The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders
Abstract
1. Introduction
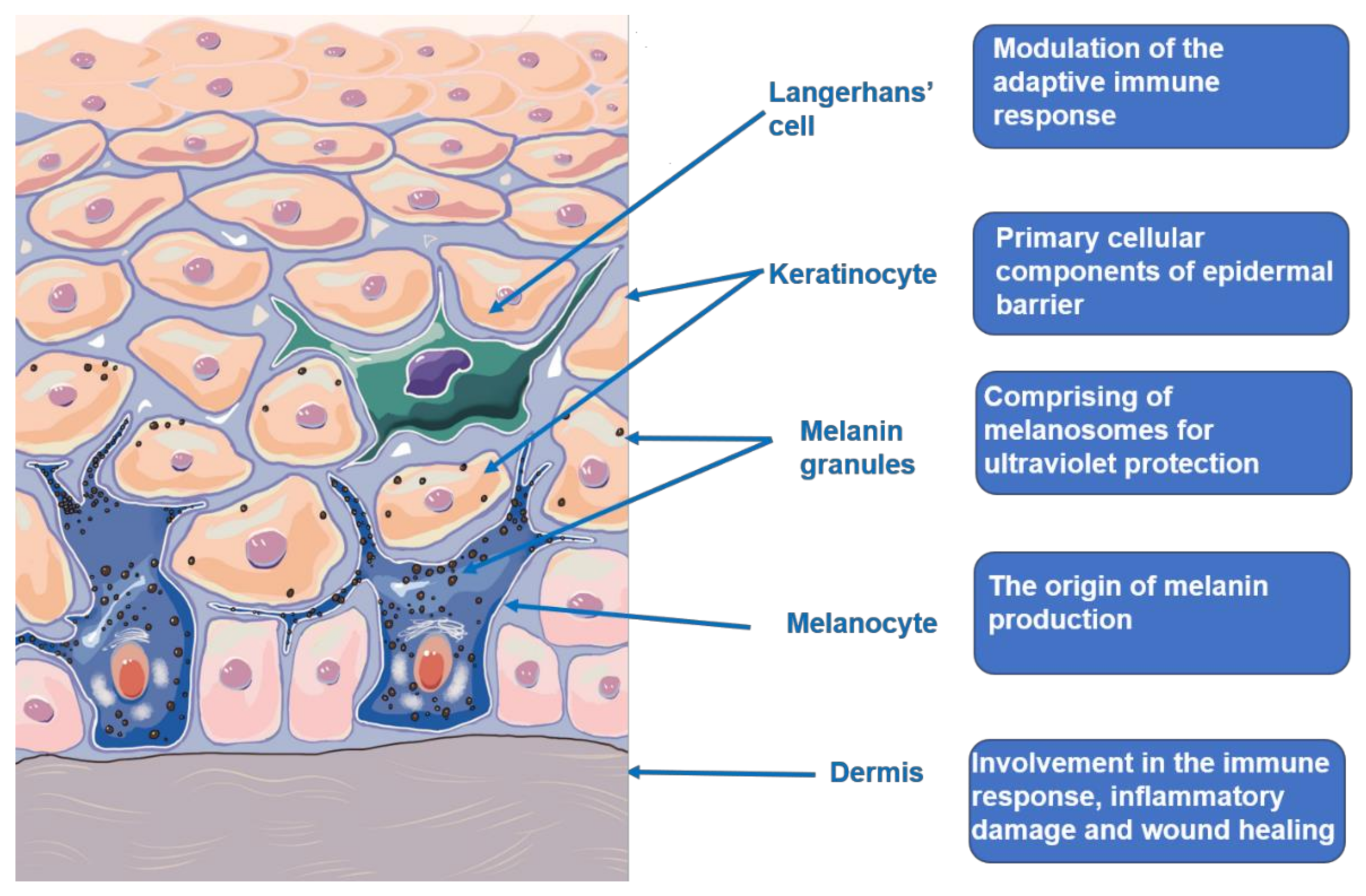
2. The Skin Structure and Function
3. The PI3K/Akt Signaling Pathway
4. Emerging Roles in Skin Homeostasis
4.1. The PI3K/AKT Pathway Is Necessary to Maintain the Epidermal Barrier Function
4.2. Activation of the PI3K/Akt Pathway Could Induce Hair Follicle Regeneration by Promoting the Differentiation and Proliferation of HFSCs
4.3. Activation of PI3K/Akt/mTOR Pathway Could Promote EMT Process and then Enhance Skin Wound Healing
4.4. PTEN/PI3K/Akt Pathway Functions in the Skin Senescence and Self-Renewal of hSKPs
4.5. Activation of PI3K/Akt Pathway Protects Melanocytes from Oxidative Stress
5. Emerging Roles in Non-Malignant Skin Disorders
5.1. The PI3K/Akt Pathway Is Involved in the Formation of Acne by Inhibiting Lipogenesis
5.2. Dysregulation of PTEN, FOXO, and mTOR of the PI3K/Akt Signaling Cascade Are Associated with the Occurrence of Psoriasis
5.3. The PI3K/Akt Pathway Is Involved in the Onset and Exacerbation of Atopic Dermatitis
5.4. The PI3K/Akt Signaling Pathway Is Associated with the Pathologic Fibrosis of Human Scleroderma
5.5. The PI3K/AKT Pathway Is Involved in the Formation of Keloid via Promoting the Proliferation, Migration, Expression Levels of EM-Related Proteins in HKFs
5.6. Activation of PI3K/Akt Protect the Melanocytes from Destruction in Vitiligo
5.7. Activation of the PI3K/Akt Pathway Is Related to the Inhibition of HFSCs Apoptosis in the Pathogenesis of Androgenic Alopecia
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koster, M.I.; Roop, D.R. Mechanisms Regulating Epithelial Stratification. Annu. Rev. Cell Dev. Biol. 2007, 23, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Eichenfield, L.F.; Fowler, J.F.; Horowitz, P.; Mcleod, R.P. Update on the Structure and Function of the Skin Barrier: Atopic Dermatitis as an Exemplar of Clinical Implications. Semin. Cutan. Med. Surg. 2013, 32, S21–S24. [Google Scholar] [CrossRef]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Pellicano, R.; Ribaldone, D.G.; Fagoonee, S.; Astegiano, M.; Saracco, G.; Mégraud, F. A 2016 panorama of Helicobacter pylori infection: Key messages for clinicians. Panminerva Med. 2016, 58, 304–317. [Google Scholar] [PubMed]
- Kawase, Y.; Yanagi, Y.; Takato, T.; Fujimoto, M.; Okochi, H. Characterization of multipotent adult stem cells from the skin: Transforming growth factor-β (TGF-β) facilitates cell growth. Exp. Cell Res. 2004, 295, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Langroudi, M.P.; Akhavan-Niaki, H. Hedgehog signaling pathway: Epigenetic regulation and role in disease and cancer development. J. Cell. Physiol. 2018, 233, 5726–5735. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. BioEssays 2005, 27, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Ekarar, J.; Emaity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar]
- Liao, Y.-X.; Zhang, Z.-P.; Zhao, J.; Liu, J.-P. Effects of Fibronectin 1 on Cell Proliferation, Senescence and Apoptosis of Human Glioma Cells through the PI3K/AKT Signaling Pathway. Cell. Physiol. Biochem. 2018, 48, 1382–1396. [Google Scholar] [CrossRef]
- Westin, J.R. Status of PI3K/Akt/mTOR Pathway Inhibitors in Lymphoma. Clin. Lymphoma Myeloma Leuk. 2014, 14, 335–342. [Google Scholar] [CrossRef]
- Liang, J.; Slingerland, J.M. Multiple Roles of the PI3K/PKB (Akt) Pathway in Cell Cycle Progression. Cell Cycle 2003, 2, 336–342. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Roy, T.; Uddin, M.B.; Banang-Mbeumi, S.; Chamcheu, R.-C.N.; Walker, A.L.; Liu, Y.-Y.; Huang, S. Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells 2019, 8, 803. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Żmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, A.; Steketee, J.D. Introduction. Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin’s Neuroendocrine System. Adv. Anat. Embryol. Cell Biol. 2012, 212. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Slominski, A.T. Neuroendocrinology of the skin. Dermato-endocrinology 2011, 3, 3–10. [Google Scholar] [CrossRef]
