Transient Receptor Potential Channel Ankyrin 1: A Unique Regulator of Vascular Function
Abstract
1. A Brief Introduction to TRP Channels
2. The Mammalian TRPA1 Channel: One of a Kind
3. Pharmacology of TRPA1 Channels
3.1. Activators
3.2. Blockers
3.3. Endogenous Regulators
4. Regulation of Vascular Tone by TRPA1 Channels
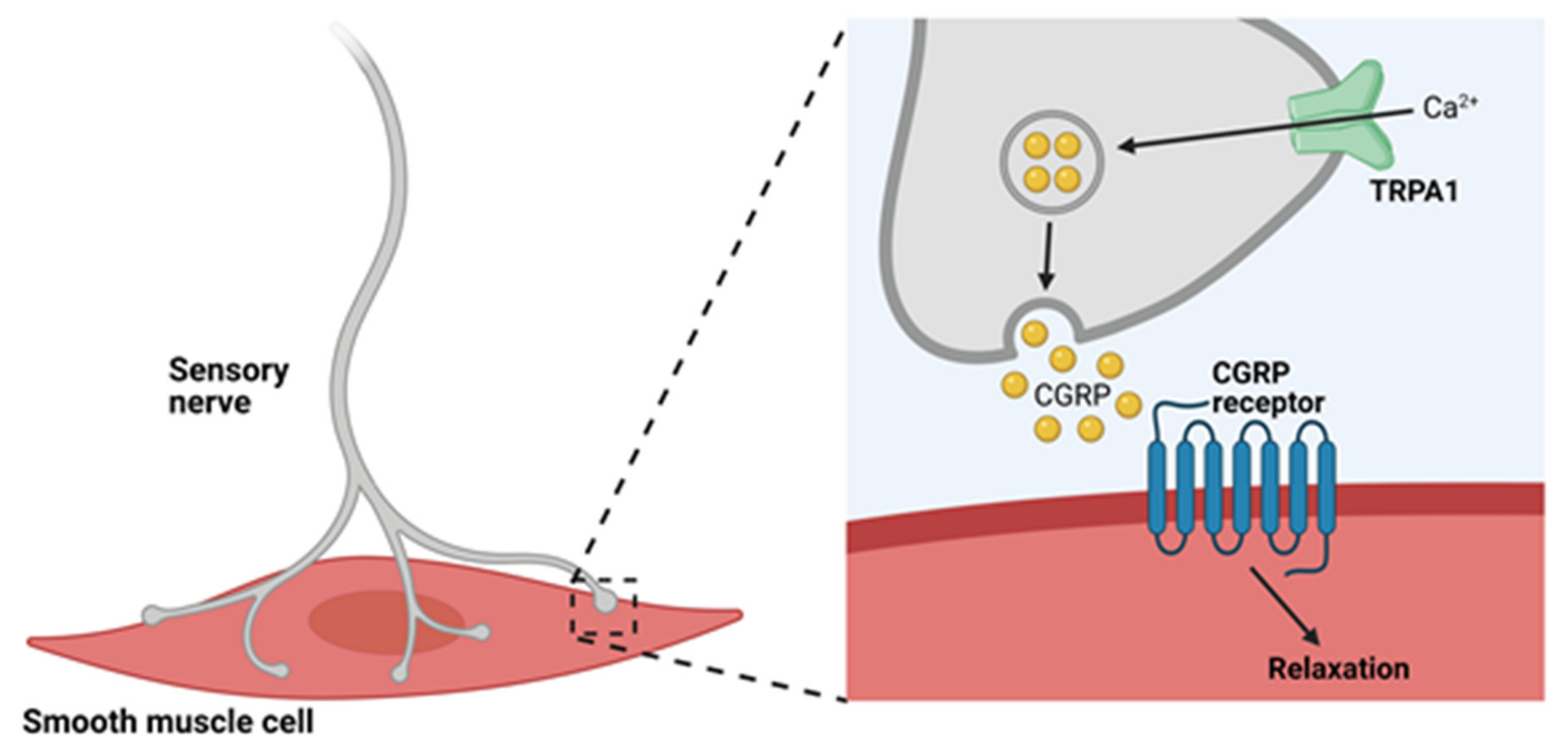
4.1. TRPA1 Channels and Neurogenic Vasodilation
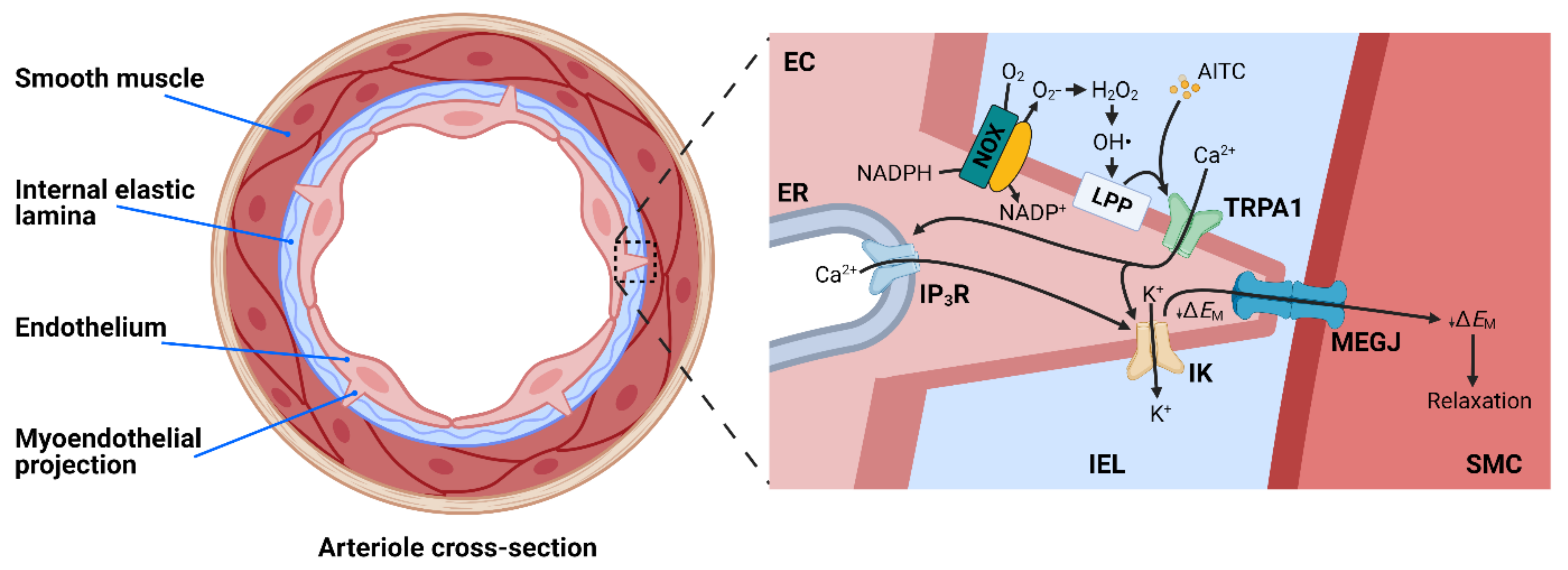
4.2. TRPA1 and Endothelium-Dependent Vasodilation
4.3. TRPA1 and Neurovascular Coupling
5. TRPA1 Channels and Vascular Disease
5.1. Inflammation
5.2. Hypertension
5.3. Stroke
6. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Runnels, L.W.; Strubing, C. The TRP ion channel family. Nat. Rev. NeuroSci. 2001, 2, 387–396. [Google Scholar] [CrossRef]
- Yu, F.H.; Catterall, W.A. The VGL-chanome: A protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE 2004, 2004, re15. [Google Scholar] [CrossRef] [PubMed]
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. BioChem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Rubin, G.M. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Earley, S.; Brayden, J.E. Transient receptor potential channels and vascular function. Clin. Sci. 2010, 119, 19–36. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Cui, K.; Yuan, X. TRP Channels and Axon Pathfinding. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Frontiers in Neuroscience; Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Thakore, P.; Earley, S. Transient Receptor Potential Channels and Endothelial Cell Calcium Signaling. Compr. Physiol. 2019, 9, 1249–1277. [Google Scholar] [CrossRef]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef]
- Gaudet, R. A primer on ankyrin repeat function in TRP channels and beyond. Mol. Biosyst. 2008, 4, 372–379. [Google Scholar] [CrossRef]
- Lee, G.; Abdi, K.; Jiang, Y.; Michaely, P.; Bennett, V.; Marszalek, P.E. Nanospring behaviour of ankyrin repeats. Nature 2006, 440, 246–249. [Google Scholar] [CrossRef]
- Sedgwick, S.G.; Smerdon, S.J. The ankyrin repeat: A diversity of interactions on a common structural framework. Trends BioChem. Sci. 1999, 24, 311–316. [Google Scholar] [CrossRef]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef]
- Hinman, A.; Chuang, H.H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef]
- Phelps, C.B.; Huang, R.J.; Lishko, P.V.; Wang, R.R.; Gaudet, R. Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 2008, 47, 2476–2484. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. NeuroPhysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Jaquemar, D.; Schenker, T.; Trueb, B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 1999, 274, 7325–7333. [Google Scholar] [CrossRef]
- Cvetkov, T.L.; Huynh, K.W.; Cohen, M.R.; Moiseenkova-Bell, V.Y. Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J. Biol. Chem. 2011, 286, 38168–38176. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Subcell BioChem. 2018, 87, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Doerner, J.F.; Gisselmann, G.; Hatt, H.; Wetzel, C.H. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 2007, 282, 13180–13189. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Duggan, A.; Kumar, G.; Garcia-Anoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. NeuroSci. 2005, 25, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Zurborg, S.; Yurgionas, B.; Jira, J.A.; Caspani, O.; Heppenstall, P.A. Direct activation of the ion channel TRPA1 by Ca2+. Nat. NeuroSci. 2007, 10, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Zayats, V.; Samad, A.; Minofar, B.; Roelofs, K.E.; Stockner, T.; Ettrich, R. Regulation of the transient receptor potential channel TRPA1 by its N-terminal ankyrin repeat domain. J. Mol. Model 2013, 19, 4689–4700. [Google Scholar] [CrossRef] [PubMed]
