate (HR), stroke volume and cardiac output. In contrast, acetylcholine produced by the parasympathetic system exerts opposite effects to those of catecholamines [1]. Like many other hormones and neurotransmitters affecting cardiac function, catecholamines and acetylcholine bind to and activate G protein-coupled receptors (GPCRs), which modulate intracellular cAMP (cyclic adenosine 3′,5′-monophosphate) signaling [3]. The intracellular concentration of this second messenger is finely tuned between the activities of adenylyl cyclases (ACs) and phosphodiesterases (PDEs), which synthetize and catalyze cAMP, respectively [4]. Cyclic AMP mediates the cellular response adaption to extracellular stimuli after activation of several specific downstream effectors such as protein kinase A (PKA), cAMP-dependent exchange protein (Epac), cyclic nucleotide-gated channels (CNGC) and Popeye domain-containing (POPDC) proteins [5]. Therefore, to ensure a high level of specificity in the cellular response to distinct stimuli, cAMP signaling needs to be tightly regulated in space and time. This is resolved by compartmentalized cAMP nanodomains that encompass signaling effectors (e.g., kinases, phosphatases and channels) and modulators (i.e., ACs and PDEs) [5]. The organization of these intracellular cAMP nanodomains is orchestrated by A-kinase anchoring proteins (AKAPs) [4].
Evidence of cardiac cAMP signaling compartmentalization relies on experiments performed in the late 1970s [6,7,8]. A heart perfused with isoproterenol (a β-AR agonist) raises the strength of contraction (i.e., inotropic response) and increases the myocardial cAMP level. The latter mobilizes PKA, which, in turn, mediates cascade activation of the phosphorylase kinase and the glycogen phosphorylase, triggering glycogen breakdown. Although PGE1 (agonist of prostaglandin E1 receptor) perfusion in the heart increases cAMP production and PKA activity, it failed to regulate cardiac contractile function and glycogen metabolism [7,9]. At the cellular level, adult rat ventricular myocytes stimulated with glucagon, glucagon like peptide-1 and β2-AR agonist exhibit, for all, an increase in the intracellular cAMP content but distinct cellular responses that are specific to each stimulus. Activation of β2-AR enhances the positive inotropic response, while the glucagon exerts positive inotropic and lusitropic (i.e., rate of myocardial relaxation) effects. In contrast, glucagon like peptide-1 ensures a modest negative inotropic response [10,11,12,13]. Therefore, restraining the cardiac cAMP signaling to distinct intracellular compartments provides means to achieve specific cardiomyocytes’ adaptation to various extracellular stimuli. Therefore, the concept of cardiac cAMP signaling compartmentalization was established (for review, see [14]).
Ischemic heart diseases or coronary heart diseases (CHDs) are the leading causes of death in industrialized countries and kill 15 million people worldwide each year [15]. CHD is a syndrome that defines heart complications with total or partial coronary artery occlusion leading to heart muscle ischemia, and blood and oxygen deprivation. CHD contains several heart conditions with various clinical features such as acute coronary syndrome (ACS), chronic angina pectoris (CAP) and chronic ischemic heart disease (CIHD) [16]. ACS, which includes unstable angina, acute myocardial infarction (AMI; with or without ST segment elevation (i.e., STEMI and non-STEMI)) and sudden cardiac death (SCD), needs intensive and acute treatment, while patients with CAP (i.e., stable angina, Prinzmetal’s variant angina, Mazzeri’s angina and angina with normal coronary arteries) or CIHD (i.e., “silent” ischemia, heart failure and ischemic cardiomyopathy) undergo chronic treatments. A prolonged cardiac ischemia characterizes MI usually triggered after a blood clot, coronary artery constriction or atherosclerosis and leads to irreversible cardiomyocyte death, affecting cardiac function [17]. To compensate the impaired cardiac function observed post-MI, the body activates an adaptive mechanism that includes the chronic sympathetic nervous system activation [18]. While this adaptation is primarily beneficial for contractile function, the chronic sympathetic activation in the long run turns out to be deleterious [18]. These harmful effects are mediated by the chronic activation of the β-AR/cAMP signaling cascade, which causes adverse cardiac remodeling, cardiac myocyte death and fibrosis, leading to fatal heart failure and arrhythmias [18,19]. Currently, there is no therapeutic option to reverse CHD or MI and mid-term mortality remains high [15]. Nevertheless, immediate treatments (e.g., angioplasty, surgery) and medication (e.g., β-blockers, angiotensin converting enzyme (ACE)) improve patient’s prognosis and quality of life [20,21,22]. Among medications, β-blockers are a preeminent therapy limiting the severity of heart damages through β-AR competitive antagonization and inhibition of chronic cAMP signaling activation [13,19,20,23,24]. However, β-blockers cause severe side effects and are ineffective in a large proportion of patients [25,26,27,28]. In this context, several studies have attempted to develop new alternative therapeutic strategies to β-blockers. Therefore, some emerging ideas propose selectively targeting cAMP modulators or effectors as innovative curatives for cardiac diseases (e.g., MI, heart failure). These would offer similar benefits to β-blockers without their side effects [29,30,31,32]. As cAMP signaling is a key regulator of cardiac physiology, this review aims at describing molecular events that mediate cAMP signaling compartmentalization in cardiomyocytes under physiological and pathophysiological conditions of MI.
2. G Protein-Coupled Receptors (GPCRs)
This section details the current knowledge about major cardiac GPCRs that directly modulate cAMP signaling and for which the regulation of cardiac function under physiological conditions and post-MI has been reported (Table 1). Other cardiac GPCRs and their function have been reviewed in [3].

Table 1.
Function of cardiac Gαs and Gαi protein-coupled receptors under physiological conditions and in myocardial infarction. α2-AR: alpha 2 adrenergic receptor; VT: ventricular tachycardia; β1-AR: beta 1 adrenergic receptor; β2-AR: beta 2 adrenergic receptor; β3-AR: beta 3 adrenergic receptor; M2R: muscarinic type 2 receptor; A1AR: A1 Adenosine Receptor; A2AAR: A2A Adenosine Receptor; A2BAR: A2B Adenosine Receptor; A3AR: A3 Adenosine receptor; GCCR: Glucagon Receptor; GLP1R: Glucagon Like Peptide 1 Receptor.
2.1. Structure
GPCRs are seven-transmembrane domain receptors with an extracellular N-terminal extremity and an intracellular C-terminal tail, which activates heterotrimeric G proteins consisting of Gα and Gβ,γ. In the inactive state, these three subunits form a heterotrimer with GDP-bound Gα. Upon GPCR stimulation, GTP replaces GDP, which leads to a conformational change and releases the Gα subunit. The nature of the Gα protein (Gαs, Gαi/o, Gαq) and Gβ,γ ensure specificity in the activation of intracellular signaling. GTP hydrolyzation in GDP by the Gα subunit triggers GPCR inactivation [52].
2.2. Adrenoreceptors
2.2.1. α-Adrenergic Receptors (α-AR)
Among α-ARs, α1-AR and α2-AR are involved in the regulation of cardiac function. The α1-AR signals through Gαq stimulation and phospholipase C activation [53]. In presynaptic nerves, α2-AR coupled to Gαi/o inhibits AC activity and reduces catecholamine release (Table 1) [54]. There is also evidence of α2-AR expression in cardiomyocytes that, under stimulation, mediates negative chronotropic (i.e., HR) and inotropic responses [33]. In a dog model of MI with left anterior descending (LAD) coronary artery ligation, the inhibition of α2-AR increases susceptibility to arrhythmia. Although the underlying mechanism of these antiarrhythmic properties remains unclear, it could involve α2-AR located in Purkinje fibers [34].
2.2.2. β-Adrenergic Receptors (β-AR)
In the heart, three β-AR isotypes are co-expressed (i.e., β1-AR, β2-AR and β3-AR), activated by catecholamines and coupled to small Gαs or Gαi proteins. While Gαs activation triggers cAMP signaling, Gαi inhibits AC activity and reduces the intracellular cAMP content. In cardiomyocytes, the β1-AR isoform is predominant and represents about 80% of total β-AR, and the β2-AR isoform represents approximately 20%, whereas β3-AR is poorly expressed [35,55]. β1-AR and β2-AR are mainly coupled to Gαs and promote an increase in cAMP production. In addition, β2-AR can couple to Gαi to reduce cAMP production and thus to temporize chronotropic effects mediated by prolonged Gαs activation [6,35]. Although β1-AR and β2-AR activations induce common cardiac effects, they also display specific responses due to their distinct subcellular localization. β1-AR is ubiquitously distributed across the plasmalemma, while cardiac β2-AR expression is restricted to the T-tubules [35]. Under physiologic conditions, β1-AR adapts cardiac contractile function through positive regulation of inotropic, chronotropic and lusitropic effects (Table 1). Similarly, β2-AR mediates positive inotropic responses. However, β2-AR regulation of the cardiac lusitropic effect remains controversial (Table 1). In highly structured adult rat ventricular cardiomyocytes, β2-AR restricted to T-tubules failed to trigger a positive lusitropic response, contrary to immature neonatal rat ventricular myocytes [35]. Interestingly, transgenic mice with β1-AR and/or β2-AR KO point to β1-AR as being sufficient and necessary to maintain catecholamine-induced positive inotropic and chronotropic effects in vivo [36]. The classic model of β-AR activation depicts initiation of the signaling from the plasmalemma; however, this has been challenged by recent findings reporting also pools of active β1-AR/Gαs and β2-AR/Gαs inside cells at the Golgi apparatus and endosomes [56,57]. The endosome-localized β2-AR ensures specific transcriptional activity, while functions associated with β1-AR distributed at the Golgi apparatus remain unexplored [58]. These findings add an additional level of complexity to the cAMP signaling compartmentalization and should be further investigated under cardiac pathophysiological conditions, as they may offer new potential therapeutic targets.
Corresponding to their specific subcellular localizations, β-ARs exhibit distinct effects on cardiac function post-MI. β1-AR induces cardiac dysfunction and cardiomyocyte apoptosis post-MI [36]. Furthermore, specific β1-AR inhibition with siRNA decreases cardiomyocyte apoptosis in the ischemic area and improves cardiac function in a rat model of MI with LAD ligation [59]. In contrast, β2-AR exhibits cardioprotective properties. In acute MI, stimulation of β2-AR during reperfusion limits infarct size and the release of cardiomyocyte injury markers such as circulating troponin I (TnI) [38]. Moreover, it also reduces deleterious cardiac remodeling and protects cardiac function in a PKA-Akt-eNOS pathway activation-dependent fashion [38]. In an ischemia/reperfusion (I/R) injury model, the stimulation of β2-AR before reperfusion partly inhibits the inflammatory response. This triggers the release of interleukin-10 (IL10), reducing leukocyte activation, which is known to increase infarct size during reperfusion [39]. However, another study reported that post-chronic MI, neither β1 nor β2-AR influences infarction size [36]. This discrepancy could be explained by differences in the MI model studied (i.e., I/R vs. permanent ligation) and/or the timing of analysis post-surgery (i.e., acute vs. chronic) [36,39]. In chronic MI, β2-AR redistributes to the plasma membrane, reflecting T-tubule network disorganization that appears in the heart failure (HF) state [60]. Although cardiac β2-AR density remains preserved, β1-AR expression decreases at the infarct and border areas [37].
GRK2 (G protein-coupled receptor kinase 2 or βARK1: β-adrenergic receptor kinase1) mediates β-AR desensitization and internalization. While the β-AR desensitization process protects the heart after acute MI, it also contributes to maintaining the vicious circle of β-AR stimulation that leads to HF [61]. Therefore, the inhibition of the desensitization process with intravenous delivery of adenoviral constructs that directs the expression of a GRK2 antagonist improves left ventricular cardiac function in a rat model of chronic MI [62]. These promising data pave the way for a putative new therapy to treat chronic MI patients.
Among β-AR gene polymorphisms reported, patients exhibiting the haplotype G16R, Q27E or T164I in ADRB2 (i.e., gene coding for β2-AR) show a reduction in MI incidence. In contrast, several ADRB1 gene variants (i.e., coding for β1-AR) in combination with β-blocker therapy display higher risks [63,64,65].
β3-AR is coupled to both Gαs and Gαi and in contrast to the other β-ARs, it ensures a negative cardiac inotropic effect [40,55]. Post-MI, the β3-AR expression level increases and shows cardioprotective properties [41]. In acute MI, pre-activation of β3-AR before the reperfusion limits the infarct size. Its activation in chronic MI protects cardiac function and reduces fibrosis [41,42]. These effects are partly mediated by the activation of β3-AR-Akt-eNOS signaling that delays mitochondrial mPTP opening and increases cell survival [42]. In addition, nNOS also appears to be involved in β3-AR stimulation-induced cardioprotection [41].
2.3. Miscellaneous Cardiac GPCRs
2.3.1. Muscarinic Receptors Type 2
Muscarinic receptor type 2 (M2R) couples to Gαi and binds acetylcholine to mediate vagal regulation of cardiac function. M2R activation inhibits adenylyl cyclase activity, which depresses cAMP production and triggers a negative chronotropic effect (Table 1) [35]. Although M2R is upregulated at the remote zone of human MI patients, its expression remains unchanged in the infarct area. It has been proposed that this upregulation could prevent arrhythmogenicity induced by sympathetic activity [43]. Similarly to β-AR, M2R exhibits polymorphisms that are associated with increased risks of cardiac death following MI [66].
2.3.2. Adenosine Receptors (or P1 Receptors)
Four different genes encode for the adenosine receptor (AR) isoforms (i.e., A1AR, A2AAR, A2BAR, A3AR), which are all expressed in the heart. A2AAR and A2BAR isoforms couple to Gαs and their stimulation produces intracellular cAMP. In contrast, A1AR and A3AR couple to Gαi/o and show opposite effects to those of A2AR isoforms. Post-MI, ARs influence cardiac fibrosis and exhibit cardioprotective effects, which has been reviewed (Table 1) [44,45].
2.3.3. Prostaglandin Receptors
In the heart, several prostaglandin receptor isotypes co-exist, which modulate the cAMP signaling differently. Prostaglandin EP2 and EP4 receptors coupled to Gαs increase AC activity and cAMP production, while prostaglandin EP3 receptor, which is coupled to Gαi, depresses cAMP signaling [35]. Post-MI, the expression levels of prostaglandin EP3 and EP4 receptors increase and ensure cardioprotective properties (Table 1) [25]. Therefore, the stimulation of prostaglandin EP4 receptor by PGE2 in an I/R model induces cardioprotective effects through the increase in IL10 secretion and inhibition of TNFα [67]. Gene therapy overexpressing prostaglandin EP4 receptor in mice subjected to MI restores cardiac function and reduces hypertrophy and fibrosis [46]. In addition, prostaglandin EP3 receptor selective activation reduces the infarct size post-MI [47].
2.3.4. Glucagon and Glucagon Like Petide-1 Receptors (GCCR and GLP1R)
Glucagon and Glucagon Like Peptide-1 (GLP1) are known to regulate glucose homeostasis and metabolism. In the heart, GCCR (glucagon receptor) couples Gαs or Gαi to modulate the cAMP production. Therefore, GCCR stimulation triggers a positive inotropic response that is blunted after Gαi coupling [48]. In MI, GCCR stimulation exacerbates the cardiac disease by increasing the apoptosis, the infarct size and the mortality rate (Table 1) [49]. Therefore, specific GCCR inhibition exhibits beneficial effects on ventricular remodeling, cardiac metabolism and function.
Upon stimulation, GLP1R triggers cAMP production, which ensures a modest negative inotropic response (Table 1). This specific GLP1 compartmentalized signaling decreases myofilament affinity for calcium [13]. Interestingly, the injection of GLP1 or a GLP1R agonist (liraglutide) before MI activates pro-survival kinase and reduces the infarct size [50,51].
3. Adenylyl Cyclases
3.1. Structure and Function
Adenylyl cyclases (ACs) are a family of enzymes that mediate intracellular cAMP production from available ATP. In mammals, nine plasmalemma-bound isoforms (i.e., AC1–9) and one soluble isoform (i.e., sAC or AC10) have been reported [3]. Stimulated GPCRs directly regulate ACs throughout heterotrimeric G proteins (e.g., Gαs or Gαi/o). GTP-bound alpha subunit of Gs (Gαs) stimulates all AC isoforms to produce cAMP, while Gαi/o inhibits the activity of several ACs (i.e., AC1, 3, 5–6 and 8–9). Plasmalemma-bound ACs are structured with two hydrophobic TM1 and TM2 domains of six transmembrane segments each. TM1 and TM2 are linked together by an intracellular C1 loop which forms the catalytic site by dimerization with the C2-terminal sequence (for review, see [29,68,69]). Soluble AC lacks the transmembrane TM regions but contains the catalytic pocket (formed by C1 and C2 domains) and regulator clusters located on the C-terminal sequence. Heterotrimeric G proteins do not modulate sAC activity, which is reported to be stimulated by calcium and bicarbonate to produce cAMP [68,69]. sAC resides in the nucleus, centriole and mitochondria [70].
Although there is consensus in the scientific community about AC1–7, 9 and sAC expression in the heart, cardiac AC8 expression remains somewhat controversial [28,71]. It is noteworthy that AC5 and AC6 represent the most abundant cardiac isoforms.
3.2. Role of Major ACs in Cardiac Physiology and During MI
Studies on knockout (KO) mice models helped to decipher the roles of AC5 and AC6 in cardiac function [72,73,74]. Upon stimulation of left ventricular cardiomyocytes, AC5 and AC6 account for approximately 35% and 60% of intracellular cAMP production, respectively [73,75]. Although AC5 and AC6 exhibit some redundancy, their respective compartmentation in cardiomyocytes also provides some specificity in their regulation of cardiac function. AC5 locates at the T-tubule network and forms a complex with caveolins to mediate the β1- and β2-AR response [74]. In contrast, AC6 distributes with β1-AR on the outside of the T-tubule network [74]. It is known that cardiac AC5 promotes inotropic and chronotropic positive responses. Under β-AR stimulation, AC5 raises intracellular cAMP levels, activating downstream effectors, which, in turn, accelerates the HR, amplifies cardiomyocyte fractional shortening and increases left ventricle pressure [72,76]. However, the implication of AC5 and AC6 in the regulation of basal contractile function remains unclear [73,76]. Similarly to AC5, AC6 also modulates the inotropic response [73].
In cardiomyocytes, the specific subcellular compartmentation provides means to achieve regulation of AC5 and AC6 activity. PDEs which strongly regulate AC5 activity in T-tubules modulate, to a lesser extent, AC6 located at the plasmalemma [74]. In the basal condition, a constitutively active Gαi inhibits AC6 to maintain low cAMP levels [77]. However, under β1-AR stimulation, this inhibition is relieved, which triggers cAMP production [77]. In contrast, AC5 turns out to be inhibited by Gαi post-β-AR activation (see Section 2.2.2).
Interestingly, cardiac fibroblasts (CF) and cardiomyocytes display a diminution in the AC expression level as well as activity after MI [78,79]. Post-MI, CF cause fibrosis and scar formation at the infarct zone [80]. It has been reported that the diminution of the AC5 and AC6 expression level in CF exacerbates collagen deposition under β-AR stimulation in a chronic MI rat model. This suggests an AC-induced antifibrotic function that would prevent deleterious cardiac remodeling after MI [81]. It is commonly recognized that AC5 participates actively in the adverse cardiac remodeling, while AC6 offers cardioprotective effects post-MI [82]. Therefore, therapeutics targeting AC-mediated cAMP production are of interest to counteract either the chronic β-AR-induced cardiac remodeling or to limit infarction. Accordingly, infusion of the AC5 inhibitor vidarabine (i.e., AraA) or C90 before or shortly after coronary reperfusion reduces β-AR signaling and infarct size in an I/R mouse model [83,84]. However, the efficacy of AC5 inhibition by vidarabine remains controversial [83,85]. Similarly, in vitro inhibition of AC5 by PMC-6 prevents cardiomyocyte apoptosis without affecting the cell contractility under chronic β-AR stimulation [86]. Strategies aiming at increasing AC6 activity exhibit beneficial effects in MI outcomes. Mice overexpressing AC6 reveal a better survival rate than wild type (WT) mice post-MI. In addition to the recovery in cardiac contractility and relaxation, these transgenic AC6 mice also exhibit reductions in left ventricular dilatation and AV block incidence [87]. Moreover, in a model of MI-induced chronic heart failure, AC6 overexpression maintains the cardiac ejection fraction, prevents myocyte apoptosis and improves cardiac function [88].
3.3. Miscellaneous Cardiac ACs
In sinoatrial node (SAN), the cardiac pacemaker activity ensures a normal heart rhythm and rate. Therefore, SAN dysfunction leads to HR disorders. Arrhythmias are common features post-MI (e.g., bradycardias, tachycardias, heart blocks) and are symptomatic of poor patient prognosis, triggering cardiac arrests and sudden deaths [89]. In SAN, calcium induces AC1 activation, which, in turn, produces cAMP and modulates the pacemaker If current [90]. Therefore, the modulation of AC1 activity in SAN has been proposed to prevent arrhythmia occurrence. In a model of atrioventricular-blocked dogs, the overexpression of AC1 by gene therapy in SAN restored pacemaker activity and sensitivity to neurohormonal regulation [91].
In CF, AC3, 5 and 6 are expressed principally in caveolae. Interestingly, in these cells, angiotensin 2 (ANG II) potentiates the β-AR response through AC3 activity [92]. ANG II binds to AT1 receptor and activates Gαq, which increases the intracellular calcium level. This induces AC3-dependent cAMP production which comes in addition to that produced by β-AR stimulation. Together, the accumulated cAMP production limits collagen synthesis [92].
AC9 represents a minor source of total cardiomyocyte cAMP production (<3%) compared to the major activity of AC5/6. However, AC9 KO mice develop bradycardia and diastolic dysfunction with a preserved ejection fraction [82]. In cardiomyocytes, a pool of AC9 complexes with AKAP9, PKA and the potassium channel KCNQ1 to favor cardiomyocyte repolarization after sympathetic stimulation (see Section 5.3) [82,93,94]. In addition, the same group reported that cardiac AC9 regulates Hsp20 phosphorylation levels [82]. Interestingly, PKA-mediated Hsp20 phosphorylation shows cardioprotective properties after cardiac I/R injury (see Section 6.2) [95].
sAC produces cAMP in the mitochondrial matrix, which triggers PKA-dependent phosphorylation of cytochrome-c, regulating ATP production [96,97]. In myocardial infarction, reperfusion leads to cardiomyocyte injury and death associated with mitochondrial dysfunction. A study performed in models mimicking I/R in cardiomyocytes highlighted sAC as a key regulator of PKA-induced Bax phosphorylation, which triggers its mitochondrial translocation, leading to cell apoptosis [86]. Controversially, a recent study reported mitochondrial sAC overexpression to exhibit cardioprotective properties, improving cardiomyocyte survival after I/R [96].