- Zbytek, B.; Mysliwski, A.; Slominski, A.; Wortsman, J.; Wei, E.T.; Mysliwska, J. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci. 2002, 70, 1013–1021. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Blazej, Z.; Tobin, D.J.; Theoharides, T.C.; Jean, R. Key Role of CRF in the Skin Stress Response System. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How ultraviolet light touches the brain and endocrine system through skin, and why. Endocrinology 2018, 159, 5. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Feingold, K.R.; Elias, P.M. Transepidermal water loss reflects permeability barrier status: Validation in human and rodent in vivo and ex vivo models. Exp. Dermatol. 2006, 15, 483–492. [Google Scholar] [CrossRef]
- Ye, J.; Garg, A.; Calhoun, C.; Feingold, K.R.; Elias, P.M.; Ghadially, R. Alterations in cytokine regulation in aged epidermis: Implications for permeability barrier homeostasis and inflammation. I. IL-1 gene family. Exp. Dermatol. 2002, 11, 209–216. [Google Scholar] [CrossRef]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Magin, T.M.; Vijayaraj, P.; Leube, R.E. Structural and regulatory functions of keratins. Exp. Cell Res. 2007, 313, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J.M. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment. Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.; Brożyna, A.A.; Janjetovic, Z.; Brooks, A.L.P.; Schwab, L.P.; Skobowiat, C.; Jóźwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1 alpha expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, G.F.; Gomaa, A.H.; Al-Dhubaibi, M.S. Highlights in pathogenesis of vitiligo. World J. Clin. Cases 2015, 3, 221–230. [Google Scholar] [CrossRef]
- Njoo, M.D.; Westerhof, W. Vitiligo. Am. J. Clin. Dermatol. 2001, 2, 167–181. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chaussade, C.; Hemmings, B.A. Evidence for functional redundancy of class IA PI3K isoforms in insulin signalling. Biochem. J. 2007, 404, 449–458. [Google Scholar] [CrossRef]
- Bozulic, L.; Hemmings, B.A. PIKKing on PKB: Regulation of PKB activity by phosphorylation. Curr. Opin. Cell Biol. 2009, 21, 256–261. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- She, Q.B.; Solit, D.B.; Ye, Q.; Reilly, K.O.; Lobo, J.; Rosen, N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 2005, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Skeen, J.; Bhaskar, P.; Chen, C.; Chen, W.; Peng, X.; Nogueira, V.; Hahn-Windgassen, A.; Kiyokawa, H.; Hay, N. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell 2006, 10, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Calnan, D.R.; Brunet, A. The FoxO code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef]
- Tia, N.; Singh, A.K.; Pandey, P.; Azad, C.S.; Chaudhary, P.; Gambhir, I.S. Role of Forkhead Box O (FOXO) transcription factor in aging and diseases. Gene 2018, 648, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef]
- Peng, X.; Xu, P.; Chen, M.; Hahn-Windgassen, A.; Skeen, J.; Jacobs, J.; Sundararajan, D.; Chen, W.; Crawford, S.; Coleman, K.; et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003, 17, 1352–1365. [Google Scholar] [CrossRef]
- Rane, M.; Pan, Y.; Singh, S.; Powell, D.; Wu, R.; Cummins, T.; Chen, Q.; Mcleish, K.; Klein, J. Heat Shock Protein 27 Controls Apoptosis by Regulating Akt Activation. J. Biol. Chem. 2003, 278, 27828–27835. [Google Scholar] [CrossRef]
- Konishi, H.; Matsuzaki, H.; Tanaka, M.; Takemura, Y.; Kuroda, S.; Ono, Y.; Kikkawa, U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997, 410, 493–498. [Google Scholar] [CrossRef]