- Sura, L.; Zima, V.; Marsakova, L.; Hynkova, A.; Barvik, I.; Vlachova, V. C-terminal acidic cluster is involved in Ca2+-induced regulation of human transient receptor potential ankyrin 1 channel. J. Biol. Chem. 2012, 287, 18067–18077. [Google Scholar] [CrossRef]
- Samad, A.; Sura, L.; Benedikt, J.; Ettrich, R.; Minofar, B.; Teisinger, J.; Vlachova, V. The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. BioChem. J. 2011, 433, 197–204. [Google Scholar] [CrossRef]
- Christensen, A.P.; Akyuz, N.; Corey, D.P. The Outer Pore and Selectivity Filter of TRPA1. PLoS ONE 2016, 11, e0166167. [Google Scholar] [CrossRef]
- Banke, T.G.; Chaplan, S.R.; Wickenden, A.D. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am. J. Physiol. Cell Physiol. 2010, 298, C1457–C1468. [Google Scholar] [CrossRef]
- Bobkov, Y.V.; Corey, E.A.; Ache, B.W. The pore properties of human nociceptor channel TRPA1 evaluated in single channel recordings. Biochim Biophys. Acta 2011, 1808, 1120–1128. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Kim, D.; Bianchi, B.R.; Cavanaugh, E.J.; Faltynek, C.R.; Kym, P.R.; Reilly, R.M. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol. Pain 2009, 5, 3. [Google Scholar] [CrossRef]
- Li, M.; Toombes, G.E.; Silberberg, S.D.; Swartz, K.J. Physical basis of apparent pore dilation of ATP-activated P2X receptor channels. Nat. NeuroSci. 2015, 18, 1577–1583. [Google Scholar] [CrossRef]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflugers Arch 2012, 464, 425–458. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Owsianik, G. Irritating channels: The case of TRPA1. J. Physiol. 2011, 589, 1543–1549. [Google Scholar] [CrossRef]
- Karashima, Y.; Prenen, J.; Talavera, K.; Janssens, A.; Voets, T.; Nilius, B. Agonist-induced changes in Ca(2+) permeation through the nociceptor cation channel TRPA1. Biophys. J. 2010, 98, 773–783. [Google Scholar] [CrossRef]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Hogestatt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibRes. through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chang, R.B.; Waters, H.N.; McKemy, D.D.; Liman, E.R. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 2008, 283, 32691–32703. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein. Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. NeuroSci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef]
- Sawada, Y.; Hosokawa, H.; Matsumura, K.; Kobayashi, S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur. J. NeuroSci. 2008, 27, 1131–1142. [Google Scholar] [CrossRef]
- Karashima, Y.; Damann, N.; Prenen, J.; Talavera, K.; Segal, A.; Voets, T.; Nilius, B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. NeuroSci. 2007, 27, 9874–9884. [Google Scholar] [CrossRef]
- Leffler, A.; Lattrell, A.; Kronewald, S.; Niedermirtl, F.; Nau, C. Activation of TRPA1 by membrane permeable local anesthetics. Mol. Pain 2011, 7, 62. [Google Scholar] [CrossRef]