4. Cyclic-AMP Downstream Effectors
4.1. Protein Kinase-A or cAMP-Dependent Protein Kinase (PKA)
4.1.1. Structure
In the heart, Protein Kinase-A (PKA) is the major cAMP downstream effector. The PKA holoenzyme is a heterotetramer composed of two regulatory (R) and two catalytic (C) subunits. R subunits contain an N-terminal docking and dimerization (D/D) domain, a linker region with a PKA C subunit inhibitor site and two cAMP binding sites (i.e., CNB-A and CNB-B). The D/D domain locates within the first 45 residues of each R subunit, which mediates R dimerization and A-kinase anchoring protein (AKAP) docking [98]. The PKA R inhibitor site consists of phosphorylation motifs that inhibit the PKA C subunit in a substrate/autoinhibitory manner [99]. Cyclic AMP molecules bind first to CNB-B sites that promote an intramolecular steric change and facilitate cAMP binding to CNB-A sites, which trigger PKA C activation [100]. The PKA C subunit phosphorylates serine and threonine residues in consensus amino acid sequences of types: Arg-Arg-X-Ser/Thr; Arg-Lys-X-Ser/Thr; Lys-Arg-X-Ser/Thr; or Lys-Lys-X-Ser/Thr (with X as hydrophobic residues) [101]. The classic binary model of PKA activation depicts the release of C subunits from the holoenzyme to phosphorylate PKA-dependent residues. However, a recent study indicated that an active PKA C subunit remains leashed to the PKA holoenzyme [102]. This suggests the phosphorylation of PKA substrates to occur within a restricted nano-compartment of 15–25 nm range inside the cell [91]. Therefore, PKA subcellular compartmentalization appears essential to ensure selective phosphorylation of its substrates (see Section 4.). Two classes of PKA holoenzymes have been identified (i.e., type I and II), which differ in their R subunits (RI and RII) [103,104]. Furthermore, each PKA subunit (RI, RII and C) exists as multiple isoforms (RIα, RIβ, RIIα, RIIβ, Cα, Cβ, Cγ and PRKX) that are encoded by distinct genes. While type I PKA is classically known to be mainly cytosolic, type II associates with organelles and specific cellular structures [4]. In the heart, the RIα, RIIα and Cα isoforms are expressed, as well as, to a lesser extent, Cβ [105].
4.1.2. PKA Modulates Cardiac Function
PKA plays a central role in the regulation of cardiac function and adaptation to stress (e.g., cardiac excitation–contraction coupling (CEC)). Upon β-AR stimulation and cAMP production, PKA phosphorylates CEC substrates and modulates positive chronotropic, inotropic and lusitropic responses [3]. In SAN, PKA triggers the positive chronotropic effect by regulating the calcium clock. This process is mediated by PKA-induced phosphorylation of L-type calcium channel (LTCC), ryanodine receptor (RyR) and phospholamban (PLB), which increase calcium release and HR [106]. Upon β-AR stimulation, these substrates turn out to also be phosphorylated by PKA in ventricular cardiomyocytes, increasing the contraction force and leading to a positive inotropic effect (Figure 1) [106]. Moreover, the positive lusitropic effect is under the control of PKA-dependent myofilament protein phosphorylation such as troponin I (TnI) and Myosin Binding Protein-C (MyBP-C) (Figure 1) [106]. Acute PKA activation mediates cardiac adaptation (e.g., fight-or-flight response). However, a chronic activation triggers adverse cardiac remodeling [18].
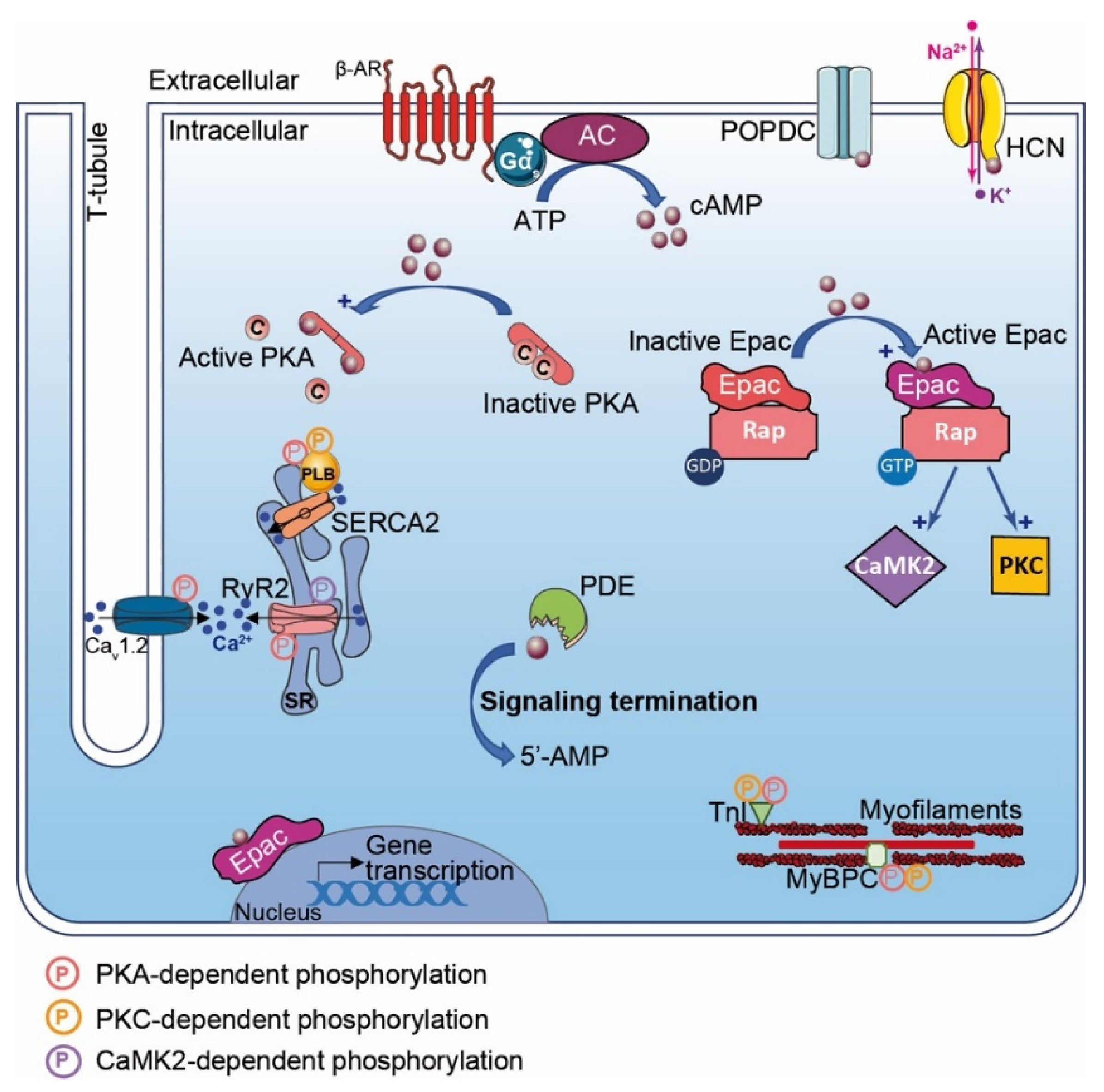
Figure 1.
β-Adrenergic Receptor and cAMP signaling pathways in the heart. β-Adrenergic Receptor (β-AR) activates adenylyl cyclase and generates pools of cAMP. cAMP (dark red-filled circles) has effects on a range of down effectors encompassing: PKA, Epac, POPDC, hyperpolarization activated cyclic nucleotide (HCN) channel and phosphodiesterases (PDEs). PKA activation leads to phosphorylation (P in pink circles) of specific substrates regulating Ca2+ flux and cardiac excitation–contraction coupling (CEC) (e.g., PLB, CaV1.2 (LTCC), RyR2, TnI, MyBPC). Cyclic AMP binding to Epac favors exchange of RAP-GDP into RAP-GTP, which activates phosphorylation by PKC and CaMK2 (P in yellow and purple circles, respectively). Activated Epac regulates gene transcription. Cyclic AMP binding to HCN channels triggers ion flux (Na+, K+) and hyperpolarization. Local concentration of cAMP gradient is limited by phosphodiesterases (PDEs), which hydrolyze cyclic nucleotide in inactive 5′-AMP, leading to termination of signaling.
4.1.3. PKA in Myocardial Infarction
PKA expression and activity post-MI remain unclear. Several studies reported an increase in type I PKA expression in acute MI in a rat model [107]. Studies performed in an I/R mice model described a decrease in type I PKA expression, while PKA C and RII subunits’ expression remained unchanged [108]. However, several studies reported neither modifications in PKA expression nor activity [109,110]. These discrepancies could be explained by models used and or by the time of study post-MI. Promptly after stress, β-AR signaling is mobilized, increasing PKA activity and pacing the heart pump counterbalancing MI-induced cardiac dysfunction [111,112]. However, sustained β-AR stimulation leads to GPCR desensitization, a compensatory mechanism diminishing PKA activity. Interestingly, PKA signaling has been described to exhibit cardioprotective effects post-MI. PKA activation in the pre-ischemic heart as a preconditioning condition limits infarct size though Rho-Kinase inhibition [113]. The antioxidant N-acetyl cysteine (NAC) shows antiarrhythmic properties. These are mediated after activation of glutathione that triggers ACs-induced cAMP production and PKA activity. Thereafter, PKA inhibits GSK3β, which abolishes connexin-43 (Cx43) internalization and occurrence of arrythmias [114]. Furthermore, adrenomedullin administration in Langendorff-perfused hearts limits infarct size in a cAMP/PKA signaling pathway activation-dependent manner favoring mitoKCa channel opening [115]. However, the molecular mechanisms underlying these processes need to be further investigated. In additional studies, it has been reported that sitagliptin (i.e., dipeptidyl-peptidase-IV inhibitor), pioglitazon (i.e., PPAR-γ agonist) and simvastatin (a statins family member) limit infarct size in a PKA-dependent manner [112,116,117].
4.2. Epac (Exchange Protein Activated by cAMP)
4.2.1. Epac Structure
Epac is a guanine nucleotide exchange factor (GEF) protein that activates Ras superfamily small GTPases Rap1 and Rap2. Two Epac isoforms (i.e., Epac1 and Epac2) are expressed in the heart. The Epac expression pattern changes during the life span. While Epac2 expression is predominant in adults, Epac1 is the major neonatal isoform [118]. The Epac N-terminus displays a regulatory region that contains one CNB (or two for Epac2) similar to that of the PKA regulatory subunit, which activates Epac upon cAMP binding. Furthermore, this region also exhibits a Disheveled/Egl-10/Pleckstrin (DEP) domain that favors Epac membrane localization. The catalytic C-terminal extremity contains three domains (i.e., REM, RA and CdC25-HD). The REM (Ras Exchange motif) domain stabilizes the Epac CdC25-HD catalytic helix and may scaffold additional regulatory proteins [119]. The Ras-association (RA) domain interacts with GTP-bound Ras and mediates Epac localization at the plasma membrane. The CdC25 homology GEF (CdC25-HD) domain triggers Epac guanine-nucleotide exchange activity [119].
4.2.2. Epac Cardiac Function
Epac enhances cardiac contractility though regulation of calcium signaling [120]. Epac activation with the specific cAMP agonist 8-CPT-2′-O-Me-cAMP (i.e., 007) mobilizes the Rap1-PLC-CAMKII signaling pathway. In this context, the activation of CAMKII phosphorylates RyR2 and induces Ca2+ released from the sarcoplasmic reticulum (SR) that is independent of PKA and the LTCC-mediated calcium-induced calcium release process (CICR) (Figure 1) [120]. Epac can also activate PKC that phosphorylates cardiac TnI and MyBPC, which enhance cell shortening (Figure 1) [120]. However, the Epac-mediated positive inotropic effect remains controversial. These divergences rely on stimulation duration, as chronic Epac stimulation induces SR calcium depletion and reduction in calcium transients [120]. In the heart, it is established that specific Epac isoform compartmentalization mediates distinct subcellular effects. While Epac2 locates at the T-tubules network and regulates CAMKII and PKC signaling, peri-nuclear Epac1 modulates gene transcription (Figure 1) [121].
Finally, the role of Epac in cardiac pathophysiology remains ambiguous as it has been reported to have effects that are both pro- and anti-apoptotic, both pro- and anti-fibrotic and both pro- and anti-hypertrophic, with pro-arrhythmic effects [122,123]. These discrepancies need to be further elucidated.
4.2.3. Epac in Myocardial Infarction
Post-MI, the profibrogenic factor TGF-β1 decreases Epac1 expression in cardiac fibroblasts located at the border zone, which inhibits fibroblast migration and induces collagen synthesis [124]. In contrast, Epac1 overexpression suppresses TGF-β1-induced collagen synthesis. Although the molecular mechanisms remain elusive and need to be further underpinned, Epac1 activation is reported to improve cardiac function by limiting infarct size, preventing atrial fibrosis, cardiac hypertrophy and remodeling [125]. Interestingly, a cell therapy approach in which mesenchymal stem cells (MSCs) are transplanted into the infarction zone to heal the diseased cardiac part needs activation of Epac-Rap1 signaling [126]. Epac activation favors homing, adhesion and differentiation of MSCs in cardiomyocytes which improves heart morphology, prevents ventricular dilatation and rescues cardiac contractility [126]. In redundancy with PKA (see above), NAC also mediates activation of Epac signaling that inhibits GSK3, limits Cx43 down-regulation and reduces arrythmia occurrence post-MI [114].
4.3. Cyclic Nucleotide-Regulated Cations Channels (CNCC)
CNCC are a heterogeneous superfamily of ion channels activated by cAMP or cGMP (Figure 1). The heart expresses two subtypes of this superfamily, which include hyperpolarization-activated cyclic nucleotide-gated ion (i.e., HCN) and cyclic nucleotide-gated ion (i.e., CNG) channels. Both HCN and CNG channels assemble at the plasmalemma in tetramer complexes. The transmembrane core consists of alpha-helical segments that form the ion-conducting pore, which in the case of the HCN channel exhibits a supplementary voltage sensor domain conferring specifically to this channel voltage-dependent gating properties [127]. The C-terminal extremity of HCN and CNG contains a CNB domain that binds cAMP and cGMP. Interestingly, HCN channels exhibit a higher affinity for cAMP binding than cGMP and vice versa for CNG channels. It is noteworthy that cyclic nucleotides binding is not required for HCN channel activation; however, it modifies channel voltage-dependent activation to more positive membrane potentials [127]. Both CNG and HCN channels are permeant to monovalent cations (i.e., Na+ and K+) and Ca2+ with distinct properties (Figure 1). The four types of HCN channels (HCN1–4) are expressed in the heart [128]. Among them, HCN4 is preferentially expressed in SAN, while HCN2 appears to be the major subtype in ventricles [129]. In SAN, HCN channels regulate the If current and trigger cardiac pacemaker activity. Therefore, HCN4 KO mice develop severe bradycardia and arrhythmic events [128]. Dynamic changes in HCN expression occurring post-MI have been reported. While the HCN4 expression level increases, favoring If current and arrhythmia occurrences in the left ventricular myocardium post-MI, the HCN1 expression level, however, diminishes [130]. The ivabradine therapeutic prescribed in patients to reduce arrhythmic events also inhibits the If current and the increase in HCN4 expression post-MI [131]. Alternatively, spironolactone that targets HCN4 expression has also been proposed as a post-MI anti-arrhythmic medication [132].
4.4. Popeye Domain-Containing Protein
Popeye domain-containing (POPDC) proteins are plasmalemma proteins that are encoded by three different genes (Figure 1) [133]. POPDC proteins consist of a short extracellular N-glycosylated N-terminal domain linked to three transmembrane domains. The cytosolic tail harbors the POPDC and a variable C-terminal domain (CTD) that can be phosphorylated (e.g., under β-AR stimulation). Interestingly, POPDC functions as a high-affinity cAMP binding site that differs from consensus CNB domains found in PKA, Epac and HCN. POPDC proteins are expressed in the heart and particularly in the conduction system (i.e., SAN and atrio ventricular nodes) [133]. POPDC knockout mice exhibit a normal basal HR but show sinus bradycardia under stress response with structural alterations of SAN myocytes [134]. Deletion of POPDC leads to cardiac arrhythmia in zebrafish [135,136]. Interestingly, patients harboring POPDC mutations present cardiac arrhythmias [135,137,138]. Post-I/R injury, POPDC-null mice show a larger infarct size and bad cardiac recovery compared with wild-type mice [139].
5. A-Kinase Anchoring Proteins (AKAP)
5.1. Structure and Function
The spatial regulation of intracellular PKA signaling is orchestrated by its compartmentalization to a precise subcellular subset mediated by direct interaction with A-kinase anchoring proteins (AKAPs) [140]. AKAPs are a large family of structurally distinct proteins (>50 identified) that are defined by their ability to provide spatial and temporal regulation of PKA signaling events. To belong to the AKAP family, proteins must exhibit two specific properties: (i) must contain an A-kinase binding (AKB) domain that consists of a 14–18 amino acid amphipathic helix region, which interacts with the dimerized PKA R-subunit D/D domain; (ii) must contain a unique subcellular targeting domain that directs PKA/AKAP complexes to a defined location inside the cell (i.e., organelle, plasmalemma, protein complexes or lipids). In addition to these features, it has been reported that several AKAPs are directly bound to or in the close vicinity of PKA-specific substrates and that they could assemble signalosome complexes by direct interactions with additional multivalent signaling proteins (e.g., kinases, PDEs, phosphatases (PPs), Epac) [4,141,142,143]. Integration of PPs and PDEs into PKA/AKAP complexes provides a supplementary regulation level with a tight control of the cAMP–PKA signaling termination.
AKAPs, which are widely expressed in various cell types and tissues, form precise macromolecular complexes to ensure the specificity in PKA-dependent signaling cascades [4,144,145,146]. Historically, it was known that most AKAPs preferentially anchored the PKA type II holoenzyme with a high affinity, while few displayed dual-specificity with the capacity of binding both PKA R subtypes [4,147,148]. In the last decade, a new class of AKAPs has been discovered and reported to specifically bind PKA type I (e.g., SKIP, smAKAP) [4,142,149,150]. AKAPs were first named according to their respective molecular mass. Although widely approved, this nomenclature turned out to be confusing due to ortholog AKAP genes encoding for proteins with various molecular masses between species. Therefore, in an attempt to standardize the nomenclature, AKAPs are now classified according to the HUGO gene nomenclature committee name (Table 2).

Table 2.
Role of cardiac AKAPs in physiological and in myocardial infarction. AKAP: A-kinase anchoring protein; MI: myocardial infarction; PP1/2B/2A: Protein Phosphatase 1/2B/2A; PDE: phosphodiesterase; Drp1: Dynamin Related Protein 1; AC5/6: adenylyl cyclase 5/6; PKC: Protein Kinase C; LTCC: L Type Calcium channel; cAMP: cyclic Adenosine Monophosphate; Epac 1: Exchange Protein Activated by cAMP; RyR: Ryanodine Receptor; NCX: Na/Ca exchange; HIF1α: Hypoxia Induce Factor 1α MEF2: Myocyte Enhancer Factor 2; NHERF: Na+/H+ Exchanger Regulatory factor; CaN: calcineurin; β-Ars: β-Arrestins; β2-AR: β2-Adrenergic Receptor.
5.2. Cardiac AKAPs with Patophysiological Function in MI
AKAP1 (i.e., D-AKAP1 or S-AKAP84, AKAP121, AKAP149) is a dual-specificity AKAP that distributes to the mitochondrial outer membrane (Table 2). The first 30 amino acids of the protein sequence correspond to a mitochondrial targeting sequence. In addition to the AKB domain, AKAP1 exhibits a PP1 (Protein Phosphatase 1) binding sequence and a KH-Tudor domain that restricts and favors translation of specific mRNAs (e.g., Star, SOD2, F0-f). Furthermore, AKAP1 anchors CaN (calcineurin, PP2B), PDE4 and Drp1 (GTPase Dynamin-related protein 1) to regulate mitochondrial fission and fusion (Table 2; Figure 2A) [140]. AKAP1 coordinates a cardioprotective macromolecular complex mediating PKA-dependent Drp1 phosphorylation, which, in turn, abolishes Drp1/FIS1 interaction and inhibits mitochondrial fission, leading to cell survival (Figure 2A) [173]. This process is counterbalanced by CaN recruitment to the AKAP1 signaling complex, which, in contrast, favors Drp1 dephosphorylation and mitochondrial fragmentation. Furthermore, AKAP1-anchored PKA enhances BAD phosphorylation that abolishes BAD-Bcl2 interaction and prevents cardiomyocyte death (Table 2) [140]. AKAP1 KO mice with permanent coronary ligation exhibit an extensive myocardial infarction size, illustrating the cardioprotective properties of this AKAP [174]. In MI, hypoxia activates Siah2 (ubiquitin-protein ligase seven in absentia homolog 2), which ubiquitinates AKAP1 and leads to its proteolysis. The loss of AKAP1 causes a decrease in PKA-dependent BAD and Drp1 phosphorylation, triggering their association with Bcl2 and FIS1, respectively, which enhances mitochondrial fission, mitochondrial ROS production, oxidative stress, cardiomyocyte death and myocardial dysfunction [151,173] (Table 2).
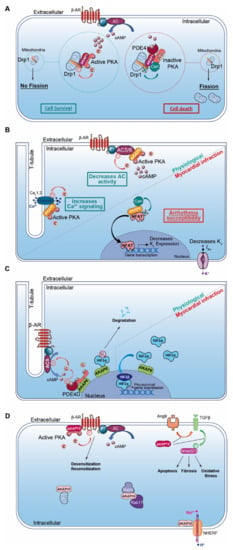
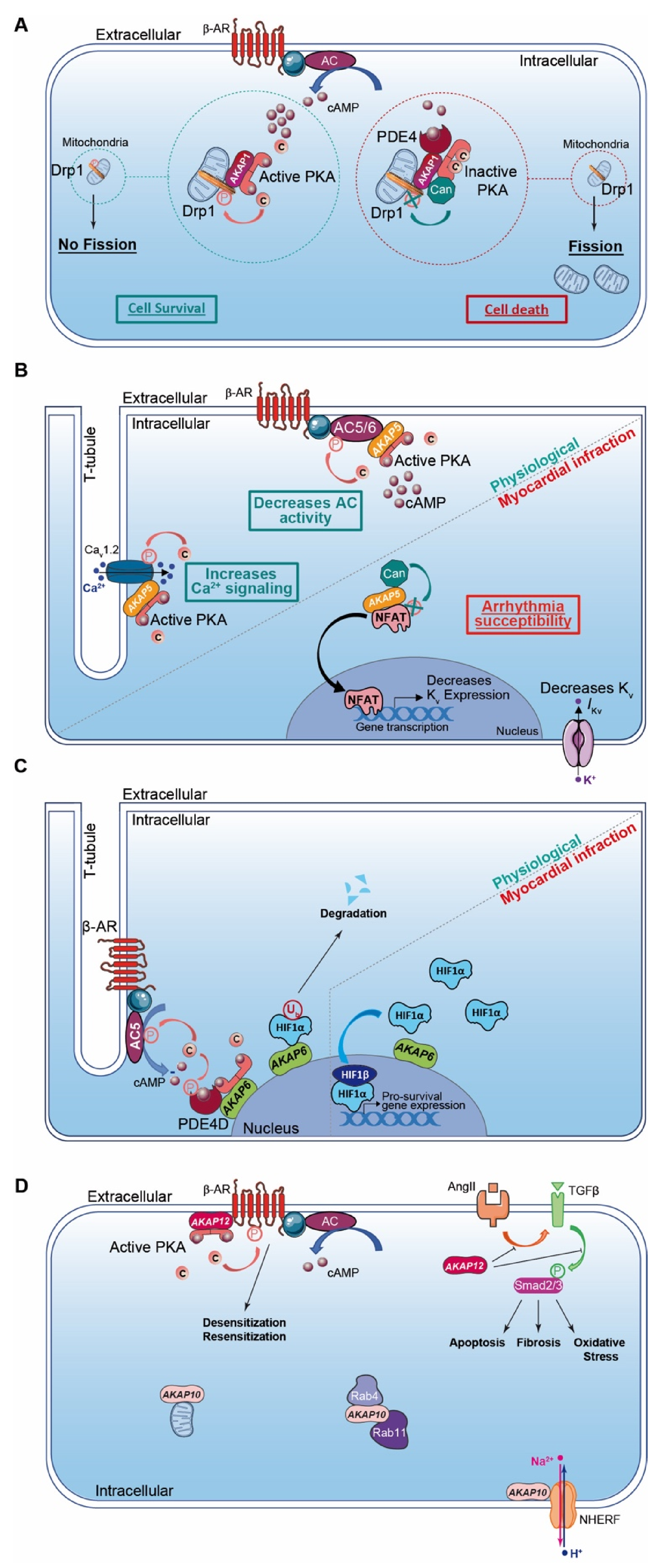
Figure 2.