- O’Shaughnessy, R.; Welti, J.; Cooke, J.; Avilion, A.; Monks, B.; Birnbaum, M.; Byrne, C. AKT-dependent HspB1 (Hsp27) Activity in Epidermal Differentiation. J. Biol. Chem. 2007, 282, 17297–17305. [Google Scholar] [CrossRef]
- O’Shaughnessy, R.; Welti, J.; Sully, K.; Byrne, C. AKT-dependent Pp2a activity is required for epidermal barrier formation during late embryonic development. Development 2009, 136, 3423–3431. [Google Scholar] [CrossRef] [PubMed]
- Elkenani, M.; Nyamsuren, G.; Raju, P.; Liakath-Ali, K.; Hamdaoui, A.; Kata, A.; Dressel, R.; Klonisch, T.; Watt, F.M.; Engel, W. Pelota Regulates Epidermal Differentiation by Modulating BMP and PI3K/AKT SignalingPathways. Journal of Investigative. Dermatology 2016, 136, 1664–1671. [Google Scholar]
- Shirai, K.; Hamada, Y.; Arakawa, N.; Yamazaki, A.; Tohgi, N.; Aki, R.; Mii, S.; Hoffman, R.M.; Amoh, Y. Hypoxia Enhances Differentiation of Hair Follicle-Associated-Pluripotent (HAP) Stem Cells to Cardiac-Muscle Cells. J. Cell. Biochem. 2016, 118, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiong, J.; Shao, S.; Xu, S.; Ni, H.; Wang, Y.; Ji, K. Hair Follicle Morphogenesis in the Treatment of Mouse Full-Thickness Skin Defects Using Composite Human Acellular Amniotic Membrane and Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 8281235. [Google Scholar]
- Chen, Y.; Fan, Z.; Wang, X.; Mo, M.; Zeng, S.B.; Xu, R.H.; Wang, X.; Wu, Y. PI3K/Akt signaling pathway is essential for de novo hair follicle regeneration. Stem Cell Res. Ther. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X.; et al. Macrophages induce AKT/β-catenin-dependent Lgr5+ stem cell activation and hair follicle regeneration through TNF. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef]
- Gingeras, T.R. Origin of phenotypes: Genes and transcripts. Genome Res. 2007, 17, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zheng, Y.; Ma, S.; Xing, Q.; Wang, X.; Yang, B.; Yin, G.; Guan, F. Long non-coding RNA regulates hair follicle stem cell proliferation and differentiation through PI3K/AKT signal pathway. Mol. Med. Rep. 2018, 17, 5477–5483. [Google Scholar] [CrossRef] [PubMed]
- Leonida, M.; Kumar, I. Wound Healing and Skin Regeneration; Springer: Berlin/Heidelberg, Germany, 2016; pp. 17–25. [Google Scholar]
- Doni, A.; D’Amico, G.; Morone, D.; Mantovani, A.; Garlanda, C. Humoral innate immunity at the crossroad between microbe and matrix recognition: The role of PTX3 in tissue damage. Semin. Cell Dev. Biol. 2017, 61, 31–40. [Google Scholar] [CrossRef]
- Gosain, A.; DiPietro, L. Aging and Wound Healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Berg, L.; Zijlstra-Willems, E.; Richters, C.; Ulrich, M.; Geijtenbeek, T. Dectin-1 activation induces proliferation and migration of human keratinocytes enhancing wound re-epithelialization. Cell. Immunol. 2014, 289, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Clark, R. Basics of Cutaneous Wound Repair. J. Dermatol. Surg. Oncol. 1993, 19, 693–706. [Google Scholar] [CrossRef]
- Chen, X.; Liang, J.; Chang, L.; Xia, X. miRNA-126 enhances viability, colony formation and migration of keratinocytes HaCaT cells by regulating PI3K/AKT signaling pathway. Cell Biol. Int. 2019, 43, 182–191. [Google Scholar]
- Jiang, Z.; You, Y.; Jiang, H.; Wang, Z.Z. MicroRNA-26a inhibits wound healing through decreased keratinocytes migration by regulating ITGA5 through PI3K/AKT signaling pathway. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Chen, T.; You, Y.; Jiang, H.; Wang, Z.Z. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation and tumorigenesis. J. Cell. Physiol. 2017, 232, 3261–3272. [Google Scholar] [CrossRef]
- Yin, S.Y.; Peng, A.P.; Huang, L.T.; Wang, Y.T.; Lan, C.W.; Yang, N.S. The Phytochemical Shikonin Stimulates Epithelial-Mesenchymal Transition (EMT) in Skin Wound Healing. Evidence-Based Complement. Altern. Med. 2013, 2013, 262796. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef]
