Neurobiological Processes Induced by Aerobic Exercise through the Endocannabinoidome
Abstract
:1. Introduction
2. Overview of the Endocannabinoid System and Ensuing Endocannabinoidome
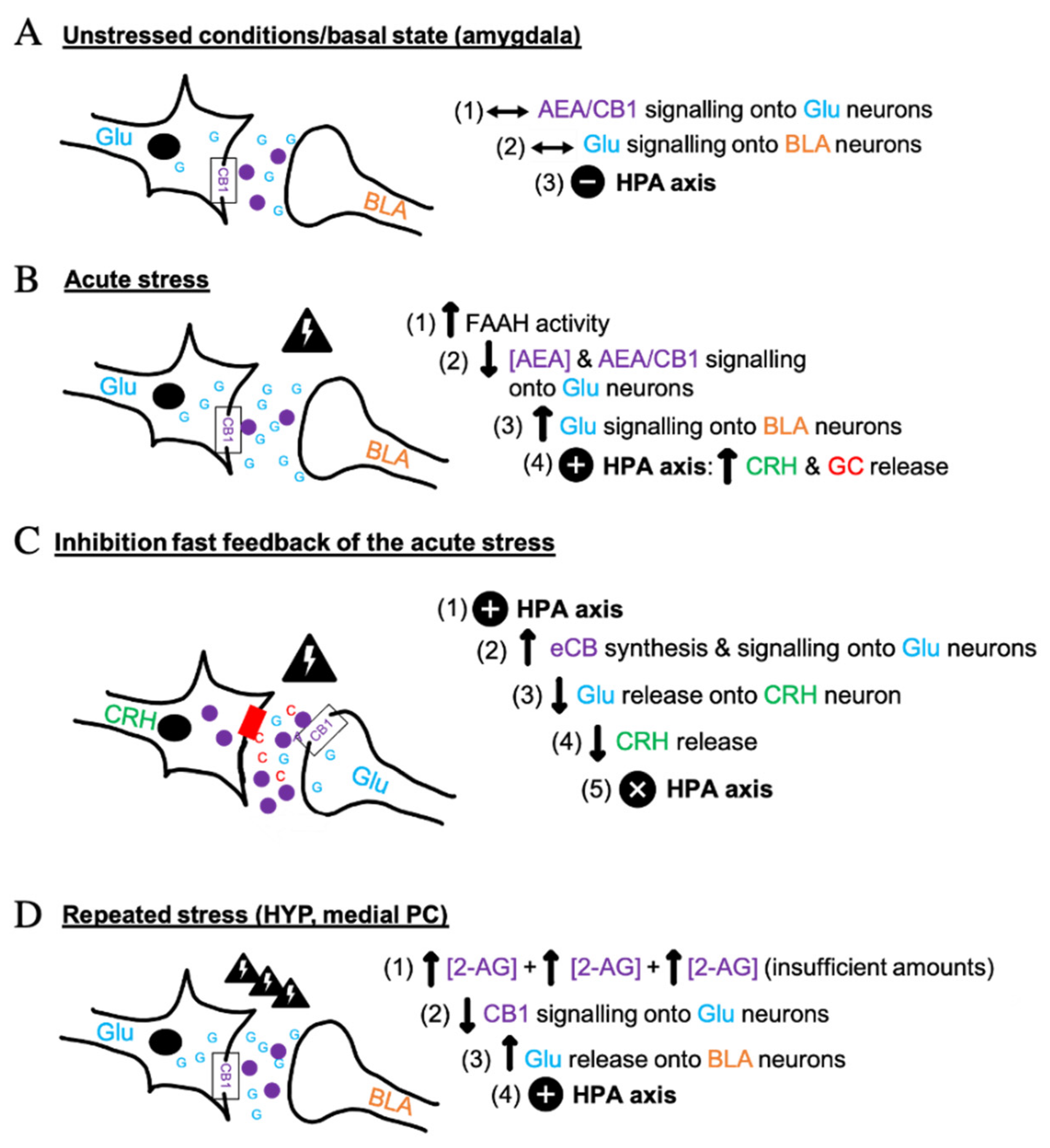
3. The Molecular and Synaptic Processes of ECS in Stress
4. Exercise and Endocannabinoids
5. Brain Endocannabinoidome Signaling
5.1. Hippocampal Endocannabinoidome
5.2. Hypothalamic Endocannabinoidome
6. Peripheral Endocannabinoidome
6.1. Mood, Depression, and Anxiety
6.2. Motivation
6.3. Inflammation
6.4. Nociception
6.5. Stress-Associated Comorbidities
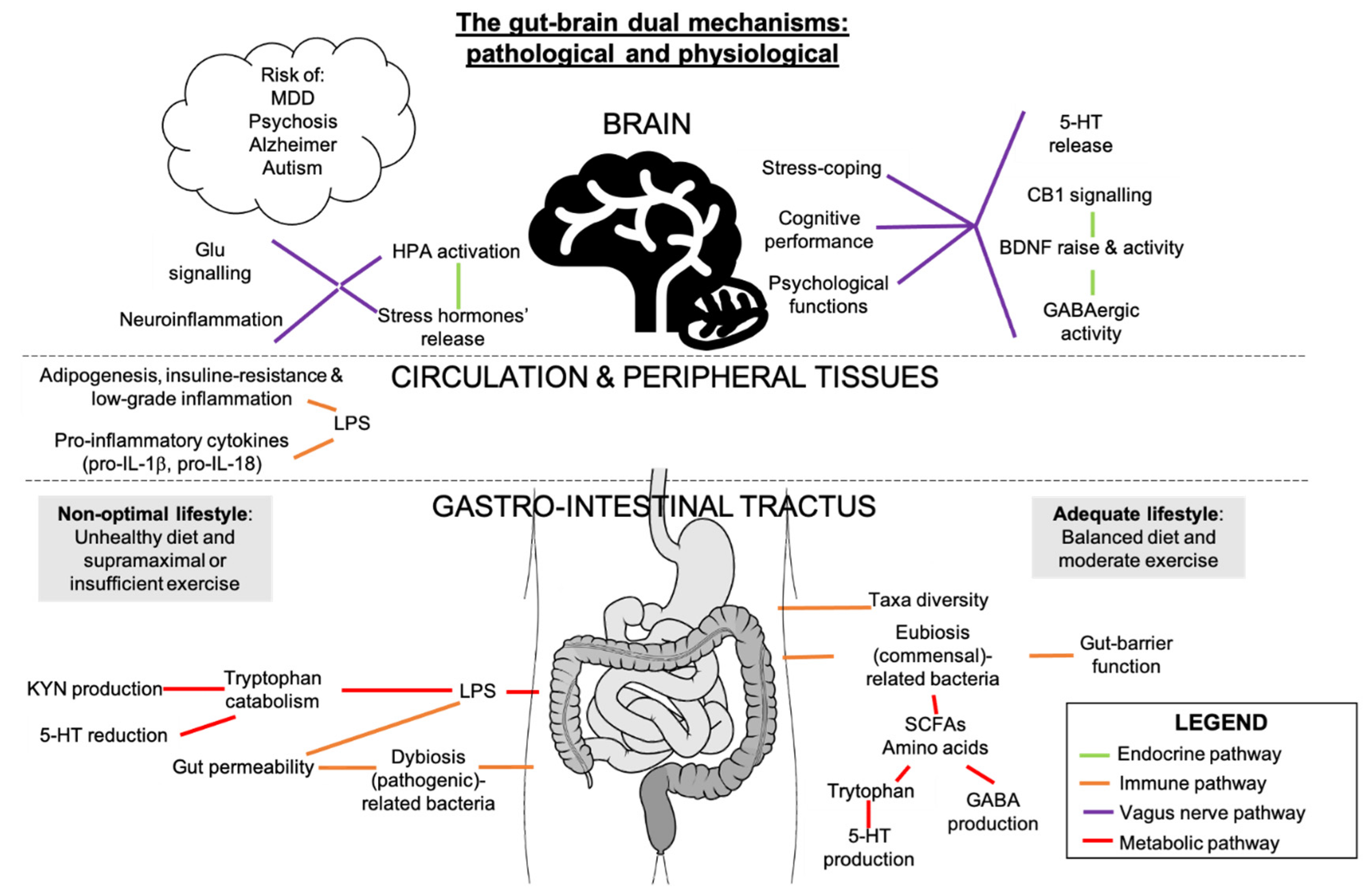
7. Potential Interactions between Gut Microbiota, Inflammation, and Endocannabinoidome in Mental Conditions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, M.N.; McEwen, B.S. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Patel, S.; Campolongo, P.; Tasker, J.G.; Wotjak, C.T.; Bains, J.S. Functional Interactions between Stress and the Endocannabinoid System: From Synaptic Signaling Behavioral Output. J. Neurosci. 2010, 30, 14980–14986. [Google Scholar] [CrossRef]
- Steiner, M.; Wotjak, C. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog. Brain Res. 2008, 170, 397–432. [Google Scholar]
- Feuerecker, M.; Hauer, D.; Toth, R.; Demetz, F.; Hölzl, J.; Thiel, M.; Kaufmann, I.; Schelling, G.; Choukèr, A. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur. J. Appl. Physiol. 2012, 112, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.N. Impairments in Endocannabinoid Signaling and Depressive Illness. JAMA 2009, 301, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Miller, G.E.; Carrier, E.J.; Gorzalka, B.B.; Hillard, C.J. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 2009, 34, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Titterness, A.K.; Morrish, A.C.; Carrier, E.J.; Lee, T.T.-Y.; Gil-Mohapel, J.; Gorzalka, B.B.; Hillard, C.J.; Christie, B.R. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 2010, 20, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkadhi, K.A. Exercise as a Positive Modulator of Brain Function. Mol. Neurobiol. 2018, 55, 3112–3130. [Google Scholar] [CrossRef]
- Maurus, I.; Hasan, A.; Röh, A.; Takahashi, S.; Rauchmann, B.; Keeser, D.; Malchow, B.; Schmitt, A.; Falkai, P. Neurobiological effects of aerobic exercise, with a focus on patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 499–515. [Google Scholar] [CrossRef]
- Dietrich, A. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Foster, A.D.; Gerdeman, G.L.; Seillier, A.; Giuffrida, A. Wired to run: Exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high”. J. Exp. Biol. 2012, 215, 1331–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koay, Y.C.; Stanton, K.; Kienzle, V.; Li, M.; Yang, J.; Celermajer, D.S.; O’Sullivan, J.F. Effect of chronic exercise in healthy young male adults: A metabolomic analysis. Cardiovasc. Res. 2021, 117, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Guo, R.; Liu, F.; Yuan, Q.; Yu, Y.; Ren, F. Gut Microbiota Regulates Depression-Like Behavior in Rats Through the Neuroendocrine-Immune-Mitochondrial Pathway. Neuropsychiatr. Dis. Treat. 