Proline Concentration and Its Metabolism Are Regulated in a Leaf Age Dependent Manner But Not by Abscisic Acid in Pea Plants Exposed to Cadmium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Total RNA Extraction and cDNA Synthesis
2.3. Identification of Nucleotide Sequences of Genes Involved in Proline Metabolism and Transport
2.4. Gene Expression Analysis
2.5. Determination of ABA and Proline Concentrations
2.6. Determination of Chlorophyll and MDA Concentrations
2.7. Statistical Analysis
3. Results
3.1. Identification of Nucleotide Sequences of Genes Involved in the Metabolism and Transport of Proline
3.2. MDA and Chlorophyll Concentration as Affected by Cadmium Stress
3.3. Proline Concentration and its Metabolism as Affected by Cadmium Stress
3.4. ABA Concentration as Affected by Cadmium Stress
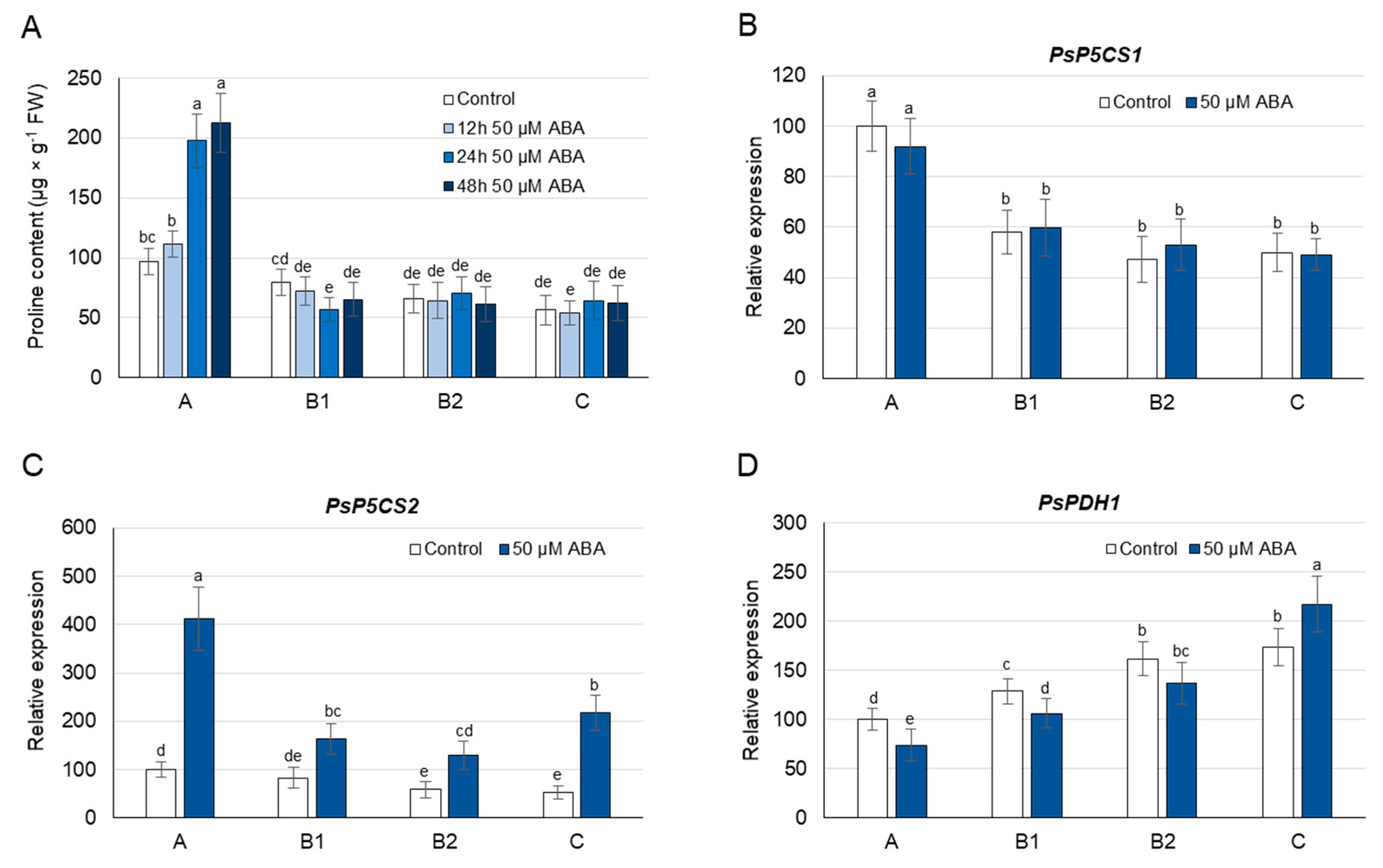
3.5. Proline Concentration and Its Metabolism as Affected by Exogenous ABA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colzi, I.; Pignattelli, S.; Giorni, E.; Papini, A.; Gonnelli, C. Linking root traits to copper exclusion mechanisms in Silene paradoxa L. (Caryophyllaceae). Plant Soil 2015, 390, 1–15. [Google Scholar] [CrossRef]
- Sychta, K.; Słomka, A.; Kuta, E. Insights into plant programmed cell death induced by heavy metals—discovering a terra incognita. Cells 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant. Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef] [Green Version]
- Gouia, H.; Suzuki, A.; Brulfert, J.; Ghorbal, H. Effect of cadmium on the coordination of nitrogen and carbon metabolism in bean seedlings. Plant Physiol. 2004, 160, 367–376. [Google Scholar] [CrossRef]
- Cuypers, A.; Smeets, K.; Ruytinx, J.; Opdenakker, K.; Keunen, E.; Remans, T.; Horemans, N.; Vanhoudt, N.; Van Sanden, S.; Van Belleghem, F.; et al. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant Physiol. 2011, 168, 309–316. [Google Scholar] [CrossRef]
- Żabka, A.; Winnicki, K.; Polit, J.T.; Wróblewski, M.; Maszewski, J. Cadmium (II)-Induced oxidative stress results in replication stress and epigenetic modifications in root meristem cell nuclei of Vicia faba. Cells 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, R.; Bueno, M.; Tiburcio, A.T. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Kishor, P.B.K.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline protects plants against abiotic oxidative stress: Biochemical and molecular mechanisms. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Ahmad, P., Ed.; Elsevier Inc.: San Diego, CA, USA; London, UK; Waltham, MA, USA, 2014; pp. 477–522. [Google Scholar]
- Strizhov, N.; Ábrahám, E.; Ökrész, L.; Blickling, S.; Zilberstein, A.; Schell, J.; Koncz, C.; Szabados, L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997, 12, 557–569. [Google Scholar] [PubMed]
