Never Change a Flowing System? The Effects of Retrograde Flow on Isolated Perfused Lungs and Vessels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Agents
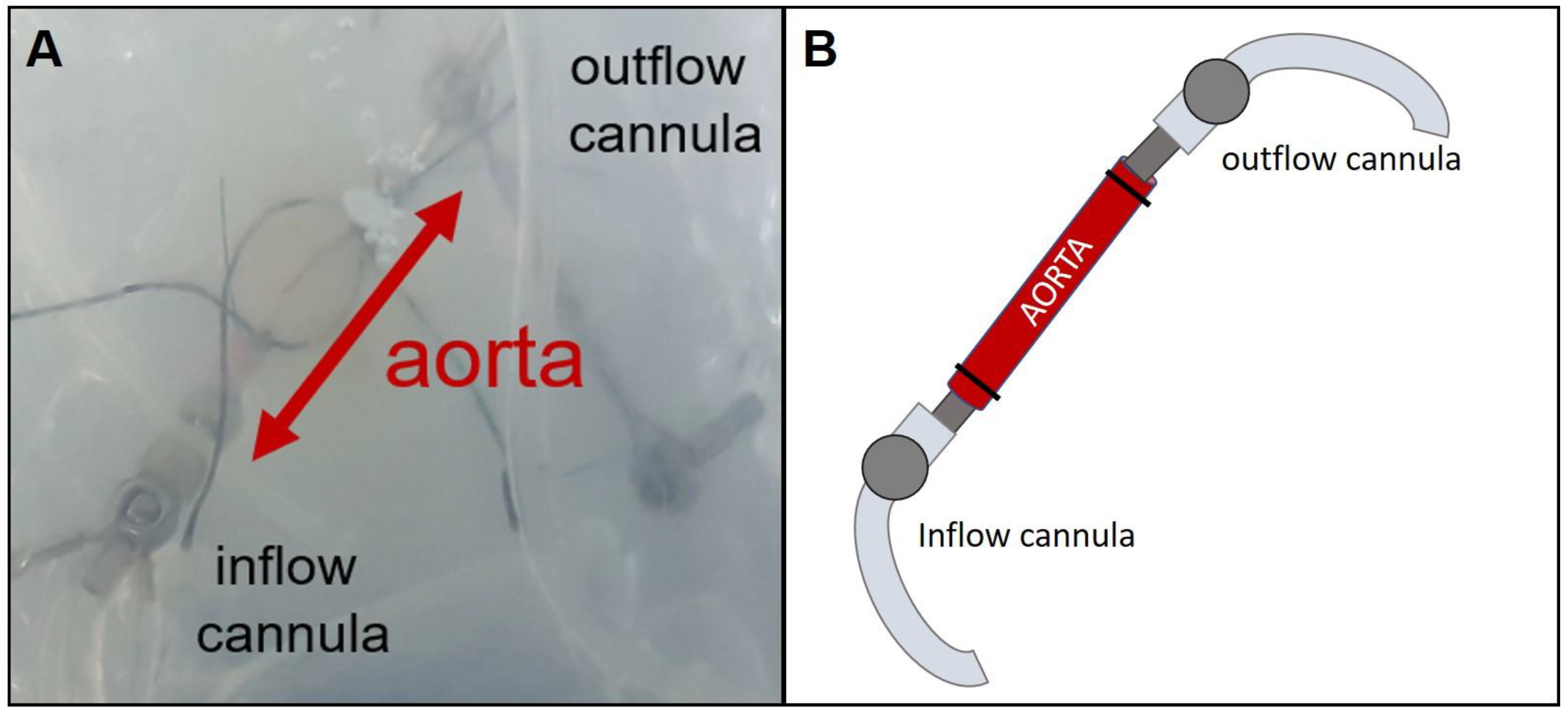
2.3. Isolated Perfused Rat Vessel Preparation (IPV)
2.4. Isolated Perfused Rat Lung Preparation (IPL)
2.5. Cytokine Determination—Electrochemiluminescence Assay
2.6. Lung Wet-to-Dry Ratio
2.7. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statistics
3. Results
3.1. IPL and IPV—Experimental Results
3.1.1. IPV—Perfusion Pressure and Cytokine Levels
3.1.2. IPL—Tidal Volume, Airway Resistance, Inflow and Perfusion Pressure
3.1.3. IPL—Edema Formation Indicated by Wet-to-Dry Ratio and Lung Weight
3.1.4. IPL—Cytokine Levels in Perfusate and BALF
3.1.5. IPL—mRNA Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kargiotis, O.; Siahos, S.; Safouris, A.; Feleskouras, A.; Magoufis, G.; Tsivgoulis, G. Subclavian Steal Syndrome with or without Arterial Stenosis: A Review. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2016, 26, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Bradley, N.A.; Roxburgh, C.; Khan, F.; Guthrie, G. Postimplantation Syndrome in Endovascular Aortic Aneurysm Repair—A Systematic Review. Vasa 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Funabashi, N.; Takaoka, H.; Ishikawa, K.; Ozawa, K.; Shimojo, N.; Kobayashi, Y. Bland-White-Garland Syndrome Diagnosed by Computed Tomography. Int. J. Cardiol. 2015, 201, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Crockett, E.S.; Batten, L.; McMurtry, I.F.; Stevens, T. Pulmonary Vascular Dysfunction Secondary to Pulmonary Arterial Hypertension: Insights Gained through Retrograde Perfusion. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L835–L845. [Google Scholar] [CrossRef]
- Krabbe, J.; Ruske, N.; Kanzler, S.; Reiss, L.K.; Ludwig, A.; Uhlig, S.; Martin, C. Retrograde Perfusion in Isolated Perfused Mouse Lungs—Feasibility and Effects on Cytokine Levels and Pulmonary Oedema Formation. Basic Clin. Pharmacol. Toxicol. 2019, 125, 279–288. [Google Scholar] [CrossRef]
- Wang, C.; Baker, B.M.; Chen, C.S.; Schwartz, M.A. Endothelial Cell Sensing of Flow Direction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2130–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballermann, B.J.; Dardik, A.; Eng, E.; Liu, A. Shear Stress and the Endothelium. Kidney Int. 1998, 54, S100–S108. [Google Scholar] [CrossRef] [Green Version]
- McCue, S.; Dajnowiec, D.; Xu, F.; Zhang, M.; Jackson, M.R.; Langille, B.L. Shear Stress Regulates Forward and Reverse Planar Cell Polarity of Vascular Endothelium In Vivo and In Vitro. Circ. Res. 2006, 98, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, N.; Ujita, S.; Sato, M. Effect of Flow Direction on the Morphological Responses of Cultured Bovine Aortic Endothelial Cells. Med. Biol. Eng. Comput. 1998, 36, 122–128. [Google Scholar] [CrossRef]
