Expression and Function of ZEB1 in the Cornea
Abstract
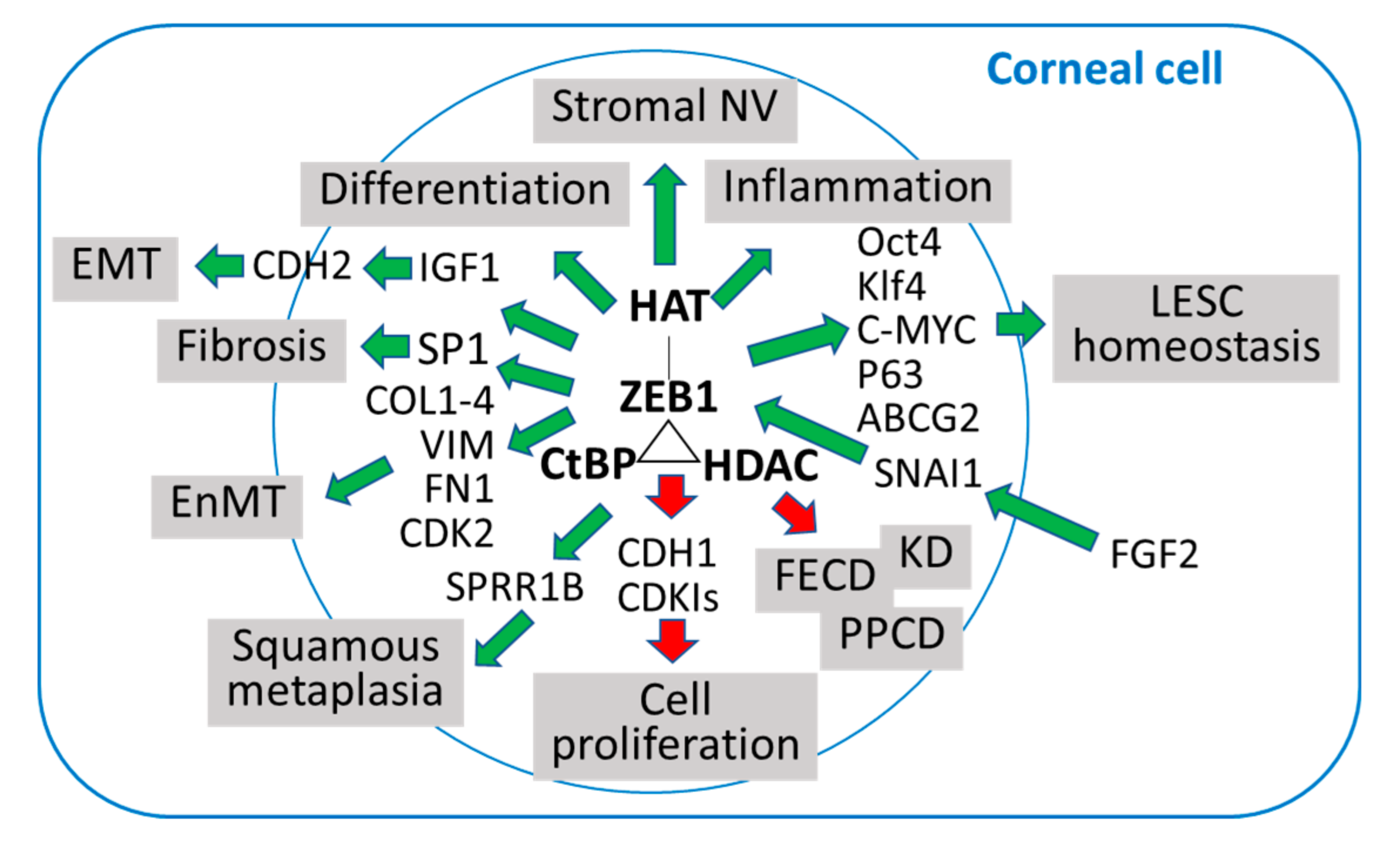
:1. ZEB1 and Epithelial to Mesenchymal Transition (EMT)
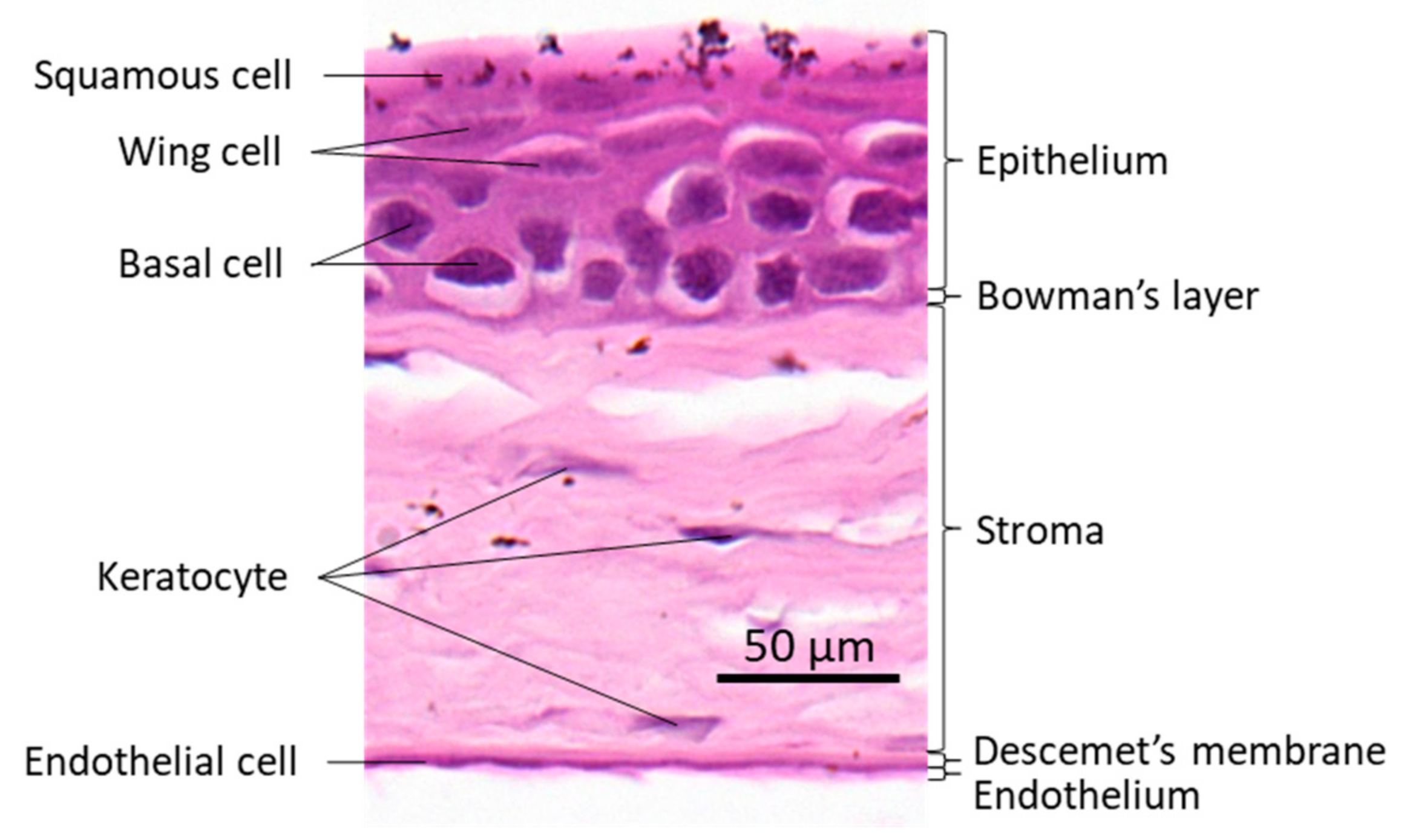
2. The Cornea and ZEB1
3. ZEB1 and Corneal Epithelium
4. ZEB1 and Corneal Endothelium
4.1. Posterior Polymorphous Corneal Dystrophy (PPCD)
4.2. Fuchs Endothelial Corneal Dystrophy (FECD)
5. ZEB1 and Keratoconus
6. ZEB1 and Corneal Wound Healing
7. ZEB1 and Corneal Neovascularization (NV)
8. ZEB1 and Corneal Inflammation
9. ZEB1 Is a Potential Therapeutical Target
10. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, C.L.; Omilusik, K.D. ZEBs: Novel Players in Immune Cell Development and Function. Trends Immunol. 2019, 40, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Funahashi, J.; Sekido, R.; Murai, K.; Kamachi, Y.; Kondoh, H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development 1993, 119, 433–446. [Google Scholar] [PubMed]
- Shirakihara, T.; Saitoh, M.; Miyazono, K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF-beta. Mol. Biol. Cell 2007, 18, 3533–3544. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, G.; Manabe, I.; Tsushima, K.; Fujiu, K.; Oishi, Y.; Imai, Y.; Maemura, K.; Miyagishi, M.; Higashi, Y.; Kondoh, H.; et al. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev. Cell 2006, 11, 93–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, L.; Li, A.; Han, X. The roles of ZEB1 in tumorigenic progression and epigenetic modifications. Biomed. Pharm. 2019, 110, 400–408. [Google Scholar] [CrossRef]
- Perez-Moreno, M.A.; Locascio, A.; Rodrigo, I.; Dhondt, G.; Portillo, F.; Nieto, M.A.; Cano, A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J. Biol. Chem. 2001, 276, 27424–27431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhao, L.; Yang, J.; Chai, D.; Zhang, M.; Zhang, J.; Ji, X.; Zhu, T. δEF1 represses BMP-2-induced differentiation of C2C12 myoblasts into the osteoblast lineage. J. Biomed. Sci. 2007, 14, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tillo, E.; Siles, L.; de Barrios, O.; Cuatrecasas, M.; Vaquero, E.C.; Castells, A.; Postigo, A. Expanding roles of ZEB factors in tumorigenesis and tumor progression. Am. J. Cancer Res. 2011, 1, 897–912. [Google Scholar]
- Wu, H.T.; Zhong, H.T.; Li, G.W.; Shen, J.X.; Ye, Q.Q.; Zhang, M.L.; Liu, J. Oncogenic functions of the EMT-related transcription factor ZEB1 in breast cancer. J. Transl. Med. 2020, 18, 51. [Google Scholar] [CrossRef]