- Xiao, W.; Tang, H.; Wu, M.; Liao, Y.; Li, K.; Li, L.; Xu, X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2017, 37, BSR20170658. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Depinho, R.A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010, 464, 520–528. [Google Scholar] [CrossRef]
- Watanabe, S.; Umehara, H.; Murayama, K.; Okabe, M.; Kimura, T.; Nakano, T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 2006, 25, 2697–2707. [Google Scholar] [CrossRef]
- Groszer, M.; Erickson, R.; Scripture-Adams, D.D.; Dougherty, J.D.; Le Belle, J.; Zack, J.A.; Geschwind, D.H.; Liu, X.; Kornblum, H.I.; Wu, H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc. Natl. Acad. Sci. USA 2006, 103, 111–116. [Google Scholar] [CrossRef]
- Sinor, A.D.; Lillien, L. Akt-1 Expression Level Regulates CNS Precursors. J. Neurosci. 2004, 24, 8531–8541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gregorian, C.; Nakashima, J.; Le Belle, J.; Ohab, J.; Kim, R.; Liu, A.; Smith, K.; Groszer, M.; Garcia, A.; Sofroniew, M.; et al. Pten Deletion in Adult Neural Stem/Progenitor Cells Enhances Constitutive Neurogenesis. J. Neurosci. 2009, 29, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.; McKenzie, I.; Mill, P.; Smith, K.; Akhavan, M.; Barnabé-Heider, F.; Biernaskie, J.; Junek, A.; Kobayashi, N.; Toma, J.; et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 2004, 6, 1082–1093. [Google Scholar] [CrossRef]
- Hunt, D.; Morris, P.; Sterling, J.; Anderson, J.; Joannides, A.; Jahoda, C.; Compston, A.; Chandran, S. A Highly Enriched Niche of Precursor Cells with Neuronal and Glial Potential Within the Hair Follicle Dermal Papilla of Adult Skin. Stem Cells 2008, 26, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Jinno, H.; Morozova, O.; Jones, K.; Biernaskie, J.; Paris, M.; Hosokawa, R.; Rudnicki, M.; Chai, Y.; Rossi, F.; Marra, M.; et al. Convergent Genesis of an Adult Neural Crest-Like Dermal Stem Cell from Distinct Developmental Origins. Stem Cells 2010, 28, 2027–2040. [Google Scholar] [CrossRef]
- Biernaskie, J.; Paris, M.; Morozova, O.; Fagan, B.; Marra, M.; Pevny, L.; Miller, F. SKPs Derive from Hair Follicle Precursors and Exhibit Properties of Adult Dermal Stem Cells. Cell Stem Cell 2009, 5, 610–623. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zhou, J.; Cao, Y.; Wang, F.; Duan, E. The PI3K-Akt pathway inhibits senescence and promotes self-renewal of human skin-derived precursors in vitro. Aging Cell. 2011, 10, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Zouboulis, C.C. Characteristics and Pathomechanisms of Endogenously Aged Skin. Dermatology 2007, 214, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Haas-Kogan, D.; Shalev, N.; Wong, M.; Mills, G.; Yount, G.; Stokoe, D. Protein kinase B (PKB/AKT) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol. 1998, 8, 1195–1198. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Eun-Mi, N.; Jinny, P.; Hwa-Ryung, S.; Jeong-Mi, K.; Minok, L.; Hyun-Kyung, S.; On-Yu, H.; Whang, P.H.; Myung-Kwan, H.; Kang-Beom, K. Skin Aging-Dependent Activation of the PI3K Signaling Pathway via Downregulation of PTEN Increases Intracellular ROS in Human Dermal Fibroblasts. Oxidative Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as Instigators and Victims of Oxidative Stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Yang, B.; Yang, Q.; Yang, X.; Yan, H.B.; Lu, Q.P. Hyperoside protects human primary melanocytes against H2O2-induced oxidative damage. Mol. Med. Rep. 2016, 13, 4613–4619. [Google Scholar] [CrossRef]
- Zhou, J.; Ling, J.; Song, J.; Wang, Y.; Feng, B.; Ping, F. Interleukin 10 protects primary melanocyte by activation of Stat-3 and PI3K/Akt/NF-kappa B signaling pathways. Cytokine 2016, 83, 275–281. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, W.; Guo, S.; Jian, Z.; Li, S.; Li, K.; Ge, R.; Dai, W.; Wang, G.; Gao, T.; et al. Oxidative stress-induced overexpression of miR-25: The mechanism underlying the degeneration of melanocytes in vitiligo. Cell Death Differ. 2016, 23, 496–508. [Google Scholar] [CrossRef]