- Eilers, H.; Cattaruzza, F.; Nassini, R.; Materazzi, S.; Andre, E.; Chu, C.; Cottrell, G.S.; Schumacher, M.; Geppetti, P.; Bunnett, N.W. Pungent general anesthetics activate transient receptor potential-A1 to produce hyperalgesia and neurogenic bronchoconstriction. Anesthesiology 2010, 112, 1452–1463. [Google Scholar] [CrossRef]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andre, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Takaya, J.; Mio, K.; Shiraishi, T.; Kurokawa, T.; Otsuka, S.; Mori, Y.; Uesugi, M. A Potent and Site-Selective Agonist of TRPA1. J. Am. Chem. Soc. 2015, 137, 15859–15864. [Google Scholar] [CrossRef]
- Xiao, B.; Dubin, A.E.; Bursulaya, B.; Viswanath, V.; Jegla, T.J.; Patapoutian, A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J. NeuroSci. 2008, 28, 9640–9651. [Google Scholar] [CrossRef]
- Bang, S.; Hwang, S.W. Polymodal ligand sensitivity of TRPA1 and its modes of interactions. J. Gen. Physiol. 2009, 133, 257–262. [Google Scholar] [CrossRef]
- Bahia, P.K.; Parks, T.A.; Stanford, K.R.; Mitchell, D.A.; Varma, S.; Stevens, S.M., Jr.; Taylor-Clark, T.E. The exceptionally high reactivity of Cys 621 is critical for electrophilic activation of the sensory nerve ion channel TRPA1. J. Gen. Physiol. 2016, 147, 451–465. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Wang, Z.; Zubcevic, L.; Hsu, A.L.; He, Q.; Borgnia, M.J.; Ji, R.R.; Lee, S.Y. Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Neuron 2020, 105, 882–894.e885. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Kiselar, J.; Pumroy, R.A.; Han, S.; Moiseenkova-Bell, V.Y. Structural insights into the molecular mechanism of mouse TRPA1 activation and inhibition. J. Gen. Physiol. 2018, 150, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef]
- Gracheva, E.O.; Ingolia, N.T.; Kelly, Y.M.; Cordero-Morales, J.F.; Hollopeter, G.; Chesler, A.T.; Sanchez, E.E.; Perez, J.C.; Weissman, J.S.; Julius, D. Molecular basis of infrared detection by snakes. Nature 2010, 464, 1006–1011. [Google Scholar] [CrossRef]
- Cordero-Morales, J.F.; Gracheva, E.O.; Julius, D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc. Natl. Acad. Sci. USA 2011, 108, E1184–E1191. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Hogestatt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. CB 2005, 15, 929–934. [Google Scholar] [CrossRef]
- Woll, K.A.; Skinner, K.A.; Gianti, E.; Bhanu, N.V.; Garcia, B.A.; Carnevale, V.; Eckenhoff, R.G.; Gaudet, R. Sites Contributing to TRPA1 Activation by the Anesthetic Propofol Identified by Photoaffinity Labeling. Biophys. J. 2017, 113, 2168–2172. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Xiao, B.; Kwan, K.Y.; Petrus, M.J.; Dubin, A.E.; Hwang, S.; Cravatt, B.; Corey, D.P.; Patapoutian, A. An ion channel essential for sensing chemical damage. J. NeuroSci. 2007, 27, 11412–11415. [Google Scholar] [CrossRef]
- Taylor-Clark, T.E.; McAlexander, M.A.; Nassenstein, C.; Sheardown, S.A.; Wilson, S.; Thornton, J.; Carr, M.J.; Undem, B.J. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibRes. by the endogenous autacoid 4-oxononenal. J. Physiol. 2008, 586, 3447–3459. [Google Scholar] [CrossRef]
- Kojima, R.; Nozawa, K.; Doihara, H.; Keto, Y.; Kaku, H.; Yokoyama, T.; Itou, H. Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur. J. Pharmacol. 2014, 723, 288–293. [Google Scholar] [CrossRef]