Cardiac AKAPs and cAMP signaling compartmentalization in myocardial infarction (MI). (A) AKAP1 coordinates at the mitochondria a cardioprotective macrocomplex that mediates phosphorylation of Drp1 by anchored PKA, which inhibits mitochondrial fission and leads to cell survival (left). This process is counterbalanced by CaN recruitment on AKAP1 signaling complex, which, in contrast, favors Drp1 dephosphorylation and mitochondrial fragmentation (right). (B) Role of cardiac AKAP5 under physiological conditions and after MI. In cardiomyocytes, AKAP5-anchored PKA mediates direct AC5 and AC6 phosphorylation to inhibit AC activity and cAMP production (top left). AKAP5 brings PKA in proximity to LTCC, which regulates Ca2+ entry (bottom left). AKAP5 anchors CaN and participates in NFATc3 activation, which down-regulates Kv channel expression level, reduces IKv, prolongs action potential duration and favors arrhythmia susceptibility post-MI (right). (C) Role of cardiac AKAP6 under physiological conditions and after MI. Activated AC5 produces a pool of cAMP that mobilizes AKAP6-anchored PKA. PKA phosphorylates AC5 and AKAP6-anchored PDE4D that, respectively, inhibit AC5-dependent cAMP production and trigger local cAMP degradation by PDE4D (left). Under physiological conditions, AKAP6 mediates HIF1-α ubiquitination and degradation, while hypoxia inhibits this process and leads to HIF1-α accumulation. HIF1-α complexes with HIF1-β and initiates transcription of pro-survival genes to favor cell survival under ischemic stress (right). (D) Cardiac AKAP10 and AKAP12 under physiological conditions. In the heart, AKAP10 distributes to the mitochondria, in the cytoplasm (with small GTPases Rab4 and Rab11) and at the plasmalemma (associated with Na/H exchanger (NHERF)). AKAP12 favors β-AR phosphorylation and triggers GPCR desensitization/resensitization cycling. In the heart, angiotensin II (AngII) activates cardiac TGFβ1 pathways, which favors oxidative stress, apoptosis and fibrosis. AKAP12 inhibits deleterious AngII and the TGFβ1 pathway and exhibits cardioprotective properties. AKAP: A-kinase anchoring protein; HIF1α: Hypoxia Induced Factor-1α; CaN: calcineurin; PDE4: phosphodiesterase 4; AC5/6: adenylyl cyclase 5/6; β-AR: β-Adrenergic Receptor.
AKAP5 (i.e., AKAP79 or AKAP75, AKAP150) is a PKA type II-specific anchoring protein for which roles and functions have been extensively investigated over the past years in cardiac physiological and pathophysiological conditions (Table 2). The AKAP5 N-terminus exhibits a membrane targeting domain distributing the AKAP to the T-tubule network or sarcolemma inner leaflets, which facilitates its interaction with several proteins (e.g., AC5 and 6, PKC, F-actin, cadherin) (Table 2) [152,153]. In cardiomyocytes, AKAP5-anchored PKA mediates direct AC5 and AC6 phosphorylation to inhibit AC activity and cAMP production (Table 2; Figure 2B) [153,175]. AKAP5 displays MAGUK and CaN binding domains in its central core. In the C-terminus, the AKB domain resides together with a leucine zipper motif that interacts with LTCC [82]. It has been reported that AKAP5 brings PKA in proximity to LTCC, caveolin-3 and β-AR [154]. This supramolecular signaling complex regulates the PKA-induced sympathetic signaling activation and LTCC-mediated Ca2+ entry in cardiomyocytes [82,154] (Table 2; Figure 2B). In chronic MI, AKAP5 KO mice develop exacerbated ventricular dilation, severe cardiac remodeling (i.e., fibrosis and large infarct), myocyte apoptosis and an enhanced hypertrophy with pulmonary edema [155]. The loss of cardiac AKAP5 leads to impaired Ca2+ signaling, a defect in CICR (i.e., Ca2+ transient and SR load), a diminution in cardiac Ca2+ regulatory protein phosphorylation and contractility deficiency (Table 2) [155]. In addition, a decrease in the cardiac AKAP5 expression level under pathological stress has been reported [155]. These data highlight AKAP5 cardioprotective functions. However, this is challenged by another study showing that AKAP5-anchored CaN participates in NFATc3 activation, which down-regulates the Kv channel expression level and reduces IKv post-MI. This prolongs the action potential duration and favors arrhythmia susceptibility (Table 2; Figure 2B) [156].
AKAP6 (i.e., mAKAP or muscle AKAP, AKAP100) is a PKA type II-specific anchoring protein which, by interaction with nesprin-1, confers a specific localization to the outer nuclear membrane (Table 2) [157]. AKAP6 has also been reported to distribute, to a lesser extent, to the SR [176]. However, this SR distribution has been challenged and it has been proposed instead that this could reflect the close proximity between the nuclear envelope and cardiac dyads (i.e., T-tubule and SR). In the heart, AKAP6, in addition to binding PKA type II and nesprin-1α, clusters a large number of proteins either constitutively or temporarily including PLCε (phospholipase Cε), AC5, Epac1, PDE4D3, RyR2, NCX, HIF1-α Hypoxia Inducible Factor 1α), MEF2 (myocyte enhancer factor-2), kinases (PKCε, PKD, MEK5, ERK5, PDK1, RSK3) and phosphatases (CaN and PP2A) (Table 2) [158]. The AKAP6-PKA-PDE4D3-AC5 complex negatively regulates the AC5-dependent cAMP production as a feedback loop (Figure 2C). Activated AC5 produces a pool of cAMP that mobilizes AKAP6-anchored PKA. Thereafter, the kinase phosphorylates both AC5 and the AKAP6-anchored PDE4D3 that, respectively, inhibit AC5-dependent cAMP production and trigger local cAMP degradation by PDE4D3 (Table 2; Figure 2C) [159]. Furthermore, AKAP6 regulates cellular oxygen homeostasis by modulating the expression level of the transcription factor HIF1-α [160] (Table 2). HIF1-α accumulation has been reported as an early MI marker [161]. Although AKAP6 in normoxia mediates HIF1-α ubiquitination and degradation, hypoxia inhibits this process and leads to HIF1-α accumulation (Figure 2C). This increases HIF1-α/HIF1-β complex formation, which initiates transcription of pro-survival genes (e.g., pro-angiogenic, metabolic and anti-apoptotic) and cell survival under ischemic stress (Table 2; Figure 2C) [160].
AKAP8 (i.e., AKAP95) is a PKA type II-specific anchoring protein highly expressed in the heart and anchors PKA RIIα with higher affinity than RIIβ (Table 2) [162,163]. AKAP8 associates with the nuclear matrix during interphase and mediates chromosome condensation at mitosis [177]. However, the role of AKAP8 remains elusive in adult cardiomyocytes that do not divide under physiological conditions. Post-MI, microRNA-21 (miR-21) is upregulated at the border of infarction zones, which exhibits cardioprotective functions against I/R-induced apoptosis [178]. Recently, it has been reported that miR-21 targets and reduces the AKAP8 expression level, leading to cardiomyocyte protection against apoptosis induced by I/R events (Table 2) [164]. However, the molecular mechanism underlying the inhibition of AKAP8 that mediates anti-apoptotic effects during I/R events needs to be further investigated.
AKAP10 (i.e., D-AKAP2) is a dual-specificity AKAP that mainly associates with mitochondria but is found, to a lesser extent, in the cytoplasm or at the plasma membrane (Table 2; Figure 2D). In addition to the AKB, the AKAP10 amino acid sequence includes two RGS (Regulators of G protein Signaling) homology domains that interact with the small GTPases Rab4 and Rab11 (Table 2; Figure 2D). Furthermore, AKAP10 also displays a PDZ (PSD-95/DlgA/ZO-1) binding motif, which docks NHERF (Na+/H+ Exchanger Regulatory Factor) isoforms and ensures the connection between the anchoring protein and the solute carrier [148,179]. AKAP10 exhibits a functional single-nucleotide polymorphism (SNP) in which the Ile in position 646 is substituted by a Val [165]. This polymorphism affects the AKB domain by increasing the affinity of AKAP10 for type I PKA anchoring without affecting the one of type II. Patients with this functional AKAP10 SNP have an increase in HR with a low HR variability predisposing to sudden cardiac death [180]. This is replicated in a mice model mutated in the AKAP10-AKB domain that displays modifications in the HR and baroreceptor function with sinoatrial and atrioventricular blocks and arrhythmias (i.e., extended P-P and P-R intervals on ECGs) [180]. These findings suggest a role for AKAP10 in the regulation of the heart rhythm. However, the underlying molecular mechanisms need to be further investigated. Interestingly, patients with a functional AKAP10 SNP without hypercholesterolemia have an increased prevalence for MI, which implies the I646V nucleotide substitution as a cardiac ischemic risk factor (Table 2) [166].
AKAP12 (i.e., gravin or AKAP250, SSeCKS) is a PKA type II-specific anchoring protein that locates in the cytoplasm or associates with the cytoskeleton network and plasma membrane (Table 2; Figure 2D). The AKAP12 N-terminus exhibits a myristoylation site and a MARCKS effector homology domain favoring the docking to the plasmalemma [167]. In the heart, AKAP12 scaffolds multiple signaling proteins including PKA type II, PKC, PLK1 (Polo-Line Kinase-1), PDE4D, CaN and β-Ars (β-Arrestins) (Table 2) [168,169]. AKAP12 binds to the β2-AR C-terminus and mediates phosphorylation of the GPCR by PKA. This triggers desensitization/resensitization cycling of the receptor (Table 2; Figure 2D) [168,169]. AKAP12-mutated mice without the ability to anchor both PKA and β2-AR exhibit a diminution in PKA-dependent β2-AR phosphorylation. This leads to the reduction in β2-AR desensitization and increases cardiac function and contractility [170]. Post-MI, cardiac Ang II levels increase which trigger cardiac remodeling and apoptosis. It has been reported that Ang-II infusion in AKAP12 KO mice mediates exacerbation of TGF-β1 signaling and Smad2/3 phosphorylation, leading to cardiac oxidative stress, apoptosis and fibrosis (Table 2; Figure 2D) [171]. In contrast, AKAP12 overexpression suppresses the Ang-II-induced TGF-β1 signaling activation and fibrosis, supporting the cardioprotective properties of AKAP12 (Table 2; Figure 2D). Of note, the cardioprotective function of AKAP12 in cardiac fibroblasts has been previously reviewed [172].
5.3. Miscellaneous Cardiac AKAPs
AKAP7 (i.e., AKAP15 or AKAP18) is a PKA type II-specific anchoring protein with several isoforms which have been reported to be present in the heart including shorter (i.e., AKAP7α, AKAP7β) and longer variants (i.e., AKAP7γ/δ (respectively, for human/rat)). All isoforms present a common C-terminus that includes the AKB domain. Differences in AKAP7 isoforms occur in the length of the N-terminus. The distribution at the plasma membrane of shorter AKAP7 isoforms is facilitated by palmitoylation and myristoylation of N-terminal residues. The leucine zipper motif present in the C-terminus mediates AKAP7α interaction with LTCC [181,182]. Upon β-AR stimulation, AKAP7α-anchored PKA mediates LTCC phosphorylation on α and β subunits to potentiate ICaL and cardiac contractility [183,184].
AKAP7γ/δ localizes at the SR membrane of cardiomyocytes and interacts with phospholamban (PLB) [185]. Upon β-AR stimulation, AKAP7γ/δ-anchored PKA phosphorylates PLB at Ser16. This phosphorylation alleviates specific PLB inhibition of SERCA2, which enhances SERCA2 ATPase activity, Ca2+ uptake in the SR and cardiac relaxation [185]. In addition, anchored PKA phosphorylates PDE3A1 bound to SERCA2, which hydrolyzes the pool of cAMP in the vicinity of the AKAP7γ/δ-PKA-PLB-SERCA2 complex to temporally regulate the PKA-induced SR Ca2+ uptake [186]. Furthermore, by anchoring Protein Phosphatase Inhibitor-1 (I-1), AKAP7γ/δ facilitates its phosphorylation by PKA at Thr35, which inhibits PP1 activity and the dephosphorylation of PLB by this phosphatase [187]. In contrast to the shorter isoforms, AKAP7γ/δ scaffolds a supramolecular signalosome complex regulating cardiac relaxation in space and time.
AKAP9 (i.e., AKAP450, AKAP350, Yotiao, CG-NAP, Hyperion) is a PKA type II-specific anchoring protein that scaffolds PDE4D and AC9 and exhibits in its C-terminus a PP1 binding region and the AKB domain. AKAP9 distributes in the cytoplasm or is found associated with the cytoskeleton network, at the plasmalemma and at the Golgi apparatus. Yotiao, which is the shortest AKAP9 isoform, interacts with KCNQ1 and its C-terminus leucine zipper motif. In cardiomyocytes, KCNQ1 (i.e., α-subunit) and KCNE1 (i.e., accessory β-subunit) form the potassium channel and trigger the slow delayed rectifier K+ current (IKs), which regulates cardiac action potential late-phase repolarization [188]. Upon β-AR stimulation, Yotiao-anchored PKA phosphorylates KCNQ1 at Ser27, increasing IKs, which shortens the action potential duration to maintain diastolic time intervals [188]. By anchoring PP1 and PDE4D in the vicinity of KCNQ1, Yotiao mediates spatiotemporal regulation of IKs by β-AR activation. Patients with mutations in the KCNQ1 and Yotiao interaction regions (e.g., G589D or Q1570L, respectively) exhibit long QT syndrome [189]. These mutations abolish the KCNQ1–Yotiao association, increasing the distancing between the potassium channel and Yotiao-PKA complex. This compromises PKA-induced KCNQ1 phosphorylation upon β-AR stimulation, which alters IKs regulation by the kinase, prolongs the action potential duration and delays cardiomyocyte repolarization. As a consequence, this extends the QT interval and favors the occurrence of fatal arrhythmias [189].
AKAP13 (i.e., AKAP-lymphoid blast crisis (AKAP-Lbc) or Ht31) is a PKA type II-specific anchoring protein expressed in the cytoplasm of cardiac fibroblasts (CF) and cardiomyocytes [190,191]. In addition to PKA type II anchoring, AKAP13 recruits additional signaling molecules including PKCη, PKD1, PDE4D, PKNα, MAPKs (i.e., MLTK, MKK3, p38α) and phosphatases (e.g., shp2) [192]. Among all known AKAPs, AKAP13 exhibits a unique feature with the capacity to activate small Rho-GTPAses (i.e., RhoA and RhoC) through a specific guanine nucleotide exchange factor (GEF) domain [190,193]. This domain is composed of a tandem of Dbl-homology (DH) and plekstrin-homology (PH) motifs located in the central core of AKAP13 [193]. The AKAP13 expression level rises in response to cardiac stress and acts as an early modulator of compensatory cardiac hypertrophy [194]. In cardiomyocytes, mechanical stress or α1-AR stimulation activates RhoA by the AKAP13 Rho-GEF domain, which, in turn, enhances the MAPK p38α signaling cascade (i.e., PKNα-MLTK-MKK3-p38α) [195,196]. Likewise, mechanical stress, α1-AR or AT1-R stimulation mobilizes AKAP13-anchored PKCη, which, in turn, phosphorylates and activates PKD1. This phosphorylation favors PKD1 dissociation from AKAP13, which consequently triggers MEF2-induced hypertrophic gene transcription and inhibition of cardiomyocyte apoptosis [197,198].
In CF, AngII stimulates AT1-R and activates the AKAP13 Rho-GEF domain that eases myofibroblast differentiation with the induction of collagen, α-SMA expression and profibrotic gene transcription [191].
Additional AKAPs are known to be expressed in the heart including AKAP11 (i.e., AKAP220), ezrin, SKIP, smAKAP and BIG2 [149,150,199,200,201]. However, their cardiac functions as PKA-anchoring proteins remain unexplored. Finally, several AKAPs have been identified in the vicinity of contractile myofilaments including synemin and myosprin, which are anchored at Z-lines, or cardiac troponin-T (cTnT) associated with thin filaments [202,203,204]. Although these AKAPs optimally regulate the contractile function by PKA, the composition of the respective protein macrocomplexes needs to be further investigated.
6. Phosphodiesterases (PDEs)
6.1. Structure and Function
The regulation of local cyclic nucleotide 3′-5′ monophosphate (cNMP; i.e., cAMP and cGMP) nanodomains in cardiomyocytes is conferred by a superfamily of enzymes that includes over 100 different PDE isoforms [205,206]. PDE ensures second messenger signaling termination by specifically hydrolyzing the cNMP phosphodiester bond to form inactive 5′-NMP (e.g., 5′-AMP or 5′-GMP). PDE isoforms result from various translation initiation sites or after alternative mRNA splicing. The PDE nomenclature includes a family number (i.e., 1–11), followed by a gene letter (i.e., A, B, C and D) and potentially a splice variant identification number. Among mammalian PDE families, three selectively hydrolyze cAMP (i.e., PDE4, 7 and 8), three are selective for cGMP (PDE5, 6 and 9) and five families hydrolyze both cyclic nucleotides with distinct properties (PDE1, 2, 3, 10 and 11) [3,206]. With the exception of PDE6, PDE7 and PDE11, all other PDE isoforms are expressed in the heart [31,207]. PDEs exhibit a conserved C-terminal catalytic domain, whereas differences occur in the N-terminal regulatory region that may include an enzyme dimerization domain, phosphorylation sites and binding sites to signalosome macrocomplexes (Figure 3A). The regulatory domain confers PDEs’ specificity. Although PKA may interact directly with some PDEs, their proximity to signaling molecules (i.e., kinases, phosphatases and AC) is generally insured by anchoring to AKAPs, which participates in intracellular signaling compartmentation. [208].
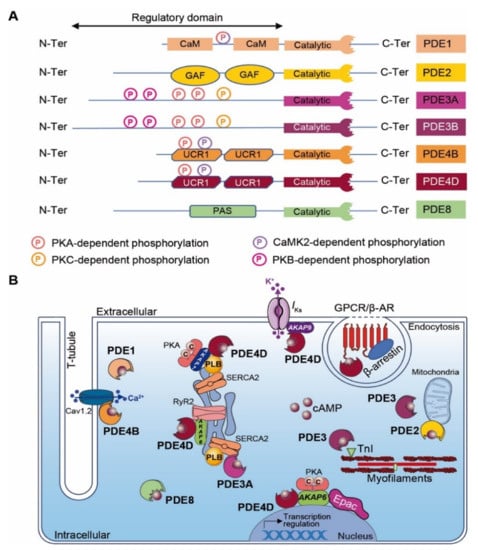
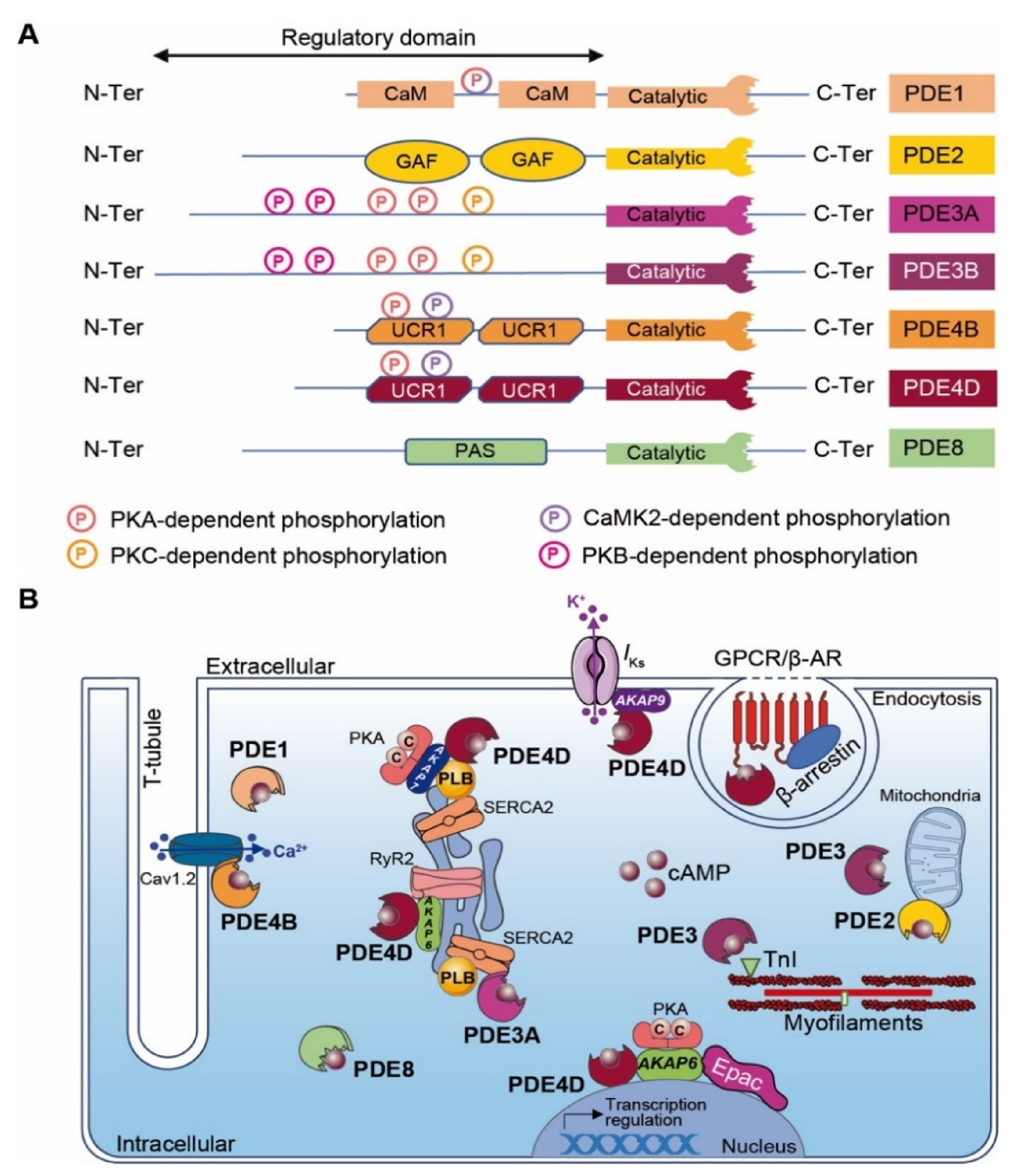
Figure 3.