- Ábrahám, E.; Rigo, G.; Székely, G.; Nagy, R.; Koncz, C.; Szabados, L. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol. Biol. 2003, 51, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Fabro, G.; Kovács, I.; Pavet, V.; Szabados, L.; Alvarez, M.E. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant Microbe Interact. 2004, 17, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.J.; Li, J.R.; Qi, S.L.; Lin, Q.F.; Jin, J.B.; Hua, X.J. Light affects salt stress-induced transcriptional memory of P5CS1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, E8335–E8343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleksza, D.; Horváth, G.V.; Sándor, G.; Szabados, L. Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiol. 2017, 175, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Kovács, H.; Aleksza, D.; Baba, A.I.; Hajdu, A.; Király, A.M.; Zsigmond, L.; Tóth, S.Z.; Kozma-Bognár, L.; Szabados, L. Light control of salt-induced proline accumulation is mediated by ELONGATED HYPOCOTYL 5 in Arabidopsis. Front. Plant Sci. 2019, 10, 1584. [Google Scholar] [CrossRef]
- Székely, G.; Abrahám, E.; Cséplo, A.; Rigó, G.; Zsigmond, L.; Csiszár, J.; Ayaydin, F.; Strizhov, N.; Jásik, J.; Schmelzer, E.; et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008, 53, 11–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armengauda, P.; Thieryc, L.; Buhota, N.; March, G.G.; Savouré, A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol. Plant. 2004, 120, 420–450. [Google Scholar] [CrossRef]
- Funck, D.; Eckard, S.; Muller, G. Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis. BMC Plant Biol. 2010, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Stein, H.; Honig, A.; Kapulnik, Y.; Zilberstein, A. Responsive modes of Medicago sativa proline dehydrogenase genes during salt stress and recovery dictate free proline accumulation. Planta 2005, 222, 70–79. [Google Scholar] [CrossRef]
- Hanson, J.; Hanssen, M.; Wiese, A.; Hendriks, M.M.W.B.; Smeekens, S. The sucrose regulated transcription factor bZIP11 affects amino acid metabolism by regulating the expression of ASPARAGINE SYNTHETASE1 and PROLINE DEHYDROGENASE2. Plant J. 2008, 53, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Fujita, Y.; Nakashima, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. A novel subgroup of bZIP proteins functions as transcriptional activators in hypoosmolarity-responsive expression of the ProDH gene in Arabidopsis. Plant Cell Physiol. 2004, 45, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Nanjo, T.; Fujita, M.; Seki, M.; Kato, T.; Tabata, S.; Shinozaki, K. Toxicity of free proline revealed in an Arabidopsis T-DNA-tagged mutant deficient in proline dehydrogenase. Plant Cell Physiol. 2003, 44, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino acid transporters in plants: Identification and function. Plants 2020, 9, 972. [Google Scholar] [CrossRef]
- Grallath, S.; Weimar, T.; Meyer, A.; Gumy, C.; Suter-Grotemeyer, M.; Neuhaus, J.M.; Rentsch, D. The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 2005, 137, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Xue, D.; Zhang, W.; Zhao, J.-M.; Xue, C.-C.; Yan, Q.; Xue, J.-Y.; Wang, H.-T.; Zhang, Y.-M.; Xing, H. Overexpression of GmProT1 and GmProT2 increases tolerance to drought and salt stresses in transgenic Arabidopsis. J. Integr. Agric. 2016, 15, 1727–1743. [Google Scholar] [CrossRef]
- Zengin, F.K.; Munzuroglu, O. Toxic effects of cadmium (Cd++) on metabolism of sunflower (Helianthus annuus L.) seedlings. Acta Agric. Scand. B Soil Plant Sci. 2006, 56, 224–229. [Google Scholar]
- Talanova, V.V.; Titov, A.F.; Boeva, N.P. Effect of increasing concentrations of lead and cadmium on cucumber seedlings. Biol. Plant. 2000, 43, 441–444. [Google Scholar] [CrossRef]