- Lu, W.; Zhao, Y.; Kong, Y.; Zhang, W.; Wang, K. Geniposide prevents H2O2-induced oxidative damage in melanocytes by activating the PI3K–Akt signalling pathway. Clin. Exp. Dermatol. 2018, 43, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lin, X.; Zhi, L.; Fang, Y.; Lin, K.; Li, K.; Wu, L. Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through PTEN pathway in vitiligo. Stem Cell Res. Ther. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Richards, J.A.; Gupta, R.; Aung, P.P.; Emley, A.; Kluger, Y.; Dogra, S.K.; Mahalingam, M.; Wajapeyee, N. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 2014, 33, 4632–4642. [Google Scholar] [CrossRef]
- Wu, H.; Goel, V.; Haluska, F.G. PTEN signaling pathways in melanoma. Oncogene 2003, 22, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.P.; Leyden, J.J.; Crerand, C.E.; Sarwer, D.B.; Margolis, D.J. Body dysmorphic disorder symptoms among patients with acne vulgaris. J. Am. Acad. Dermatol. 2007, 57, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.H.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Cong, T.X.; Hao, D.; Wen, X.; Li, X.H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef]
- Fan, W.; Yanase, T.; Morinaga, H.; Okabe, T.; Nomura, M.; Daitoku, H.; Fukamizu, A.; Kato, S.; Takayanagi, R.; Nawata, H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J. Biol. Chem. 2007, 282, 7329–7338. [Google Scholar] [CrossRef]
- Yasutomi, K.; Shinji, M.; Takayoshi, S.; Fumiko, A.; Sayaka, K.; Satoshi, S.; Aki, K.; Hiroyuki, A.; Unterman, T.G.; Osamu, E. Regulation of SREBP1c gene expression in skeletal muscle: Role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology 2008, 5, 2293. [Google Scholar]
- Qiuping, M.; Wei, F.; Pengfei, L.; Nicosia, S.V.; Guido, J.; Xiaohong, Z.; Wenlong, B. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol. Endocrinol. 2009, 23, 213. [Google Scholar]
- Chen, C.; Jeon, S.; Bhaskar, P.T.; Nogueira, V.; Sundararajan, D.; Tonic, I.; Park, Y.; Hay, N. FoxOs Inhibit mTORC1 and Activate Akt by Inducing the Expression of Sestrin3 and Rictor. Dev. Cell 2010, 18, 592–604. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Im, M.; Kim, S.Y.; Sohn, K.C.; Choi, D.K.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Hwang, Y.L.; Zouboulis, C.C.; Lee, J.H. Epigallocatechin-3-gallate suppresses IGF-I-induced lipogenesis and cytokine expression in SZ95 sebocytes. J. Investig. Dermatol. 2012, 132, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.S.; Krowchuk, D.P.; Leyden, J.J.; Lucky, A.W.; Shalita, A.R.; Siegfried, E.C.; Thiboutot, D.M.; Van Voorhees, A.S.; Beutner, K.A.; Sieck, C.K.; et al. Guidelines of care for acne vulgaris management. J. Am. Acad. Dermatol. 2007, 56, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Agamia, N.F.; Roshdy, O.H.; Abdelmaksoud, R.E.; Abdalla, D.M.; Talaat, I.M.; Zaki, E.I.; El Tawdy, A.; Melnik, B.C. Effect of oral isotretinoin on the nucleo-cytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp. Dermatol. 2018, 27, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Mpione, E.C.; Mazzotta, A.; Bianchi, L.; Chimenti, S. Severe acne successfully treated with etanercept. Acta Derm. Venereol. 2006, 86, 256–257. [Google Scholar]
- Guy, R.; Kealey, T. The effects of inflammatory cytokines on the isolated human sebaceous infundibulum. J. Investig. Dermatol. 1998, 110, 410–415. [Google Scholar] [CrossRef][Green Version]
- Downie, M.; Sanders, D.; Kealey, T. Modelling the remission of individual acne lesion in vitro. Br. J. Dermatol. 2002, 147, 869–878. [Google Scholar] [CrossRef]