Cardiac cAMP-PDEs. (A) Structure of major cardiac cAMP-PDEs. PDEs exhibit a conserved C-terminal catalytic domain and a variable N-terminal regulatory domain. Kinase-dependent phosphorylation sites are indicated as circled P (light pink for PKA, yellow for PKC, purple for CaMK2, dark pink for PKB and mixed with light pink and purple for PKA and CaMK2). CaM: calmodulin binding domain; GAF: GAF (i.e., cGMP-dependent PDE, Anabaena adenylyl cyclases and E. Coli FhlA) domain; UCR: upstream conserved region; PAS: Per-Arnt-Sim domain; and PDE: phosphodiesterase. (B) Scheme of major cardiac cAMP-PDE compartmentalization. GPCR: G protein-coupled receptor; β-AR: β-Adrenergic Receptor; TnI: troponin I; RyR2: Ryanodine receptor-2; Epac: Exchange Protein Activated by cAMP, PKA: Protein Kinase-A; SERCA2: Sarco/Endoplasmic Reticulum Ca2+-ATPase; PLB: Phospholamban; AKAP: A-kinase anchoring protein.
6.2. Cardiac PDEs with Physiological Function in MI
PDE1 isoforms are encoded by three different genes (i.e., PDE1A, PDE1B and PDE1C). Among cardiac PDE1 isoforms, PDE1A expression is upregulated in diseased hearts, PDE1B expression remains low and PDE1C, which represents the major PDE1 isoform, displays a striated expression pattern [209]. PDE1 exhibits two Ca2+/CaM binding domains positioned at the N-terminus region. Ca2+/CaM binding induces PDE1 conformational change, which enhances PDE hydrolytic activity [210]. In contrast, PKA- and CaMKII-dependent PDE1 phosphorylation reduces Ca2+/CaM binding and phosphodiesterase activity [209,211]. In ischemic cardiomyopathy, PDE1A expression increases in cardiomyocytes and in α-SMA (α-Smooth Muscle Actine)-positive myofibroblasts of the infarct border zone, but the expression level remains low in normal fibroblasts [212]. In myofibroblasts, PDE1A upregulation inhibits cAMP-Epac1-Rap1 and cGMP-PKG signaling pathways, leading to fibroblast transformation, extracellular matrix synthesis and collagen deposition [212].
PDE2A isoforms (i.e., PDE2A1, PDE2A2 and PDE2A3) are encoded by a unique PDE2A gene. The cAMP hydrolytic activity is controlled by cGMP binding to a tandem of regulatory GAF sites located in the PDE2A N-terminus region (Figure 3A) [213]. The PDE2A protein expression level upregulates in ischemic cardiomyopathy [214]. Interestingly, PDE2A overexpression in primary cardiac fibroblast culture leads to the degradation of cAMP and to an increase in α-SMA and CTGF (Connective Tissue Growth Factor) expression, which induce myofibroblast conversion and fibrosis [215].
Although it is clear that cardiac PDE2A compartmentalization modulates cardiac function, its role in cardiomyocytes remains controversial. In cardiac myocytes, the inhibition of mitochondrial PDE2A leads to a local accumulation of cAMP, which increases PKA-dependent phosphorylation of Drp1 and improves cell survival [216]. Furthermore, PDE2A exhibits cardioprotective effects by regulating the HR under chronic β-AR stress conditions [217]. Pharmacological inhibition of PDE2A activity increases the HR in WT mice, whereas its cardiac overexpression does the opposite in transgenic mice. The latter is explained by the cardiac PDE2A overexpression that mediates the reduction in β-AR/cAMP signaling and diminution of LTCC activity in ventricular cardiomyocytes and SAN (i.e., calcium clock) [217]. Post-MI, cardiac PDE2A overexpression blunts arrhythmia susceptibility and maintains proper cardiac contractile function under acute β-AR stimulation [217]. Additional studies performed under healthy conditions reported that the stimulation of β3-AR located in the T-tubule network induces NO synthesis by eNOS, which, in turn, triggers cGMP production [218]. This neo-synthetized cGMP activates a pool of PDE2A located in the T-tubule caveolin-rich region, which locally hydrolyzes the pool of cAMP produced after β1- and β2-AR stimulation and attenuates the inotropic response [218]. In chronic MI, the T-tubule network is disrupted and β3-AR redistributes at the cardiomyocyte plasmalemma [219]. This disorganizes β3-AR/cGMP/PDE2A signaling and consequently abolishes the PDE2A regulation on T-tubule cAMP levels [219].
In contrast to the regulation of PDE2, cGMP binding to PDE3 inhibits the cAMP hydrolysis activity. The PDE3 family is encoded by two different genes (i.e., PDE3A and PDE3B), which generate all isoforms (i.e., PDE3A1, PDE3A2, PDE3A3 and PDE3B) (Figure 3A) [163,220,221]. Although PDE3A and PDE3B are both expressed in cardiomyocytes, PDE3A isoforms are more abundant [222]. The phosphodiesterase catalytic domain locates at the C-terminus of all PDE3 variants. The longest PDE3 isoforms (i.e., PDE3A1 and PDE3B) exhibit several phosphorylation sites (e.g., PKA, PKB and PKC) and hydrophobic loops favoring PDE3 insertion into lipid membranes at the N-terminal region (Figure 3A) [223]. PDE3A1 locates mainly at the SR subset, while the other PDE3A isoforms distribute in the cytoplasm. The PDE3B isoform is found in proximity to mitochondria at cardiomyocyte Z-bands and T-tubules (Figure 3B) [224]. Under the physiological condition of β-AR stimulation, PDE3 tightly regulates cardiac contractile function by temporally limiting chronotropic, inotropic and lusitropic effects. These effects are mediated after PDE3-dependent cAMP degradation that restricts PKA activity and reduces PLB and TnI phosphorylation (Figure 3B) [225]. PDE3A KO mice exhibit enhanced basal contractility due to an accumulation of cardiac cAMP, which increases Ca2+ transient amplitudes, SR Ca2+ load, SERCA2a activity and PKA-dependent PLB phosphorylation. Furthermore, the AKAP18/PKA/SERCA2a/PLB/PDE3A supramolecular complex located at the SR subset mediates the spatiotemporal control of basal cardiac contractility in an LTCC-independent fashion (Figure 3B) [226]. In mice, PI3Kγ orchestrates a cardiac signaling macrocomplex with PKA and PDE3B that regulates local cAMP signaling in space and time [227,228]. However, PDE3B KO mice present normal cardiac function [229]. In comparison to PDE3A, this points to an auxiliary regulation role for PDE3B in the heart. It is admitted that all PDE3 isoforms exhibit cardioprotective functions in a rodent model with I/R injury. PDE3A1 transgenic mice with MI triggered post-I/R injuries present reductions in infarct size and myocyte apoptosis. Furthermore, these mice also display a conserved contractile function with a preserved ejection fraction post-MI [225]. Inhibition of ICER (Inducible cAMP Early Repressor) and Bcl2 expression mediates these cardioprotective effects [225]. PDE3B KO mice present in vitro and in vivo cardiac protection (e.g., reduced infarct size and a preserved cardiac function) after acute MI in a cAMP-induced preconditioning process [224]. It has been proposed that PDE3B knockout mediates cardioprotective effects by favoring a local pool of cAMP-PKA signaling, which mediates mitoKca channel activation, ICEF (Ischemia-Induced Caveolin-3–Enriched Fractions) signalosome assembly, the decrease in ROS production and resistance to Ca2+-induced mPTP pore opening [224].
Of note, pan-PDE3 inhibitors (e.g., amrinone, milrinone) have been clinically used to treat heart failure patients [230]. Despite the short-term symptomatic improvement in heart function, long-term treatment leads to an increase in patient mortality due to sudden cardiac death [231,232]. Considering the roles of cardiac PDE3A vs. PDE3B, isoform-specific PDE3 inhibitors could pave the way to diversification of the MI therapeutic scheme.
Among PDEs, cAMP-specific PDE4s are by far the largest PDE family encompassing more than 20 different isoforms encoded by four distinct genes (i.e., PDE4A, PDE4B, PDE4C and PDE4D) that generate all alternatively spliced variants. These alternative splicings provide PDE4s with different sizes and molecular masses that are classified into long, short, super-short and dead-short isoforms. PDE4s exhibit a unique N-terminal domain (TD), a central region containing upstream conserved regions 1 and/or 2 (UCR1 and UCR2) and a highly conserved C-terminal catalytic domain. The N-terminal domain confers to PDE4s a specific subcellular location, binding to signalosome complexes and the modulation of enzymatic activity (e.g., kinase phosphorylation) (Figure 3A) [233]. PDE4A, PDE4B and PDE4D isoforms are expressed in the cardiovascular system [234,235]. Although PDE4s have limited effects in basal cardiac function, they modulate the HR and cardiac contractility in response to β-AR stimulation [233,236]. In mice, a fraction of cardiac PDE4B is recruited to the LTCC/AKAP18/PKA signalosome in the T-tubule network controlling LTCC under β-AR stimulation [235]. Similarly to PDE3A, cardiac PDE4D regulates SERCA2a activity and SR Ca2+ load and leak (Figure 3B) [235,237,238]. This redundancy between both PDE subtypes highlights the necessity to tightly control the cAMP concentration in proximity to the SR to ensure a proper regulation of calcium re-uptake and relaxation. Furthermore, it has been reported that AKAP9 orchestrates a supramolecular signaling complex encompassing PKA, PP1 and PDE4D at the plasmalemma in the vicinity of the cardiac potassium channel (see Section 5.3). PDE integration in this complex modulates, in time, the cAMP signaling that controls the cardiac action potential repolarization current [239]. PDE4D plays also a central role in β-Ars’ desensitization process by directly interacting with β-arrestin [240].
PDE4D KO mice exhibit an increase in the cardiac cAMP level at the Z-line, which triggers PKA-dependent RyR2 hyperphosphorylation and calcium leak [233]. Interestingly, the crossing of these PDE4D KO mice with mice harboring a mutation in the RyR2 PKA-dependent phosphorylation site (RyR2-S2808A) produces offspring that show protection against MI-induced sudden cardiac death [241]. This indicates a key role of PDE4D in the regulation of the dyadic cAMP concentration, maintaining a proper level of RyR2 PKA-dependent phosphorylation and activity (Figure 3B).
Finally, the PKA-dependent phosphorylation of Hsp20 (at Ser16) has been reported in rats to provide cardioprotection and to reduce myocardial apoptosis after I/R injury [242,243,244,245]. PDE4D directly interacts with Hsp20 and finely tunes its phosphorylation by locally modulating the cAMP level and PKA activity [246]. A protein–protein interaction (PPI)-disrupting peptide displacing PDE4D from Hsp20 binding in neonatal rat ventricular cardiomyocytes increases PKA-dependent Hsp20 phosphorylation and shows cardioprotective functions. These PPIs could be of interest as new therapeutic agents in MI [247].
6.3. Miscellaneous Cardiac PDEs
In addition to the major types, other PDEs hydrolyzing cAMP co-exist in the heart but exhibit lower expression (i.e., PDE8A and PDE10). Their roles in the regulation of cardiac physiology or post-MI remain unclear and need to be further characterized. Beside its detailed role in CF, PDE1 also modulates the cAMP-PKA signaling pathway in the SAN and regulates pacemaker activity [248]. PDE8A regulates the calcium current and SR leak in cardiomyocytes [249]. However, the molecular mechanisms underlying this regulation need to be further investigated. The dual-activity PDE10 has recently been described to co-distribute in CF and cardiomyocytes. In response to pathological stimuli, PDE10 triggers cardiomyocyte hypertrophy, myofibroblast transformation and cardiac remodeling [207].
7. Discussion
Cyclic AMP signaling is one of the best characterized signaling pathways and critically involved in many cellular and physiological processes. It is now widely accepted that in the heart, cAMP plays a central role in the regulation of cardiac function. This includes the binding of an extracellular ligand (i.e., hormones and neurotransmitters) to GPCR, which, via G proteins and AC, leads to an intracellular increase in cAMP content. Next, this triggers activation of intracellular downstream effectors (i.e., PKA, Epac, CNCCs, POPDC), which, in a synergistic process, mediate the adaptation of cardiomyocytes to distinct stimuli. The temporal regulation of the cAMP signaling depends mainly on AC and cAMP-PDE activities. AKAPs provide the means to achieve both spatial and temporal regulation of PKA signaling. By scaffolding signaling effectors and modulators in the vicinity of substrates, AKAPs ensure spatial specificity in signal transduction. In addition, they also facilitate temporal regulation of the cAMP signaling pathway by placing the kinase optimally vs. the cAMP nanodomains. Furthermore, by assembling multiprotein signal complexes including phosphatases and PDEs, AKAPs facilitate discrete temporal control of the cAMP signalosome [4].
Acute β-AR signaling is triggered to compensate MI-induced loss of cardiac pump efficacy. This process increases myocardial cAMP, which favors positive cardiac inotropic effects and left ventricular function. Historically, considering these beneficial effects of acute β-AR signaling, the use of β-adrenergic agonists or PDE inhibitors has been speculated to be beneficial for patients with MI and heart failure [231,250]. Despite the short-term symptomatic improvement in heart function, long-term treatment with β-AR signaling activators leads to an increase in patient mortality due to sudden cardiac death [231,250]. While catecholamines and myocardial cAMP initially help the stressed heart, chronically at high levels, they promote adverse cardiac remodeling, cardiac myocyte death, fibrosis replacement and progressive deterioration of cardiac function (i.e., the vicious circle of heart failure) [19,251]. In the same period, several studies reported the deleterious effects of increased cardiac cAMP appearing post-acute MI that causes myocardial perfusion–contraction mismatching, increases infract size and promotes ventricular arrhythmias [252,253]. Therefore, various therapeutic strategies have been envisioned among which β-blocker adjunction evokes remarkable beneficial effects and reduces mortality [254]. Several β-blocker classes have been developed with specific physical and biochemical properties, which are differentially recommended in the treatment of distinct cardiac pathologies (e.g., non-selective β-blockers and cardio-selective β1-blockers with or without intrinsic sympathomimetic activity (ISA)). Post-MI, the beneficial cardiac effects of β-blockers are primarily mediated by their negative chronotropic and inotropic features, which decrease myocardial oxygen demands, increase coronary blood flow (by extending the diastolic filling time) and favor perfusion in ischemic regions [255]. Despite the fact that β-blockers are generally considered well tolerable, they may cause severe side effects, leading to low compliance in patients. These effects are multiple and affect cardiovascular functions as much as other physiological functions, leading to fatigue, depression, cold extremities and erectile dysfunction. Cardiovascular unwanted effects include bradycardia, AV block, hypotension and bronchoconstriction. Although β-blockers are administrated to patients post-MI or with hypertension or arrhythmias, they are contraindicated in patients with hypotension, bradycardia, Raynaud phenomena, severe pneumopathy, renal insufficiency or diabetes mellitus [16]. Despite current medications, no cure exists for MI and mortality remains high. Contraindications and side effects forced the scientific and medical community to consider therapeutic alternatives to β-blockers. In consequence, it was envisioned to target β-AR/cAMP signaling downstream effectors and/or modulators to increase the specificity and efficacy of therapeutics in cardiac pathologies. Therefore, inhibition of PKA has become of interest as a novel putative therapeutic target to counteract the β-AR activation signaling cascade observed [256]. Initially, small molecules exhibiting a PKA inhibition (i.e., H89 and KT-5720) were envisioned [243,257]. These two compounds antagonize ATP docking in the PKA C subunit nucleotide binding pocket. In vitro, these PKA inhibitors reveal promising effects by reducing cardiomyocyte death induced by chronic β-AR stimulation [257,258]. Unfortunately, these molecules present a deficit in specificity of PKA inhibition [259]. Consequently, alternative PKA inhibitor tools have been conceived and tested. PKI peptides are derivative analogs from the endogenous PKI (protein kinase-A inhibitor) protein, which encompass a PKA-specific inhibitory domain [259,260]. These peptides exhibit potent and highly specific PKA inhibition properties [259]. Interestingly, β-AR stimulation failed to induce cardiac hypertrophy, fibrosis, myocyte apoptosis and decreased cardiac function in transgenic mice specifically overexpressing in the heart PKI peptide [29]. In one study, the authors observed a superior cardioprotective potential with selective inhibition of PKA compared to β-blocker therapy after myocardial infarction [29]. Despite a substantial diminution of cardiac adaptation to exercise, PKI seems a promising path that could be developed in the design of alternative therapies to β-blockers post-MI [29,30,106].
Almost two decades ago, the overexpression of cardiac AC6 in mice revealed unexpected promising outcomes post-MI and for which a reduction in the mortality rate, a decrease in cardiac remodeling and protection of cardiac function were reported [87]. Precise mechanisms underlying cardioprotective outcomes remain uncertain. However, the authors suggested that the positive effects observed with respect to contractile function could be the consequence of an increase in the PLB phosphorylation level and SR calcium load [87]. Therefore, gene therapy directing specific cardiac AC6 overexpression could be a possible therapeutic solution post-MI.
Human clinical trials demonstrated in various pathologies benefit from small interfering RNAs mediating a specific gene knockdown (reviewed in [261]). However, targeting cAMP signaling modulators or regulators may be drastic as these are involved in the regulation of key processes regulating the function of cardiomyocytes. However, silencing PKA substrates triggering adverse cardiac remodeling post-MI could be a possible envisioned therapeutic.
An elegant alternative with specific PDE activators would increase myocardial cAMP hydrolysis in distinct cAMP nanodomains and thus inhibit deleterious PKA signaling post-MI. However, such molecules are still in development or under characterization and have not yet been tested under cardiac pathological conditions [262].
Finally, targeting protein–protein interactions (PPI) in macromolecular signaling complexes orchestrated by AKAPs with PPI disruptors (e.g., peptides and small molecules) offers new exciting therapeutic options [142]. Peptides are easy to synthesize, highly specific and exhibit strong affinity with minimal toxicity and immune responses. Several PPI disruptors have been designed to target interactions between AKAP/PKA or AKAP/PKA substrates and their efficacy evaluated in the heart (reviewed in [142]). As the PKA/AKAP interaction domain is structurally common to all complexes, PPI disruptors (e.g., Ht31, RIAD, SuperAKAP-IS) would lack specificity and should not be considered as potential therapeutics. Alternatively, PPI disruptors targeting the anchoring domain between AKAPs and PKA substrates demonstrate higher specificities and efficiencies. Among them, a PPI-disrupting peptide competing in the AKAP18δ/PLB interaction in cardiomyocytes alters PLB phosphorylation and calcium re-uptake [185]. In addition, a PPI-disrupting peptide targeting the mAKAP/CaN interaction decreases in vitro cardiac hypertrophy [263]. Finally, TAC mice overexpressing a peptide disrupting the AKAP-Lbc/p38 interaction exhibit in vivo a reduction in cardiac hypertrophy [196]. Therefore, PPI-disrupting peptides provide new exciting and specific therapeutics that can be used post-MI as an alternative to β-blocker therapy.
The beginning of this century has seen the emergence of innovative therapeutic approaches focusing on the engineering of allosteric modulators and “biased” ligands to selectively activate one of GPCR-associated intracellular signaling events (for review, see [264]). Therefore, the discovery of β-Ars-biased agonists has been expected in the cardiology field to selectively trigger cardioprotective β-Ars signaling over the β-AR Gαs coupling. In this context, β2-AR-pepducin ICL1–9, a cell penetrating lipopeptides derived from the amino acid sequence of the first β2-AR intracellular loop, exhibits promising β-Ars-biased agonist properties. It selectively activates β2-AR-associated β-Ars signaling, internalizes β2-AR and promotes adult cardiomyocyte contractility [265,266]. In addition, intramyocardial injection of β2-AR-pepducin ICL1–9 during I/R injuries reduces infarct size, maintains cardiomyocyte survival, improves cardiac function and therefore may offer an elegant alternative to β-blocker treatment post-MI [267].
It is noteworthy that cAMP signaling displays ubiquitous distribution in all cells and organs over the body, and caution should be noted as the design of medications regulating such signaling could introduce side effects. To avoid adverse effects, new therapeutic molecules developed to treat cardiac diseases could, with benefit, be delivered efficiently and rapidly directly to the myocardium in a tissue-specific manner. Such a strategy would improve the treatment of cardiovascular diseases in general (i.e., CHD, hypertrophic cardiomyopathies, acute or chronic HF, rhythm disorders) and would abolish or minimize undesirable side effects. In addition, heart-targeting systems would support the development of new therapeutic agent classes, such as PPI disruptors (peptides, small compounds) or nucleic acids (siRNA) [268,269,270]. Alternatively, gene therapy strategies using associated adenoviruses (AAVs) exhibiting preferential cardiac tropism would specifically direct expression of cAMP modulators/regulators (e.g., PDE, AC), peptides (e.g., PKI, PPI disruptors) or siRNA in the heart and could be considered as therapeutic options [259,271].
In conclusion, although the management of patients post-MI is well established, strategies to improve disease outcome are still under investigation. Even if β-blockers remain the cornerstone therapy in CHD, they also exhibit unwanted effects and are inappropriate for some patients [272]. As described here, the cAMP signaling and its compartmentalization play a crucial role in cardiac physiology and are extensively modified post-MI. Therefore, new therapeutic options targeting cAMP signaling would deserve further investigations and might offer beneficial avenues to prevent fibrosis in CHD or susceptibility to arrhythmias, but also to reduce infarct size and/or to preserve cardiac contractile function.