- Zengin, F.K.; Munzuroglu, O. Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol. Crac. Ser. Bot. 2005, 47, 157–164. [Google Scholar]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Farooq, M.A.; Liu, D.; Daud, M.K.; Ali, S.; Zhou, W. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 2015, 10, e0123328. [Google Scholar] [CrossRef]
- Szalai, G.; Tajti, J.; Hamow, K.A.; Ildikó, D.; Khalil, R.; Vanková, R.; Dobrev, P.; Misheva, S.P.; Janda, T.; Pál, M. Molecular background of cadmium tolerance in Rht dwarf wheat mutant is related to a metabolic shift from proline and polyamine to phytochelatin synthesis. Environ. Sci. Pollut. Res. 2020, 27, 23664–23676. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, N.; Kojic, D.; Pilipovic, A.; Pajevic, S.; Krstic, B.; Borisev, M.; Orlovic, S. Responses of hybrid poplar to cadmium stress: Photosynthetic characteristics, cadmium and proline accumulation, and antioxidant enzyme activity. Acta Biol. Crac. Ser. Bot. 2008, 50, 95–103. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.-M.; Xi, H.-X.; Ai, K.-J.; Hou, H.-S. Overexpression of wheat TaNCED gene in Arabidopsis enhances tolerance to drought stress and delays seed germination. Biol. Plant. 2017, 61, 64–72. [Google Scholar] [CrossRef]
- Estrada-Melo, A.C.; Ma, C.; Reid, M.S.; Jiang, C.-Z. Overexpression of an ABA biosynthesis gene using a stress-inducible promoter enhances drought resistance in petunia. Hortic. Res. 2015, 2, 15013. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Wu, L.; Wu, M.; Zhu, C.; Jin, Q.; Zhang, J. Abscisic acid mediated proline biosynthesis and antioxidant ability in roots of two different rice genotypes under hypoxic stress. BMC Plant Biol. 2020, 20, 198. [Google Scholar] [CrossRef]
- Verslues, P.E.; Bray, E.A. Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J. Exp. Bot. 2006, 57, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Savouré, A.; Hua, X.J.; Bertauche, N.; Van Montagu, M.; Verbruggen, N. Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Mol. Gen. Genet. 1997, 254, 104–109. [Google Scholar] [CrossRef]
- Sharma, S.; Verslues, P.E. Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ. 2010, 33, 1838–1851. [Google Scholar] [CrossRef]
- McDonnell, E.M.; Coughlan, S.J.; Wyn Jones, R.G. Differential effects of abscisic acid on glycinebetaine and proline accumulation in three plant species. Z. Panzenphysiol. 1983, 109, 207–213. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Univ. Calif. Agric. Exp. Stn. Circ. 1938, 347, 1–39. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of total RNA isolation by a single extraction with an acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔ CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Die, J.V.; Román, B.; Nadal, S.; González-Verdejo, C.I. Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta 2010, 232, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Zdunek-Zastocka, E.; Grabowska, A. The interplay of PsABAUGT1 with other abscisic acid metabolic genes in the regulation of ABA homeostasis during the development of pea seeds and germination in the presence of H2O2. Plant Sci. 2019, 285, 79–90. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Verdoy, D.; De La Peña, T.C.; Redondo, F.J.; Lucas, M.M.; Pueyo, J.J. Transgenic Medicago truncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ. 