- Seelan, R.S.; Mukhopadhyay, P.; Pisano, M.M.; Greene, R.M. Developmental epigenetics of the murine secondary palate. ILAR J. 2012, 53, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Lencinas, A.; Chhun, D.C.; Dan, K.P.; Ross, K.D.; Hoover, E.A.; Antin, P.B.; Runyan, R.B. Olfactomedin-1 activity identifies a cell invasion checkpoint during epithelial-mesenchymal transition in the chick embryonic heart. Dis. Models Mech. 2013, 6, 632–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, G.; Croci, A.; Dowling, A.; Zhang, S.; Zoeller, R.T.; Darling, D.S. Developmental and functional evidence of a role for Zfhep in neural cell development. Brain Res. Mol. Brain Res. 2001, 96, 59–67. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.Y.; Fattet, L.; Yang, J. Molecular pathways: Linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin. Cancer Res. 2015, 21, 962–968. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; El-Naggar, S.; Darling, D.S.; Higashi, Y.; Dean, D.C. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development 2008, 135, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Gallup, M.; Chen, Y.T.; McNamara, N.A. Molecular mechanism of proinflammatory cytokine-mediated squamous metaplasia in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2466–2475. [Google Scholar] [CrossRef] [Green Version]
- Secker, G.A.; Daniels, J.T. Limbal Epithelial Stem Cells of the Cornea; StemBook: Cambridge, MA, USA, 2008. [Google Scholar]
- Fini, M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 1999, 18, 529–551. [Google Scholar] [CrossRef]
- Pinnamaneni, N.; Funderburgh, J.L. Concise review: Stem cells in the corneal stroma. Stem Cells 2012, 30, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Zavala, J.; Lopez Jaime, G.R.; Rodriguez Barrientos, C.A.; Valdez-Garcia, J. Corneal endothelium: Developmental strategies for regeneration. Eye 2013, 27, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Zhang, Y.; Liang, W.; Lu, X.; Piri, N.; Wang, W.; Kaplan, H.J.; Dean, D.C.; Zhang, L.; Liu, Y. Zeb1 promotes corneal neovascularization by regulation of vascular endothelial cell proliferation. Commun. Biol. 2020, 3, 349. [Google Scholar] [CrossRef]