Author Contributions
Conceptualization, A.-S.C. and G.P.; writing—original draft preparation, A.-S.C.; writing—review and editing, G.P.; visualization, A.-S.C.; supervision, G.P.; funding acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work has benefitted funds from Inserm (ASC and GP), ANR (ANR-20-CE18-0017-01 to GP) and the LabEx Lermit fellowship (ANR-10-LABX-33 to ASC). UMR-S1180 is a member of the Laboratory of Excellence in Research on Medication and Innovative Therapeutics supported by the Agence Nationale de la Recherche (ANR-10-LABX-33) under the program “Investissements d’Avenir” (ANR-11-IDEX-0003-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We are grateful to members of Inserm UMR-S1180 and Kjetil Tasken (Institute of Cancer Research, Oslo University Hospital, Norway) for the critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| α-AR | Alpha adrenergic receptor |
| α1-AR | Alpha 1 adrenergic receptor |
| α2-AR | Alpha 2 adrenergic receptor |
| α-SMA | Alpha smooth muscle actinin |
| A2AR | Adenosine receptor A2 |
| A2BAR | Adenosine receptor A2B |
| A3AR | Adenosine receptor A3A |
| AC | Adenylyl cyclase |
| ACE | Angiotensin converting enzyme |
| ACS | Acute coronary syndrome |
| AKAP | A-kinase anchoring protein |
| AMI | Acute myocardial infarction |
| ANG II | Angiotensin II |
| AV block | Atrio ventricular block |
| BAD | Bcl2 associated death promoter |
| β-AR | Beta adrenergic receptor |
| β1-AR | Beta 1 adrenergic receptor |
| β2-AR | Beta 2 adrenergic receptor |
| β3-AR | Beta 3 adrenergic receptor |
| βARK1 | Beta adrenergic receptor kinase 1 |
| β-Ars | Beta arrestins |
| CAMKII | Ca2+/Calmodulin-dependent protein kinase II |
| cAMP | Cyclic adenosine 3′-5′-monophosphate |
| CaN | Calcineurin |
| CEC | Cardiac excitation–contraction coupling |
| CF | Cardiac fibroblasts |
| cGMP | Cyclic guanosine-3′,5′-monophosphate |
| CHD | Coronary heart diseases |
| CHID | Chronic ischemic heart disease |
| CICR | Calcium-induced calcium release |
| CNCC | Cyclic nucleotide regulates cations channel |
| CNGC | Cyclic nucleotide-gated channels |
| CNG | Cyclic nucleotide-gated ion channels |
| cNMP | Cyclic nucleotide-3′,5′-monophosphate |
| CTGF | Connective tissue growth factor |
| cTnT | Cardiac Troponin T |
| Drp1 | GTPase dynamin related protein 1 |
| eNOS | Endothelial NO synthase |
| EP3 | Prostaglandin EP3 receptor |
| EP4 | Prostaglandin EP4 receptor |
| Epac | Exchange protein activated by cAMP |
| ERK5 | Extracellular signal-regulated kinase 5 |
| FIS1 | Mitochondrial fission 1 protein |
| GCCR | Glucagon receptor |
| GEF | Guanosine exchange factor |
| GLP1 | Glucagon-like peptide 1 |
| GLP1R | Glucagon-like peptide 1 receptor |
| GPCR | G protein-coupled receptor |
| GRK2 | G protein-coupled receptor kinase 2 |
| GSK3β | Glycogen synthase kinase 3 beta |
| HCN | Hyperpolarization-activated cyclic nucleotide–gated channels |
| HF | Heart failure |
| HIF1α | Hypoxia inducible factor 1alpha |
| HR | Heart rate |
| I/R | Ischemia/reperfusion |
| ICEF | Ischemia-induced caveolin 3 enriched fractions |
| ICER | Inducible cAMP early repressor |
| KCNQ1 | Potassium voltage-gated channel subfamily-Q member 1 |
| LAD | Left anterior descending artery ligation |
| LTCC | L-type calcium channel |
| M2R | Muscarinic receptor type 2 |
| MEF2 | Myocyte enhancer factor 2 |
| MEK5 | Mitogen-activated protein kinase kinase 5 |
| MI | Myocardial infarction |
| miR-21 | miRNA-21 |
| MKK3 | Mitogen-activated protein kinase 3 |
| MLTK | MLK-like mitogen-activated protein triple kinase |
| mPTP | Mitochondrial permeability transition pore |
| MSCs | Mesenchymal stem cells |
| MyBPC | Myosin binding protein C |
| NAC | N acetyl cysteine |
| NCX | Na/Ca exchanger |
| NFATc3 | Nuclear factor of activated T-cells, cytoplasmic 3 |
| NO | Nitric oxide |
| PDE | Phosphodiesterase |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidyl inositol-3 kinase |
| PKA | Protein kinase-A |
| PKC | Protein kinase-C |
| PKD | Protein kinase-D |
| PKD1 | Polycystic kidney disease 1 |
| PKG | Protein kinase G |
| PKI | Protein kinase-A inhibitor |
| PLB | Phospholamban |
| PLC | Phospholipase C |
| PLK1 | Polo-like kinase1 |
| POPDC | Popeye domain containing protein |
| PP1 | Protein phosphatase 1 |
| PP2B | Protein phosphatase 2B |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| PPI disruptors | Protein–protein interaction disruptors |
| PPs | Protein phosphatases |
| ROS | Reactive oxygen species |
| RSK3 | Ribosomal S6 kinase |
| RyR | Ryanodin receptor |
| sAC | Soluble adenylyl cyclase |
| SAN | Sino atrial node |
| SCD | Sudden cardiac death |
| SERCA2 | Sarco/endoplasmic reticulum Ca2+-ATPase |
| Siah2 | Ubiquitin protein ligase seven in absentia homolog 2 |
| SKIP | Sphingosine kinase type 1-interacting protein |
| smAKAP | Small membrane A-kinase anchoring protein |
| SNP | Single-nucleotide polymorphism |
| SR | Sarcoplasmic reticulum |
| STEMI | ST elevation myocardial infarction |
| TAC | Transverse aortic constriction |
| TnI | Troponin I |
References
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and Endocrine Control of Cardiovascular Function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Stress-Activated Cytokines and The Heart: From Adaptation to Maladaptation. Annu. Rev. Physiol. 2003, 65, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Boularan, C.; Gales, C. Cardiac CAMP: Production, Hydrolysis, Modulation and Detection. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Pidoux, G.; Taskén, K. Specificity and Spatial Dynamics of Protein Kinase A Signaling Organized by A-Kinase-Anchoring Proteins. J. Mol. Endocrinol. 2010, 44, 271–284. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Surdo, N.C.; Pantano, S.; Zaccolo, M. Imaging CAMP Nanodomains in the Heart. Biochem. Soc. Trans. 2019, 47, 1383–1392. [Google Scholar] [CrossRef]
- Corbin, J.D.; Sugden, P.H.; Lincoln, T.M.; Keely, S.L. Compartmentalization of Adenosine 3’:5’-Monophosphate and Adenosine 3’:5’-Monophosphate-Dependent Protein Kinase in Heart Tissue. J. Biol. Chem. 1977, 252, 3854–3861. [Google Scholar] [CrossRef]
- Brunton, L.L.; Hayes, J.S.; Mayer, S.E. Hormonally Specific Phosphorylation of Cardiac Troponin I and Activation of Glycogen Phosphorylase. Nature 1979, 280, 78–80. [Google Scholar] [CrossRef]
- Hayes, J.S.; Brunton, L.L.; Brown, J.H.; Reese, J.B.; Mayer, S.E. Hormonally Specific Expression of Cardiac Protein Kinase Activity. Proc. Natl. Acad. Sci. USA 1979, 76, 1570–1574. [Google Scholar] [CrossRef]
- Buxton, I.L.; Brunton, L.L. Compartments of Cyclic AMP and Protein Kinase in Mammalian Cardiomyocytes. J. Biol. Chem. 1983, 258, 10233–10239. [Google Scholar] [CrossRef]
- Xiao, R.P.; Lakatta, E.G. Beta 1-Adrenoceptor Stimulation and Beta 2-Adrenoceptor Stimulation Differ in Their Effects on Contraction, Cytosolic Ca2+, and Ca2+ Current in Single Rat Ventricular Cells. Circ. Res. 1993, 73, 286–300. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Pak, E.; Robinson, R.B.; Steinberg, S.F. Beta 2-Adrenergic Receptor Actions in Neonatal and Adult Rat Ventricular Myocytes. Circ. Res. 1995, 76, 40–52. [Google Scholar] [CrossRef]
- Farah, A.E. Glucagon and the Circulation. Pharmacol. Rev. 1983, 35, 181–217. [Google Scholar]
- Vila Petroff, M.G.; Egan, J.M.; Wang, X.; Sollott, S.J. Glucagon-like Peptide-1 Increases CAMP but Fails to Augment Contraction in Adult Rat Cardiac Myocytes. Circ. Res. 2001, 89, 445–452. [Google Scholar] [CrossRef]
- Fischmeister, R.; Castro, L.R.V.; Abi-Gerges, A.; Rochais, F.; Jurevicius, J.; Leroy, J.; Vandecasteele, G. Compartmentation of Cyclic Nucleotide Signaling in the Heart: The Role of Cyclic Nucleotide Phosphodiesterases. Circ. Res. 2006, 99, 816–828. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef]
- Boudonas, G.E. β-Blockers in Coronary Artery Disease Management. Hippokratia 2010, 14, 231–235. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task. Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Gabriel-Costa, D. The Pathophysiology of Myocardial Infarction-Induced Heart Failure. Pathophysiology 2018, 25, 277–284. [Google Scholar] [CrossRef]
- Francis, G.S. Pathophysiology of Chronic Heart Failure. Am. J. Med. 2001, 110 (Suppl 7A), 37S–46S. [Google Scholar] [CrossRef]
- Effect of Carvedilol on Mortality and Morbidity in Patients with Chronic Heart Failure. Circulation 1996, 94, 592. [CrossRef]
- López-Sendón, J.; Swedberg, K.; McMurray, J.; Tamargo, J.; Maggioni, A.P.; Dargie, H.; Tendera, M.; Waagstein, F.; Kjekshus, J.; Lechat, P.; et al. Expert Consensus Document on Beta-Adrenergic Receptor Blockers. Eur. Heart J. 2004, 25, 1341–1362. [Google Scholar] [CrossRef]
- Kezerashvili, A.; Marzo, K.; De Leon, J. Beta Blocker Use after Acute Myocardial Infarction in the Patient with Normal Systolic Function: When Is It “Ok” to Discontinue? Curr. Cardiol. Rev. 2012, 8, 77–84. [Google Scholar] [CrossRef]
- Stewart, S.; Jenkins, A.; Buchan, S.; McGuire, A.; Capewell, S.; McMurray, J.J.J.V. The Current Cost of Heart Failure to the National Health Service in the UK. Eur. J. Heart Fail. 2002, 4, 361–371. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement from the American Heart Association. Circ. Heart. Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Hampton, J.R. Choosing the Right β-Blocker. Drugs 1994, 48, 549–568. [Google Scholar] [CrossRef]
- Ko, D.T.; Hebert, P.R.; Coffey, C.S.; Curtis, J.P.; Foody, J.M.; Sedrakyan, A.; Krumholz, H.M. Adverse Effects of Beta-Blocker Therapy for Patients with Heart Failure: A Quantitative Overview of Randomized Trials. Arch. Intern. Med. 2004, 164, 1389–1394. [Google Scholar] [CrossRef]
- Arnold, S.V.; Spertus, J.A.; Lipska, K.J.; Lanfear, D.E.; Tang, F.; Grodzinsky, A.; McGuire, D.K.; Gore, M.O.; Goyal, A.; Maddox, T.M.; et al. Type of Beta-Blocker Use Among Patients with Versus without Diabetes after Myocardial Infarction. Am. Heart J. 2014, 168, 273–279. [Google Scholar] [CrossRef]
- Koren, G.; Norton, G.; Radinsky, K.; Shalev, V. Chronic Use of β-Blockers and the Risk of Parkinson’s Disease. Clin. Drug Investig. 2019, 39, 463–468. [Google Scholar] [CrossRef]
- Zhang, X.; Szeto, C.; Gao, E.; Tang, M.; Jin, J.; Fu, Q.; Makarewich, C.; Ai, X.; Li, Y.; Tang, A.; et al. Cardiotoxic and Cardioprotective Features of Chronic β-Adrenergic Signaling. Circ. Res. 2013, 112, 498–509. [Google Scholar] [CrossRef]
- Whelan, R.S.; Konstantinidis, K.; Xiao, R.-P.; Kitsis, R.N. Cardiomyocyte Life-Death Decisions in Response to Chronic β-Adrenergic Signaling. Circ. Res. 2013, 112, 408–410. [Google Scholar] [CrossRef]
- Kim, G.E.; Kass, D.A. Cardiac Phosphodiesterases and Their Modulation for Treating Heart Disease. Handb. Exp. Pharmacol. 2017, 243, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Dema, A.; Perets, E.; Schulz, M.S.; Deák, V.A.; Klussmann, E. Pharmacological Targeting of AKAP-Directed Compartmentalized CAMP Signalling. Cell Signal. 2015, 27, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, N.I.; Kuptsova, A.M.; Faskhutdinov, L.I.; Zefirov, A.L.; Zefirov, T.L. Effect of A2-Adrenoceptor Stimulation on Functional Parameters of Langendorff-Isolated Rat Heart. Bull. Exp. Biol. Med. 2018, 165, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Arnar, D.O.; Xing, D.; Martins, J.B. Alpha-2 Adrenergic Antagonism Enhances Risk of Ventricular Tachycardia during Acute Ischemia. Scand. Cardiovasc. J. 2007, 41, 378–385. [Google Scholar] [CrossRef]
- Agarwal, S.R.; Ostrom, R.S.; Harvey, R.D. Membrane Microdomains and cAMP Compartmentation in Cardiac Myocytes. In Microdomains in the Cardiovascular System; Nikolaev, V., Zaccolo, M., Eds.; Cardiac and Vascular Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 17–35. ISBN 978-3-319-54579-0. [Google Scholar]
- Yoo, B.; Lemaire, A.; Mangmool, S.; Wolf, M.J.; Curcio, A.; Mao, L.; Rockman, H.A. B1-Adrenergic Receptors Stimulate Cardiac Contractility and CaMKII Activation in Vivo and Enhance Cardiac Dysfunction Following Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1377–H1386. [Google Scholar] [CrossRef]
- Kompa, A.R.; Gu, X.; Evans, B.A.; Summers, R.J. Desensitization of Cardiac β -Adrenoceptor Signaling with Heart Failure Produced by Myocardial Infarction in the Rat. Evidence for the Role of Gi but Not Gs or Phosphorylating Proteins. J. Mol. Cell. Cardiol. 1999, 31, 1185–1201. [Google Scholar] [CrossRef]
- Bhushan, S.; Kondo, K.; Predmore, B.L.; Zlatopolsky, M.; King, A.L.; Pearce, C.; Huang, H.; Tao, Y.-X.; Condit, M.E.; Lefer, D.J. Selective Β2-Adrenoreceptor Stimulation Attenuates Myocardial Cell Death and Preserves Cardiac Function after Ischemia-Reperfusion Injury. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1865–1874. [Google Scholar] [CrossRef]
- Tian, Y.; Miao, B.; Charles, E.; Kron, I.L.; French, B.A.; Yang, Z. Stimulation of the Beta2 Adrenergic Receptor at Reperfusion Limits Myocardial Reperfusion Injury via IL-10 Dependent Anti-Inflammatory Pathway in the Spleen. Circ. J. 2018, 82, 2829–2836. [Google Scholar] [CrossRef]
- Gauthier, C.; Tavernier, G.; Charpentier, F.; Langin, D.; Le Marec, H. Functional Beta3-Adrenoceptor in the Human Heart. J. Clin. Investig. 1996, 98, 556–562. [Google Scholar] [CrossRef]
- Niu, X.; Zhao, L.; Li, X.; Xue, Y.; Wang, B.; Lv, Z.; Chen, J.; Sun, D.; Zheng, Q. Β3-Adrenoreceptor Stimulation Protects against Myocardial Infarction Injury via ENOS and NNOS Activation. PLoS ONE 2014, 9, e98713. [Google Scholar] [CrossRef]
- García-Prieto, J.; García-Ruiz, J.M.; Sanz-Rosa, D.; Pun, A.; García-Alvarez, A.; Davidson, S.M.; Fernández-Friera, L.; Nuno-Ayala, M.; Fernández-Jiménez, R.; Bernal, J.A.; et al. B3 Adrenergic Receptor Selective Stimulation during Ischemia/Reperfusion Improves Cardiac Function in Translational Models through Inhibition of MPTP Opening in Cardiomyocytes. Basic Res. Cardiol. 2014, 109, 422. [Google Scholar] [CrossRef]
- Mazzadi, A.N.; Pineau, J.; Costes, N.; Le Bars, D.; Bonnefoi, F.; Croisille, P.; Porcher, R.; Chevalier, P. Muscarinic Receptor Upregulation in Patients With Myocardial Infarction: A New Paradigm. Circ. Cardiovasc. Imaging 2009, 2, 365–372. [Google Scholar] [CrossRef]
- Headrick, J.P.; Lasley, R.D. Adenosine Receptors and Reperfusion Injury of the Heart. In Adenosine Receptors in Health and Disease; Wilson, C.N., Mustafa, S.J., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 189–214. ISBN 978-3-540-89614-2. [Google Scholar]
- Forman, M.B.; Stone, G.W.; Jackson, E.K. Role of Adenosine as Adjunctive Therapy in Acute Myocardial Infarction. Cardiovasc. Drug Rev. 2006, 24, 116–147. [Google Scholar] [CrossRef]
- Bryson, T.D.; Gu, X.; Khalil, R.M.; Khan, S.; Zhu, L.; Xu, J.; Peterson, E.; Yang, X.-P.; Harding, P. Overexpression of Prostaglandin E2 EP4 Receptor Improves Cardiac Function after Myocardial Infarction. J. Mol. Cell Cardiol. 2018, 118, 1–12. [Google Scholar] [CrossRef]
- Zacharowski, K.; Olbrich, A.; Piper, J.; Hafner, G.; Kondo, K.; Thiemermann, C. Selective Activation of the Prostanoid EP 3 Receptor Reduces Myocardial Infarct Size in Rodents. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2141–2147. [Google Scholar] [CrossRef]
- Gonzalez-Muñoz, C.; Nieto-Cerón, S.; Cabezas-Herrera, J.; Hernández-Cascales, J. Glucagon Increases Contractility in Ventricle but Not in Atrium of the Rat Heart. Eur. J. Pharmacol. 2008, 587, 243–247. [Google Scholar] [CrossRef]
- Ali, S.; Ussher, J.R.; Baggio, L.L.; Kabir, M.G.; Charron, M.J.; Ilkayeva, O.; Newgard, C.B.; Drucker, D.J. Cardiomyocyte Glucagon Receptor Signaling Modulates Outcomes in Mice with Experimental Myocardial Infarction. Mol. Metab. 2015, 4, 132–143. [Google Scholar] [CrossRef]
- Bose, A.K.; Mocanu, M.M.; Carr, R.D.; Brand, C.L.; Yellon, D.M. Glucagon-like Peptide 1 Can Directly Protect the Heart Against Ischemia/Reperfusion Injury. Diabetes 2005, 54, 146–151. [Google Scholar] [CrossRef]
- Noyan-Ashraf, M.H.; Momen, M.A.; Ban, K.; Sadi, A.-M.; Zhou, Y.-Q.; Riazi, A.M.; Baggio, L.L.; Henkelman, R.M.; Husain, M.; Drucker, D.J. GLP-1R Agonist Liraglutide Activates Cytoprotective Pathways and Improves Outcomes After Experimental Myocardial Infarction in Mice. Diabetes 2009, 58, 975–983. [Google Scholar] [CrossRef]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and Dynamics of GPCR Signaling Complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef]
- Kaykı-Mutlu, G.; Papazisi, O.; Palmen, M.; Danser, A.H.J.; Michel, M.C.; Arioglu-Inan, E. Cardiac and Vascular A1-Adrenoceptors in Congestive Heart Failure: A Systematic Review. Cells 2020, 9, 2412. [Google Scholar] [CrossRef] [PubMed]
- Giovannitti, J.A.; Thoms, S.M.; Crawford, J.J. Alpha-2 Adrenergic Receptor Agonists: A Review of Current Clinical Applications. Anesth. Prog. 2015, 62, 31–38. [Google Scholar] [CrossRef] [PubMed]
- de Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Irannejad, R.; Tomshine, J.C.; Tomshine, J.R.; Chevalier, M.; Mahoney, J.P.; Steyaert, J.; Rasmussen, S.G.F.; Sunahara, R.K.; El-Samad, H.; Huang, B.; et al. Conformational Biosensors Reveal GPCR Signalling from Endosomes. Nature 2013, 495, 534–538. [Google Scholar] [CrossRef]
- Irannejad, R.; Pessino, V.; Mika, D.; Huang, B.; Wedegaertner, P.B.; Conti, M.; von Zastrow, M. Functional Selectivity of GPCR-Directed Drug Action through Location Bias. Nat. Chem. Biol. 2017, 13, 799–806. [Google Scholar] [CrossRef]
- Tsvetanova, N.G.; von Zastrow, M. Spatial Encoding of Cyclic AMP Signaling Specificity by GPCR Endocytosis. Nat. Chem. Biol. 2014, 10, 1061–1065. [Google Scholar] [CrossRef]
- Arnold, A.-S.; Tang, Y.L.; Qian, K.; Shen, L.; Valencia, V.; Phillips, M.I.; Zhang, Y.C. Specific Β1-Adrenergic Receptor Silencing with Small Interfering RNA Lowers High Blood Pressure and Improves Cardiac Function in Myocardial Ischemia. J. Hypertens. 2007, 25, 197–205. [Google Scholar] [CrossRef]
- Nikolaev, V.O.; Moshkov, A.; Lyon, A.R.; Miragoli, M.; Novak, P.; Paur, H.; Lohse, M.J.; Korchev, Y.E.; Harding, S.E.; Gorelik, J. Β2-Adrenergic Receptor Redistribution in Heart Failure Changes CAMP Compartmentation. Science 2010, 327, 1653–1657. [Google Scholar] [CrossRef]
- El-Armouche, A.; Eschenhagen, T. Beta-Adrenergic Stimulation and Myocardial Function in the Failing Heart. Heart Fail. Rev. 2009, 14, 225–241. [Google Scholar] [CrossRef]