- Choi, J.J.; Park, M.Y.; Lee, H.J.; Yoon, D.Y.; Lim, Y.; Hyun, J.W.; Zouboulis, C.C.; Jin, M. TNF-α increases lipogenesis via JNK and PI3K/Akt pathways in SZ95 human sebocytes. J. Dermatol. Sci. 2012, 65, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Nograles, E.K.; Davidovici, B.; Krueger, J.G. New insights in the immunologic basis of psoriasis. Semin. Cutan. Med. Surg. 2010, 29, 3–9. [Google Scholar] [CrossRef]
- Hong, K.K.; Gwak, M.J.; Song, J.; Kim, N.I. Nuclear factor-κB pathway activation and phosphatase and tensin homolog downregulation in psoriasis. Br. J. Dermatol. 2016, 174, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.Y.; Pearline, R.V.; Grueneberg, D.; Hsia, M.; Ravichandran, P.; Kothari, N.; Natesan, S.; Brugge, J.S. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial–mesenchymal transition. J. Cell Biol. 2005, 171, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Murayama, K.; Kimura, T.; Tarutani, M.; Tomooka, M.; Hayashi, R.; Okabe, M.; Nishida, K.; Itami, S.; Katayama, I.; Nakano, T. Akt activation induces epidermal hyperplasia and proliferation of epidermal progenitors. Oncogene 2007, 26, 4882–4888. [Google Scholar] [CrossRef]
- Balato, A.; Lembo, S.; Ayala, F.; Balato, N.; Caiazzo, G.; Raimondo, A.; Caprio, R.D.; Monfrecola, G. Mechanistic target of rapamycin complex 1 is involved in psoriasis and regulated by anti-TNF-α treatment. Exp. Dermatol. 2017, 26, 325–327. [Google Scholar] [CrossRef]
- Buerger, C.; Malisiewicz, B.; Eiser, A.; Hardt, K.; Boehncke, W.H. Mammalian target of rapamycin and its downstream signalling components are activated in psoriatic skin. Br. J. Dermatol. 2013, 169, 156–159. [Google Scholar] [CrossRef]
- Shirsath, N.; Mayer, G.; Singh, T.P.; Wolf, P. 8-methoxypsoralen plus UVA (PUVA) therapy normalizes signalling of phosphorylated component of mTOR pathway in psoriatic skin of K5.hTGF beta 1 transgenic mice. Exp. Dermatol. 2015, 24, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Buerger, C.; Shirsath, N.; Lang, V. Inflammation dependent mTORC1 signaling interferes with the switch from keratinocyte proliferation to differentiation. PLoS ONE 2017, 12, e0180853. [Google Scholar] [CrossRef]
- Mitra, A.D.; Raychaudhuri, S.P.; Abria, C.J.; Mitra, A.; A Wright, R.; Ray, R.; Kundu-Raychaudhuri, S. 1α,25-Dihydroxyvitamin-D3-3-Bromoacetate Regulates AKT/mTOR Signaling Cascades: A Therapeutic Agent for Psoriasis. J. Investig. Dermatol. 2013, 133, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Raychaudhuri, S.K.; Raychaudhuri, S.P. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine 2012, 60, 38–42. [Google Scholar] [CrossRef]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Shin, D.M.; Yuk, J.M.; Shi, G.; Choi, D.K.; Lee, S.H.; Huang, S.M.; Kim, J.M.; Kim, C.D.; Lee, J.-H.; et al. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J. Immunol. 2010, 186, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, X.; Meng, X.; Lin, M. Phosphoinositide-3 Kinase/Protein Kinase-B/Mammalian Target of Rapamycin Pathway in Psoriasis Pathogenesis. A Potential Therapeutic Target? Acta Derm. Venereol. 2014, 94, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, W.; Chen, S. Comparison of gene expression profiles reveals aberrant expression of FOXO1, Aurora A/B and EZH2 in lesional psoriatic skins. Mol. Biol. Rep. 2010, 38, 4219–4224. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.N.; Xue, R.Z.; Luo, G.P. The expressions of p-Akt and p-FoxO1 in the lesions of psoriasis vulgaris. China J. Lepr. Skin Dis. 2014, 30, 737–739. [Google Scholar]