- Tseng, H.; Green, H. Basonuclin: A keratinocyte protein with multiple paired zinc fingers. Proc. Natl. Acad. Sci. USA 1992, 89, 10311–10315. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Loughner, C.L.; Swamynathan, S.; Swamynathan, S.K. KLF4 Plays an Essential Role in Corneal Epithelial Homeostasis by Promoting Epithelial Cell Fate and Suppressing Epithelial-Mesenchymal Transition. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2785–2795. [Google Scholar] [CrossRef]
- Ortiz-Melo, M.T.; Garcia-Murillo, M.J.; Salazar-Rojas, V.M.; Campos, J.E.; Castro-Munozledo, F. Transcriptional profiles along cell programming into corneal epithelial differentiation. Exp. Eye Res. 2020, 202, 108302. [Google Scholar] [CrossRef]
- Ko, J.A.; Yanai, R.; Nishida, T. IGF-1 released by corneal epithelial cells induces up-regulation of N-cadherin in corneal fibroblasts. J. Cell Physiol. 2009, 221, 254–261. [Google Scholar] [CrossRef]
- Sanchez-Tillo, E.; Liu, Y.; de Barrios, O.; Siles, L.; Fanlo, L.; Cuatrecasas, M.; Darling, D.S.; Dean, D.C.; Castells, A.; Postigo, A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell Mol. Life Sci. 2012, 69, 3429–3456. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.; Dash, D.P.; Muszynska, D.; Hosseini, M.; Segev, F.; George, S.; Frazer, D.G.; Moore, J.E.; Kaye, S.B.; Young, T.; et al. Mutational spectrum of the ZEB1 gene in corneal dystrophies supports a genotype-phenotype correlation. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3215–3223. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.S.; Moller, H.U.; Aldave, A.J.; Seitz, B.; Bredrup, C.; Kivela, T.; Munier, F.L.; Rapuano, C.J.; Nischal, K.K.; Kim, E.K.; et al. IC3D classification of corneal dystrophies--edition 2. Cornea 2015, 34, 117–159. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Peng, X.; Tan, J.; Darling, D.S.; Kaplan, H.J.; Dean, D.C. Zeb1 mutant mice as a model of posterior corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1843–1849. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiari, P.; Frausto, R.F.; Roldan, A.N.; Wang, C.; Yu, F.; Aldave, A.J. Exclusion of pathogenic promoter region variants and identification of novel nonsense mutations in the zinc finger E-box binding homeobox 1 gene in posterior polymorphous corneal dystrophy. Mol. Vis. 2013, 19, 575–580. [Google Scholar]