- Liu, C.; Reese, R.; Vu, S.; Rouge, L.; Shields, S.D.; Kakiuchi-Kiyota, S.; Chen, H.; Johnson, K.; Shi, Y.P.; Chernov-Rogan, T.; et al. A Non-covalent Ligand Reveals Biased Agonism of the TRPA1 Ion Channel. Neuron 2021, 109, 273–284.e274. [Google Scholar] [CrossRef]
- Lin King, J.V.; Emrick, J.J.; Kelly, M.J.S.; Herzig, V.; King, G.F.; Medzihradszky, K.F.; Julius, D. A Cell-Penetrating Scorpion Toxin Enables Mode-Specific Modulation of TRPA1 and Pain. Cell 2019, 178, 1362–1374.e1316. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Hwang, S.W.; Miyamoto, T.; Dubin, A.E.; Patapoutian, A.; Story, G.M. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol. Cell NeuroSci. 2006, 32, 335–343. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Wei, H.; Chapman, H.; Saarnilehto, M.; Kuokkanen, K.; Koivisto, A.; Pertovaara, A. Roles of cutaneous versus spinal TRPA1 channels in mechanical hypersensitivity in the diabetic or mustard oil-treated non-diabetic rat. Neuropharmacology 2010, 58, 578–584. [Google Scholar] [CrossRef]
- Defalco, J.; Steiger, D.; Gustafson, A.; Emerling, D.E.; Kelly, M.G.; Duncton, M.A. Oxime derivatives related to AP18: Agonists and antagonists of the TRPA1 receptor. Bioorg Med. Chem. Lett. 2010, 20, 276–279. [Google Scholar] [CrossRef]
- Petrus, M.; Peier, A.M.; Bandell, M.; Hwang, S.W.; Huynh, T.; Olney, N.; Jegla, T.; Patapoutian, A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. Pain 2007, 3, 40. [Google Scholar] [CrossRef]
- McGaraughty, S.; Chu, K.L.; Perner, R.J.; Didomenico, S.; Kort, M.E.; Kym, P.R. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflaMed. rats. Mol. Pain 2010, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Copeland, K.W.; Boezio, A.A.; Cheung, E.; Lee, J.; Olivieri, P.; Schenkel, L.B.; Wan, Q.; Wang, W.; Wells, M.C.; Youngblood, B.; et al. Development of novel azabenzofuran TRPA1 antagonists as in vivo tools. Bioorg. Med. Chem. Lett. 2014, 24, 3464–3468. [Google Scholar] [CrossRef] [PubMed]
- Rooney, L.; Vidal, A.; D’Souza, A.M.; Devereux, N.; Masick, B.; Boissel, V.; West, R.; Head, V.; Stringer, R.; Lao, J.; et al. Discovery, optimization, and biological evaluation of 5-(2-(trifluoromethyl)phenyl)indazoles as a novel class of transient receptor potential A1 (TRPA1) antagonists. J. Med. Chem. 2014, 57, 5129–5140. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hamalainen, M.M.; Saarnilehto, M.; Koivisto, A.; Pertovaara, A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology 2009, 111, 147–154. [Google Scholar] [CrossRef]
- Takahashi, N.; Kuwaki, T.; Kiyonaka, S.; Numata, T.; Kozai, D.; Mizuno, Y.; Yamamoto, S.; Naito, S.; Knevels, E.; Carmeliet, P.; et al. TRPA1 underlies a sensing mechanism for O2. Nat. Chem. Biol. 2011, 7, 701–711. [Google Scholar] [CrossRef]
- Bessac, B.F.; Jordt, S.E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology 2008, 23, 360–370. [Google Scholar] [CrossRef]
- Miyamoto, T.; Dubin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 2009, 4, e7596. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suarez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef]
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985, 313, 54–56. [Google Scholar] [CrossRef]
- Pozsgai, G.; Bodkin, J.V.; Graepel, R.; Bevan, S.; Andersson, D.A.; Brain, S.D. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc. Res. 2010, 87, 760–768. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Kodji, X.; Abdul-Kader, N.; Heads, R.; Fernandes, E.S.; Bevan, S.; Brain, S.D. TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. [Google Scholar] [CrossRef]
