Pericoronary Adipose Tissue Attenuation Is Associated with High-Risk Plaque and Subsequent Acute Coronary Syndrome in Patients with Stable Coronary Artery Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Coronary CTA Protocol
2.3. Plaque Analysis
2.4. PCAT Analysis
2.5. Statistical Analysis
3. Results
3.1. Plaque and Vessel Characteristics
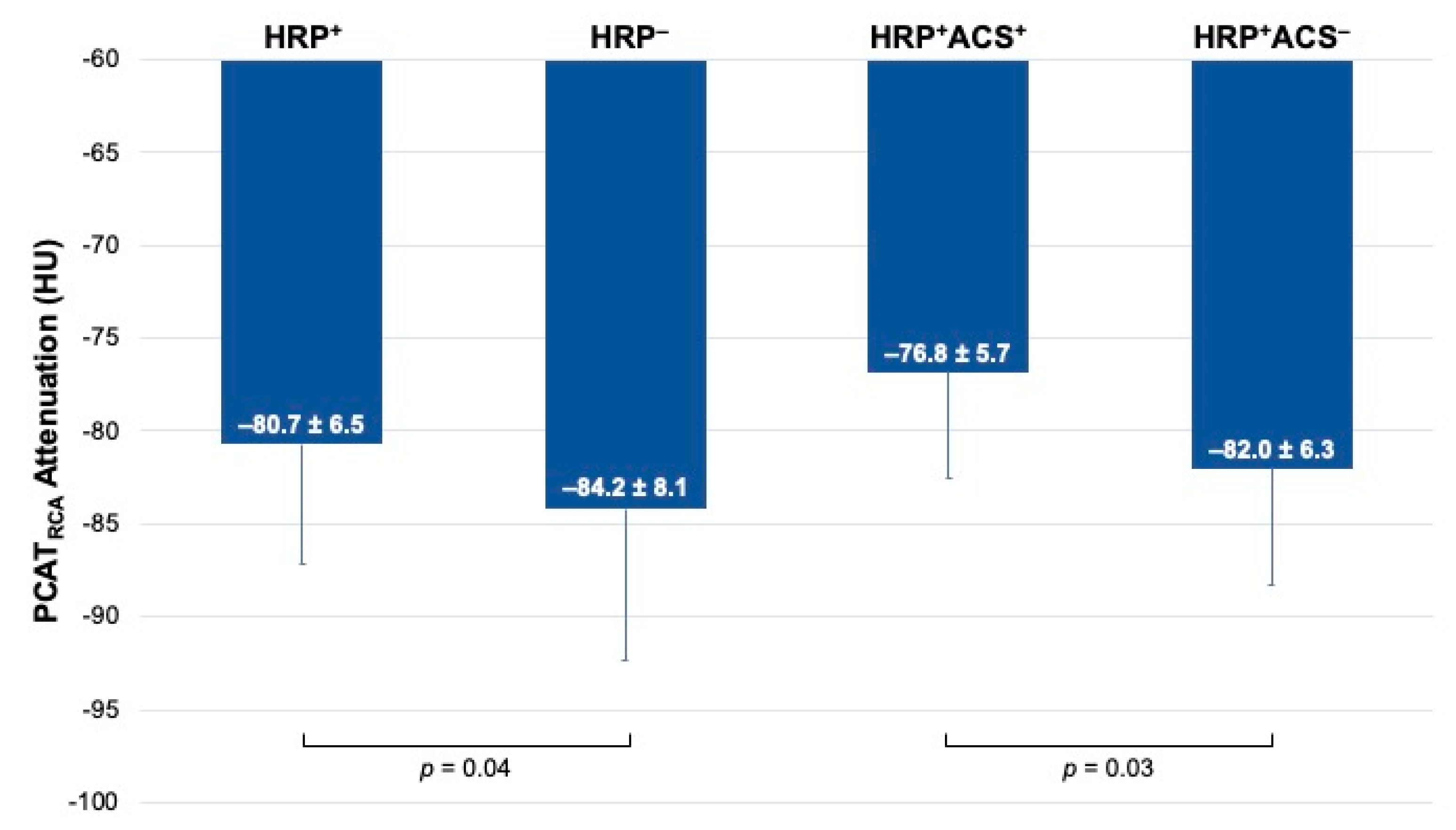
3.2. Relationship between PCAT and HRP
3.3. Relationship between PCAT and ACS
3.4. Sex-Specific Differences in PCAT
3.5. Multivariable Regression Analysis
3.6. Time-Dependent Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fishbein, M.C.; Siegel, R.J. How big are coronary atherosclerotic plaques that rupture? Circulation 1996, 94, 2662–2666. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Kondo, T.; Sarai, M.; Sugiura, A.; Harigaya, H.; Sato, T.; Inoue, K.; Okumura, M.; Ishii, J.; Anno, H.; et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 2007, 50, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Sarai, M.; Harigaya, H.; Anno, H.; Inoue, K.; Hara, T.; Naruse, H.; Ishii, J.; Hishida, H.; Wong, N.D.; et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 2009, 54, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nerlekar, N.; Ha, F.J.; Cheshire, C.; Rashid, H.; Cameron, J.D.; Wong, D.T.; Seneviratne, S.; Brown, A.J. Computed Tomographic Coronary Angiography-Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2018, 11, e006973. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Goeller, M.; Tamarappoo, B.K.; Kwan, A.C.; Cadet, S.; Commandeur, F.; Razipour, A.; Slomka, P.J.; Gransar, H.; Chen, X.; Otaki, Y.; et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2019. [Google Scholar] [CrossRef]
- Goeller, M.; Rahman Ihdayhid, A.; Cadet, S.; Lin, A.; Adams, D.; Thakur, U.; Yap, G.; Marwan, M.; Achenbach, S.; Dey, D.; et al. Pericoronary adipose tissue and quantitative global non-calcified plaque characteristics from CT angiography do not differ in matched South Asian, East Asian and European-origin Caucasian patients with stable chest pain. Eur. J. Radiol. 2020, 125, 108874. [Google Scholar] [CrossRef]
- Lin, A.; Nerlekar, N.; Yuvaraj, J.; Fernandes, K.; Jiang, C.; Dey, D.; Nicholls, S.J.; Wong, D.T.L. Pericoronary Adipose Tissue Computed Tomography Attenuation in Different Stages of Coronary Artery Disease: A Cross-Sectional Study. J. Am. Coll. Cardiol. 2020, 75, 1718. [Google Scholar] [CrossRef]