- Vincent, A.L.; Niederer, R.L.; Richards, A.; Karolyi, B.; Patel, D.V.; McGhee, C.N. Phenotypic characterisation and ZEB1 mutational analysis in posterior polymorphous corneal dystrophy in a New Zealand population. Mol. Vis. 2009, 15, 2544–2553. [Google Scholar]
- Jang, M.S.; Roldan, A.N.; Frausto, R.F.; Aldave, A.J. Posterior polymorphous corneal dystrophy 3 is associated with agenesis and hypoplasia of the corpus callosum. Vis. Res. 2014, 100, 88–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, D.D.; Frausto, R.F.; Lin, B.R.; Hanser, E.M.; Cohen, Z.; Aldave, A.J. Transcriptomic Profiling of Posterior Polymorphous Corneal Dystrophy. Invest. Ophthalmol. Vis. Sci. 2017, 58, 3202–3214. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.W.; Frausto, R.F.; Ann, L.B.; Jang, M.S.; Aldave, A.J. Functional impact of ZEB1 mutations associated with posterior polymorphous and Fuchs’ endothelial corneal dystrophies. Invest. Ophthalmol. Vis. Sci. 2014, 55, 6159–6166. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.W.; Frausto, R.F.; Chiu, S.; Lin, B.R.; Aldave, A.J. Investigating the Molecular Basis of PPCD3: Characterization of ZEB1 Regulation of COL4A3 Expression. Invest. Ophthalmol. Vis. Sci. 2016, 57, 4136–4143. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Tillo, E.; Fanlo, L.; Siles, L.; Montes-Moreno, S.; Moros, A.; Chiva-Blanch, G.; Estruch, R.; Martinez, A.; Colomer, D.; Gyorffy, B.; et al. The EMT activator ZEB1 promotes tumor growth and determines differential response to chemotherapy in mantle cell lymphoma. Cell Death Differ. 2014, 21, 247–257. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, Y.; Zhou, Y.; Xie, Y.; Ju, P.; Long, Y.; Liu, J.; Ni, D.; Cao, F.; Lyu, Z.; et al. Zeb1 Is a Potential Regulator of Six2 in the Proliferation, Apoptosis and Migration of Metanephric Mesenchyme Cells. Int. J. Mol. Sci. 2016, 17, 1283. [Google Scholar] [CrossRef] [Green Version]
- Eneling, K.; Brion, L.; Pinto, V.; Pinho, M.J.; Sznajder, J.I.; Mochizuki, N.; Emoto, K.; Soares-da-Silva, P.; Bertorello, A.M. Salt-inducible kinase 1 regulates E-cadherin expression and intercellular junction stability. FASEB J. 2012, 26, 3230–3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzunhan, Y.; Bernard, O.; Marchant, D.; Dard, N.; Vanneaux, V.; Larghero, J.; Gille, T.; Clerici, C.; Valeyre, D.; Nunes, H.; et al. Mesenchymal stem cells protect from hypoxia-induced alveolar epithelial-mesenchymal transition. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L439–L451. [Google Scholar] [CrossRef] [Green Version]