- Zhang, M.; Zhang, X. The role of PI3K/AKT/FOXO signaling in psoriasis. Arch. Dermatol. Res. 2019, 311, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bürger, C.; Shirsath, N.; Lang, V.; Diehl, S.; Wolf, P. Blocking mTOR Signalling with Rapamycin Ameliorates Imiquimod-induced Psoriasis in Mice. Investig. Dermatol. 2016, 136, S221. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Satyshur, K.A.; Syed, D.N.; Dodwad, S.; Chaves-Rodriquez, M.I.; Longley, B.J. Dual Inhibition of PI3K/Akt and mTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features In Vitro and in an Imiquimod-Induced Psoriasis-Like Disease in Mice. Antioxidants Redox Signal. 2017, 26, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Pal, H.C.; Siddiqui, I.A.; Adhami, V.M.; Ayehunie, S.; Boylan, B.T.; Noubissi, F.K.; Khan, N.; Syed, D.N.; Elmets, C.A. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Ski. Pharmacol. Physiol. 2015, 28, 177–188. [Google Scholar] [CrossRef]
- Yue, L.; Ailin, W.; Jinwei, Z.; Leng, L.; Jianan, W.; Li, L.; Haiming, C.; Ling, H.; Chuanjian, L. PSORI-CM02 ameliorates psoriasis in vivo and in vitro by inducing autophagy via inhibition of the PI3K/Akt/mTOR pathway. Phytomedicine 2019, 64, 153054. [Google Scholar] [CrossRef]
- Li, Q.; Huang, H.; He, Z.; Sun, Y.; Tang, Y.; Shang, X.; Wang, C. Regulatory effects of antitumor agent matrine on FOXO and PI3K-AKT pathway in castration-resistant prostate cancer cells. Sci. China Life Sci. 2017, 61, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Imai-Sumida, M.; Chiyomaru, T.; Majid, S.; Saini, S.; Nip, H.; Dahiya, R.; Tanaka, Y.; Yamamura, S. Silibinin suppresses bladder cancer through down-regulation of actin cytoskeleton and PI3K/Akt signaling pathways. Oncotarget 2017, 8, 92032–92042. [Google Scholar] [CrossRef]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.Q.; Xu, P.H.; Deng, H.; Chen, L.S.; Ding, X.A.; Bai, M.; Li, P.; Yuan, D.F. The characterization and clinical significance of PI3K/Akt signaling pathway activation in the peripheral T cells of pediatric patients with atopic dermatitis. Int. J. Clin. Exp. Med. 2017, 10, 2904–2910. [Google Scholar]
- Moriya, C.; Jinnin, M.; Yamane, K.; Maruo, K.; Muchemwa, F.C.; Igata, T.; Makino, T.; Fukushima, S.; Ihn, H. Expression of matrix metalloproteinase-13 is controlled by IL-13 via PI3K/Akt3 and PKC-δ in normal human dermal fibroblasts. J. Investig. Dermatol. 2011, 131, 655. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Mitoma, C.; Mitoma, H.; Tsuji, G.; Chiba, T.; Nakahara, T.; Uchi, H.; Kadono, T. Pathogenesis of systemic sclerosis—current concept and emerging treatments. Immunol. Res. 2017, 65, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Singh, A.R.; Durden, D.L. MDM2 Regulates Hypoxic Hypoxia-inducible Factor 1α Stability in an E3 Ligase, Proteasome, and PTEN-Phosphatidylinositol 3-Kinase-AKT-dependent Manner. J. Biol. Chem. 2014, 289, 22785–22797. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C.; Lu, J.; Zhu, L.; Li, M. 2-Methoxyestradiol inhibits hypoxia-induced scleroderma fibroblast collagen synthesis by phosphatidylinositol 3-kinase/Akt/mTOR signalling. Rheumatology 2018, 57, 1675–1684. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 553–562. [Google Scholar] [CrossRef]
- Makino, K.; Makino, T.; Stawski, L. Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis Res. 2017, 19, 134. [Google Scholar] [CrossRef]
- Zhu, L.; Song, Y.; Li, M. 2-Methoxyestradiol inhibits bleomycin-induced systemic sclerosis through suppression of fibroblast activation. J. Dermatol. Sci. 2015, 77, 63–70. [Google Scholar] [CrossRef]
- Kudo, A. Periostin in fibrillogenesis for tissue regeneration: Periostin actions inside and outside the cell. Cell. Mol. Life Sci. 2011, 68, 3201–3207. [Google Scholar] [CrossRef]