2006, 29, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, I.; Stein, H.; Kapulnik, Y.; Szabados, L.; Strizhov, N.; Schell, J.; Koncz, C.; Zilberstein, A. Isolation and characterization of two different cDNAs of delta (1)-pyrroline-5-carboxylate synthase in alfalfa, transcriptionally induced upon salt stress. Plant Mol. Biol. 1998, 38, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Ribarits, A.; Abdullaev, A.; Tashpulatov, A.; Richter, A.; Heberle-Bors, E.; Touraev, A. Two tobacco proline dehydrogenases are differentially regulated and play a role in early plant development. Planta 2007, 225, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Yoshiba, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 1996, 8, 1323–1335. [Google Scholar]
- Zdunek-Zastocka, E.; Grabowska, A.; Branicki, T.; Michniewska, B. Biochemical characterization of the triticale TsPAP1, a new type of plant prolyl aminopeptidase, and its impact on proline content and flowering time in transgenic Arabidopsis plants. Plant Physiol. Biochem. 2017, 116, 18–26. [Google Scholar] [CrossRef]
- Dellero, Y.; Clouet, V.; Marnet, N.; Pellizzaro, A.; Dechaumet, S.; Niogret, M.-F.; Bouchereau, A. Leaf status and environmental signals jointly regulate proline metabolism in winter oilseed rape. J. Exp. Bot. 2020, 71, 2098–2111. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing salt tolerance of plants: From metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef] [PubMed]
- Naliwajski, M.; Skłodowska, M. The relationship between the antioxidant system and proline metabolism in the leaves of cucumber plants acclimated to salt stress. Cells 2021, 10, 609. [Google Scholar] [CrossRef]
- Gutsch, A.; Hendrix, S.; Guerriero, G.; Renaut, J.; Lutts, S.; Alseekh, S.; Fernie, A.R.; Hausman, J.-F.; Vangronsveld, J.; Ann Cuypers, A.; et al. Long-Term Cd exposure alters the metabolite profile in stem tissue of Medicago sativa. Cells 2020, 9, 2707. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.E.; Carpena-Ruiz, R.; Gárate, A. Alterations in the mineral nutrition of pea seedlings exposed to cadmium. J. Plant Nutr. 1996, 19, 1581–1598. [Google Scholar] [CrossRef]
- Hernández, L.E.; Gárate, A.; Carpena-Ruiz, R. Effects of cadmium on the uptake, distribution and assimilation of nitrate in Pisum sativum. Plant Soil 1997, 189, 97–106. [Google Scholar] [CrossRef]
- Neuberg, M.; Pavlikova, D.; Pavlik, M.; Balik, J. The effect of different nitrogen nutrition on proline and asparagine content in plant. Plant Soil Environ. 2010, 56, 305–311. [Google Scholar] [CrossRef]
- Walker, M.C.; van der Donk, W.A. The Many Roles of Glutamate in Metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabassa-Hourton, C.; Schertl, P.; Bordenave-Jacquemin, M.; Saadallah, K.; Guivarc’h, A.; Lebreton, S.; Planchais, S.; Klodmann, J.; Eubel, H.; Crilat, E.; et al. Proteomic and functional analysis of proline dehydrogenase 1 link proline catabolism to mitochondrial electron transport in Arabidopsis thaliana. Biochem. J. 2016, 473, 2623–2634. [Google Scholar] [CrossRef]