- Lin, A.; Nerlekar, N.; Munnur, R.K.; Kataoka, Y.; Andrews, J.; Dey, D.; Nicholls, S.J.; Wong, D.T.L. Cholesterol crystal-induced coronary inflammation: Insights from optical coherence tomography and pericoronary adipose tissue computed tomography attenuation. J. Cardiovasc. Comput. Tomogr. 2020, 14, 277–278. [Google Scholar] [CrossRef]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Marwan, M.; Hell, M.; Schuhback, A.; Gauss, S.; Bittner, D.; Pflederer, T.; Achenbach, S. CT Attenuation of Pericoronary Adipose Tissue in Normal Versus Atherosclerotic Coronary Segments as Defined by Intravascular Ultrasound. J. Comput. Assist. Tomogr. 2017, 41, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Dey, D.; Cadet, S.; Lee, S.E.; Otaki, Y.; Huynh, P.T.; Doris, M.K.; Eisenberg, E.; Yun, M.; Jansen, M.A.; et al. Peri-Coronary Adipose Tissue Density Is Associated With (18)F-Sodium Fluoride Coronary Uptake in Stable Patients with High-Risk Plaques. JACC Cardiovasc. Imaging 2019, 12, 2000–2010. [Google Scholar] [CrossRef]
- Wong, D.T.L.; Soh, S.Y.; Ko, B.S.; Cameron, J.D.; Crossett, M.; Nasis, A.; Troupis, J.; Meredith, I.T.; Seneviratne, S.K. Superior CT coronary angiography image quality at lower radiation exposure with second generation 320-detector row CT in patients with elevated heart rate: A comparison with first generation 320-detector row CT. Cardiovasc. Diagn. Ther. 2014, 4, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF. Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Glob. Heart 2012, 7, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef]
- Min, J.K.; Shaw, L.J.; Devereux, R.B.; Okin, P.M.; Weinsaft, J.W.; Russo, D.J.; Lippolis, N.J.; Berman, D.S.; Callister, T.Q. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J. Am. Coll. Cardiol. 2007, 50, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Kolossvary, M.; Szilveszter, B.; Edes, I.F.; Nardai, S.; Voros, V.; Hartyanszky, I.; Merkely, B.; Voros, S.; Maurovich-Horvat, P. Comparison of Quantity of Coronary Atherosclerotic Plaques Detected by Computed Tomography Versus Angiography. Am. J. Cardiol. 2016, 117, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Virmani, R.; Burke, A.P.; Farb, A.; Weber, D.K.; Kutys, R.; Finn, A.V.; Gold, H.K. Pathologic assessment of the vulnerable human coronary plaque. Heart 2004, 90, 1385–1391. [Google Scholar] [CrossRef]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Hedgire, S.; Baliyan, V.; Zucker, E.J.; Bittner, D.O.; Staziaki, P.V.; Takx, R.A.P.; Scholtz, J.E.; Meyersohn, N.; Hoffmann, U.; Ghoshhajra, B. Perivascular Epicardial Fat Stranding at Coronary CT Angiography: A Marker of Acute Plaque Rupture and Spontaneous Coronary Artery Dissection. Radiology 2018, 287, 808–815. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Martini, C.; Botti, A.; Pinazzi, A.; Bottazzi, B.; Palumbo, A.A. Coronary Inflammation by Computed Tomography Pericoronary Fat Attenuation in MINOCA and Tako-Tsubo Syndrome. J. Am. Heart Assoc. 2019, 8, e013235. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Desai, M.Y.; Marwan, M.; Kotanidis, C.P.; Antonopoulos, A.S.; Schottlander, D.; Channon, K.M.; Neubauer, S.; Achenbach, S.; Antoniades, C. Perivascular Fat Attenuation Index Stratifies Cardiac Risk Associated With High-Risk Plaques in the CRISP-CT Study. J. Am. Coll. Cardiol. 