- Shyu, H.Y.; Ko, C.J.; Luo, Y.C.; Lin, H.Y.; Wu, S.R.; Lan, S.W.; Cheng, T.S.; Hu, S.H.; Lee, M.S. Ketamine Increases Permeability and Alters Epithelial Phenotype of Renal Distal Tubular Cells via a GSK-3beta-Dependent Mechanism. J. Cell Biochem. 2016, 117, 881–893. [Google Scholar] [CrossRef]
- Zakharevich, M.; Kattan, J.M.; Chen, J.L.; Lin, B.R.; Cervantes, A.E.; Chung, D.D.; Frausto, R.F.; Aldave, A.J. Elucidating the molecular basis of PPCD: Effects of decreased ZEB1 expression on corneal endothelial cell function. Mol. Vis. 2017, 23, 740–752. [Google Scholar]
- Frausto, R.F.; Chung, D.D.; Boere, P.M.; Swamy, V.S.; Duong, H.N.V.; Kao, L.; Azimov, R.; Zhang, W.; Carrigan, L.; Wong, D.; et al. ZEB1 insufficiency causes corneal endothelial cell state transition and altered cellular processing. PLoS ONE 2019, 14, e0218279. [Google Scholar] [CrossRef] [Green Version]
- Yellore, V.S.; Rayner, S.A.; Nguyen, C.K.; Gangalum, R.K.; Jing, Z.; Bhat, S.P.; Aldave, A.J. Analysis of the role of ZEB1 in the pathogenesis of posterior polymorphous corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 2012, 53, 273–278. [Google Scholar] [CrossRef]
- Aldave, A.J.; Ann, L.B.; Frausto, R.F.; Nguyen, C.K.; Yu, F.; Raber, I.M. Classification of posterior polymorphous corneal dystrophy as a corneal ectatic disorder following confirmation of associated significant corneal steepening. JAMA Ophthalmol. 2013, 131, 1583–1590. [Google Scholar] [CrossRef] [Green Version]
- Cunnusamy, K.; Bowman, C.B.; Beebe, W.; Gong, X.; Hogan, R.N.; Mootha, V.V. Congenital Corneal Endothelial Dystrophies Resulting From Novel De Novo Mutations. Cornea 2016, 35, 281–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzotta, C.; Traversi, C.; Raiskup, F.; Rizzo, C.L.; Renieri, A. First identification of a triple corneal dystrophy association: Keratoconus, epithelial basement membrane corneal dystrophy and fuchs’ endothelial corneal dystrophy. Case Rep. Ophthalmol. 2014, 5, 281–288. [Google Scholar] [CrossRef]