2020, 16, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Tantimonaco, M.; Ceci, R.; Sabatini, S.; Catani, M.V.; Rossi, A.; Gasperi, V.; Maccarrone, M. Physical activity and the endocannabinoid system: An overview. Cell. Mol. Life Sci. 2014, 71, 2681–2698. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crombie, K.M.; Brellenthin, A.G.; Hillard, C.J.; Koltyn, K.F. Psychobiological Responses to Aerobic Exercise in Individuals With Posttraumatic Stress Disorder: Psychobiological Responses to Exercise in PTSD. J. Trauma. Stress 2018, 31, 134–145. [Google Scholar] [CrossRef]
- Watkins, B.A. Diet, endocannabinoids, and health. Nutr. Res. 2019, 70, 32–39. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Azua, I.; Lutz, B. Multiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissues. Cell. Mol. Life Sci. 2019, 76, 1341–1363. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015, 16, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Di Marzo, V. Endocannabinoid signaling in the brain: Biosynthetic mechanisms in the limelight. Nat. Neurosci. 2011, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef]
- Di Marzo, V.; Silvestri, C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Wang, J. (Eds.) The Endocannabinoidome, 1st ed.; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-420126-2. [Google Scholar]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef]
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules 2014, 19, 17078–17106. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 Activation Improves Exercise Endurance and Energy Metabolism through PGC-1α Upregulation in Mice. Cell Res. 2012, 22, 551–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorzalka, B.B.; Hill, M.N.; Hillard, C.J. Regulation of endocannabinoid signaling by stress: Implications for stress-related affective disorders. Neurosci. Biobehav. Rev. 2008, 32, 1152–1160. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Hohmann, A.G.; Suplita, R.L.; Bolton, N.M.; Neely, M.H.; Fegley, D.; Mangieri, R.; Krey, J.F.; Michael Walker, J.; Holmes, P.V.; Crystal, J.D.; et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 2005, 435, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Rada, P.; Pieruzzini, P.R.; Hsueh, B.; Gould, E. Physical Exercise Prevents Stress-Induced Activation of Granule Neurons and Enhances Local Inhibitory Mechanisms in the Dentate Gyrus. J. Neurosci. 2013, 33, 7770–7777. [Google Scholar] [CrossRef]