2020, 76, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, A.S.; Vroegindewey, M.M.; Kardys, I.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; van Geuns, R.J.; Regar, E.; Van Mieghem, N.M.; Ligthart, J.; Serruys, P.W.; et al. Prognostic Value of Intravascular Ultrasound in Patients With Coronary Artery Disease. J. Am. Coll. Cardiol. 2018, 72, 2003–2011. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kanaji, Y.; Hoshino, M.; Yamaguchi, M.; Hada, M.; Ohya, H.; Sumino, Y.; Hirano, H.; Kanno, Y.; Horie, T.; et al. Determinants of Pericoronary Adipose Tissue Attenuation on Computed Tomography Angiography in Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e016202. [Google Scholar] [CrossRef] [PubMed]
- Plank, F.; Beyer, C.; Friedrich, G.; Wildauer, M.; Feuchtner, G. Sex differences in coronary artery plaque composition detected by coronary computed tomography: Quantitative and qualitative analysis. Neth. Heart J. 2019, 27, 272–280. [Google Scholar] [CrossRef]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 144–152. [Google Scholar] [CrossRef]
- Lansky, A.J.; Ng, V.G.; Maehara, A.; Weisz, G.; Lerman, A.; Mintz, G.S.; De Bruyne, B.; Farhat, N.; Niess, G.; Jankovic, I.; et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc. Imaging 2012, 5, S62–S72. [Google Scholar] [CrossRef] [PubMed]
- Hell, M.M.; Achenbach, S.; Schuhbaeck, A.; Klinghammer, L.; May, M.S.; Marwan, M. CT-based analysis of pericoronary adipose tissue density: Relation to cardiovascular risk factors and epicardial adipose tissue volume. J. Cardiovasc. Comput. Tomogr. 2016, 10, 52–60. [Google Scholar] [CrossRef] [PubMed]




| HRP (n = 41) | No HRP (n = 41) | p-Value | |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Age, years | 59.8 (1.45) | 59.2 (1.60) | 0.77 |
| Male sex | 27 (65.9) | 27 (65.9) | 1.0 |
| Hypertension | 16 (39.0) | 15 (36.6) | 0.82 |
| Hypercholesterolaemia | 22 (53.7) | 16 (39.0) | 0.18 |
| Diabetes mellitus | 6 (14.6) | 3 (7.3) | 0.48 |
| Smoker | 7 (17.1) | 4 (9.8) | 0.52 |
| Family history of IHD | 22 (53.7) | 22 (53.7) | 1.0 |
| Obesity | 3 (7.30) | 2 (4.90) | 1.0 |
| ACS | 10 (24.4) | 3 (7.30) | 0.07 |
| Vessel localisation | |||
| LAD | 26 (63.4) | 34 (82.9) | |
| LCx | 4 (9.76) | 2 (4.88) | |
| RCA | 11 (26.8) | 5 (12.2) | |
| Plaque characteristics | |||
| PR | 41 (100) | - | |
| LAP | 24 (58.5) | - | |
| SC | 22 (53.7) | - | |
| Remodelling index * | 1.5 (1.31, 1.73) | - | |
| Obstructive CAD | 15 (36.6) | 1 (2.44) | <0.001 |
| Total plaque volume | 55.6 (6.93) | - | |
| Total plaque burden | 152 (64.6) | - | |
| CCTA segment scores * | |||
| Segment involvement score | 4.5 (3, 6) | 1.0 (0, 4.5) | <0.001 |