- Deurs, B.V.; Roepstorff, K.; Hommelgaard, A.M.; Sandvig, K. Caveolae: Anchored, Multifunctional Platforms in the Lipid Ocean. Trends Cell Biol. 2003, 13, 92–100. [Google Scholar] [CrossRef]
- Schubert, W.; Frank, P.G.; Woodman, S.E.; Hyogo, H.; Cohen, D.E.; Chow, C.-W.; Lisanti, M.P. Microvascular Hyperpermeability in Caveolin-1 (−/−) Knock-out Mice Treatment With A Specific Nitric-Oxide Synthase Inhibitor, L-Name, Restores Normal Microvascular Permeability In Cav-1 Null Mice. J. Biol. Chem. 2002, 277, 40091–40098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkachenko, E.; Gutierrez, E.; Saikin, S.K.; Fogelstrand, P.; Kim, C.; Groisman, A.; Ginsberg, M.H. The Nucleus of Endothelial Cell as a Sensor of Blood Flow Direction. Biol. Open 2013, 2, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.; Joly, P.; Bergmann, C.; Korus, G.; Duda, G.N. The Impact of Substrate Stiffness and Mechanical Loading on Fibroblast-Induced Scaffold Remodeling. Tissue Eng. Part A 2012, 18, 1804–1817. [Google Scholar] [CrossRef] [PubMed]
- Trichet, L.; Le Digabel, J.; Hawkins, R.J.; Vedula, S.R.K.; Gupta, M.; Ribrault, C.; Hersen, P.; Voituriez, R.; Ladoux, B. Evidence of a Large-Scale Mechanosensing Mechanism for Cellular Adaptation to Substrate Stiffness. Proc. Natl. Acad. Sci. USA 2012, 109, 6933–6938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlig, S.; Yang, Y.; Waade, J.; Wittenberg, C.; Babendreyer, A.; Kuebler, W. Differential regulation of lung endothelial permeability in vitro and in situ. Cell Physiol. Biochem. 2014, 34, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, J.; Ruske, N.; Braunschweig, T.; Kintsler, S.; Spillner, J.W.; Schröder, T.; Kalverkamp, S.; Kanzler, S.; Rieg, A.D.; Uhlig, S.; et al. The Effects of Hydroxyethyl Starch and Gelatine on Pulmonary Cytokine Production and Oedema Formation. Sci. Rep. 2018, 8, 5123. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, S.; Klassen, S.; Katz, I.; Balakirski, G.; Krabbe, J.; von Stillfried, S.; Kintsler, S.; Braunschweig, T.; Babendreyer, A.; Spillner, J.; et al. Argon Reduces the Pulmonary Vascular Tone in Rats and Humans by GABA-Receptor Activation. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Townsley, M.I. Structure and Composition of Pulmonary Arteries, Capillaries and Veins. Compr. Physiol. 2012, 2, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Jaecklin, T.; Engelberts, D.; Otulakowski, G.; O’Brodovich, H.; Post, M.; Kavanagh, B.P. Lung-Derived Soluble Mediators Are Pathogenic in Ventilator-Induced Lung Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L648–L658. [Google Scholar] [CrossRef] [Green Version]