- Rong, Z.; Hu, J.; Corey, D.R.; Mootha, V.V. Quantitative Studies of Muscleblind Proteins and Their Interaction With TCF4 RNA Foci Support Involvement in the Mechanism of Fuchs’ Dystrophy. Invest. Ophthalmol. Vis. Sci. 2019, 60, 3980–3991. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Weisenberger, D.J.; Zheng, S.; Wolf, M.; Hwang, D.G.; Rose-Nussbaumer, J.R.; Jurkunas, U.V.; Chan, M.F. Aberrant DNA methylation of miRNAs in Fuchs endothelial corneal dystrophy. Sci. Rep. 2019, 9, 16385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Liu, X.; Chen, Y.; Liu, J.Y.; Lu, H.; Wang, W.; Lu, X.; Dean, K.C.; Gao, L.; Kaplan, H.J.; et al. Sphere-induced reprogramming of RPE cells into dual-potential RPE stem-like cells. EBioMedicine 2020, 52, 102618. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Kumawat, B.L.; Paliwal, P.; Tandon, R.; Sharma, N.; Sen, S.; Kashyap, S.; Nag, T.C.; Vajpayee, R.B.; Sharma, A. Association of ZEB1 and TCF4 rs613872 changes with late onset Fuchs endothelial corneal dystrophy in patients from northern India. Mol. Vis. 2015, 21, 1252–1260. [Google Scholar]
- Burdon, K.P.; Vincent, A.L. Insights into keratoconus from a genetic perspective. Clin Exp. Optom. 2013, 96, 146–154. [Google Scholar] [CrossRef]
- Bykhovskaya, Y.; Margines, B.; Rabinowitz, Y.S. Genetics in Keratoconus: Where are we? Eye Vis. 2016, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Wen, L.; Roufas, A.; Madigan, M.C.; Sutton, G. Expression of SFRP Family Proteins in Human Keratoconus Corneas. PLoS ONE 2013, 8, e66770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.G.; Jung, E.; Heur, M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J. Biol. Chem. 2018, 293, 3758–3769. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jung, E.; Heur, M. Injury induces endothelial to mesenchymal transition in the mouse corneal endothelium in vivo via FGF2. Mol. Vis. 2019, 25, 22–34. [Google Scholar]
- Lee, J.; Jung, E.; Gestoso, K.; Heur, M. ZEB1 Mediates Fibrosis in Corneal Endothelial Mesenchymal Transition Through SP1 and SP3. Invest. Ophthalmol. Vis. Sci. 2020, 61, 41. [Google Scholar] [CrossRef]
- Singh, K.; Sinha, M.; Pal, D.; Tabasum, S.; Gnyawali, S.C.; Khona, D.; Sarkar, S.; Mohanty, S.K.; Soto-Gonzalez, F.; Khanna, S.; et al. Cutaneous Epithelial to Mesenchymal Transition Activator ZEB1 Regulates Wound Angiogenesis and Closure in a Glycemic Status-Dependent Manner. Diabetes 2019, 68, 2175–2190. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Haensel, D.; Sun, P.; MacLean, A.L.; Ma, X.; Zhou, Y.; Stemmler, M.P.; Brabletz, S.; Berx, G.; Plikus, M.V.; Nie, Q.; et al. An Ovol2-Zeb1 transcriptional circuit regulates epithelial directional migration and proliferation. EMBO Rep. 2019, 20, e46273. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, D.; Kim, Y.S.; Kim, S.; Lee, H.K.; Yang, J.W.; Hur, D.Y. The Epstein-Barr virus causes epithelial-mesenchymal transition in human corneal epithelial cells via Syk/src and Akt/Erk signaling pathways. Invest. Ophthalmol. Vis. Sci. 2014, 55, 1770–1779. [Google Scholar] [CrossRef] [Green Version]
- Straza, M.W.; Paliwal, S.; Kovi, R.C.; Rajeshkumar, B.; Trenh, P.; Parker, D.; Whalen, G.F.; Lyle, S.; Schiffer, C.A.; Grossman, S.R. Therapeutic targeting of C-terminal binding protein in human cancer. Cell Cycle 2010, 9, 3740–3750. [Google Scholar] [CrossRef] [Green Version]
- Dcona, M.M.; Morris, B.L.; Ellis, K.C.; Grossman, S.R. CtBP- an emerging oncogene and novel small molecule drug target: Advances in the understanding of its oncogenic action and identification of therapeutic inhibitors. Cancer Biol. Ther. 2017, 18, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Moribe, H.; Kondoh, H.; Higashi, Y. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development 1998, 125, 21–31. [Google Scholar] [PubMed]
- Sabourin, J.C.; Ackema, K.B.; Ohayon, D.; Guichet, P.O.; Perrin, F.E.; Garces, A.; Ripoll, C.; Charite, J.; Simonneau, L.; Kettenmann, H.; et al. A mesenchymal-like ZEB1(+) niche harbors dorsal radial glial fibrillary acidic protein-positive stem cells in the spinal cord. Stem Cells 2009, 27, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Dean, K.C.; Huang, L.; Chen, Y.; Lu, X.; Liu, Y. An Rb1-dependent amplification loop between Ets1 and Zeb1 is evident in thymocyte differentiation and invasive lung adenocarcinoma. BMC Mol. Biol. 2015, 16, 8. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, X.; Liang, W.; Dean, D.C.; Zhang, L.; Liu, Y. Expression and Function of ZEB1 in the Cornea. Cells 2021, 10, 925. https://doi.org/10.3390/cells10040925
Zhang Y, Liu X, Liang W, Dean DC, Zhang L, Liu Y. Expression and Function of ZEB1 in the Cornea. Cells. 2021; 10(4):925. https://doi.org/10.3390/cells10040925
Chicago/Turabian StyleZhang, Yingnan, Xiao Liu, Wei Liang, Douglas C. Dean, Lijun Zhang, and Yongqing Liu. 2021. "Expression and Function of ZEB1 in the Cornea" Cells 10, no. 4: 925. https://doi.org/10.3390/cells10040925