- Miao, Y.; Li, M.; Wang, C.; Li, H.; Chen, H. Effect of β-Adrenergic Receptor Kinase Inhibitor on Post-Myocardial Infarction Heart Failure in Rats. Int. J. Clin. Exp. Pathol. 2017, 10, 9858–9865. [Google Scholar]
- Zee, R.Y.L.; Cook, N.R.; Reynolds, R.; Cheng, S.; Ridker, P.M. Haplotype Analysis of the Beta2 Adrenergic Receptor Gene and Risk of Myocardial Infarction in Humans. Genetics 2005, 169, 1583–1587. [Google Scholar] [CrossRef][Green Version]
- Schürks, M.; Kurth, T.; Ridker, P.M.; Buring, J.E.; Zee, R.Y.L. Association between Polymorphisms in the Β2-Adrenergic Receptor Gene with Myocardial Infarction and Ischaemic Stroke in Women. Thromb Haemost 2009, 101, 351–358. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Heckbert, S.R.; Sotoodehnia, N.; Bis, J.C.; Smith, N.L.; Marciante, K.D.; Hindorff, L.A.; Lange, L.A.; Lumley, T.S.; Rice, K.M.; et al. Β1- and Β2-Adrenergic Receptor Gene Variation, β-Blocker Use and Risk of Myocardial Infarction and Stroke. Am. J. Hypertens 2008, 21, 290–296. [Google Scholar] [CrossRef]
- Hautala, A.J.; Tulppo, M.P.; Kiviniemi, A.M.; Rankinen, T.; Bouchard, C.; Mäkikallio, T.H.; Huikuri, H.V. Acetylcholine Receptor M2 Gene Variants, Heart Rate Recovery, and Risk of Cardiac Death after an Acute Myocardial Infarction. Ann. Med. 2009, 41, 197–207. [Google Scholar] [CrossRef]
- Xiao, C.-Y.; Yuhki, K.; Hara, A.; Fujino, T.; Kuriyama, S.; Yamada, T.; Takayama, K.; Takahata, O.; Karibe, H.; Taniguchi, T.; et al. Prostaglandin E 2 Protects the Heart From Ischemia-Reperfusion Injury via Its Receptor Subtype EP 4. Circulation 2004, 109, 2462–2468. [Google Scholar] [CrossRef]
- Steegborn, C. Structure, Mechanism, and Regulation of Soluble Adenylyl Cyclases—Similarities and Differences to Transmembrane Adenylyl Cyclases. Biochim. Biophys. Acta 2014, 1842, 2535–2547. [Google Scholar] [CrossRef]
- Sadana, R.; Dessauer, C.W. Physiological Roles for G Protein-Regulated Adenylyl Cyclase Isoforms: Insights from Knockout and Overexpression Studies. Neurosignals 2009, 17, 5–22. [Google Scholar] [CrossRef]
- Schirmer, I.; Bualeong, T.; Budde, H.; Cimiotti, D.; Appukuttan, A.; Klein, N.; Steinwascher, P.; Reusch, P.; Mügge, A.; Meyer, R.; et al. Soluble Adenylyl Cyclase: A Novel Player in Cardiac Hypertrophy Induced by Isoprenaline or Pressure Overload. PLoS ONE 2018, 13, e0192322. [Google Scholar] [CrossRef]
- Mougenot, N.; Mika, D.; Czibik, G.; Marcos, E.; Abid, S.; Houssaini, A.; Vallin, B.; Guellich, A.; Mehel, H.; Sawaki, D.; et al. Cardiac Adenylyl Cyclase Overexpression Precipitates and Aggravates Age-Related Myocardial Dysfunction. Cardiovasc. Res. 2019, 115, 1778–1790. [Google Scholar] [CrossRef]
- Tang, T.; Lai, N.C.; Roth, D.M.; Drumm, J.; Guo, T.; Lee, K.-W.; Han, P.-L.; Dalton, N.; Gao, M.H. Adenylyl Cyclase Type V Deletion Increases Basal Left Ventricular Function and Reduces Left Ventricular Contractile Responsiveness to Beta-Adrenergic Stimulation. Basic Res. Cardiol. 2006, 101, 117–126. [Google Scholar] [CrossRef]
- Tang, T.; Gao, M.H.; Lai, N.C.; Firth, A.L.; Takahashi, T.; Guo, T.; Yuan, J.X.-J.; Roth, D.M.; Hammond, H.K. Adenylyl Cyclase Type 6 Deletion Decreases Left Ventricular Function via Impaired Calcium Handling. Circulation 2008, 117, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Timofeyev, V.; Myers, R.E.; Kim, H.J.; Woltz, R.L.; Sirish, P.; Heiserman, J.P.; Li, N.; Singapuri, A.; Tang, T.; Yarov-Yarovoy, V.; et al. Adenylyl Cyclase Subtype-Specific Compartmentalization: Differential Regulation of L-Type Ca2+ Current in Ventricular Myocytes. Circ. Res. 2013, 112, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Takagi, G.; Kawabe, J.; Yang, G.; Lee, M.-C.; Hong, C.; Liu, J.; Vatner, D.E.; Sadoshima, J.; Vatner, S.F.; et al. Disruption of Type 5 Adenylyl Cyclase Gene Preserves Cardiac Function against Pressure Overload. Proc. Natl. Acad. Sci. USA 2003, 100, 9986–9990. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Kawabe, J.; Yatani, A.; Takagi, G.; Lee, M.-C.; Hong, C.; Liu, J.; Takagi, I.; Sadoshima, J.; Vatner, D.E.; et al. Type 5 Adenylyl Cyclase Disruption Alters Not Only Sympathetic But Also Parasympathetic and Calcium-Mediated Cardiac Regulation. Circ. Res. 2003, 93, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Bull Melsom, C.; Cosson, M.-V.; Ørstavik, Ø.; Lai, N.C.; Hammond, H.K.; Osnes, J.-B.; Skomedal, T.; Nikolaev, V.; Levy, F.O.; Krobert, K.A. Constitutive Inhibitory G Protein Activity upon Adenylyl Cyclase-Dependent Cardiac Contractility Is Limited to Adenylyl Cyclase Type 6. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Boucher, M.; Nim, S.; de Montigny, C.; Rousseau, G. Alterations of β-Adrenoceptor Responsiveness in Postischemic Myocardium after 72 h of Reperfusion. Eur. J. Pharmacol. 2004, 495, 185–191. [Google Scholar] [CrossRef]
- Bräunig, J.H.; Albrecht-Küpper, B.; Seifert, R. Adenylyl Cyclase Regulation in Heart Failure Due to Myocardial Infarction in Rats. Naunyn-Schmiedeberg’s Arch. Pharm. 2014, 387, 389–398. [Google Scholar] [CrossRef]
- Ma, Y.; Iyer, R.P.; Jung, M.; Czubryt, M.P.; Lindsey, M.L. Cardiac Fibroblast Activation Post-Myocardial Infarction: Current Knowledge Gaps. Trends Pharmacol. Sci. 2017, 38, 448–458. [Google Scholar] [CrossRef]
- Swaney, J.S.; Patel, H.H.; Yokoyama, U.; Lai, N.C.; Spellman, M.; Insel, P.A.; Roth, D.M. Adenylyl Cyclase Activity and Function Are Decreased in Rat Cardiac Fibroblasts after Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3216–H3220. [Google Scholar] [CrossRef]
- Baldwin, T.A.; Dessauer, C.W. Function of Adenylyl Cyclase in Heart: The AKAP Connection. J. Cardiovasc Dev. Dis. 2018, 5, 2. [Google Scholar] [CrossRef]
- Bravo, C.A.; Vatner, D.E.; Pachon, R.; Zhang, J.; Vatner, S.F. A Food and Drug Administration–Approved Antiviral Agent That Inhibits Adenylyl Cyclase Type 5 Protects the Ischemic Heart Even When Administered after Reperfusion. J. Pharm. Exp. 2016, 357, 331–336. [Google Scholar] [CrossRef]
- Zhang, J.; Levy, D.; Oydanich, M.; Bravo, C.A.; Yoon, S.; Vatner, D.E.; Vatner, S.F. A Novel Adenylyl Cyclase Type 5 Inhibitor That Reduces Myocardial Infarct Size Even When Administered after Coronary Artery Reperfusion. J. Mol. Cell. Cardiol. 2018, 121, 13–15. [Google Scholar] [CrossRef]
- Seifert, R. Vidarabine Is Neither a Potent nor a Selective AC5 Inhibitor. Biochem. Pharm. 2014, 87, 543–546. [Google Scholar] [CrossRef]
- Appukuttan, A.; Kasseckert, S.A.; Micoogullari, M.; Flacke, J.-P.; Kumar, S.; Woste, A.; Abdallah, Y.; Pott, L.; Reusch, H.P.; Ladilov, Y. Type 10 Adenylyl Cyclase Mediates Mitochondrial Bax Translocation and Apoptosis of Adult Rat Cardiomyocytes under Simulated Ischaemia/Reperfusion. Cardiovasc. Res. 2012, 93, 340–349. [Google Scholar] [CrossRef]
- Toshiyuki, T.; Tong, T.; Chin, L.N.; Roth David, M.; Brian, R.; Miho, S.; Lew Wilbur, Y.W.; Paul, C.; Kirk, H.H. Increased Cardiac Adenylyl Cyclase Expression Is Associated With Increased Survival After Myocardial Infarction. Circulation 2006, 114, 388–396. [Google Scholar] [CrossRef]
- Lai, N.C.; Tang, T.; Gao, M.H.; Saito, M.; Takahashi, T.; Roth, D.M.; Hammond, H.K. Activation of Cardiac Adenylyl Cyclase Expression Increases Function of the Failing Ischemic Heart in Mice. J. Am. Coll. Cardiol. 2008, 51, 1490–1497. [Google Scholar] [CrossRef]
- Granrud, G.A.; Vatterott, P.J. Arrhythmias and Acute Myocardial Infarction. Postgrad. Med. 1991, 90, 85–88. [Google Scholar] [CrossRef]
- Mattick, P.; Parrington, J.; Odia, E.; Simpson, A.; Collins, T.; Terrar, D. Ca2+-Stimulated Adenylyl Cyclase Isoform AC1 Is Preferentially Expressed in Guinea-Pig Sino-Atrial Node Cells and Modulates the I(f) Pacemaker Current. J. Physiol. 2007, 582, 1195–1203. [Google Scholar] [CrossRef]
- Boink, G.J.J.; Nearing, B.D.; Shlapakova, I.N.; Duan, L.; Kryukova, Y.; Bobkov, Y.; Tan, H.L.; Cohen, I.S.; Danilo, P.; Robinson, R.B.; et al. Ca 2+ -Stimulated Adenylyl Cyclase AC1 Generates Efficient Biological Pacing as Single Gene Therapy and in Combination With HCN2. Circulation 2012, 126, 528–536. [Google Scholar] [CrossRef]
- Ostrom, R.S.; Naugle, J.E.; Hase, M.; Gregorian, C.; Swaney, J.S.; Insel, P.A.; Brunton, L.L.; Meszaros, J.G. Angiotensin II Enhances Adenylyl Cyclase Signaling via Ca2+/Calmodulin Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J. Biol. Chem. 2003, 278, 24461–24468. [Google Scholar] [CrossRef]
- Li, Y.; Hof, T.; Baldwin, T.A.; Chen, L.; Kass, R.S.; Dessauer, C.W. Regulation of IKs Potassium Current by Isoproterenol in Adult Cardiomyocytes Requires Type 9 Adenylyl Cyclase. Cells 2019, 8, 981. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Kass, R.S.; Dessauer, C.W. The A-Kinase Anchoring Protein Yotiao Facilitates Complex Formation between Adenylyl Cyclase Type 9 and the IKs Potassium Channel in Heart. J. Biol. Chem. 2012, 287, 29815–29824. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ren, X.; Wang, X.; Zhang, P.; Jones, W.K.; Molkentin, J.D.; Fan, G.-C.; Kranias, E.G. Blockade of Hsp20 Phosphorylation Exacerbates Cardiac Ischemia/Reperfusion Injury by Suppressed Autophagy and Increased Cell Death. Circ. Res. 2009, 105, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Pozdniakova, S.; Jayarajan, V.; Troidl, C.; Abdallah, Y.; Aslam, M.; Ladilov, Y. Protective Role of Soluble Adenylyl Cyclase against Reperfusion-Induced Injury of Cardiac Cells. Biochim. Et Biophys. Acta Mol. Basis Dis. 2019, 1865, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Acin-Perez, R.; Salazar, E.; Kamenetsky, M.; Buck, J.; Levin, L.R.; Manfredi, G. Cyclic AMP Produced inside Mitochondria Regulates Oxidative Phosphorylation. Cell Metab. 2009, 9, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Newlon, M.G.; Roy, M.; Morikis, D.; Carr, D.W.; Westphal, R.; Scott, J.D.; Jennings, P.A. A Novel Mechanism of PKA Anchoring Revealed by Solution Structures of Anchoring Complexes. EMBO J. 2001, 20, 1651–1662. [Google Scholar] [CrossRef]
- Haushalter, K.J.; Casteel, D.E.; Raffeiner, A.; Stefan, E.; Patel, H.H.; Taylor, S.S. Phosphorylation of Protein Kinase A (PKA) Regulatory Subunit RIα by Protein Kinase G (PKG) Primes PKA for Catalytic Activity in Cells. J. Biol. Chem. 2018, 293, 4411–4421. [Google Scholar] [CrossRef]
- Kopperud, R.; Christensen, A.E.; Kjarland, E.; Viste, K.; Kleivdal, H.; Doskeland, S.O. Formation of Inactive CAMP-Saturated Holoenzyme of CAMP-Dependent Protein Kinase under Physiological Conditions. J. Biol. Chem. 2002, 277, 13443–13448. [Google Scholar] [CrossRef]
- Francis, S.H.; Corbin, J.D. Structure and Function of Cyclic Nucleotide-Dependent Protein Kinases. Annu Rev. Physiol. 1994, 56, 237–272. [Google Scholar] [CrossRef]
- Smith, F.D.; Esseltine, J.L.; Nygren, P.J.; Veesler, D.; Byrne, D.P.; Vonderach, M.; Strashnov, I.; Eyers, C.E.; Eyers, P.A.; Langeberg, L.K.; et al. Local Protein Kinase A Action Proceeds through Intact Holoenzymes. Science 2017, 356, 1288–1293. [Google Scholar] [CrossRef]
- Reimann, E.M.; Walsh, D.A.; Krebs, E.G. Purification and Properties of Rabbit Skeletal Muscle Adenosine 3’,5’-Monophosphate-Dependent Protein Kinases. J. Biol. Chem. 1971, 246, 1986–1995. [Google Scholar] [CrossRef]
- Corbin, J.D.; Keely, S.L.; Park, C.R. The Distribution and Dissociation of Cyclic Adenosine 3’:5’-Monophosphate-Dependent Protein Kinases in Adipose, Cardiac, and Other Tissues. J. Biol. Chem. 1975, 250, 218–225. [Google Scholar] [CrossRef]
- Uhler, M.D.; Chrivia, J.C.; McKnight, G.S. Evidence for a Second Isoform of the Catalytic Subunit of CAMP-Dependent Protein Kinase. J. Biol. Chem. 1986, 261, 15360–15363. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.E.; Zhang, X.; Li, Y.; Chen, B.; Liu, C.; Ai, X.; Zhang, X.; Tian, Y.; Zhang, C.; et al. Cardiomyocyte PKA Ablation Enhances Basal Contractility While Eliminates Cardiac β-Adrenergic Response Without Adverse Effects on the Heart. Circ. Res. 2019, 124, 1760–1777. [Google Scholar] [CrossRef]
- Feng, G.; Yan, Z.; Li, C.; Hou, Y. MicroRNA-208a in an Early Stage Myocardial Infarction Rat Model and the Effect on CAMP-PKA Signaling Pathway. Mol. Med. Rep. 2016, 14, 1631–1635. [Google Scholar] [CrossRef]
- Haushalter, K.J.; Schilling, J.M.; Song, Y.; Sastri, M.; Perkins, G.A.; Strack, S.; Taylor, S.S.; Patel, H.H. Cardiac Ischemia-Reperfusion Injury Induces ROS-Dependent Loss of PKA Regulatory Subunit RIα. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1231–H1242. [Google Scholar] [CrossRef]
- Han, Y.S.; Arroyo, J.; Ogut, O. Human Heart Failure Is Accompanied by Altered Protein Kinase A Subunit Expression and Post-Translational State. Arch. Biochem. Biophys. 2013, 538, 25–33. [Google Scholar] [CrossRef]
- Kronenbitter, A.; Funk, F.; Hackert, K.; Gorreßen, S.; Glaser, D.; Boknik, P.; Poschmann, G.; Stühler, K.; Isić, M.; Krüger, M.; et al. Impaired Ca2+ Cycling of Nonischemic Myocytes Contributes to Sarcomere Dysfunction Early after Myocardial Infarction. J. Mol. Cell. Cardiol. 2018, 119, 28–39. [Google Scholar] [CrossRef]
- Piddo, A.M.; Sánchez, M.I.; Sapag-Hagar, M.; Corbalán, R.; Foncea, R.; Ebensperger, R.; Godoy, I.; Meléndez, J.; Jalil, J.E.; Lavandero, S. Cyclic AMP-Dependent Protein Kinase and Mechanical Heart Function in Ventricular Hypertrophy Induced by Pressure Overload or Secondary to Myocardial Infarction. J. Mol. Cell. Cardiol. 1996, 28, 1073–1083. [Google Scholar] [CrossRef]
- Li, X.-D.; Cheng, Y.-T.; Yang, Y.-J.; Meng, X.-M.; Zhao, J.-L.; Zhang, H.-T.; Wu, Y.-J.; You, S.-J.; Wu, Y.-L. PKA-Mediated ENOS Phosphorylation in the Protection of Ischemic Preconditioning against No-Reflow. Microvasc. Res. 2012, 84, 44–54. [Google Scholar] [CrossRef]
- Sanada, S.; Asanuma, H.; Tsukamoto, O.; Minamino, T.; Node, K.; Takashima, S.; Fukushima, T.; Ogai, A.; Shinozaki, Y.; Fujita, M.; et al. Protein Kinase A as Another Mediator of Ischemic Preconditioning Independent of Protein Kinase C. Circulation 2004, 110, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Lin, S.-Z.; Chang, N.-C. Both PKA and Epac Pathways Mediate N-Acetylcysteine-Induced Connexin43 Preservation in Rats with Myocardial Infarction. PLoS ONE 2013, 8, e71878. [Google Scholar] [CrossRef]
- Nishida, H.; Sato, T.; Miyazaki, M.; Nakaya, H. Infarct Size Limitation by Adrenomedullin: Protein Kinase A but Not PI3-Kinase Is Linked to Mitochondrial KCa Channels. Cardiovasc. Res. 2008, 77, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Keyes, K.T.; Zhang, C.; Perez-Polo, J.R.; Lin, Y.; Birnbaum, Y. The Myocardial Infarct Size-Limiting Effect of Sitagliptin Is PKA-Dependent, Whereas the Protective Effect of Pioglitazone Is Partially Dependent on PKA. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1454–H1465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Geng, Y.; Zhao, J.; Zhang, H.; Cheng, Y.; Wu, Y. Phosphorylation of Endothelial NOS Contributes to Simvastatin Protection against Myocardial No-Reflow and Infarction in Reperfused Swine Hearts: Partially via the PKA Signaling Pathway. Acta Pharmacol. Sin. 2012, 33, 879–887. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulucan, C.; Wang, X.; Baljinnyam, E.; Bai, Y.; Okumura, S.; Sato, M.; Minamisawa, S.; Hirotani, S.; Ishikawa, Y. Developmental Changes in Gene Expression of Epac and Its Upregulation in Myocardial Hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1662–H1672. [Google Scholar] [CrossRef]
- Bos, J.L. Epac Proteins: Multi-Purpose CAMP Targets. Trends Biochem. Sci. 2006, 31, 680–686. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, G.; Morel, E.; Domínguez-Rodríguez, A.; Llach, A.; Lezoualc’h, F.; Benitah, J.-P.; Gomez, A.M. Epac in Cardiac Calcium Signaling. J. Mol. Cell Cardiol. 2013, 58, 162–171. [Google Scholar] [CrossRef]
- Pereira, L.; Rehmann, H.; Lao, D.H.; Erickson, J.R.; Bossuyt, J.; Chen, J.; Bers, D.M. Novel Epac Fluorescent Ligand Reveals Distinct Epac1 vs. Epac2 Distribution and Function in Cardiomyocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 3991–3996. [Google Scholar] [CrossRef]
- Fujita, T.; Umemura, M.; Yokoyama, U.; Okumura, S.; Ishikawa, Y. The Role of Epac in the Heart. Cell. Mol. Life Sci. 2017, 74, 591–606. [Google Scholar] [CrossRef]
- Kryzhanovsky, S.A.; Nikiforova, T.D.; Durnev, A.D. Epac Proteins and Their Role in the Physiological and Pathological Processes in the Cardiovascular System. Part 1: The Role of Epac Proteins in the Physiological and Pathological Processes of the Vasculature. Hum. Physiol. 2020, 46, 200–215. [Google Scholar] [CrossRef]
- Yokoyama, U.; Patel, H.H.; Lai, N.C.; Aroonsakool, N.; Roth, D.M.; Insel, P.A. The Cyclic AMP Effector Epac Integrates Pro- and Anti-Fibrotic Signals. Proc. Natl. Acad. Sci. USA 2008, 105, 6386–6391. [Google Scholar] [CrossRef]
- Surinkaew, S.; Aflaki, M.; Takawale, A.; Chen, Y.; Qi, X.-Y.; Gillis, M.-A.; Shi, Y.-F.; Tardif, J.-C.; Chattipakorn, N.; Nattel, S. Exchange Protein Activated by Cyclic-Adenosine Monophosphate (Epac) Regulates Atrial Fibroblast Function and Controls Cardiac Remodelling. Cardiovasc Res. 2019, 115, 94–106. [Google Scholar] [CrossRef]
- Khan, I.; Ali, A.; Akhter, M.A.; Naeem, N.; Chotani, M.A.; Iqbal, H.; Kabir, N.; Atiq, M.; Salim, A. Epac-Rap1-Activated Mesenchymal Stem Cells Improve Cardiac Function in Rat Model of Myocardial Infarction. Cardiovasc 2017, 35. [Google Scholar] [CrossRef]
- Biel, M. Cyclic Nucleotide-Regulated Cation Channels. J. Biol. Chem. 2009, 284, 9017–9021. [Google Scholar] [CrossRef]
- Scicchitano, P.; Carbonara, S.; Ricci, G.; Mandurino, C.; Locorotondo, M.; Bulzis, G.; Gesualdo, M.; Zito, A.; Carbonara, R.; Dentamaro, I.; et al. HCN Channels and Heart Rate. Molecules 2012, 17, 4225–4235. [Google Scholar] [CrossRef]
- Baruscotti, M.; Difrancesco, D. Pacemaker Channels. Ann. N.Y. Acad. Sci. 2004, 1015, 111–121. [Google Scholar] [CrossRef]
- Xia, S.; Wang, Y.; Zhang, Y.; Deng, S.-B.; Du, J.-L.; Wang, X.-C.; She, Q. Dynamic Changes in HCN2, HCN4, KCNE1, and KCNE2 Expression in Ventricular Cells from Acute Myocardial Infarction Rat Hearts. Biochem. Biophys. Res. Commun. 2010, 395, 330–335. [Google Scholar] [CrossRef]
- Mackiewicz, U.; Gerges, J.Y.; Chu, S.; Duda, M.; Dobrzynski, H.; Lewartowski, B.; Mączewski, M. Ivabradine Protects Against Ventricular Arrhythmias in Acute Myocardial Infarction in the Rat. J. Cell. Physiol. 2014, 229, 813–823. [Google Scholar] [CrossRef]