- Gunduz-Cinar, O.; Hill, M.N.; McEwen, B.S.; Holmes, A. Amygdala FAAH and anandamide: Mediating protection and recovery from stress. Trends Pharmacol. Sci. 2013, 34, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Patel, S.; Carrier, E.J.; Rademacher, D.J.; Ormerod, B.K.; Hillard, C.J.; Gorzalka, B.B. Downregulation of Endocannabinoid Signaling in the Hippocampus Following Chronic Unpredictable Stress. Neuropsychopharmacology 2005, 30, 508–515. [Google Scholar] [CrossRef]
- Patel, S.; Roelke, C.T.; Rademacher, D.J.; Cullinan, W.E.; Hillard, C.J. Endocannabinoid Signaling Negatively Modulates Stress-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis. Endocrinology 2004, 145, 5431–5438. [Google Scholar] [CrossRef]
- Hill, M.N.; McLaughlin, R.J.; Pan, B.; Fitzgerald, M.L.; Roberts, C.J.; Lee, T.T.-Y.; Karatsoreos, I.N.; Mackie, K.; Viau, V.; Pickel, V.M.; et al. Recruitment of Prefrontal Cortical Endocannabinoid Signaling by Glucocorticoids Contributes to Termination of the Stress Response. J. Neurosci. 2011, 31, 10506–10515. [Google Scholar] [CrossRef]
- Hill, M.N.; Carrier, E.J.; McLaughlin, R.J.; Morrish, A.C.; Meier, S.E.; Hillard, C.J.; Gorzalka, B.B. Regional alterations in the endocannabinoid system in an animal model of depression: Effects of concurrent antidepressant treatment. J. Neurochem. 2008, 106, 2322–2336. [Google Scholar] [CrossRef] [Green Version]
- McPartland, J.M.; Guy, G.W.; Di Marzo, V. Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System. PLoS ONE 2014, 9, e89566. [Google Scholar] [CrossRef] [PubMed]
- Thompson, Z.; Argueta, D.; Garland, T.; DiPatrizio, N. Circulating levels of endocannabinoids respond acutely to voluntary exercise, are altered in mice selectively bred for high voluntary wheel running, and differ between the sexes. Physiol. Behav. 2017, 170, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. Neuroreport 2003, 14, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the Effects of Exercise, Training and Gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H. Neurotrophins and Neuronal Plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Heyman, E.; Gamelin, F.-X.; Goekint, M.; Piscitelli, F.; Roelands, B.; Leclair, E.; Di Marzo, V.; Meeusen, R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—Possible implications for reward and depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Duman, R.S. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef]
- Ferreira, F.F.; Ribeiro, F.F.; Rodrigues, R.S.; Sebastião, A.M.; Xapelli, S. Brain-Derived Neurotrophic Factor (BDNF) Role in Cannabinoid-Mediated Neurogenesis. Front. Cell. Neurosci. 2018, 12, 441. [Google Scholar] [CrossRef] [Green Version]
- Maison, P.; Walker, D.J.; Walsh, F.S.; Williams, G.; Doherty, P. BDNF regulates neuronal sensitivity to endocannabinoids. Neurosci. Lett. 2009, 467, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review: Physical activity and BDNF. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Rossi, S.; De Chiara, V.; Musella, A.; Kusayanagi, H.; Mataluni, G.; Bernardi, G.; Usiello, A.; Centonze, D. Chronic Psychoemotional Stress Impairs Cannabinoid-Receptor-Mediated Control of GABA Transmission in the Striatum. J. Neurosci. 