- Yang, L.; Serada, S.; Fujimoto, M.; Terao, M.; Kotobuki, Y.; Kitaba, S.; Matsui, S.; Kudo, A.; Naka, T.; Murota, H.; et al. Periostin Facilitates Skin Sclerosis via PI3K/Akt Dependent Mechanism in a Mouse Model of Scleroderma. PLoS ONE 2012, 7, e41994. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Wu, M.; Ren, Y.; Luo, X.; Hu, W.; Zhang, Q.; Wu, Y. Treatment of keloids through Runx2 siRNA-induced inhibition of the PI3K/AKT signaling pathway. Mol. Med. Rep. 2020, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wu, X.; Yang, B.; Yao, X.; Cui, X.; Xu, P.; Chen, X. miR-188-5p regulates proliferation and invasion via PI3K/Akt/MMP-2/9 signaling in keloids. Acta Biochim. Biophys. Sin. 2019, 51, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Prashiela, M.; Nada, E.; Orlow, S.J. Recent advances in understanding vitiligo. F1000research 2016, 5, 2234. [Google Scholar]
- Zhang, Y.; Liu, L.; Jin, L.; Yi, X.; Dang, E.; Yang, Y.; Li, C.; Gao, T. Oxidative Stress–Induced Calreticulin Expression and Translocation: New Insights into the Destruction of Melanocytes. J. Investig. Dermatol. 2014, 134, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Z. PI3K/Akt-Nrf2 and Anti-Inflammation Effect of Macrolides in Chronic Obstructive Pulmonary Disease. Curr. Drug Metab. 2019, 20, 301–304. [Google Scholar]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, C.S.; Lee, A. Reduced Nrf2 activation in PI3K phosphorylation-impaired vitiliginous keratinocytes increases susceptibility to ROS-generating chemical-induced apoptosis. Environ. Toxicol. 2017, 32, 2481–2491. [Google Scholar] [CrossRef]
- Wan, J.; Lin, F.; Zhang, W.; Xu, A.; Degiorgis, J.; Lu, H.; Wan, Y. Novel approaches to vitiligo treatment via modulation of mTOR and NF-κB pathways in human skin melanocytes. Int. J. Biol. Sci. 2017, 13, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Rebora, A. Pathogenesis of androgenetic alopecia. J. Am. Acad. Dermatol. 2004, 50, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Ashrafuzzaman, M.; Yamamoto, T.; Shibata, N.; Hirayama, T.; Kobayashi, M. Potential Involvement of the Stem Cell Factor Receptor c-kit in Alopecia Areata and Androgenetic Alopecia: Histopathological, Immunohistochemical, and Semiquantitative Investigations. Acta Histochem. Cytochem. 2010, 43, 9–17. [Google Scholar] [CrossRef]
- Hoang, P.M.; Keady, M.; Mahalingam, M. Stem cell markers (cytokeratin 15, CD34 and nestin) in primary scarring and nonscarring alopecia. Br. J. Dermatol. 2009, 160, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Garza, L.; Yang, C.; Zhao, T.; Blatt, H.; Lee, M.; He, H.; Stanton, D.; Carrasco, L.; Spiegel, J.; Tobias, J.; et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J. Clin. Investig. 2011, 121, 613–622. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Ma, T.; Liu, Q. Vascular Endothelial Growth Factor Protects CD200-Rich and CD34-Positive Hair Follicle Stem Cells Against Androgen-Induced Apoptosis Through the Phosphoinositide 3-Kinase/Akt Pathway in Patients With Androgenic Alopecia. Dermatol. Surg. 2020, 46, 358–368. [Google Scholar] [CrossRef]

| Non-Malignant Skin Disorder | PI3K/Akt Pathway-Related Cytokines or Proteins |
|---|---|
| Acne | IGF-1, FoxO1, mTORC1, SREBP-1c, AMPK, TNF-a |
| Psoriasis | PTEN, IL-12, IL-17, mTOR, FOXO |
| Atopic dermatitis | IL-6, IL-10, IL-13, MMP-13 |
| Scleroderma | Periostin, HIF-1a, CTGF and collagen I |
| Keloid | MMP-2, MMP-9 |
| Vitiligo | PTEN, Nrf2, mTORC1 |
| Androgenic alopecia | VEGF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells 2021, 10, 1219. https://doi.org/10.3390/cells10051219
Teng Y, Fan Y, Ma J, Lu W, Liu N, Chen Y, Pan W, Tao X. The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells. 2021; 10(5):1219. https://doi.org/10.3390/cells10051219
Chicago/Turabian StyleTeng, Yan, Yibin Fan, Jingwen Ma, Wei Lu, Na Liu, Yingfang Chen, Weili Pan, and Xiaohua Tao. 2021. "The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders" Cells 10, no. 5: 1219. https://doi.org/10.3390/cells10051219
APA StyleTeng, Y., Fan, Y., Ma, J., Lu, W., Liu, N., Chen, Y., Pan, W., & Tao, X. (2021). The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells, 10(5), 1219. https://doi.org/10.3390/cells10051219