| Segment stenosis score | 5.5 (3, 9.5) | 2.0 (0, 6) | 0.001 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | Beta | 95%CI | p-value | Beta | 95%CI | p-value |
| Age | −0.032 | −0.204, 0.140 | 0.712 | |||
| Male sex | 5.631 | 2.367, 8.895 | 0.001 | 5.085 | 1.986, 8.814 | 0.002 |
| Presence of HRP | 3.437 | 0.210, 6.663 | 0.037 | 3.017 | 0.087, 5.947 | 0.044 |
| Presentation with ACS | 5.238 | 0.851, 9.626 | 0.020 | 0.156 | −0.970, 7.338 | 0.131 |
| Hypertension | 0.469 | −2.948, 3.887 | 0.785 | |||
| Hypercholesterolaemia | 1.232 | −2.082, 4.545 | 0.462 | |||
| Diabetes | −0.174 | −5.478, 5.130 | 0.948 | |||
| Smoker | 7.146 | 2.548, 11.744 | 0.003 | 5.733 | 1.396, 10.070 | 0.010 |
| Family history of IHD | 1.366 | −1.945, 4.677 | 0.414 | |||
| Obesity | −2.505 | −9.412, 4.401 | 0.472 | |||
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95%CI | p-value | OR | 95%CI | p-value |
| Age | 1.007 | 0.963, 1.053 | 0.766 | |||
| Male sex | 1.0 | 0.401, 2.491 | 1.0 | |||
| PCATRCA | 1.066 | 1.003, 1.134 | 0.041 | 1.064 | 1.000, 1.132 | 0.049 |
| Hypertension | 1.109 | 0.454, 2.710 | 0.820 | |||
| Hypercholesterolaemia | 1.809 | 0.752, 4.352 | 0.186 | 1.723 | 0.701, 4.238 | 0.236 |
| Diabetes | 2.171 | 0.504, 9.350 | 0.298 | |||
| Smoker | 1.904 | 0.512, 7.085 | 0.337 | |||
| Family history of IHD | 1.0 | 0.420, 2.382 | 1.0 | |||
| Obesity | 1.539 | 0.243, 9.733 | 0.647 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuvaraj, J.; Lin, A.; Nerlekar, N.; Munnur, R.K.; Cameron, J.D.; Dey, D.; Nicholls, S.J.; Wong, D.T.L. Pericoronary Adipose Tissue Attenuation Is Associated with High-Risk Plaque and Subsequent Acute Coronary Syndrome in Patients with Stable Coronary Artery Disease. Cells 2021, 10, 1143. https://doi.org/10.3390/cells10051143
Yuvaraj J, Lin A, Nerlekar N, Munnur RK, Cameron JD, Dey D, Nicholls SJ, Wong DTL. Pericoronary Adipose Tissue Attenuation Is Associated with High-Risk Plaque and Subsequent Acute Coronary Syndrome in Patients with Stable Coronary Artery Disease. Cells. 2021; 10(5):1143. https://doi.org/10.3390/cells10051143
Chicago/Turabian StyleYuvaraj, Jeremy, Andrew Lin, Nitesh Nerlekar, Ravi K. Munnur, James D. Cameron, Damini Dey, Stephen J. Nicholls, and Dennis T. L. Wong. 2021. "Pericoronary Adipose Tissue Attenuation Is Associated with High-Risk Plaque and Subsequent Acute Coronary Syndrome in Patients with Stable Coronary Artery Disease" Cells 10, no. 5: 1143. https://doi.org/10.3390/cells10051143
APA StyleYuvaraj, J., Lin, A., Nerlekar, N., Munnur, R. K., Cameron, J. D., Dey, D., Nicholls, S. J., & Wong, D. T. L. (2021). Pericoronary Adipose Tissue Attenuation Is Associated with High-Risk Plaque and Subsequent Acute Coronary Syndrome in Patients with Stable Coronary Artery Disease. Cells, 10(5), 1143. https://doi.org/10.3390/cells10051143