2008, 28, 7284–7292. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The Role of the Dorsal Striatum in Reward and Decision-Making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meadows, A.; Lee, J.H.; Wu, C.-S.; Wei, Q.; Pradhan, G.; Yafi, M.; Lu, H.-C.; Sun, Y. Deletion of G-protein-coupled receptor 55 promotes obesity by reducing physical activity. Int. J. Obes. 2016, 40, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yang, L.; Shi, W.; Wang, L.; Zhou, S.; Guan, S.; Zhao, M.; Yang, Q. The novel cannabinoid receptor GPR55 mediates anxiolytic-like effects in the medial orbital cortex of mice with acute stress. Mol. Brain 2017, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Ghafouri, N.; Ghafouri, B.; Fowler, C.J.; Larsson, B.; Turkina, M.V.; Karlsson, L.; Gerdle, B. Effects of two different specific neck exercise interventions on palmitoylethanolamide and stearoylethanolamide concentrations in the interstitium of the trapezius muscle in women with chronic neck shoulder pain. Pain Med. 2014, 15, 1379–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallard, A.; Briskey, D.; Richards, A.; Mills, D.; Rao, A. The Effect of Orally Dosed Levagen+TM (palmitoylethanolamide) on Exercise Recovery in Healthy Males-A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients 2020, 12, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyman, E.; Gamelin, F.-X.; Aucouturier, J.; Di Marzo, V. The role of the endocannabinoid system in skeletal muscle and metabolic adaptations to exercise: Potential implications for the treatment of obesity: Exercise and the endocannabinoid system. Obes. Rev. 2012, 13, 1110–1124. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Li, Q.; Dong, X.; Hu, H.; Hu, R.; Ye, H.; Wu, Y.; Hu, R.; Li, Y. Exercise improved rat metabolism by raising PPAR-α. Int. J. Sports Med. 2011, 32, 568–573. [Google Scholar] [CrossRef]
- GTEx Consortium The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [CrossRef]
- GTEx Consortium Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [Green Version]
- Dubreucq, S.; Koehl, M.; Abrous, D.N.; Marsicano, G.; Chaouloff, F. CB1 receptor deficiency decreases wheel-running activity: Consequences on emotional behaviours and hippocampal neurogenesis. Exp. Neurol. 2010, 224, 106–113. [Google Scholar] [CrossRef]
- Gamelin, F.-X.; Aucouturier, J.; Iannotti, F.A.; Piscitelli, F.; Mazzarella, E.; Aveta, T.; Leriche, M.; Dupont, E.; Cieniewski-Bernard, C.; Leclair, E.; et al. Exercise training and high-fat diet elicit endocannabinoid system modifications in the rat hypothalamus and hippocampus. J. Physiol. Biochem. 2016, 73, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13105–13108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Vieira, T.H.; Bastos, C.P.; Pereira, G.S.; Moreira, F.A.; Massensini, A.R. A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice: Endocannabinoid System Mediates Promnesic Effect of Exercise. Hippocampus 2014, 24, 79–88. [Google Scholar] [CrossRef]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef] [PubMed]