- Graepel, R.; Fernandes, E.S.; Aubdool, A.A.; Andersson, D.A.; Bevan, S.; Brain, S.D. 4-oxo-2-nonenal (4-ONE): Evidence of transient receptor potential ankyrin 1-dependent and -independent nociceptive and vasoactive responses in vivo. J. Pharmacol. Exp. Ther. 2011, 337, 117–124. [Google Scholar] [CrossRef]
- Kunkler, P.E.; Ballard, C.J.; Oxford, G.S.; Hurley, J.H. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011, 152, 38–44. [Google Scholar] [CrossRef]
- Hogestatt, E.D.; Johansson, R.; Andersson, D.A.; Zygmunt, P.M. Involvement of sensory nerves in vasodilator responses to acetylcholine and potassium ions in rat hepatic artery. Br. J. Pharmacol 2000, 130, 27–32. [Google Scholar] [CrossRef][Green Version]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Dowell, F.J.; Martin, W. The effects of peroxynitrite on rat aorta: Interaction with glucose and related substances. Eur. J. Pharmacol. 1997, 338, 43–53. [Google Scholar] [CrossRef]
- Casey, D.B.; Pankey, E.A.; Badejo, A.M.; Bueno, F.R.; Bhartiya, M.; Murthy, S.N.; Uppu, R.M.; Nossaman, B.D.; Kadowitz, P.J. Peroxynitrite has potent pulmonary vasodilator activity in the rat. Can J. Physiol. Pharmacol. 2012, 90, 485–500. [Google Scholar] [CrossRef]
- Keatinge, W.R. The effect of general chilling on the vasodilator response to cold. J. Physiol. 1957, 139, 497–507. [Google Scholar] [CrossRef]
- Daanen, H.A. Finger cold-induced vasodilation: A review. Eur. J. Appl Physiol. 2003, 89, 411–426. [Google Scholar] [CrossRef]
- Karashima, Y.; Talavera, K.; Everaerts, W.; Janssens, A.; Kwan, K.Y.; Vennekens, R.; Nilius, B.; Voets, T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1273–1278. [Google Scholar] [CrossRef]
- Sawada, Y.; Hosokawa, H.; Hori, A.; Matsumura, K.; Kobayashi, S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007, 1160, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef]
- Caspani, O.; Heppenstall, P.A. TRPA1 and cold transduction: An unresolved issue? J. Gen. Physiol. 2009, 133, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Gonzales, A.L.; Crnich, R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res. 2009, 104, 987–994. [Google Scholar] [CrossRef]
- Qian, X.; Francis, M.; Solodushko, V.; Earley, S.; Taylor, M.S. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation 2013, 20, 138–148. [Google Scholar] [CrossRef]
- Hansted, A.K.; Bhatt, D.K.; Olesen, J.; Jensen, L.J.; Jansen-Olesen, I. Effect of TRPA1 activator allyl isothiocyanate (AITC) on rat dural and pial arteries. Pharmacol. Rep. 2019, 71, 565–572. [Google Scholar] [CrossRef]
- Hansted, A.K.; Jensen, L.J.; Olesen, J.; Jansen-Olesen, I. Localization of TRPA1 channels and characterization of TRPA1 mediated responses in dural and pial arteries in vivo after intracarotid infusion of Na2S. Cephalalgia 2020, 40, 1310–1320. [Google Scholar] [CrossRef]
- Sullivan, M.N.; Gonzales, A.L.; Pires, P.W.; Bruhl, A.; Leo, M.D.; Li, W.; Oulidi, A.; Boop, F.A.; Feng, Y.; Jaggar, J.H.; et al. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci. Signal 2015, 8, ra2. [Google Scholar] [CrossRef]
- Sullivan, M.N.; Earley, S. TRP channel Ca(2+) sparklets: Fundamental signals underlying endothelium-dependent hyperpolarization. Am. J. Physiol. Cell Physiol. 2013, 305, C999–C1008. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Richmond, R.; Halliwell, B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. BioChem. J. 1979, 184, 469–472. [Google Scholar] [CrossRef]