- Song, T.; Yang, J.; Yao, Y.; Li, H.; Chen, Y.; Zhang, J.; Huang, C. Spironolactone Diminishes Spontaneous Ventricular Premature Beats by Reducing HCN4 Protein Expression in Rats with Myocardial Infarction. Mol. Med. Rep. 2011, 4, 569–573. [Google Scholar] [CrossRef][Green Version]
- Brand, T. POPDC Proteins and Cardiac Function. Biochem. Soc. Trans. 2019, 47, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Froese, A.; Breher, S.S.; Waldeyer, C.; Schindler, R.F.R.; Nikolaev, V.O.; Rinné, S.; Wischmeyer, E.; Schlueter, J.; Becher, J.; Simrick, S.; et al. Popeye Domain Containing Proteins Are Essential for Stress-Mediated Modulation of Cardiac Pacemaking in Mice. J. Clin. Invest. 2012, 122, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.F.R.; Scotton, C.; Zhang, J.; Passarelli, C.; Ortiz-Bonnin, B.; Simrick, S.; Schwerte, T.; Poon, K.-L.; Fang, M.; Rinné, S.; et al. POPDC1(S201F) Causes Muscular Dystrophy and Arrhythmia by Affecting Protein Trafficking. J. Clin. Investig. 2016, 126, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Kirchmaier, B.C.; Poon, K.L.; Schwerte, T.; Huisken, J.; Winkler, C.; Jungblut, B.; Stainier, D.Y.; Brand, T. The Popeye Domain Containing 2 (Popdc2) Gene in Zebrafish Is Required for Heart and Skeletal Muscle Development. Dev. Biol. 2012, 363, 438–450. [Google Scholar] [CrossRef]
- De Ridder, W.; Nelson, I.; Asselbergh, B.; De Paepe, B.; Beuvin, M.; Ben Yaou, R.; Masson, C.; Boland, A.; Deleuze, J.-F.; Maisonobe, T.; et al. Muscular Dystrophy with Arrhythmia Caused by Loss-of-Function Mutations in BVES. Neurol. Genet. 2019, 5, e321. [Google Scholar] [CrossRef]
- Meinke, P.; Kerr, A.R.W.; Czapiewski, R.; de Las Heras, J.I.; Dixon, C.R.; Harris, E.; Kölbel, H.; Muntoni, F.; Schara, U.; Straub, V.; et al. A Multistage Sequencing Strategy Pinpoints Novel Candidate Alleles for Emery-Dreifuss Muscular Dystrophy and Supports Gene Misregulation as Its Pathomechanism. EBioMedicine 2020, 51, 102587. [Google Scholar] [CrossRef]
- Alcalay, Y.; Hochhauser, E.; Kliminski, V.; Dick, J.; Zahalka, M.A.; Parnes, D.; Schlesinger, H.; Abassi, Z.; Shainberg, A.; Schindler, R.F.R.; et al. Popeye Domain Containing 1 (Popdc1/Bves) Is a Caveolae-Associated Protein Involved in Ischemia Tolerance. PLoS ONE 2013, 8, e71100. [Google Scholar] [CrossRef]
- Marin, W. A-Kinase Anchoring Protein 1 (AKAP1) and Its Role in Some Cardiovascular Diseases. J. Mol. Cell. Cardiol. 2020, 138, 99–109. [Google Scholar] [CrossRef]
- Carr, D.W.; Stofko-Hahn, R.E.; Fraser, I.D.; Bishop, S.M.; Acott, T.S.; Brennan, R.G.; Scott, J.D. Interaction of the Regulatory Subunit (RII) of CAMP-Dependent Protein Kinase with RII-Anchoring Proteins Occurs through an Amphipathic Helix Binding Motif. J. Biol. Chem. 1991, 266, 14188–14192. [Google Scholar] [CrossRef]
- Calejo, A.I.; Taskén, K. Targeting Protein-Protein Interactions in Complexes Organized by A Kinase Anchoring Proteins. Front. Pharmacol. 2015, 6, 192. [Google Scholar] [CrossRef]
- Dodge-Kafka, K.L.; Soughayer, J.; Pare, G.C.; Carlisle Michel, J.J.; Langeberg, L.K.; Kapiloff, M.S.; Scott, J.D. The Protein Kinase A Anchoring Protein MAKAP Coordinates Two Integrated CAMP Effector Pathways. Nature 2005, 437, 574–578. [Google Scholar] [CrossRef]
- Taskén, K.; Aandahl, E.M. Localized Effects of CAMP Mediated by Distinct Routes of Protein Kinase A. Physiol. Rev. 2004, 84, 137–167. [Google Scholar] [CrossRef]
- Esseltine, J.L.; Scott, J.D. AKAP Signaling Complexes: Pointing towards the next Generation of Therapeutic Targets? Trends Pharm. Sci 2013, 34, 648–655. [Google Scholar] [CrossRef]
- Scott, J.D.; Dessauer, C.W.; Taskén, K. Creating Order from Chaos: Cellular Regulation by Kinase Anchoring. Annu Rev. Pharmacol. Toxicol. 2013, 53, 187–210. [Google Scholar] [CrossRef]
- Huang, L.J.; Durick, K.; Weiner, J.A.; Chun, J.; Taylor, S.S. Identification of a Novel Protein Kinase A Anchoring Protein That Binds Both Type I and Type II Regulatory Subunits. J. Biol. Chem. 1997, 272, 8057–8064. [Google Scholar] [CrossRef]
- Huang, L.J.; Durick, K.; Weiner, J.A.; Chun, J.; Taylor, S.S. D-AKAP2, a Novel Protein Kinase A Anchoring Protein with a Putative RGS Domain. Proc. Natl. Acad. Sci. USA 1997, 94, 11184–11189. [Google Scholar] [CrossRef]
- Means, C.K.; Lygren, B.; Langeberg, L.K.; Jain, A.; Dixon, R.E.; Vega, A.L.; Gold, M.G.; Petrosyan, S.; Taylor, S.S.; Murphy, A.N.; et al. An Entirely Specific Type I A-Kinase Anchoring Protein That Can Sequester Two Molecules of Protein Kinase A at Mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, E1227–E1235. [Google Scholar] [CrossRef]
- Burgers, P.P.; Ma, Y.; Margarucci, L.; Mackey, M.; van der Heyden, M.A.G.; Ellisman, M.; Scholten, A.; Taylor, S.S.; Heck, A.J.R. A Small Novel A-Kinase Anchoring Protein (AKAP) That Localizes Specifically Protein Kinase A-Regulatory Subunit I (PKA-RI) to the Plasma Membrane. J. Biol. Chem. 2012, 287, 43789–43797. [Google Scholar] [CrossRef]
- Perrino, C.; Feliciello, A.; Schiattarella, G.G.; Esposito, G.; Guerriero, R.; Zaccaro, L.; Del Gatto, A.; Saviano, M.; Garbi, C.; Carangi, R.; et al. AKAP121 Downregulation Impairs Protective CAMP Signals, Promotes Mitochondrial Dysfunction, and Increases Oxidative Stress. Cardiovasc. Res. 2010, 88, 101–110. [Google Scholar] [CrossRef]
- Sanderson, J.L.; Dell’Acqua, M.L. AKAP Signaling Complexes in Regulation of Excitatory Synaptic Plasticity. Neuroscientist 2011, 17, 321–336. [Google Scholar] [CrossRef]
- Efendiev, R.; Samelson, B.K.; Nguyen, B.T.; Phatarpekar, P.V.; Baameur, F.; Scott, J.D.; Dessauer, C.W. AKAP79 Interacts with Multiple Adenylyl Cyclase (AC) Isoforms and Scaffolds AC5 and -6 to Alpha-Amino-3-Hydroxyl-5-Methyl-4-Isoxazole-Propionate (AMPA) Receptors. J. Biol. Chem. 2010, 285, 14450–14458. [Google Scholar] [CrossRef]
- Nichols, C.B.; Rossow, C.F.; Navedo, M.F.; Westenbroek, R.E.; Catterall, W.A.; Santana, L.F.; McKnight, G.S. Sympathetic Stimulation of Adult Cardiomyocytes Requires Association of AKAP5 with a Subpopulation of L-Type Calcium Channels. Circ. Res. 2010, 107, 747–756. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Drum, B.M.; Chen, Y.; Yin, H.; Guo, X.; Luckey, S.W.; Gilbert, M.L.; McKnight, G.S.; Scott, J.D.; et al. Loss of AKAP150 Promotes Pathological Remodelling and Heart Failure Propensity by Disrupting Calcium Cycling and Contractile Reserve. Cardiovasc. Res. 2017, 113, 147–159. [Google Scholar] [CrossRef]
- Nieves-Cintrón, M.; Hirenallur-Shanthappa, D.; Nygren, P.J.; Hinke, S.A.; Dell’Acqua, M.L.; Langeberg, L.K.; Navedo, M.; Santana, L.F.; Scott, J.D. AKAP150 Participates in Calcineurin/NFAT Activation during the down-Regulation of Voltage-Gated K+ Currents in Ventricular Myocytes Following Myocardial Infarction. Cell. Signal. 2016, 28, 733–740. [Google Scholar] [CrossRef]
- Kapiloff, M.S.; Schillace, R.V.; Westphal, A.M.; Scott, J.D. MAKAP: An A-Kinase Anchoring Protein Targeted to the Nuclear Membrane of Differentiated Myocytes. J. Cell Sci. 1999, 112 (Pt 16), 2725–2736. [Google Scholar]
- Passariello, C.L.; Li, J.; Dodge-Kafka, K.; Kapiloff, M.S. MAKAP-a Master Scaffold for Cardiac Remodeling. J. Cardiovasc. Pharm. 2015, 65, 218–225. [Google Scholar] [CrossRef]
- Kapiloff, M.S.; Piggott, L.A.; Sadana, R.; Li, J.; Heredia, L.A.; Henson, E.; Efendiev, R.; Dessauer, C.W. An Adenylyl Cyclase-MAKAPbeta Signaling Complex Regulates CAMP Levels in Cardiac Myocytes. J. Biol. Chem. 2009, 284, 23540–23546. [Google Scholar] [CrossRef]
- Jansen, S.; Jorgensen, J.; Caplehorn, J.; Hunt, D. Preoperative Ultrasound to Predict Conversion in Laparoscopic Cholecystectomy. Surg. Laparosc. Endosc. 1997, 7, 121–123. [Google Scholar] [CrossRef]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early Expression of Angiogenesis Factors in Acute Myocardial Ischemia and Infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- Hausken, Z.E.; Dell’Acqua, M.L.; Coghlan, V.M.; Scott, J.D. Mutational Analysis of the A-Kinase Anchoring Protein (AKAP)-Binding Site on RII. Classification Of Side Chain Determinants for Anchoring and Isoform Selective Association with AKAPs. J. Biol. Chem. 1996, 271, 29016–29022. [Google Scholar] [CrossRef]
- Meacci, E.; Taira, M.; Moos, M.; Smith, C.J.; Movsesian, M.A.; Degerman, E.; Belfrage, P.; Manganiello, V. Molecular Cloning and Expression of Human Myocardial CGMP-Inhibited CAMP Phosphodiesterase. Proc. Natl. Acad. Sci. USA 1992, 89, 3721–3725. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yao, Z.; Zhao, W.; Zhang, Y.; Yao, C.; Tong, C. MiR-21 Enhances the Protective Effect of Loperamide on Rat Cardiomyocytes against Hypoxia/Reoxygenation, Reactive Oxygen Species Production and Apoptosis via Regulating Akap8 and Bard1 Expression. Exp. Med. 2019, 17, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, S.; Burns-Hamuro, L.L.; Ma, Y.; Hamon, S.C.; Canaves, J.M.; Shi, M.M.; Nelson, M.R.; Sing, C.F.; Cantor, C.R.; Taylor, S.S.; et al. Amino Acid Variant in the Kinase Binding Domain of Dual-Specific A Kinase-Anchoring Protein 2: A Disease Susceptibility Polymorphism. Proc. Natl. Acad. Sci. USA 2003, 100, 4066–4071. [Google Scholar] [CrossRef] [PubMed]
- Nishihama, K.; Yamada, Y.; Matsuo, H.; Segawa, T.; Watanabe, S.; Kato, K.; Yajima, K.; Hibino, T.; Yokoi, K.; Ichihara, S.; et al. Association of Gene Polymorphisms with Myocardial Infarction in Individuals with or without Conventional Coronary Risk Factors. Int. J. Mol. Med. 2007, 19, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Malbon, C.C.; Tao, J.; Shumay, E.; Wang, H.-Y. AKAP (A-Kinase Anchoring Protein) Domains: Beads of Structure-Function on the Necklace of G-Protein Signalling. Biochem. Soc. Trans. 2004, 32, 861–864. [Google Scholar] [CrossRef]
- Shih, M.; Lin, F.; Scott, J.D.; Wang, H.Y.; Malbon, C.C. Dynamic Complexes of Beta2-Adrenergic Receptors with Protein Kinases and Phosphatases and the Role of Gravin. J. Biol. Chem. 1999, 274, 1588–1595. [Google Scholar] [CrossRef]
- Lin, F.; Wang, H.Y.; Malbon, C.C. Gravin-Mediated Formation of Signaling Complexes in Beta 2-Adrenergic Receptor Desensitization and Resensitization. J. Biol. Chem. 2000, 275, 19025–19034. [Google Scholar] [CrossRef]
- Guillory, A.N.; Yin, X.; Wijaya, C.S.; Diaz Diaz, A.C.; Rababa’h, A.; Singh, S.; Atrooz, F.; Sadayappan, S.; McConnell, B.K. Enhanced Cardiac Function in Gravin Mutant Mice Involves Alterations in the β-Adrenergic Receptor Signaling Cascade. PLoS ONE 2013, 8, e74784. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Q.-H.; Chu, Y.; Wu, W.-M.; Song, J.-X.; Zhu, X.-B.; Wang, Q. Blockage of AKAP12 Accelerates Angiotensin II (Ang II)-Induced Cardiac Injury in Mice by Regulating the Transforming Growth Factor Β1 (TGF-Β1) Pathway. Biochem. Biophys. Res. Commun. 2018, 499, 128–135. [Google Scholar] [CrossRef]
- Diviani, D.; Osman, H.; Delaunay, M.; Kaiser, S. The Role of A-Kinase Anchoring Proteins in Cardiac Oxidative Stress. Biochem. Soc. Trans. 2019, 47, 1341–1353. [Google Scholar] [CrossRef]
- Kim, H.; Scimia, M.C.; Wilkinson, D.; Trelles, R.D.; Wood, M.R.; Bowtell, D.; Dillin, A.; Mercola, M.; Ronai, Z.A. Fine-Tuning of Drp1/Fis1 Availability by AKAP121/Siah2 Regulates Mitochondrial Adaptation to Hypoxia. Mol. Cell. 2011, 44, 532–544. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Cattaneo, F.; Pironti, G.; Magliulo, F.; Carotenuto, G.; Pirozzi, M.; Polishchuk, R.; Borzacchiello, D.; Paolillo, R.; Oliveti, M.; et al. Correction: Akap1 Deficiency Promotes Mitochondrial Aberrations and Exacerbates Cardiac Injury Following Permanent Coronary Ligation via Enhanced Mitophagy and Apoptosis. PLoS ONE 2016, 11, e0158934. [Google Scholar] [CrossRef]
- Bauman, A.L.; Soughayer, J.; Nguyen, B.T.; Willoughby, D.; Carnegie, G.K.; Wong, W.; Hoshi, N.; Langeberg, L.K.; Cooper, D.M.F.; Dessauer, C.W.; et al. Dynamic Regulation of CAMP Synthesis through Anchored PKA-Adenylyl Cyclase V/VI Complexes. Mol. Cell. 2006, 23, 925–931. [Google Scholar] [CrossRef]
- Ruehr, M.L.; Russell, M.A.; Bond, M. A-Kinase Anchoring Protein Targeting of Protein Kinase A in the Heart. J. Mol. Cell. Cardiol. 2004, 37, 653–665. [Google Scholar] [CrossRef]
- Collas, P.; Le Guellec, K.; Taskén, K. The A-Kinase-Anchoring Protein AKAP95 Is a Multivalent Protein with a Key Role in Chromatin Condensation at Mitosis. J. Cell. Biol. 1999, 147, 1167–1180. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 Prevents Excessive Inflammation and Cardiac Dysfunction after Myocardial Infarction through Targeting KBTBD7. Cell. Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef]
- Sarma, G.N.; Moody, I.S.; Ilouz, R.; Phan, R.H.; Sankaran, B.; Hall, R.A.; Taylor, S.S. D-AKAP2:PKA RII:PDZK1 Ternary Complex Structure: Insights from the Nucleation of a Polyvalent Scaffold. Protein. Sci. 2015, 24, 105–116. [Google Scholar] [CrossRef]
- Tingley, W.G.; Pawlikowska, L.; Zaroff, J.G.; Kim, T.; Nguyen, T.; Young, S.G.; Vranizan, K.; Kwok, P.-Y.; Whooley, M.A.; Conklin, B.R. Gene-Trapped Mouse Embryonic Stem Cell-Derived Cardiac Myocytes and Human Genetics Implicate AKAP10 in Heart Rhythm Regulation. Proc. Natl. Acad. Sci. USA 2007, 104, 8461–8466. [Google Scholar] [CrossRef]
- Fraser, I.D.; Tavalin, S.J.; Lester, L.B.; Langeberg, L.K.; Westphal, A.M.; Dean, R.A.; Marrion, N.V.; Scott, J.D. A Novel Lipid-Anchored A-Kinase Anchoring Protein Facilitates CAMP-Responsive Membrane Events. EMBO J. 1998, 17, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Hulme, J.T.; Lin, T.W.-C.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Beta-Adrenergic Regulation Requires Direct Anchoring of PKA to Cardiac CaV1.2 Channels via a Leucine Zipper Interaction with A Kinase-Anchoring Protein 15. Proc. Natl. Acad. Sci. USA 2003, 100, 13093–13098. [Google Scholar] [CrossRef]
- Hulme, J.T.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Phosphorylation of Serine 1928 in the Distal C-Terminal Domain of Cardiac CaV1.2 Channels during Beta1-Adrenergic Regulation. Proc. Natl. Acad. Sci. USA 2006, 103, 16574–16579. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, M.; Gerhardstein, B.L.; Gao, T.; Hosey, M.M. Functional Regulation of L-Type Calcium Channels via Protein Kinase A-Mediated Phosphorylation of the Beta(2) Subunit. J. Biol. Chem. 1999, 274, 33851–33854. [Google Scholar] [CrossRef] [PubMed]
- Lygren, B.; Carlson, C.R.; Santamaria, K.; Lissandron, V.; McSorley, T.; Litzenberg, J.; Lorenz, D.; Wiesner, B.; Rosenthal, W.; Zaccolo, M.; et al. AKAP Complex Regulates Ca2+ Re-Uptake into Heart Sarcoplasmic Reticulum. EMBO Rep. 2007, 8, 1061–1067. [Google Scholar] [CrossRef]
- Ahmad, F.; Shen, W.; Vandeput, F.; Szabo-Fresnais, N.; Krall, J.; Degerman, E.; Goetz, F.; Klussmann, E.; Movsesian, M.; Manganiello, V. Regulation of Sarcoplasmic Reticulum Ca2+ ATPase 2 (SERCA2) Activity by Phosphodiesterase 3A (PDE3A) in Human Myocardium: Phosphorylation-Dependent Interaction of PDE3A1 with SERCA2. J. Biol. Chem. 2015, 290, 6763–6776. [Google Scholar] [CrossRef]
- Singh, A.; Redden, J.M.; Kapiloff, M.S.; Dodge-Kafka, K.L. The Large Isoforms of A-Kinase Anchoring Protein 18 Mediate the Phosphorylation of Inhibitor-1 by Protein Kinase A and the Inhibition of Protein Phosphatase 1 Activity. Mol. Pharm. 2011, 79, 533–540. [Google Scholar] [CrossRef]
- Marx, S.O.; Kurokawa, J.; Reiken, S.; Motoike, H.; D’Armiento, J.; Marks, A.R.; Kass, R.S. Requirement of a Macromolecular Signaling Complex for Beta Adrenergic Receptor Modulation of the KCNQ1-KCNE1 Potassium Channel. Science 2002, 295, 496–499. [Google Scholar] [CrossRef]
- Ponomarenko, M.P.; Beniukh, D.N.; Orlov, I.L.; Kolchanov, N.A. [Precise recognition method of structure-function determinants of protein molecules]. Biofizika 1991, 36, 943–956. [Google Scholar]
- Diviani, D.; Soderling, J.; Scott, J.D. AKAP-Lbc Anchors Protein Kinase A and Nucleates Galpha 12-Selective Rho-Mediated Stress Fiber Formation. J. Biol. Chem. 2001, 276, 44247–44257. [Google Scholar] [CrossRef]
- Cavin, S.; Maric, D.; Diviani, D. A-Kinase Anchoring Protein-Lbc Promotes pro-Fibrotic Signaling in Cardiac Fibroblasts. Biochim Biophys Acta 2014, 1843, 335–345. [Google Scholar] [CrossRef]
- Diviani, D.; Osman, H.; Reggi, E. A-Kinase Anchoring Protein-Lbc: A Molecular Scaffold Involved in Cardiac Protection. J. Cardiovasc. Dev. Dis. 2018, 5. [Google Scholar] [CrossRef]
- Abdul Azeez, K.R.; Knapp, S.; Fernandes, J.M.P.; Klussmann, E.; Elkins, J.M. The Crystal Structure of the RhoA-AKAP-Lbc DH-PH Domain Complex. Biochem. J. 2014, 464, 231–239. [Google Scholar] [CrossRef]
- Appert-Collin, A.; Cotecchia, S.; Nenniger-Tosato, M.; Pedrazzini, T.; Diviani, D. The A-Kinase Anchoring Protein (AKAP)-Lbc-Signaling Complex Mediates Alpha1 Adrenergic Receptor-Induced Cardiomyocyte Hypertrophy. Proc. Natl. Acad. Sci. USA 2007, 104, 10140–10145. [Google Scholar] [CrossRef]
- Cariolato, L.; Cavin, S.; Diviani, D. A-Kinase Anchoring Protein (AKAP)-Lbc Anchors a PKN-Based Signaling Complex Involved in A1-Adrenergic Receptor-Induced P38 Activation. J. Biol. Chem. 2011, 286, 7925–7937. [Google Scholar] [CrossRef]
- Pérez López, I.; Cariolato, L.; Maric, D.; Gillet, L.; Abriel, H.; Diviani, D. A-Kinase Anchoring Protein Lbc Coordinates a P38 Activating Signaling Complex Controlling Compensatory Cardiac Hypertrophy. Mol. Cell. Biol. 2013, 33, 2903–2917. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, G.K.; Smith, F.D.; McConnachie, G.; Langeberg, L.K.; Scott, J.D. AKAP-Lbc Nucleates a Protein Kinase D Activation Scaffold. Mol. Cell. 2004, 15, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, G.K.; Soughayer, J.; Smith, F.D.; Pedroja, B.S.; Zhang, F.; Diviani, D.; Bristow, M.R.; Kunkel, M.T.; Newton, A.C.; Langeberg, L.K.; et al. AKAP-Lbc Mobilizes a Cardiac Hypertrophy Signaling Pathway. Mol. Cell. 2008, 32, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lester, L.B.; Coghlan, V.M.; Nauert, B.; Scott, J.D. Cloning and Characterization of a Novel A-Kinase Anchoring Protein. AKAP 220, Association with Testicular Peroxisomes. J. Biol. Chem. 1996, 271, 9460–9465. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, D.T.; Bradford, A.J.; Smith, J.; Martin, M.; Roy, C.; Mangeat, P.H.; Goldenring, J.R. Ezrin Is a Cyclic AMP-Dependent Protein Kinase Anchoring Protein. EMBO J. 1997, 16, 35–43. [Google Scholar] [CrossRef]