- Cota, D. CB1 receptors: Emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health. Diabetes/Metab. Res. Rev. 2007, 23, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Mock, E.D.; Mustafa, M.; Gunduz-Cinar, O.; Cinar, R.; Petrie, G.N.; Kantae, V.; Di, X.; Ogasawara, D.; Varga, Z.V.; Paloczi, J.; et al. Discovery of a NAPE-PLD inhibitor that modulates emotional behavior in mice. Nat. Chem. Biol. 2020, 16, 667–675. [Google Scholar] [CrossRef]
- Marsicano, G.; Bisogno, T.; Hermann, H.; Tang, J.; Hofmann, C. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef]
- Edwards, A.; Abizaid, A. Driving the need to feed: Insight into the collaborative interaction between ghrelin and endocannabinoid systems in modulating brain reward systems. Neurosci. Biobehav. Rev. 2016, 66, 33–53. [Google Scholar] [CrossRef]
- King-Himmelreich, T.S.; Möser, C.V.; Wolters, M.C.; Schmetzer, J.; Schreiber, Y.; Ferreirós, N.; Russe, O.Q.; Geisslinger, G.; Niederberger, E. AMPK contributes to aerobic exercise-induced antinociception downstream of endocannabinoids. Neuropharmacology 2017, 124, 134–142. [Google Scholar] [CrossRef]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Fatty Acid Modulation of the Endocannabinoid System and the Effect on Food Intake and Metabolism. Int. J. Endocrinol. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.D.; Crombie, K.M.; Cook, D.B.; Hillard, C.J.; Koltyn, K.F. Serum Endocannabinoid and Mood Changes after Exercise in Major Depressive Disorder. Med. Sci. Sports Exerc. 2019, 51, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Brellenthin, A.G.; Crombie, K.M.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and Mood Responses to Exercise in Adults with Varying Activity Levels. Med. Sci. Sports Exerc. 2017, 49, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; Millar, S.A.; Herrod, P.J.J.; Barrett, D.A.; Ortori, C.A.; Mellon, V.A.; O’Sullivan, S.E. An Analysis of Endocannabinoid Concentrations and Mood Following Singing and Exercise in Healthy Volunteers. Front. Behav. Neurosci. 2018, 12, 269. [Google Scholar] [CrossRef]
- Jaromin, E.; Sadowska, E.T.; Koteja, P. Is Experimental Evolution of an Increased Aerobic Exercise Performance in Bank Voles Mediated by Endocannabinoid Signaling Pathway? Front. Physiol. 2019, 10, 640. [Google Scholar] [CrossRef]
- Fernández-Aranda, F.; Sauchelli, S.; Pastor, A.; Gonzalez, M.L.; de la Torre, R.; Granero, R.; Jiménez-Murcia, S.; Baños, R.; Botella, C.; Fernández-Real, J.M.; et al. Moderate-Vigorous Physical Activity across Body Mass Index in Females: Moderating Effect of Endocannabinoids and Temperament. PLoS ONE 2014, 9, e104534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: A review. J. Adv. Res. 2018, 11, 43–55. [Google Scholar] [CrossRef]