- Launay, A.; Cabassa-Hourton, C.; Eubel, H.; Maldiney, R.; Guivarc’h, A.; Crilat, E.; Planchais, S.; Lacoste, J.; Bordenave-Jacquemin, M.; Clément, G.; et al. Proline oxidation fuels mitochondrial respiration during dark-induced leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2019, 70, 6203–6214. [Google Scholar] [CrossRef] [Green Version]
- Stroiński, A.; Giżewska, K.; Zielezińska, M. Abscisic acid is required in transduction of cadmium signal to potato roots. Biol. Plant. 2013, 57, 121–127. [Google Scholar] [CrossRef]
- Yan, H.; Filardo, F.; Hu, X.; Zhao, X.; Fu, D. Cadmium stress alters the redox reaction and hormone balance in oilseed rape (Brassica napus L.) leaves. Environ. Sci. Pollut. Res. Int. 2016, 23, 3758–3769. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Song, J.; Yue, S.; Yang, H. Cd2+ uptake inhibited by MhNCED3 from Malus hupehensis alleviates Cd-induced cell death. Environ. Exp. Bot. 2019, 166, 103802. [Google Scholar] [CrossRef]
- Fediuc, E.; Lips, S.H.; Erdei, L. O-acetylserine (thiol) lyase activity in Phragmites and Typha plants under cadmium and NaCl stress conditions and the involvement of ABA in the stress response. J. Plant Physiol. 2005, 162, 865–872. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ. 2003, 26, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Pál, M.; Tajti, J.; Szalai, G.; Peeva, V.; Végh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef]
- Sripinyowanich, S.; Klomsakul, P.; Boonburapong, B.; Bangyeekhun, T.; Asami, T.; Gu, H.Y.; Buaboocha, T.; Chadchawan, S. Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): The role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ. Exp. Bot. 2013, 86, 94–105. [Google Scholar] [CrossRef]
- Xue, X.; Liu, A.; Hua, X. Proline accumulation and transcriptional regulation of proline biosynthesis and degradation in Brassica napus. BMB Rep. 2009, 42, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Satoh, R.; Kiyosue, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A gene encoding proline dehydrogenase is not only induced by proline and hypoosmolarity, but is also developmentally regulated in the reproductive organs of Arabidopsis. Plant Physiol. 1998, 118, 1233–1241. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdunek-Zastocka, E.; Grabowska, A.; Michniewska, B.; Orzechowski, S. Proline Concentration and Its Metabolism Are Regulated in a Leaf Age Dependent Manner But Not by Abscisic Acid in Pea Plants Exposed to Cadmium Stress. Cells 2021, 10, 946. https://doi.org/10.3390/cells10040946
Zdunek-Zastocka E, Grabowska A, Michniewska B, Orzechowski S. Proline Concentration and Its Metabolism Are Regulated in a Leaf Age Dependent Manner But Not by Abscisic Acid in Pea Plants Exposed to Cadmium Stress. Cells. 2021; 10(4):946. https://doi.org/10.3390/cells10040946
Chicago/Turabian StyleZdunek-Zastocka, Edyta, Agnieszka Grabowska, Beata Michniewska, and Sławomir Orzechowski. 2021. "Proline Concentration and Its Metabolism Are Regulated in a Leaf Age Dependent Manner But Not by Abscisic Acid in Pea Plants Exposed to Cadmium Stress" Cells 10, no. 4: 946. https://doi.org/10.3390/cells10040946
APA StyleZdunek-Zastocka, E., Grabowska, A., Michniewska, B., & Orzechowski, S. (2021). Proline Concentration and Its Metabolism Are Regulated in a Leaf Age Dependent Manner But Not by Abscisic Acid in Pea Plants Exposed to Cadmium Stress. Cells, 10(4), 946. https://doi.org/10.3390/cells10040946