- Girouard, H.; Iadecola, C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006, 100, 328–335. [Google Scholar] [CrossRef]
- Nippert, A.R.; Biesecker, K.R.; Newman, E.A. Mechanisms Mediating Functional Hyperemia in the Brain. Neuroscientist 2018, 24, 73–83. [Google Scholar] [CrossRef]
- Grubb, S.; Lauritzen, M.; Aalkjaer, C. Brain capillary pericytes and neurovascular coupling. Comp. BioChem. Physiol. A. Mol. Integr. Physiol. 2021, 254, 110893. [Google Scholar] [CrossRef]
- Blinder, P.; Tsai, P.S.; Kaufhold, J.P.; Knutsen, P.M.; Suhl, H.; Kleinfeld, D. The cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nat. NeuroSci. 2013, 16, 889–897. [Google Scholar] [CrossRef]
- Coelho-Santos, V.; Shih, A.Y. Postnatal development of cerebrovascular structure and the neurogliovascular unit. Wiley Interdiscip Rev. Dev. Biol. 2020, 9, e363. [Google Scholar] [CrossRef]
- Longden, T.A.; Dabertrand, F.; Koide, M.; Gonzales, A.L.; Tykocki, N.R.; Brayden, J.E.; Hill-Eubanks, D.; Nelson, M.T. Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. NeuroSci. 2017, 20, 717–726. [Google Scholar] [CrossRef]
- Longden, T.A.; Nelson, M.T. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 2015, 22, 183–196. [Google Scholar] [CrossRef]
- Thakore, P.; Alvarado, M.G.; Ali, S.; Mughal, A.; Pires, P.W.; Yamasaki, E.; Pritchard, H.A.; Isakson, B.E.; Tran, C.H.T.; Earley, S. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. Elife 2021, 10, e63040. [Google Scholar] [CrossRef]
- Girouard, H.; Bonev, A.D.; Hannah, R.M.; Meredith, A.; Aldrich, R.W.; Nelson, M.T. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. USA 2010, 107, 3811–3816. [Google Scholar] [CrossRef]
- Park, L.; Koizumi, K.; El Jamal, S.; Zhou, P.; Previti, M.L.; Van Nostrand, W.E.; Carlson, G.; Iadecola, C. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 2014, 45, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.L.; Klug, N.R.; Moshkforoush, A.; Lee, J.C.; Lee, F.K.; Shui, B.; Tsoukias, N.M.; Kotlikoff, M.I.; Hill-Eubanks, D.; Nelson, M.T. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc. Natl. Acad. Sci. USA 2020, 117, 27022–27033. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, M.; Sasaki, T.; Sadakari, J.; Gengyo-Ando, K.; Kagawa-Nagamura, Y.; Kobayashi, C.; Ikegaya, Y.; Nakai, J. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS ONE 2012, 7, e51286. [Google Scholar] [CrossRef] [PubMed]
- Andre, E.; Campi, B.; Materazzi, S.; Trevisani, M.; Amadesi, S.; Massi, D.; Creminon, C.; Vaksman, N.; Nassini, R.; Civelli, M.; et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 2008, 118, 2574–2582. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Andre, E.; Sartiani, L.; Aldini, G.; Trevisani, M.; Carnini, C.; Massi, D.; Pedretti, P.; Carini, M.; et al. Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 4904–4916. [Google Scholar] [CrossRef]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernandez-Pena, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef]