- Grunewald, Z.I.; Lee, S.; Kirkland, R.; Ross, M.; de La Serre, C.B. Cannabinoid receptor type-1 partially mediates metabolic endotoxemia-induced inflammation and insulin resistance. Physiol. Behav. 2019, 199, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Galdino, G.; Romero, T.; da Silva, J.F.P.; Aguiar, D.; de Paula, A.M.; Cruz, J.; Parrella, C.; Piscitelli, F.; Duarte, I.; Di Marzo, V.; et al. Acute Resistance Exercise Induces Antinociception by Activation of the Endocannabinoid System in Rats. Anesth. Analg. 2014, 119, 702–715. [Google Scholar] [CrossRef] [Green Version]
- Galdino, G.; Romero, T.R.L.; Silva, J.F.P.; Aguiar, D.C.; de Paula, A.M.; Cruz, J.S.; Parrella, C.; Piscitelli, F.; Duarte, I.D.; Di Marzo, V.; et al. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology 2014, 77, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ludtke, D.D.; Siteneski, A.; Galassi, T.d.O.; Buffon, A.C.; Cidral-Filho, F.J.; Reed, W.R.; Salgado, A.S.I.; Santos, A.R.S.; Martins, D.F. High-intensity swimming exercise reduces inflammatory pain in mice by activation of the endocannabinoid system. Scand. J. Med. Sci. Sports 2020, 30, 1369–1378. [Google Scholar] [CrossRef]
- Stensson, N.; Grimby-Ekman, A. Altered relationship between anandamide and glutamate in circulation after 30 min of arm cycling: A comparison of chronic pain subject with healthy controls. Mol. Pain 2019, 15, 1744806919898360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Wilker, S.; Pfeiffer, A.; Elbert, T.; Ovuga, E.; Karabatsiakis, A.; Krumbholz, A.; Thieme, D.; Schelling, G.; Kolassa, I.-T. Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology 2016, 67, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.M.; Huang, S.M. Cannabinoid analgesia. Pharmacol. Ther. 2002, 95, 127–135. [Google Scholar] [CrossRef]
- Roman, P.; Estévez, A.F.; Miras, A.; Sánchez-Labraca, N.; Cañadas, F.; Vivas, A.B.; Cardona, D. A Pilot Randomized Controlled Trial to Explore Cognitive and Emotional Effects of Probiotics in Fibromyalgia. Sci. Rep. 2018, 8, 10965. [Google Scholar] [CrossRef]
- Järbrink-Sehgal, E.; Andreasson, A. The gut microbiota and mental health in adults. Curr. Opin. Neurobiol. 2020, 62, 102–114. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Ahmad, M.I.; Hussain, M.; Khan, I.A.; Zhao, D.; Li, C. Meat Protein in High-Fat Diet Induces Adipogensis and Dyslipidemia by Altering Gut Microbiota and Endocannabinoid Dysregulation in the Adipose Tissue of Mice. J. Agric. Food Chem. 2020, 68, 3933–3946. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Shen, M.; Boubertakh, B.; Martin, C.; Flamand, N.; Silvestri, C.; Di Marzo, V. Alterations of brain endocannabinoidome signaling in germ-free mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158786. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Boubertakh, B.; Leblanc, N.; Deschênes, T.; Lacroix, S.; Martin, C.; Houde, A.; Veilleux, A.; Flamand, N.; Muccioli, G.G.; et al. Germ-free mice exhibit profound gut microbiota-dependent alterations of intestinal endocannabinoidome signaling. J. Lipid Res. 2020, 61, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Pechereau, F.; Leblanc, N.; Boubertakh, B.; Houde, A.; Martin, C.; Flamand, N.; Silvestri, C.; Raymond, F.; Di Marzo, V.; et al. Rapid and Concomitant Gut Microbiota and Endocannabinoidome Response to Diet-Induced Obesity in Mice. mSystems 2019, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castonguay-Paradis, S.; Lacroix, S.; Rochefort, G.; Parent, L.; Perron, J.; Martin, C.; Lamarche, B.; Raymond, F.; Flamand, N.; Di Marzo, V.; et al. Dietary fatty acid intake and gut microbiota determine circulating endocannabinoidome signaling beyond the effect of body fat. Sci. Rep. 2020, 10, 15975. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegh; Geerlings; Knol; Roeselers; Belzer Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [CrossRef] [Green Version]
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. The role of the gut microbiome in the development of schizophrenia. Schizophr. Res. 2020, in press. [Google Scholar] [CrossRef]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Pedersini, P.; Turroni, S.; Villafañe, J.H. Gut microbiota and physical activity: Is there an evidence-based link? Sci. Total Environ. 2020, 727, 138648. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Nicoletti, F.; Mango, D.; Saidi, A.; Orlando, R.; Scaccianoce, S. Stress as risk factor for Alzheimer’s disease. Pharmacol. Res. 2018, 132, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; You, X.; Wang, C.; Li, X.; Sheng, Y.; Zhuang, P.; Zhang, Y. Bidirectional Brain-gut-microbiota Axis in increased intestinal permeability induced by central nervous system injury. CNS Neurosci. Ther. 2020, 26, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G559–G571. [Google Scholar] [CrossRef] [Green Version]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51–52, 80–85. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forteza, F.; Giorgini, G.; Raymond, F. Neurobiological Processes Induced by Aerobic Exercise through the Endocannabinoidome. Cells 2021, 10, 938. https://doi.org/10.3390/cells10040938
Forteza F, Giorgini G, Raymond F. Neurobiological Processes Induced by Aerobic Exercise through the Endocannabinoidome. Cells. 2021; 10(4):938. https://doi.org/10.3390/cells10040938
Chicago/Turabian StyleForteza, Fabiola, Giada Giorgini, and Frédéric Raymond. 2021. "Neurobiological Processes Induced by Aerobic Exercise through the Endocannabinoidome" Cells 10, no. 4: 938. https://doi.org/10.3390/cells10040938
APA StyleForteza, F., Giorgini, G., & Raymond, F. (2021). Neurobiological Processes Induced by Aerobic Exercise through the Endocannabinoidome. Cells, 10(4), 938. https://doi.org/10.3390/cells10040938