- Hirano, S.; Zhou, Q.; Furuyama, A.; Kanno, S. Differential Regulation of IL-1β and IL-6 Release in Murine Macrophages. Inflammation 2017, 40, 1933–1943. [Google Scholar] [CrossRef]
- Page, I.; Gaillard, F. Descriptive Neuroradiology: Beyond the Hummingbird. Pract. Neurol. 2020, 20, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Skrindo, I.; Ballke, C.; Gran, E.; Johansen, F.-E.; Baekkevold, E.S.; Jahnsen, F.L. IL-5 Production by Resident Mucosal Allergen-Specific T Cells in an Explant Model of Allergic Rhinitis. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Gamrekelashvili, J.; Giagnorio, R.; Jussofie, J.; Soehnlein, O.; Duchene, J.; Briseño, C.G.; Ramasamy, S.K.; Krishnasamy, K.; Limbourg, A.; Häger, C.; et al. Regulation of Monocyte Cell Fate by Blood Vessels Mediated by Notch Signalling. Nat. Commun. 2016, 7, 12597. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, M.; Weinreich, D.M.; Farma, J.M.; Carroll, N.M.; Turner, E.M.; Alexander, H.R. Interleukin-1β Induced Vascular Permeability Is Dependent on Induction of Endothelial Tissue Factor (TF) Activity. J. Transl. Med. 2005, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjærgaard, B.; Honge, J.L.; Magnusdottir, S.O.; Rasmussen, B.S.; Baandrup, U.T.; Hasenkam, J.M.; Kristensen, S.R. Retrograde Lung Perfusion in the Treatment of Massive Pulmonary Embolism. A Randomised Porcine Study. Thromb. Res. 2015, 135, 410–414. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zeng, M.; Curry, F.E. CGMP Modulates Basal and Activated Microvessel Permeability Independently of [Ca2+]i. Am. J. Physiol. 1998, 274, H1865–H1874. [Google Scholar] [CrossRef]
- Draijer, R.; Atsma, D.E.; van der Laarse, A.; van Hinsbergh, V.W. CGMP and Nitric Oxide Modulate Thrombin-Induced Endothelial Permeability. Regulation via Different Pathways in Human Aortic and Umbilical Vein Endothelial Cells. Circ. Res. 1995, 76, 199–208. [Google Scholar] [CrossRef]
- Tsukahara, H.; Noiri, E.; Jiang, M.Z.; Hiraoka, M.; Mayumi, M. Role of Nitric Oxide in Human Pulmonary Microvascular Endothelial Cell Adhesion. Life Sci. 2000, 67, 1–11. [Google Scholar] [CrossRef]
- Ting, L.H.; Jahn, J.R.; Jung, J.I.; Shuman, B.R.; Feghhi, S.; Han, S.J.; Rodriguez, M.L.; Sniadecki, N.J. Flow Mechanotransduction Regulates Traction Forces, Intercellular Forces, and Adherens Junctions. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2220–H2229. [Google Scholar] [CrossRef]
- Kantrow, S.; deBoisblanc, B.P. Mysteries of the Drunken Lung. Crit. Care Med. 2004, 32, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Weifeng, Y.; Li, L.; Yujie, H.; Weifeng, L.; Zhenhui, G.; Wenjie, H. Inhibition of Acute Lung Injury by TNFR-Fc through Regulation of an Inflammation-Oxidative Stress Pathway. PLoS ONE 2016, 11, e0151672. [Google Scholar] [CrossRef] [PubMed]