- Zhao, J.F.; Shyue, S.K.; Kou, Y.R.; Lu, T.M.; Lee, T.S. Transient Receptor Potential Ankyrin 1 Channel Involved in Atherosclerosis and Macrophage-Foam Cell Formation. Int. J. Biol. Sci. 2016, 12, 812–823. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Plump, A.S.; Smith, J.D.; Hayek, T.; Aalto-Setala, K.; Walsh, A.; Verstuyft, J.G.; Rubin, E.M.; Breslow, J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992, 71, 343–353. [Google Scholar] [CrossRef]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258, 468–471. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. BioMed. Res. Int. 2016, 2016, 9582430. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Wu, C.K.; Wu, C.L.; Su, T.C.; Kou, Y.R.; Kor, C.T.; Lee, T.S.; Tarng, D.C. Renal Tubular TRPA1 as a Risk Factor for Recovery of Renal Function from Acute Tubular Necrosis. J. Clin. Med. 2019, 8, 2187. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakayama, M.; Iwabuchi, M.; Terawaki, H.; Sato, T.; Kohno, M.; Ito, S. Plasma alpha-oxoaldehyde levels in diabetic and nondiabetic chronic kidney disease patients. Am. J. Nephrol. 2008, 28, 871–878. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal stress, the glyoxalase system, and diabetic chronic kidney disease. Curr. Opin. Nephrol. Hypertens 2019, 28, 26–33. [Google Scholar] [CrossRef]
- Eberhardt, M.J.; Filipovic, M.R.; Leffler, A.; de la Roche, J.; Kistner, K.; Fischer, M.J.; Fleming, T.; Zimmermann, K.; Ivanovic-Burmazovic, I.; Nawroth, P.P.; et al. Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): A possible mechanism of metabolic neuropathies. J. Biol. Chem. 2012, 287, 28291–28306. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Light, E.; Vastani, N.; Vallortigara, J.; Bierhaus, A.; Fleming, T.; Bevan, S. Methylglyoxal evokes pain by stimulating TRPA1. PLoS ONE 2013, 8, e77986. [Google Scholar] [CrossRef]
- Taler, S.J. Initial Treatment of Hypertension. N. Engl. J. Med. 2018, 378, 636–644. [Google Scholar] [CrossRef]
- Gangula, P.R.; Zhao, H.; Supowit, S.C.; Wimalawansa, S.J.; Dipette, D.J.; Westlund, K.N.; Gagel, R.F.; Yallampalli, C. Increased blood pressure in alpha-calcitonin gene-related peptide/calcitonin gene knockout mice. Hypertension 2000, 35, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Smillie, S.J.; King, R.; Kodji, X.; Outzen, E.; Pozsgai, G.; Fernandes, E.; Marshall, N.; de Winter, P.; Heads, R.J.; Dessapt-Baradez, C.; et al. An ongoing role of alpha-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension 2014, 63, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Bodkin, J.V.; Thakore, P.; Aubdool, A.A.; Liang, L.; Fernandes, E.S.; Nandi, M.; Spina, D.; Clark, J.E.; Aaronson, P.I.; Shattock, M.J.; et al. Investigating the potential role of TRPA1 in locomotion and cardiovascular control during hypertension. Pharmacol. Res. Perspect 2014, 2, e00052. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, Y.; He, K.; Wang, P.; Wang, D.H. Knockout of TRPA1 exacerbates angiotensin II-induced kidney injury. Am. J. Physiol. Renal. Physiol. 2019, 317, F623–F631. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat 2018, 2018, 3238165. [Google Scholar] [CrossRef] [PubMed]
- Kontos, H.A.; Wei, E.P.; Raper, A.J.; Rosenblum, W.I.; Navari, R.M.; Patterson, J.L., Jr. Role of tissue hypoxia in local regulation of cerebral microcirculation. Am. J. Physiol. 1978, 234, H582–H591. [Google Scholar] [CrossRef]
- Pires, P.W.; Earley, S. Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. Elife 2018, 7. [Google Scholar] [CrossRef]
- Liu, S.; Shi, H.; Liu, W.; Furuichi, T.; Timmins, G.S.; Liu, K.J. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2004, 24, 343–349. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Reyes, R.C.; Parpura, V. TRP channels coordinate ion signalling in astroglia. Rev. Physiol. BioChem. Pharmacol. 2014, 166, 1–22. [Google Scholar] [CrossRef]