- Li, H.; Adamik, R.; Pacheco-Rodriguez, G.; Moss, J.; Vaughan, M. Protein Kinase A-Anchoring (AKAP) Domains in Brefeldin A-Inhibited Guanine Nucleotide-Exchange Protein 2 (BIG2). Proc. Natl. Acad. Sci. USA 2003, 100, 1627–1632. [Google Scholar] [CrossRef]
- Russell, M.A.; Lund, L.M.; Haber, R.; McKeegan, K.; Cianciola, N.; Bond, M. The Intermediate Filament Protein, Synemin, Is an AKAP in the Heart. Arch. Biochem. Biophys. 2006, 456, 204–215. [Google Scholar] [CrossRef]
- Reynolds, J.G.; McCalmon, S.A.; Tomczyk, T.; Naya, F.J. Identification and Mapping of Protein Kinase A Binding Sites in the Costameric Protein Myospryn. Biochim. Biophys. Acta 2007, 1773, 891–902. [Google Scholar] [CrossRef][Green Version]
- Sumandea, C.A.; Garcia-Cazarin, M.L.; Bozio, C.H.; Sievert, G.A.; Balke, C.W.; Sumandea, M.P. Cardiac Troponin T, a Sarcomeric AKAP, Tethers Protein Kinase A at the Myofilaments. J. Biol. Chem. 2011, 286, 530–541. [Google Scholar] [CrossRef]
- Baillie, G.S.; Scott, J.D.; Houslay, M.D. Compartmentalisation of Phosphodiesterases and Protein Kinase A: Opposites Attract. FEBS Lett. 2005, 579, 3264–3270. [Google Scholar] [CrossRef]
- Lugnier, C. Cyclic Nucleotide Phosphodiesterase (PDE) Superfamily: A New Target for the Development of Specific Therapeutic Agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Lighthouse, J.K.; Mickelsen, D.M.; Wu, J.; Yao, P.; Small, E.M.; Yan, C. A Novel Role of Cyclic Nucleotide Phosphodiesterase 10A in Pathological Cardiac Remodeling and Dysfunction. Circulation 2020, 141, 217–233. [Google Scholar] [CrossRef]
- Dell’Acqua, M.L.; Scott, J.D. Protein Kinase A Anchoring. J. Biol. Chem. 1997, 272, 12881–12884. [Google Scholar] [CrossRef]
- Chen, S.; Knight, W.E.; Yan, C. Roles of PDE1 in Pathological Cardiac Remodeling and Dysfunction. J. Cardiovasc. Dev. Dis. 2018, 5, 22. [Google Scholar] [CrossRef]
- Kincaid, R.L.; Stith-Coleman, I.E.; Vaughan, M. Proteolytic Activation of Calmodulin-Dependent Cyclic Nucleotide Phosphodiesterase. J. Biol. Chem. 1985, 260, 9009–9015. [Google Scholar] [CrossRef]
- Ang, K.-L.; Antoni, F.A. Reciprocal Regulation of Calcium Dependent and Calcium Independent Cyclic AMP Hydrolysis by Protein Phosphorylation. J. Neurochem. 2002, 81, 422–433. [Google Scholar] [CrossRef]
- Miller, C.L.; Cai, Y.; Oikawa, M.; Thomas, T.; Dostmann, W.R.; Zaccolo, M.; Fujiwara, K.; Yan, C. Cyclic Nucleotide Phosphodiesterase 1A: A Key Regulator of Cardiac Fibroblast Activation and Extracellular Matrix Remodeling in the Heart. Basic Res. Cardiol. 2011, 106, 1023–1039. [Google Scholar] [CrossRef]
- Martins, T.J.; Mumby, M.C.; Beavo, J.A. Purification and Characterization of a Cyclic GMP-Stimulated Cyclic Nucleotide Phosphodiesterase from Bovine Tissues. J. Biol. Chem. 1982, 257, 1973–1979. [Google Scholar] [CrossRef]
- Mehel, H.; Emons, J.; Vettel, C.; Wittköpper, K.; Seppelt, D.; Dewenter, M.; Lutz, S.; Sossalla, S.; Maier, L.S.; Lechêne, P.; et al. Phosphodiesterase-2 Is up-Regulated in Human Failing Hearts and Blunts β-Adrenergic Responses in Cardiomyocytes. J. Am. Coll. Cardiol. 2013, 62, 1596–1606. [Google Scholar] [CrossRef]
- Vettel, C.; Lämmle, S.; Ewens, S.; Cervirgen, C.; Emons, J.; Ongherth, A.; Dewenter, M.; Lindner, D.; Westermann, D.; Nikolaev, V.O.; et al. PDE2-Mediated CAMP Hydrolysis Accelerates Cardiac Fibroblast to Myofibroblast Conversion and Is Antagonized by Exogenous Activation of CGMP Signaling Pathways. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1246–H1252. [Google Scholar] [CrossRef]
- Monterisi, S.; Lobo, M.J.; Livie, C.; Castle, J.C.; Weinberger, M.; Baillie, G.; Surdo, N.C.; Musheshe, N.; Stangherlin, A.; Gottlieb, E.; et al. PDE2A2 Regulates Mitochondria Morphology and Apoptotic Cell Death via Local Modulation of CAMP/PKA Signalling. eLife 2017, 6, e21374. [Google Scholar] [CrossRef]
- Vettel, C.; Lindner, M.; Dewenter, M.; Lorenz, K.; Schanbacher, C.; Riedel, M.; Lämmle, S.; Meinecke, S.; Mason, F.E.; Sossalla, S.; et al. Phosphodiesterase 2 Protects Against Catecholamine-Induced Arrhythmia and Preserves Contractile Function After Myocardial Infarction. Circ. Res. 2017, 120, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Mongillo, M.; Tocchetti, C.G.; Terrin, A.; Lissandron, V.; Cheung, Y.-F.; Dostmann, W.R.; Pozzan, T.; Kass, D.A.; Paolocci, N.; Houslay, M.D.; et al. Compartmentalized Phosphodiesterase-2 Activity Blunts β-Adrenergic Cardiac Inotropy via an NO/CGMP-Dependent Pathway. Circ. Res. 2006, 98, 226–234. [Google Scholar] [CrossRef]
- Schobesberger, S.; Wright, P.T.; Poulet, C.; Sanchez Alonso Mardones, J.L.; Mansfield, C.; Friebe, A.; Harding, S.E.; Balligand, J.-L.; Nikolaev, V.O.; Gorelik, J. Β3-Adrenoceptor Redistribution Impairs NO/CGMP/PDE2 Signalling in Failing Cardiomyocytes. eLife 2020, 9, e52221. [Google Scholar] [CrossRef]
- Miki, T.; Taira, M.; Hockman, S.; Shimada, F.; Lieman, J.; Napolitano, M.; Ward, D.; Taira, M.; Makino, H.; Manganiello, V.C. Characterization of the CDNA and Gene Encoding Human PDE3B, the CGIP1 Isoform of the Human Cyclic GMP-Inhibited Cyclic Nucleotide Phosphodiesterase Family. Genomics 1996, 36, 476–485. [Google Scholar] [CrossRef]
- Wechsler, J.; Choi, Y.-H.; Krall, J.; Ahmad, F.; Manganiello, V.C.; Movsesian, M.A. Isoforms of Cyclic Nucleotide Phosphodiesterase PDE3A in Cardiac Myocytes. J. Biol. Chem. 2002, 277, 38072–38078. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.R.; Chin, E.; Zhou, J.; Taira, M.; Murata, T.; Manganiello, V.C.; Bondy, C.A. Distinctive Anatomical Patterns of Gene Expression for CGMP-Inhibited Cyclic Nucleotide Phosphodiesterases. J. Clin. Investig. 1995, 95, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Kenan, Y.; Murata, T.; Shakur, Y.; Degerman, E.; Manganiello, V.C. Functions of the N-Terminal Region of Cyclic Nucleotide Phosphodiesterase 3 (PDE 3) Isoforms. J. Biol. Chem. 2000, 275, 12331–12338. [Google Scholar] [CrossRef]
- Chung, Y.W.; Lagranha, C.; Chen, Y.; Sun, J.; Tong, G.; Hockman, S.C.; Ahmad, F.; Esfahani, S.G.; Bae, D.H.; Polidovitch, N.; et al. Targeted Disruption of PDE3B, but Not PDE3A, Protects Murine Heart from Ischemia/Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2015, 112, E2253–E2262. [Google Scholar] [CrossRef]
- Oikawa, M.; Wu, M.; Lim, S.; Knight, W.E.; Miller, C.L.; Cai, Y.; Lu, Y.; Blaxall, B.C.; Takeishi, Y.; Abe, J.; et al. Cyclic Nucleotide Phosphodiesterase 3A1 Protects the Heart against Ischemia-Reperfusion Injury. J. Mol. Cell. Cardiol. 2013, 64, 11–19. [Google Scholar] [CrossRef]
- Beca, S.; Ahmad, F.; Shen, W.; Liu, J.; Makary, S.; Polidovitch, N.; Sun, J.; Hockman, S.; Chung, Y.W.; Movsesian, M.; et al. Phosphodiesterase Type 3A Regulates Basal Myocardial Contractility Through Interacting With Sarcoplasmic Reticulum Calcium ATPase Type 2a Signaling Complexes in Mouse Heart. Circ. Res. 2013, 112, 289–297. [Google Scholar] [CrossRef]
- Patrucco, E.; Notte, A.; Barberis, L.; Selvetella, G.; Maffei, A.; Brancaccio, M.; Marengo, S.; Russo, G.; Azzolino, O.; Rybalkin, S.D.; et al. PI3Kgamma Modulates the Cardiac Response to Chronic Pressure Overload by Distinct Kinase-Dependent and -Independent Effects. Cell 2004, 118, 375–387. [Google Scholar] [CrossRef]
- Perino, A.; Ghigo, A.; Ferrero, E.; Morello, F.; Santulli, G.; Baillie, G.S.; Damilano, F.; Dunlop, A.J.; Pawson, C.; Walser, R.; et al. Integrating Cardiac PIP3 and CAMP Signaling through a PKA Anchoring Function of P110γ. Mol. Cell. 2011, 42, 84–95. [Google Scholar] [CrossRef]
- Sun, B.; Li, H.; Shakur, Y.; Hensley, J.; Hockman, S.; Kambayashi, J.; Manganiello, V.C.; Liu, Y. Role of Phosphodiesterase Type 3A and 3B in Regulating Platelet and Cardiac Function Using Subtype-Selective Knockout Mice. Cell. Signal. 2007, 19, 1765–1771. [Google Scholar] [CrossRef]
- Baim, D.S.; McDowell, A.V.; Cherniles, J.; Monrad, E.S.; Parker, J.A.; Edelson, J.; Braunwald, E.; Grossman, W. Evaluation of a New Bipyridine Inotropic Agent--Milrinone--in Patients with Severe Congestive Heart Failure. N. Engl. J. Med. 1983, 309, 748–756. [Google Scholar] [CrossRef]
- Packer, M.; Carver, J.R.; Rodeheffer, R.J.; Ivanhoe, R.J.; DiBianco, R.; Zeldis, S.M.; Hendrix, G.H.; Bommer, W.J.; Elkayam, U.; Kukin, M.L. Effect of Oral Milrinone on Mortality in Severe Chronic Heart Failure. The PROMISE Study Research Group. N. Engl. J. Med. 1991, 325, 1468–1475. [Google Scholar] [CrossRef]
- Packer, M. Effect of Phosphodiesterase Inhibitors on Survival of Patients with Chronic Congestive Heart Failure. Am. J. Cardiol. 1989, 63, 41A–45A. [Google Scholar] [CrossRef]
- Fertig, B.A.; Baillie, G.S. PDE4-Mediated CAMP Signalling. J. Cardiovasc. Dev. Dis. 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Kostic, M.M.; Erdogan, S.; Rena, G.; Borchert, G.; Hoch, B.; Bartel, S.; Scotland, G.; Huston, E.; Houslay, M.D.; Krause, E.G. Altered Expression of PDE1 and PDE4 Cyclic Nucleotide Phosphodiesterase Isoforms in 7-Oxo-Prostacyclin-Preconditioned Rat Heart. J. Mol. Cell. Cardiol. 1997, 29, 3135–3146. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.; Richter, W.; Mika, D.; Castro, L.R.V.; Abi-Gerges, A.; Xie, M.; Scheitrum, C.; Lefebvre, F.; Schittl, J.; Mateo, P.; et al. Phosphodiesterase 4B in the Cardiac L-Type Ca2+ Channel Complex Regulates Ca2+ Current and Protects against Ventricular Arrhythmias in Mice. J. Clin. Investig. 2011, 121, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Greenstein, J.L.; Winslow, R.L. Interaction between Phosphodiesterases in the Regulation of the Cardiac β-Adrenergic Pathway. J. Mol. Cell. Cardiol. 2015, 88, 29–38. [Google Scholar] [CrossRef]
- Huang, H.; Xie, M.; Gao, L.; Zhang, W.; Zhu, X.; Wang, Y.; Li, W.; Wang, R.; Chen, K.; Boutjdir, M.; et al. Rolipram, a PDE4 Inhibitor, Enhances the Inotropic Effect of Rat Heart by Activating SERCA2a. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Beca, S.; Helli, P.B.; Simpson, J.A.; Zhao, D.; Farman, G.P.; Jones, P.; Tian, X.; Wilson, L.S.; Ahmad, F.; Chen, S.R.W.; et al. Phosphodiesterase 4D Regulates Baseline Sarcoplasmic Reticulum Ca2+ Release and Cardiac Contractility, Independently of L-Type Ca2+ Current. Circ. Res. 2011, 109, 1024–1030. [Google Scholar] [CrossRef]
- Terrenoire, C.; Houslay, M.D.; Baillie, G.S.; Kass, R.S. The Cardiac IKs Potassium Channel Macromolecular Complex Includes the Phosphodiesterase PDE4D3. J. Biol. Chem. 2009, 284, 9140–9146. [Google Scholar] [CrossRef]
- Shi, Q.; Li, M.; Mika, D.; Fu, Q.; Kim, S.; Phan, J.; Shen, A.; Vandecasteele, G.; Xiang, Y.K. Heterologous Desensitization of Cardiac β-Adrenergic Signal via Hormone-Induced ΒAR/Arrestin/PDE4 Complexes. Cardiovasc. Res. 2017, 113, 656–670. [Google Scholar] [CrossRef]
- Lehnart, S.E.; Wehrens, X.H.T.; Reiken, S.; Warrier, S.; Belevych, A.E.; Harvey, R.D.; Richter, W.; Jin, S.-L.C.; Conti, M.; Marks, A.R. Phosphodiesterase 4D Deficiency in the Ryanodine-Receptor Complex Promotes Heart Failure and Arrhythmias. Cell 2005, 123, 25–35. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, T.; Wang, X. Gene Transfer of Heat-Shock Protein 20 Protects against Ischemia/Reperfusion Injury in Rat Hearts. Acta Pharm. Sin. 2005, 26, 1193–1200. [Google Scholar] [CrossRef]
- Nicolaou, P.; Knöll, R.; Haghighi, K.; Fan, G.-C.; Dorn, G.W.; Hasenfub, G.; Kranias, E.G. Human Mutation in the Anti-Apoptotic Heat Shock Protein 20 Abrogates Its Cardioprotective Effects. J. Biol. Chem. 2008, 283, 33465–33471. [Google Scholar] [CrossRef]
- Fan, G.-C.; Chu, G.; Mitton, B.; Song, Q.; Yuan, Q.; Kranias, E.G. Small Heat-Shock Protein Hsp20 Phosphorylation Inhibits Beta-Agonist-Induced Cardiac Apoptosis. Circ. Res. 2004, 94, 1474–1482. [Google Scholar] [CrossRef]
- Edwards, H.V.; Scott, J.D.; Baillie, G.S. PKA Phosphorylation of the Small Heat-Shock Protein Hsp20 Enhances Its Cardioprotective Effects. Biochem. Soc. Trans. 2012, 40, 210–214. [Google Scholar] [CrossRef]
- Sin, Y.Y.; Edwards, H.V.; Li, X.; Day, J.P.; Christian, F.; Dunlop, A.J.; Adams, D.R.; Zaccolo, M.; Houslay, M.D.; Baillie, G.S. Disruption of the Cyclic AMP Phosphodiesterase-4 (PDE4)-HSP20 Complex Attenuates the β-Agonist Induced Hypertrophic Response in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2011, 50, 872–883. [Google Scholar] [CrossRef]
- Martin, T.P.; Hortigon-Vinagre, M.P.; Findlay, J.E.; Elliott, C.; Currie, S.; Baillie, G.S. Targeted Disruption of the Heat Shock Protein 20-Phosphodiesterase 4D (PDE4D) Interaction Protects against Pathological Cardiac Remodelling in a Mouse Model of Hypertrophy. FEBS Open Bio. 2014, 4, 923–927. [Google Scholar] [CrossRef]
- Lukyanenko, Y.O.; Younes, A.; Lyashkov, A.E.; Tarasov, K.V.; Riordon, D.R.; Lee, J.; Sirenko, S.G.; Kobrinsky, E.; Ziman, B.; Tarasova, Y.S.; et al. Ca2+/Calmodulin-Activated Phosphodiesterase 1A Is Highly Expressed in Rabbit Cardiac Sinoatrial Nodal Cells and Regulates Pacemaker Function. J. Mol. Cell Cardiol. 2016, 98, 73–82. [Google Scholar] [CrossRef]
- Patrucco, E.; Albergine, M.S.; Santana, L.F.; Beavo, J.A. Phosphodiesterase 8A (PDE8A) Regulates Excitation–Contraction Coupling in Ventricular Myocytes. J. Mol. Cell. Cardiol. 2010, 49, 330–333. [Google Scholar] [CrossRef]
- Movsesian, M.A. Beta-Adrenergic Receptor Agonists and Cyclic Nucleotide Phosphodiesterase Inhibitors: Shifting the Focus from Inotropy to Cyclic Adenosine Monophosphate. J. Am. Coll. Cardiol. 1999, 34, 318–324. [Google Scholar] [CrossRef]
- Kemp, C.D.; Conte, J.V. The Pathophysiology of Heart Failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Schulz, R.; Rose, J.; Martin, C.; Brodde, O.E.; Heusch, G. Development of Short-Term Myocardial Hibernation. Its Limitation by the Severity of Ischemia and Inotropic Stimulation. Circulation 1993, 88, 684–695. [Google Scholar] [CrossRef]
- Lubbe, W.F.; Podzuweit, T.; Opie, L.H. Potential Arrhythmogenic Role of Cyclic Adenosine Monophosphate (AMP) and Cytosolic Calcium Overload: Implications for Prophylactic Effects of Beta-Blockers in Myocardial Infarction and Proarrhythmic Effects of Phosphodiesterase Inhibitors. J. Am. Coll. Cardiol. 1992, 19, 1622–1633. [Google Scholar] [CrossRef]
- Freemantle, N.; Cleland, J.; Young, P.; Mason, J.; Harrison, J. Beta Blockade after Myocardial Infarction: Systematic Review and Meta Regression Analysis. BMJ 1999, 318, 1730–1737. [Google Scholar] [CrossRef]
- Dézsi, C.A.; Szentes, V. The Real Role of β-Blockers in Daily Cardiovascular Therapy. Am. J. Cardiovasc. Drugs 2017, 17, 361–373. [Google Scholar] [CrossRef]
- Bristow, M.R. Treatment of Chronic Heart Failure with β-Adrenergic Receptor Antagonists: A Convergence of Receptor Pharmacology and Clinical Cardiology. Circ. Res. 2011, 109, 1176–1194. [Google Scholar] [CrossRef]
- Iwai-Kanai, E.; Hasegawa, K.; Araki, M.; Kakita, T.; Morimoto, T.; Sasayama, S. Alpha- and Beta-Adrenergic Pathways Differentially Regulate Cell Type-Specific Apoptosis in Rat Cardiac Myocytes. Circulation 1999, 100, 305–311. [Google Scholar] [CrossRef]
- Communal, C.; Singh, K.; Pimentel, D.R.; Colucci, W.S. Norepinephrine Stimulates Apoptosis in Adult Rat Ventricular Myocytes by Activation of the Beta-Adrenergic Pathway. Circulation 1998, 98, 1329–1334. [Google Scholar] [CrossRef]
- Liu, C.; Ke, P.; Zhang, J.; Zhang, X.; Chen, X. Protein Kinase Inhibitor Peptide as a Tool to Specifically Inhibit Protein Kinase A. Front. Physiol. 2020, 11, 574030. [Google Scholar] [CrossRef]
- Scott, J.D.; Fischer, E.H.; Demaille, J.G.; Krebs, E.G. Identification of an Inhibitory Region of the Heat-Stable Protein Inhibitor of the CAMP-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1985, 82, 4379–4383. [Google Scholar] [CrossRef]
- Wittrup, A.; Lieberman, J. Knocking down Disease: A Progress Report on SiRNA Therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef]
- Omar, F.; Findlay, J.E.; Carfray, G.; Allcock, R.W.; Jiang, Z.; Moore, C.; Muir, A.L.; Lannoy, M.; Fertig, B.A.; Mai, D.; et al. Small-Molecule Allosteric Activators of PDE4 Long Form Cyclic AMP Phosphodiesterases. Proc. Natl. Acad. Sci. USA 2019, 116, 13320–13329. [Google Scholar] [CrossRef]
- Li, J.; Vargas, M.A.X.; Kapiloff, M.S.; Dodge-Kafka, K.L. Regulation of MEF2 Transcriptional Activity by Calcineurin/MAKAP Complexes. Exp. Cell. Res. 2013, 319, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Lefkowitz, R.J.; Rajagopal, S. Biased Signalling: From Simple Switches to Allosteric Microprocessors. Nat. Rev. Drug Discov. 2018, 17, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.; Du, Y.; Quoyer, J.; Panettieri, R.A.; Janz, J.M.; Bouvier, M.; Kobilka, B.K.; Benovic, J.L. Development and Characterization of Pepducins as Gs-Biased Allosteric Agonists. J. Biol. Chem. 2014, 289, 35668–35684. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.; Schilling, J.; Song, J.; Carter, R.L.; Du, Y.; Yoo, S.M.; Traynham, C.J.; Koch, W.J.; Cheung, J.Y.; Tilley, D.G.; et al. β-Arrestin-Biased Signaling through the Β2-Adrenergic Receptor Promotes Cardiomyocyte Contraction. Proc. Natl. Acad. Sci. USA 2016, 113, E4107–E4116. [Google Scholar] [CrossRef]
- Grisanti, L.A.; Thomas, T.P.; Carter, R.L.; de Lucia, C.; Gao, E.; Koch, W.J.; Benovic, J.L.; Tilley, D.G. Pepducin-Mediated Cardioprotection via β-Arrestin-Biased Β2-Adrenergic Receptor-Specific Signaling. Theranostics 2018, 8, 4664–4678. [Google Scholar] [CrossRef]
- Dimmeler, S. Cardiovascular Disease Review Series. EMBO Mol. Med. 2011, 3, 697. [Google Scholar] [CrossRef]
- Ruvinov, E.; Dvir, T.; Leor, J.; Cohen, S. Myocardial Repair: From Salvage to Tissue Reconstruction. Expert. Rev. Cardiovasc. 2008, 6, 669–686. [Google Scholar] [CrossRef]
- Maheshwari, R.; Tekade, M.; Sharma, P.A.; Tekade, R.K. Nanocarriers Assisted SiRNA Gene Therapy for the Management of Cardiovascular Disorders. Curr. Pharm. Des. 2015, 21, 4427–4440. [Google Scholar] [CrossRef]
- Karam, S.; Margaria, J.P.; Bourcier, A.; Mika, D.; Varin, A.; Bedioune, I.; Lindner, M.; Bouadjel, K.; Dessillons, M.; Gaudin, F.; et al. Cardiac Overexpression of PDE4B Blunts β-Adrenergic Response and Maladaptive Remodeling in Heart Failure. Circulation 2020, 142, 161–174. [Google Scholar] [CrossRef]
- Stoschitzky’, ’Kurt Individual Beta-Blockers for Individual Patients. e-J. Esc. Counc. Cardiol. Pract. 2018, 6, 8. [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).