- Petecchia, L.; Sabatini, F.; Usai, C.; Caci, E.; Varesio, L.; Rossi, G.A. Cytokines Induce Tight Junction Disassembly in Airway Cells via an EGFR-Dependent MAPK/ERK1/2-Pathway. Lab. Investig. 2012, 92, 1140–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute Respiratory Distress Syndrome. Nat. Rev. Dis. Primer 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]








| Gene | Sequence | Annealing Temperature | |
|---|---|---|---|
| Cysltr1 | forward: | ACTCCACAATGTACTCTATGATCTC | 61 °C |
| reverse: | TCACCAAAGAACCACTTGCCT | ||
| Efnb2 | forward: | AAGCCAAATCCAGGTTCTAGCA | 56 °C |
| reverse: | GCGGTACTTGAGCAGCAG | ||
| Nos3 | forward: | GATGAATACGATGTGGTATCCCT | 60 °C |
| reverse: | TTTGTAACTCTTGTGCTGCT | ||
| EphB4 | forward: | TGGATGAGAGCGAGAGTTGG | 63 °C |
| reverse: | GTGCCCGATGAGATATTGCC | ||
| Ednra | forward: | GACAGGTACAGAGCAGTGG | 60 °C |
| reverse: | GCAGAAGTAGAATCCAAAGAGCC | ||
| Nos2 | forward: | GTCTTGGTGAAAGCGGTG | 60 °C |
| reverse: | AGCAGTAGTTGTTCCTCTTCC | ||
| Klf2 | forward: | CTATCTTGCCGTCCTTTGCC | 63 °C |
| reverse: | CTGTTTAGGTCCTCATCCGT | ||
| Ptgis | forward: | GCCGTGTTATTACTGTTGCTG | 60 °C |
| reverse: | TAAATATGTCACCGTGCTTCTCCT | ||
| Tbxa2r | forward: | CTGTGAGGTGGAGATGATGG | 57 °C |
| reverse: | CGGAAGAGGATGTAGACCC | ||
| Thbd | forward: | CGGTCTCAACAGCAACAG | 59 °C |
| reverse: | CAGGATCTCGGGTATTCAC | ||
| Vcam1 | forward: | GTGGACATCTACTCATTCCCT | 61 °C |
| reverse: | GTAAACATCAGGAGCCAAACAC | ||
| Gapdh | forward: | CGGGGCTCTCCAGAACATCATCC | 56 °C |
| reverse: | CCAGCCCCAGCGTCAAAGGTG | ||
| Rplp0 | forward: | ACAGTACCTGCTCAGAACACC | 56 °C |
| reverse: | TGCCATTGTCAAACACCTGCT | ||
| Tbp | forward: | TCTTGGCTGTAAACTTGACC | 57 °C |
| reverse: | CTGGATTGTTCTTCACTCTTGG | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krabbe, H.; Klassen, S.; Bleidorn, J.; Jacobs, M.J.; Krabbe, J.; Babendreyer, A.; Martin, C. Never Change a Flowing System? The Effects of Retrograde Flow on Isolated Perfused Lungs and Vessels. Cells 2021, 10, 1210. https://doi.org/10.3390/cells10051210
Krabbe H, Klassen S, Bleidorn J, Jacobs MJ, Krabbe J, Babendreyer A, Martin C. Never Change a Flowing System? The Effects of Retrograde Flow on Isolated Perfused Lungs and Vessels. Cells. 2021; 10(5):1210. https://doi.org/10.3390/cells10051210
Chicago/Turabian StyleKrabbe, Hanif, Sergej Klassen, Johannes Bleidorn, Michael J. Jacobs, Julia Krabbe, Aaron Babendreyer, and Christian Martin. 2021. "Never Change a Flowing System? The Effects of Retrograde Flow on Isolated Perfused Lungs and Vessels" Cells 10, no. 5: 1210. https://doi.org/10.3390/cells10051210
APA StyleKrabbe, H., Klassen, S., Bleidorn, J., Jacobs, M. J., Krabbe, J., Babendreyer, A., & Martin, C. (2021). Never Change a Flowing System? The Effects of Retrograde Flow on Isolated Perfused Lungs and Vessels. Cells, 10(5), 1210. https://doi.org/10.3390/cells10051210