- Shigetomi, E.; Tong, X.; Kwan, K.Y.; Corey, D.P.; Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. NeuroSci. 2011, 15, 70–80. [Google Scholar] [CrossRef]
- Shigetomi, E.; Jackson-Weaver, O.; Huckstepp, R.T.; O’Dell, T.J.; Khakh, B.S. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. NeuroSci. 2013, 33, 10143–10153. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, Y.S.; Kim, T.H.; Jin, M.U.; Ahn, D.K.; Noguchi, K.; Bae, Y.C. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J. Chem. NeuroaNat. 2012, 45, 45–49. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, S.; Teng, S.; Jin, M.; Zhou, Z. Ca(2+)-Dependent and Ca(2+)-Independent ATP Release in Astrocytes. Front Mol. NeuroSci. 2018, 11, 224. [Google Scholar] [CrossRef]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Duchen, M.R. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philos Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 2309–2314. [Google Scholar] [CrossRef]



| Agonist | Source | EC50; Isoform | Reference |
|---|---|---|---|
| Allyl isothiocyanate (AITC) | Mustard | 33 μM; mTRPA1 11 μM; rTRPA1 64 μM; hTRPA1 | [17,38,47] |
| Cinnamaldehyde | Cinnamon | 100 µM; mTRPA1 | [47] |
| Allicin | Garlic | 1.3 µM; mTRPA1 1.9 µM; hTRPA1 | [58,59] |
| Propofol | Anesthetic | 17 μM; mTRPA1 | [60] |
| Lidocaine | Anesthetic | 5.7 mM; rTRPA1 24 mM; hTRPA1 | [44] |
| 4-HNE | Reactive oxygen species | 13–20 µM; mTRPA1 27 µM; rTRPA1 5 µM; hTRPA1 | [41,46,61,62] |
| 4-ONE | Reactive oxygen species | 1.9 µM; mTRPA1 5.8 µM; hTRPA1 | [41,62] |
| 4-HHE | Reactive oxygen species | 38.9 µM; mTRPA1 ≥4.3 µM; hTRPA1 | [41,62] |
| H2O2 | Reactive oxygen species | 230 μM; mTRPA1 | [41] |
| 15-deoxy-delta(12,14)-prostaglandin J(2) [15d-PGJ(2)] | Reactive oxygen species | 5.6 μM; mTRPA1 | [41] |
| ASP7663 | Synthetic | 0.50 µM; mTRPA1 0.54 µM; rTRPA1 0.51 µM; hTRPA1 | [63] |
| JT010 | Synthetic | 0.65 nM; hTRPA1 | [48] |
| GNE551 | Synthetic | 254 nM; hTRPA1 | [64] |
| WaTx | Peptidergic toxin | 6 nM; mTRPA1 15 nM; rTRPA1, 16nM; hTRPA1 | [65]. |
| Antagonist | IC50; isoform | Reference |
|---|---|---|
| HC-030031 | 7.6 μM; rTRPA1 5.3–6.2 μM; hTRPA1 | [68] |
| Chembridge-5861528 | 14.3–18.7 µM; hTRPA1 | [69,75] |
| AP-18 | 4.5 μM; mTRPA1 3.1 μM; hTRPA1 | [70,71] |
| A-967079 | 289 nM; rTRPA1 67 nM; hTRPA1 | [72] |
| Compound 10 | 45 nM; rTRPA1 170 nM; hTRPA1 | [73] |
| Compound 31 | 85 nM; rTRPA1 15 nM; hTRPA1 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado, M.G.; Thakore, P.; Earley, S. Transient Receptor Potential Channel Ankyrin 1: A Unique Regulator of Vascular Function. Cells 2021, 10, 1167. https://doi.org/10.3390/cells10051167
Alvarado MG, Thakore P, Earley S. Transient Receptor Potential Channel Ankyrin 1: A Unique Regulator of Vascular Function. Cells. 2021; 10(5):1167. https://doi.org/10.3390/cells10051167
Chicago/Turabian StyleAlvarado, Michael G., Pratish Thakore, and Scott Earley. 2021. "Transient Receptor Potential Channel Ankyrin 1: A Unique Regulator of Vascular Function" Cells 10, no. 5: 1167. https://doi.org/10.3390/cells10051167
APA StyleAlvarado, M. G., Thakore, P., & Earley, S. (2021). Transient Receptor Potential Channel Ankyrin 1: A Unique Regulator of Vascular Function. Cells, 10(5), 1167. https://doi.org/10.3390/cells10051167






